Abstract
Lewy body diseases (LBD) comprise a group of complex neurodegenerative conditions originating from accumulation of misfolded alpha-synuclein (α-syn) in the form of Lewy bodies. LBD pathologies are characterized by α-syn deposition in association with other proteins such as Amyloid β (Aβ), Tau, and TAR-DNA-binding protein. To investigate the complex interactions of these proteins, we constructed 2 novel transgenic overexpressing (OE) C. elegans strains (α-synA53T;Taupro-agg (OE) and α-synA53T;Aβ1-42;Taupro-agg (OE)) and compared them with previously established Parkinson’s, Alzheimer’s, and Lewy Body Dementia disease models. The LBD models presented here demonstrate impairments including uncoordinated movement, egg-laying deficits, altered serotonergic and cholinergic signaling, memory and posture deficits, as well as dopaminergic neuron damage and loss. Expression levels of total and prone to aggregation α-syn protein were increased in α-synA53T;Aβ1-42 but decreased in α-synA53T;Taupro-agg animals when compared to α-synA53T animals suggesting protein interactions. These alterations were also observed at the mRNA level suggesting a pre-transcriptional mechanism. miRNA-seq revealed that cel-miR-1018 was upregulated in LBD models α-synA53T, α-synA53T;Aβ1-42, and α-synA53T;Taupro-agg compared with WT. cel-miR-58c was upregulated in α-synA53T;Taupro-agg but downregulated in α-synA53T and α-synA53T;Aβ1-42 compared with WT. cel-miR-41-3p and cel-miR-355-5p were significantly downregulated in 3 LBD models. Our results obtained in a model organism provide evidence of interactions between different pathological proteins and alterations in specific miRNAs that may further exacerbate or ameliorate LBD pathology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lewy body diseases (LBD) are neurodegenerative conditions caused by accumulation of misfolded alpha-synuclein protein (α-syn) that form Lewy bodies in the central nervous system [1]. LBD can be divided into at least 3 clinicopathologic entities: Parkinson’s disease (PD), Parkinson’s disease dementia (PDD), and Dementia with Lewy bodies (DLB) based on the clinical symptoms and varying distribution of Lewy bodies. PD is the most common neurodegenerative movement disorder with central features including motor symptoms caused by neuronal loss in the substantia nigra and striatum. PD can be characterized by tremors, stiffness, bradykinesia, and neuropsychiatric symptoms including autonomic dysfunction and sleep disturbances. It is estimated that about 80% of PD patients eventually develop to PDD [2]. In PDD and DLB, Lewy bodies form in the cytoplast of cortical neurons and are characterized by cognitive impairment with cognitive fluctuation and parkinsonism. DLB and PDD are distinguishable based on the order and time interval of dementia and motor symptoms. Specifically, DLB is defined as patients with dementia before motor symptoms or dementia occurring within one year after motor symptoms. PDD is defined as patients who do not develop dementia until one year after motor symptoms appear. Furthermore, PDD with severe motor symptoms show neuronal loss in the substantia nigra which is similar to PD. However, 25% of patients with DLB do not present any motor symptoms and this subset shows preserved neurons in the substantia nigra [3].
The major component of Lewy bodies is α-syn, a 14.5 kDa natively unfolded protein whose monomers can aggregate to form soluble prefibrillar intermediate oligomers that convert to highly ordered β-sheet fibrils [4]. In patients with LBD, the ratio of α-syn tetramers-oligomers decreases [5]; subsequently, α-syn monomers bind to vesicle and lipid membranes to promote the aggregation of α-syn [6]. The Lewy body is a complex inclusion that consists of α-syn and > 90 identified components including organelles such as impaired mitochondria, lysosomes, and vesicles [7]. Subsets of LBD are related by links with several proteins like α-syn, Amyloid β (Aβ), Tau, as well as TAR-DNA-binding protein 43 (TDP-43). In the cases with dementia, 19.2% of patients displayed quadruple misfolded proteins including α-syn, Aβ, Tau and TDP-43 whereas 20.6% of patients presented with misfolded α-syn, Aβ, and Tau [8]. In the participants without clinical symptoms of dementia the following was observed: 12% presented the misfolded α-syn and Aβ; 11% of patients presented the misfolded α-syn and Tau; 3% of patients presented the misfolded α-syn and TDP-43; more than 19% of patients presented the misfolded α-syn, Aβ and Tau; and 2% of patients presented with α-syn, Tau, and TDP-43 or α-syn, Aβ, and TDP-43 [9]. Aβ plaques were observed in 50% of patients with DLB [10].Tau aggregation is observed in 25% cases of DLB [11] and the co-aggregation of α-syn and Tau were found in the same tangles [12]. Aβ can interact with α-syn and then induce the aggregation of α-syn which can lead to neurodegeneration [13]. α-Syn aggregation also induces disassembly of microtubules and causes the aggregation of Tau protein [14]. Moreover, TDP-43 frequently co-occurs with LBD. More than 30% of patients with DLB [15], 7.2% of PD patients and 19% of patients with PDD [16] presented TDP-43 pathology with co-pathology of α-syn and TDP-43 inducing a more severe neurodegeneration in PD [17]. Thus, the pathologies of PD, PDD and DLB and AD overlap [18].
Caenorhabditis elegans (C. elegans) is an invertebrate model organism utilized in the research of neurodegenerative diseases due to the high conservation of basic neuronal functions and many homologous pathologic PD genes, although not SNCA (α-syn). Previous studies by us and others have shown the ability of overexpressing transgenic animals to recapitulate some features of PD [19,20,21,22]. These transgenic models have been invaluable in understanding the molecular basis for many of the processes involved in neurodegeneration. One of the genetic effectors of neurodegenerative diseases are miRNAs, which are a type of non-coding RNA with a length of 19–24 nucleotides. MicroRNA is involved in the occurrence of Lewy body disease and have even been proposed as biomarkers or for therapy [23,24,25,26,27,28,29]. miRNA can affect PD and AD by binding to pathological proteins directly, interfering with the production, modification, aggregation, and degradation of pathological proteins as well [30, 31]. Nonetheless, the role of miRNAs in PDD and DLB remain largely unexplored.
In order to further understand the influence of miRNA in LBD, and to gain insight into protein interactions, we constructed 2 novel LBD models based on the pathological proteins (α-synA53T;Taupro-agg (OE) and α-synA53T;Aβ1-42; Taupro-agg (OE)) in C. elegans. We also utilized our 3 previously published LBD models (α-synA53T (OE), α-synA53T;Aβ1-42 (OE), and α-synA53T;TDP-43(OE)). We studied the molecular neuropathology and behavior of the different LBD models and observed interactions between α-synA53T with Aβ1-42 and Taupro-agg. We also observed dysregulation of miRNAs in LBD models including α-synA53T, α-synA53T;Aβ1-42, and α-synA53T;Taupro-agg. In the present work, we constructed and analyzed LBD model animals to provide insight into the disease pathology. Our findings suggest an intimate connection between the multiple proteins involved in LBD and suggests a complex dysregulation at the miRNA level.
Methods
C. elegans strains and maintenance
All strains of C. elegans were maintained on nematode growth medium (NGM) plates inoculated with Escherichia coli (E. coli) OP50 at 16°C unless otherwise indicated. Upon reaching the L4 developmental stage which were marked as day 0 stage of C. elegans in this study, the worms were transferred to an elevated temperature of 23°C to induce the expression of the Aβ protein and to conduct subsequent experiments. All experiments were carried out under conditions ensuring adequate supply of E. coli OP50. Strains N2, CB1111, CB1112, CL2355, BR5270, CL6049 were obtained from the Caenorhabditis Genetics Center (CGC). UM0001, UM0010, UM0017, UM0022 were previously published as indicated in Table 1.
Genetic crosses
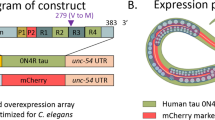
N2 males were mated with UM0010 Is[aex-3::α-synA53T + dat-1::GFP] hermaphrodites to produce males carrying aex-3::α-syn(A53T). The male worms carrying the transgene were further mated with UM0001 Is[snb-1p::Aβ1-42 + mtl-2p::GFP; rab-3p::F3(delta)K280 + myo- 2p::mCherry] and BR5270 byIs[rab-3p::F3(delta)K280 + myo-2p::mCherry] hermaphrodites. BR5270 is an integrated transgenic strain overexpressing a pro-aggregating Tau fragment. The Tau pro-aggregating fragment comprises amino acids 258–360 of the full-length tau with a K280 mutation [32]. K280 has been previously found in FTLD patients [33]. When expressed, this fragment with the mutation promotes the aggregation of wildtype and mutant Tau and thus is considered highly toxic [34, 35]. The offspring that contained all three markers including human Aβ peptide; human pro-aggregating tau and human α-synA53T were allowed to self-fertilize to homozygosity and selected under fluorescence microscopy. The genotypes of target worms were then verified by single-worm PCR (Figure S1).Markers of transgenic strains are shown in Figure S2. The primers are as follows: human α-syn: 5′-GGTGTTCTCTATGTAGGCTCCA, 3′-TAGGCTTCAGGTTCGTAGTCTT; human Aβ peptide (APP): 5′-CCGACATGACTCAGGATATG, 3′-GCTCACGCTATGACAACA; and human Tau (MAPT): 5′-CTCCACTGAGAACCTGAAG, 3′-GCCTAATGAGCCACACTT.
Body bend assay
The number of body bends in 30 s were observed in adult day 1 and day 5 stage animals under a dissecting microscope. The number of body bends were only counted when head oscillation of worms was over half of the worms’ body. Thirty worms were counted per experiment and each experiment was performed in triplicate.
Thrashing assay
Worms developed to adult day 1 and day 5 stage were randomly retrieved from the M9 buffer and subjected to recuperation for a duration of 30 s, following which the body bending frequency within that period was assayed. Thirty animals were counted per experiment, and each experiment was performed in triplicate.
Developmental stages assay
C. elegans were subjected to agitation in M9 buffer for a continuous 20 h period after synchronization which ensured all worms were in the same L1 stage and subsequently transferred to fresh NGM at a temperature of 20°C. The developmental phases of C. elegans were identified based on the progression of the worms’ ecdysis. The time of transfer to the NGM dish was designated as 0 h, and the development phases of worms were counted for 26 h with over 200 animals evaluated per interval. The counting of the worms’ developmental progression was continued every 6 h after 40 h until all the individuals had reached the adult stages.
Locomotion assay
Worms that developed to day 1 stage were transferred to 23°C until adult stage of day 1. Approximately 30 worms were randomly selected to the center of 35 mm plates seeded with 20 μL E. coli OP50. After 5 min of acclimation, the number of worms that had moved away from E. coli OP50 were documented every 5 min at room temperature, and the assay was conducted for 1 h. Each experiment was performed in 5 replicates.
Tracking of C. elegans
A single animal developed to day 1 adult stage was picked in a 13 × 13 mm2 circle boundary shielded with Vaseline and inoculated with E. coli OP50. The locomotive progress of the organism for 1 h under room temperature was monitored using a camera (Tiannuoxiang, China). The tracks and movements of the organism were then measured using Tracker software (https://physlets.org/tracker). Each experiment was conducted in triplicate with 10 worms per trial.
Chemotaxis assay
Animals developed to day 1 adult stage were washed gently with M9 three times. Approximately 2000 animals were chosen for the training experiment, transferred to fresh NGM with 3 circles having a diameter of 14 mm, and left to train for 1 h at room temperature. For the 3 circles, the first served as the starting point, the second was administered 1 μL 1 M sodium azide and 2 μL 100% EtOH, and the last received 1 μL 1 M sodium azide and 2 μL 10% butanone. Approximately 3000 animals were starved in M9 for 1 h at room temperature. Thereafter, these animals were transferred to fresh NGM with E. coli OP50, as well as 2 μL 10% butanone, for 1 h at room temperature. Following this, the animals were washed 3 times gently in M9 and transferred to the fresh NGM with the aforementioned three circles and trained for 1 h at room temperature. The number of nematodes in different areas including 3 circles and other area of plates were counted to determine the learning index of animals using the formula \(\frac{{\# {\text{Attractant}} - \# {\text{Control}}}}{{\# {\text{Total}}}}\).
Basal slowing response
Animals developed to day 1 adult stage were washed twice gently in M9 and then transferred to E. coil OP50 seeded NGM plates. Residual M9 was removed by wicking action using a paper napkin and the locomotion of worms was recorded by camera (Tiannuoxiang, China). The locomotion of animals both inside and outside the E. coil OP50 lawn for 30 s was counted. Each experiment was conducted in triplicate and 30 worms were counted in each experiment.
Dopaminergic neuron impairment assay
C. elegans UM0010 Is[aex-3::α-synA53T + dat-1::GFP], UMH6 Is[snb-1p::Aβ1-42 + mtl-2p::GFP; aex-3::α-synA53T + dat-1::GFP], UM0017 Is[snb-1::TDP-43 + mtl-2::GFP; aex-3::α-synA53T + dat-1::GFP], UMR1 Is[snb-1p::Aβ1-42 + mtl-2p::GFP; rab-3p::F3(delta)K280 + myo-2p::mCherry; aex-3::α- syn(A53T)], and UMR3 Is[rab-3p::F3(delta)K280 + myo-2p::mCherry; aex-3::α-synA53T + dat-1::GFP] developed to day 5 stage were picked onto an agarose pad and anesthetized with 15 mM NaN3. Micrographs were taken at 400× magnification with a Zeiss confocal microscope LSM710. The images were analyzed using ZEN imaging software (Zeiss, 8.1.0.484). Each experiment consisted of at least 30 animals and was performed in 3 independent replicates.
Viability assay
Hermaphrodite animals at adult stage day 2 were transferred from plates into 96-well plates with two animals added to each well. One-hundred μL of 5 mg/mL serotonin, or 10 mg/mL levamisole, or M9 buffer was pipetted to each well. The number of eggs in each well was counted using a stereomicroscope (Nikon, TLBD4.1) after a treatment period of 1 h. Forty-eight worms were assessed per experiment, and each experiment was conducted in triplicate.
Worm bagging assay
Worms bagging refers to the phenomenon wherein nematodes are unable to deposit their eggs externally, which results in the hatching of larvae within the hermaphrodite body. Animals developed to day 5 adult stage under 23°C and 20°C were observed under a fluorescence microscope (Nikon, SMZ18), and the hermaphrodite worms whose eggs hatched internally due to dystocia were recorded. Each trial was undertaken in triplicate with 100 animals examined per trial.
Lifespan assay
The worms developed to the L4 stage at 16°C were marked as day 0. Upon transfer to a temperature of 23°C for activation, the mortality of the worms was monitored daily until all animals had perished. During this period, the worms were provided with fresh NGM plates at regular intervals of two days. The entire life cycle was carried out at 23°C and with sufficient food. Any worm that failed to respond to mechanical stimulation via platinum wire was recorded as deceased. Worms that perished due to stress-induced factors, such as climbing to the edge of the NGM plates or internal deformation were censored. At least 60 worms were counted for every experiment, and at least 3 experimental replicates were performed for each experiment.
Western Blotting
For SDS-PAGE western blotting, the worms developed to day 1 adult stage were collected and dissolved in Tris-Urea-SDS buffer (1 × TBS, 5% SDS, 8 M Urea, 50 mM dithiothreitol), which was sonicated using the Bioruptor® Plus sonication device. Then the suspension was collected and stored at –81°C. About 20 μg of proteins were loaded onto 12% SDS–polyacrylamide gel, which was blotted onto PVDF membranes (Bio-Rad). These membranes were then blocked for 1 h at room temperature. Subsequently, primary antibodies including anti-α-syn (1:1000, Thermo/PA5-85,343) and anti-β-actin (1:1000, Santa Cruz/sc-47778) were incubated overnight at 4°C. Peroxidase AffiniPure Goat Anti-Rabbit IgG (1:5000, Jackson ImmunoResearch/111035144) and Peroxidase AffiniPure Goat Anti-Mouse IgG (1:5000, Jackson ImmunoResearch/115035146) secondary antibodies were used for detection and incubated for 1 h at room temperature. Finally, the proteins were visualized using the Clarity Western ECL Substrate kit (Bio-Rad) and imaged using ChemiDoc™ MP imaging system (Bio-Rad).
For Native-PAGE western blotting, the collected worms at day 1 adult stage were then dissolved using RAB buffer (750 mM NaCl, 100 mM MES, 1 mM EGTA, 0.5 mM MgSO4, 20 mM NaF, 1 mM PMSF, 10 mM Protease Inhibitor cocktail), and the resulting suspension was subjected to grinding through pellet pestles and stored under –81°C. Twenty μg of proteins were loaded onto 12% Native-PAGE gel (12% Acrylamide/Bis, 0.375 M Tris with pH 8.8, 0.05% APS, and 5% TEMED for resolving; 4% Acrylamide/Bis, 126 mM Tris with pH 6.8, 0.05% APS, and 10% TEMED for stacking), which was later blotted onto PVDF membranes (Bio-Rad). Membranes with proteins were blocked 1 h at room temperature. Subsequently, primary antibodies including anti-α-syn (1:1000, Thermo/PA5-85,343) and anti-β-actin (1:1000, Santa Cruz/sc-47778) were incubated overnight at 4°C. Then, Peroxidase AffiniPure Goat Anti-Rabbit IgG (1:5000, Jackson ImmunoResearch/111035144) and Peroxidase AffiniPure Goat Anti-Mouse IgG (1:5000, Jackson ImmunoResearch/115035146) secondary antibodies were used to incubate for 1 h at room temperature. Finally, the proteins were visualized using the Clarity Western ECL Substrate kit (Bio-Rad) and imaged using ChemiDocTM MP imaging system (Bio-Rad).
RNA-sequencing
For Small RNA-sequencing, worms at day 1 adult stage were rinsed 3 times with M9 buffer and stored in Trizol at –81°C. RNA was precipitated using isopropanol following the sample extraction via chloroform. The RNA was washed with ethanol and dissolved in water. Ethanol-washed RNA was dried before being dissolved in nuclease-free water with ribonuclease inhibitor. NanoDrop was utilized for assessing the concentration and quality of isolated RNA. RNA samples were considered qualified with threshold OD260/OD280 ≥ 1.95 and concentration ≥ 17 ng/μL. RNA integrity was visualized with 1.2% gel electrophoresis, and RNA samples without degradation were considered acceptable. Three replicates were performed for each strain of C. elegans. NEBNext® Small RNA Library Prep Set for Illumina® (Biolab) was used to construct Small RNA libraries. microRNAs (~140 bp) were screened by running and cutting fragments from a 6% polyacrylamide gel and Agilent 2100 Bioanalyzer was used to check the cDNA of each sample for size, purity, and concentration. Single-end sequencing of qualified samples was performed at the HisqSE50 platform by Novogene.
For RNA-sequencing, worms at day 1 adult stage were rinsed 3 times with M9 buffer and stored in Trizol at –81°C. NanoDrop was applied to measure the concentration and quality of isolated, and RNA samples were considered qualified with threshold OD260/OD280 ≥ 2.0 and concentration ≥ 20 ng/μL. At the same time, the integrity of RNA was examined by 1.2% gel electrophoresis, and RNA samples without degradation were considered accepted. Three replicates were performed for each strain of C. elegans. NEBNext Ultra RNA Library Prep Kit for Illumina (Biolab) was employed to construct RNA library. mRNA was isolated utilizing Agencourt AMPure XP Beads, and purity and concentration of the cDNA of each sample were measured through the Agilent 2100 Bioanalyzer. Finally, paired-end sequencing was performed on qualified samples utilizing the Novaseq 6000 PE150 platform by Novogene.
Bioinformatics analysis
The raw data obtained from Novogene and internal core laboratory (Table S1) was preprocessed by trimmomatic and trim_galore and the clean data was checked by fastqc. Subsequently, all the clean data was aligned with the genome of C. elegans (PRJNA13758) and Homo sapiens (GRCh38.p14) by bowtie and hisat2. Samtools was employed to generate alignment files. Counts of every gene and miRNAs were calculated by feature count based on the annotation files of PRJNA13758 and GRCh38.p14. The differential expressed genes (DEGs) and miRNAs (DE-miRNAs) were obtained by DESeq2 and visualized by R package ggplot2 and pheatmap. Package VennDiagram was employed in R to finely visualize the Venn diagrams of DE-miRNAs. The batch effect of samples were checked by R package factoextra and FactoMineR and then were corrected by R package sva. The counts of genes and miRNAs were normalized based on the definitions of Fragments Per Kilobase of transcript per Million mapped reads (FPKM) and Count Per Million (CPM) by R. The miRTarBase database and TargetScanWorm 6.2 were utilized to identify the target genes of DE-miRNAs. The targets which were DEGs of DE-miRNAs were selected and the targets-miRNA networks were visualized by Cytoscape 3.10.0. The GO functions were conducted by DAVID database and the enrichment analysis of Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway were conducted by GSEA 4.3.2. Sequencing data for this study are available from NCBI SRA database under the project accession number PRJNA1070381.
Real-Time Quantitative Reverse Transcription PCR
Worms at day 1 adult stage were rinsed 3 times with M9 buffer and stored in Trizol at -81℃. RNA was isolated and NanoDrop was utilized to detect the concentration and assess the quality of RNA. RNA samples were considered qualified with threshold OD260/OD280 ≥ 2.0. The reverse transcription was performed by High-Capacity cDNA Reverse Transcription Kit (Thermo, 4,368,813). Power SYBR Green PCR Master Mix (Applied Biosystems, 4,309,155) and LifeTech ABI7500 Fast Realtime PCR system were employed to perform the qRT-PCR and relative expression levels of mRNA was calculated by 2−ΔΔCt and normalized to the expression of gene cdc-42. The list of primers are in Table S2.
Mitochondrial mass measurement
Worms developed to adult day 1 were transferred to plates with 5 μM MitoTracker™ Green (Thermo/ M7514) which was diluted by 1 mM MitoTracker™ stock solution (dissolved in dimethyl sulfoxide) and kept for 24 h at 23°C in the dark. Worms were then transferred to clean plates with no MitoTracker™ Green and allowed to crawl for 1 h. Animals were picked onto an agarose pad and anesthetized with 15 mM NaN3. Micrographs were taken at 630× magnification with a Zeiss confocal microscope LSM710. The images were analyzed using ZEN imaging software (Zeiss, 8.1.0.484) and Image J. Each experiment consisted of 20 animals and was performed in 3 independent replicates.
Statistical analysis
Data were analyzed and visualized by GraphPad Prism, SPSS Statistics 22 and R and presented as mean ± SEM in this study. Differences between two groups were evaluated by Student’s t-test, while more than two groups were tested by one-way ANOVA with Tukey post-hoc. Differences were considered significant when the adjusted P value < 0.05. The mean lifespan of different strains was analyzed via Kaplan–Meier and finally visualized by OriginPro 8.
Results
LBD models show phenotypic defects in C. elegans
To obtain LBD models, we crossed α-synA53T with Taupro-agg, and with Aβ1-42; Taupro-agg, then screened homozygotes according to the different markers corresponding to different proteins. The marker of Aβ1-42 is GFP in intestines (Figure S2B). The marker of Taupro-agg is mCherry in pharyngeal muscles (Figure S2D). The marker of α-synA53T is GFP in dopaminergic neurons (Figure S2E). Finally, the genotypes of homozygotes were confirmed by single-worm PCR, and the results are shown in Figure S1. In addition, we also referenced the LBD models constructed within our laboratory, which included α-synA53T[19], α-synA53T;Aβ1-42[38], and α-synA53T;TDP-43[39]. Both α-synA53T;Aβ1-42 and α-synA53T;TDP-43 contains GFP in dopaminergic neurons and intestines (Figure S2F-G). For convenience, we divided the C. elegans strains used in this study into three main categories: wild type (WT), control group (which included Aβ1-42, TDP-43, Taupro-agg and Aβ1-42;Taupro-agg), and LBD group (which included α-synA53T, α-synA53T;Aβ1-42, α-synA53T;TDP-43, α-synA53T;Taupro-agg, α-synA53T;Aβ1-42;Taupro-agg).
To determine whether over-expression of exogenous proteins could affect the phenotypes of worms, the different ages postures of WT and all transgenic strains were observed. For WT and control group at day 1 stage, the postures of WT, strains overexpressing Taupro-agg, Aβ1-42;Taupro-agg and α-synA53T;Taupro-agg were normal (Fig. 1A). Worms that overexpressed Aβ1-42 and TDP-43 exhibited nontypical postures (Fig. 1B–C). Regarding the LBD group, α-synA53T and α-synA53T;Aβ1-42 demonstrated severe uncoordinated phenotypes, including non-sinusoidal and slow movement (Fig. 1D–F). Moreover, α-synA53T;TDP-43 and α-synA53T;Aβ1-42;Taupro-agg also exhibited uncoordinated phenotypes, albeit with a lesser degree compared to α-synA53T and α-synA53T;Aβ1-42 (Fig. 1G). Notably, all transgenic strains showed strongly defective postures compared to WT when animals were older (Fig. 1G). These results suggest that our overexpression transgenic models recapitulate the postural deficits often observed in PD and in some forms of LBD patients.
The postural observation of wild type (WT) and transgenic strains. A Typical normal posture of C. elegans. B–F Examples of defective postures of C. elegans. G The percentage of worms with defective postures. One-hundred (100) worms were counted per experiment, and each experiment was performed in triplicate (N ≥ 300 animals). Values in the panel are the average ± S.E.M. Differences between groups were evaluated by One-way ANOVA with Tukey Post-Hoc (*, comparison with WT; #, comparison with α-synA53T; ****, Adjust P < 0.0001; ####, Adjust P < 0.0001). Static posture images of C. elegans were shown in Figure S3. Details of group comparisons are shown in Table S3
LBD models demonstrate developmental delay in C. elegans
In order to determine whether the exogenous proteins including α-synA53T, Aβ1-42, Taupro-agg and TDP-43 could affect C. elegans at early development, we monitored the development cycle of all transgenic strains. Five developmental stages of C. elegans were scored. Egg stage, L1 larva stage, L2 larva stage, L3 larva stage, L4 larva stage. The larva stage can further be divided into three sub-stages namely early, middle, and late stages (Fig. 2A). Wild type worm eggs usually enter the L3 stage between 26 to 30 h at 20°C. An additional 34 to 46 h is required for the worms to enter the L4 larva stage. At 58 h, the worms undergo metaplasia and transition to adulthood (Fig. 2A).
Control group and LBD group displayed developmental delays. The extent of developmental delays in the control group were as follows: Aβ1-42;Taupro-agg > Taupro-agg > Aβ1-42 > TDP-43 (Fig. 2C–E, I, L). Moreover, Aβ1-42;Taupro-agg and Taupro-agg showed strong individual difference in ontogeny. In the LBD group, all LBD models, except for α-synA53T;TDP-43, experienced severe developmental delays (Fig. 2F–H,J–L). Furthermore, α-synA53T;Aβ1-42, α-synA53T;Taupro-agg, and α-synA53T;Aβ1-42; Taupro-agg displayed severe individual difference in ontogeny as well. These results indicate that the co-expression of Aβ1-42 and α-synA53T, and the expression of Taupro-agg may adversely affect the animal’s developmental progress.
L The percentage of animals developed to late L3 or older at 40 h, developed to late L4 or older at 58 h, and developed to young adult or older at 70 h; different colors indicate different strains. All strains were incubated in M9 buffer to L1 stage. Time in hours was counted after bleaching and synchronization. The developmental stages of each of the 10 strains were counted after 26 h, followed by every 4 h up until 40. Thereafter, the animals’ developmental stages were counted every 6 h until all animals developed to adult stage. A–L More than 200 animals were counted at each time point, and each experiment was performed in triplicate (N ≥ 600 animals). L Values in the panel are the average ± S.E.M. Differences between groups were evaluated by One-way ANOVA with Tukey Post-Hoc (*comparison with WT; #, comparison with α-synA53T; *Adjust P < 0.05; ****Adjust P < 0.0001; #Adjust P < 0.05; ####Adjust P < 0.0001). Details of group comparisons are shown in Table S4.
Movement, behavioral, and lifespan deficits were observed in LBD models
Thrashing and locomotion assays measure the movement ability of C. elegans that may recapitulate some features of the motor symptoms of LBD. The thrashing assay, where animals are placed in a liquid droplet of buffer and body bends across the body axis are counted determines ability to move freely in liquid and does not require any fine motor skills. The locomotion assay, where animals are placed on a solid agar plate with food and the media provides resistance, also measures body bends but requires more strength and coordination. While both assays measure movement, they are complementary where thrashing measures simple gross movement and locomotion measures stronger coordinated movement. To determine whether the exogenous proteins could lead to motor impairment, thrashing and locomotion of C. elegans assays were conducted. In the control group, the movement capacity of TDP-43 demonstrated a marked reduction, while the movement capacities of Aβ1-42, Taupro-agg, and Aβ1-42;Taupro-agg showed significant elevation in comparison to the WT. In LBD group, movement capacities of α-synA53T, α-synA53T; Aβ1-42, α-synA53T; TDP-43 and α-synA53T; Aβ1-42;Taupro-agg were significantly decreased, and the movement capacity of TDP-43 showed noteworthy increase compared with WT. Additionally, the movement capacity of α-synA53T;Aβ1-42 was significantly weaker than α-synA53T; but the capacities of α-synA53T;TDP-43,α-synA53T;Taupro-agg, and α-synA53T;Aβ1-42;Taupro-agg considerably increased compared with α-synA53T. The motility of C. elegans showed significant reduction with age. The capacities of TDP-43, α-synA53T, α-synA53T;Aβ1-42, α-synA53T;TDP-43, an α-synA53T;Taupro-agg demonstrated significant decrease with age. Moreover, α-synA53T; Aβ1-42 essentially lost motility at day 5 adult stage (Fig. 3A) (Table S5).
The phenotypic characteristics of wild type (WT) strain and transgenic strains. A Thrashing abilities of WT and transgenic strains were tested by bending in the M9 buffer. The number of body bends of animals developed to day 1 adult stage and day 5 adult stage were counted for 30 s. B Locomotion of WT and transgenic strains developed to day 1 adult stage and day 5 adult stage were tested by counting the body bends of animals in NGM with a lawn of live E. coli OP50 in 30 s. C Animals whose larvae developed inside their bodies were labeled as Worms’ bagging. The fractions of such animals kept under 20°C and 16°C to 23°C were counted (N ≥ 100 animals analyzed in 3 replicates). D The serotoninergic (5-HT) and cholinergic signaling pathways of WT strain and transgenic strains developed to day 2 adult stage were measured by counting the number of eggs of animals when exposed to M9 buffer, 5 mg/mL serotonin dissolved in M9 buffer and 10 mg/mL levamisole dissolved in M9 buffer for 1 h at room temperature (N ≥ 48 animals analyzed for each of 3 replicates). Details are shown in the Figure S4. E Learning index of different strains were measured by Chemotaxis assays. A higher learning index of animals indicates a stronger memory ability (N ≥ 1500 animals analyzed for each of 3 replicates). F–G Longevities of WT and transgenic strains. Different colors represent different strains. At least 60 worms were counted for every experiment, and at least 3 experimental replicates were performed for each experiment (N ≥ 180 animals, Mantel-Cox log-rank test; * comparison with WT, # comparison with α-synA53T, ***, P < 0.001; ****, P < 0.0001; ####, P < 0.0001; details of groups comparison are shown in Table S10). A–B 30 worms were counted per experiment, and each experiment was performed in triplicate (N ≥ 90 animals). Movement videos of C. elegans developed to day 1 and day 5 stage are provided in the supplementary material. A–E Values in the panel are the average ± S.E.M. Differences between groups were evaluated by One-ANOVA with Tukey Post-Hoc (*, comparison with WT; #, comparison with α-synA53T; ns, not significant; *, Adjust P < 0.05; **, Adjust P < 0.01; ***, Adjust P < 0.001; ****, Adjust P < 0.0001; #, Adjust P < 0.05; ###, Adjust P < 0.001; ####, Adjust P < 0.0001). Details of group comparisons are shown in Tables S5–S10
In the day1 adult stage, the activity levels of Aβ1-42;Taupro-agg, α-synA53T;Taupro-agg were higher than WT, while the activity levels of other transgenic animals were significantly lower than WT. The activity levels of α-synA53T; Aβ1-42, α-synA53T;TDP-43 and α-synA53T;Aβ1-42; Taupro-agg were lower than the activity level of α-synA53T. However, α-synA53T;Taupro-agg had significantly higher activity levels than α-synA53T. Although the activity of WT did not change significantly with age, the activity levels of all transgenic animals decreased significantly with age, with the activities of TDP-43, α-synA53T, Aβ1-42;Taupro-agg and α-synA53T;Taupro-agg showing significant reductions. In the adult stage day 5, the activity of Aβ1-42;Taupro-agg was comparable to WT, but other transgenic animals exhibited lower activity levels than WT. Specifically, TDP-43, α-synA53T, and α-synA53T;Aβ1-42 were almost incapacitated. However, α-synA53T;TDP-43, α-synA53T;Taupro-agg, and α-synA53T;Aβ1-42;Taupro-agg showed considerably higher activity levels compared to α-synA53T in day5 adult stage (Fig. 3B) (Table S6).
Transgenic animals exhibited deficiencies in their egg-laying. Egg-laying is a complex process that involves the regulation of the serotoninergic (5-HT) and cholinergic signaling pathways [40]. The egg laying assay is a simple and convenient, yet powerful assay to measure the status of these neuronal systems in a living animal. At normal resting stages, wildtype animals will lay few eggs. However, when stimulated by the neurotransmitter serotonin or the acetylcholine agonist levamisole, animals will respond by laying a large number of eggs if the respective neurotransmitter system is functioning well. The “worm bagging” phenotype is a worst case scenario where animals are unable to lay eggs. The fertilized eggs from the hermaphrodite remain inside the worm body, hatch, grow, and can be seen moving inside the mother’s body resembling a “bag of worms”.
To measure this phenotype, we counted worm bagging proportions in animals that developed to day 5 adult stage at 20°C and 23°C. WT, Aβ1-42, Taupro-agg, and α-synA53T presented few worms with a bagging phenotype, with Aβ1-42;Taupro-agg displaying a low percentage of dystocia syndrome. Conversely, TDP-43 and LBD group (excluding α-synA53T) demonstrated deficiencies in their egg-laying, among which the most severe was α-synA53T;Taupro-agg (78.25%), followed by α-synA53T;Aβ1-42;Taupro-agg (43.13%) and α-synA53T;TDP-43 (42.42%), then TDP-43 (21.12%) at 20°C. TDP-43 and Aβ1-42;Taupro-agg in the control group and α-synA53T and α-synA53T;Aβ1-42 in the LBD group significantly increased the worms bagging ratio with temperature elevation. At 23℃, the worms bagging ratios of α-synA53T;TDP-43 and α-synA53T;Aβ1-42;Taupro-agg were comparable with α-synA53T, but α-synA53T;Aβ1-42, and α-synA53T;Taupro-agg showed stronger egg-laying deficits than α-synA53T (Fig. 3C) (Table S7).
The serotoninergic pathway response of all transgenic animals were impaired (Fig. 3D) (Tables S8–S9). Serotoninergic signaling in egg-laying of α-synA53T;Aβ1-42;Taupro-agg was basically absent. However, in the LBD group, worms α-synA53T;TDP-43 and α-synA53T;Taupro-agg significantly improved serotoninergic stimulated egg-laying compared to α-synA53T. In contrast, α-synA53T;Aβ1-42 and α-synA53T;Aβ1-42;Taupro-agg did not show any significant effect on serotonergic signaling compared to α-synA53T. Notably, among all transgenic animals, strains TDP-43, α-synA53T;TDP-43 and α-synA53T;Aβ1-42;Taupro-agg were found to have healthy and responsive cholinergic signaling compared to WT, while other transgenic animals had impaired cholinergic signaling. Specifically, α-synA53T;Aβ1-42 showed the most serious deficit in the cholinergic signaling. In the LBD group, α-synA53T;TDP-43, α-synA53T;Taupro-agg and α-synA53T;Aβ1-42;Taupro-agg significantly improved the cholinergic signaling, whereas α-synA53T;Aβ1-42 exhibited more severe impairment of the cholinergic signaling compared to α-synA53T (Fig. 3D) (Tables S8–S9).
Dementia is one of the clinical symptoms of LBD. To investigate the memory capacity of different transgenic animals, we performed chemotaxis assays on the LBD group, with the exception of α-synA53T and α-synA53T;Aβ1-42 due to their poor motor abilities. In comparison with WT, all transgenic animals exhibited considerably impaired memory capacities (Fig. 3E) which suggested α-synA53T;TDP-43, α-synA53T;Taupro-agg and α-synA53T;Aβ1-42;Taupro-agg manifested a dementia phenotype.
Compared with WT, the average lifespan of all transgenic animals decreased. In the LBD group, compared with α-synA53T, the average lifespan of α-synA53T;Aβ1-42 was reduced by 12.08%, while α-synA53T;TDP-43 was increased by 34.66%, and the average lifespan of α-synA53T;Taupro-agg increased by 20.78%, and α-synA53T;Aβ1-42;Taupro-agg increased lifespan by 50.72% (Fig. 3G). Furthermore, the survival curves showed α-synA53T, α-synA53T;TDP-43 and α-synA53T;Taupro-agg exhibited small individual differences in lifespan statistics; while α-synA53T;Aβ1-42 and α-synA53T;Aβ1-42;Taupro-agg showed strong individual differences. The longest lifespan of α-synA53T;Aβ1-42 was 24 days, and the longest lifespan of α-synA53T;Aβ1-42;Taupro-agg was 35 days (Fig. 3F).
Dopaminergic neuron functional deficits and degeneration observed in LBD models
One of the clinical symptoms in PD patients is bradykinesia, which refers to the inability to flexibly control one’s body. To assess whether animals could recapitulate these symptoms, we conducted an analysis of the capabilities of WT and transgenic animal responses to food. CAT-1 and CAT-2 are involved in dopamine metabolic process [37]. Using cat-1 and cat-2 null mutants as positive controls, we observed that animals in the WT and control group had a greater tendency to remain in E. coli OP50, while animals in LBD group had a greater tendency of staying outside the E. coli OP50 food lawn. The LBD group also displayed a variable ability for locomotion, with α-synA53T;Aβ1-42 < α-synA53T < α-synA53T;TDP-43 < α-synA53T;Aβ1-42;Taupro-agg < α-synA53T;Taupro-agg (Fig. 4A) (Table S11). Interestingly, however, WT, control group as well as α-synA53T;Aβ1-42;Taupro-agg in LBD group could control their locomotion flexibly. This was evident in their capacity to adjust their speed and consequently slow down once food had been detected. Moreover, compared with WT, α-synA53T, α-synA53T;TDP-43, α-synA53T;Taupro-agg presented weaker sensitivity of move for food. Conversely, in LBD group, α-synA53T;TDP-43, α-synA53T;Taupro-agg and α-synA53T;Aβ1-42;Taupro-agg showed stronger sensitivity to move for food, compared to α-synA53T, (Fig. 4B) (Table S12).
The dopaminergic neuron deficits of wild type (WT) and transgenic strains. A The basic locomotion based upon food sensing was measured by counting the number of worms located out of food in small NGM plates (35 mm2) with a lawn of live E. coli OP50 per 5 min for 1 h. Animals moving from the center of the food was recorded as – 5 min. cat-1 and cat-2 strains were used as the positive assay controls (Tukey Post-Hoc; details of groups comparison in time points 15th, 30th, 45th, and 60th minute were shown in Figure S3 and Table S8). B The locomotor rates of animals which were in different conditions (E. coli OP50 ( +) and no E. coli OP50 ( – )) were counted. The decreasing locomotor rate of animals when animals were on a lawn of E. coli OP50 suggests functional dopaminergic neurons. To ensure the validity of this assay, cat-1 and cat-2 strains were set as positive controls (two sample t-test; ns, not significant; *, P < 0.05; **, P < 0.01; ****, P < 0.0001). C–D The movement videos of animals for 1 h in 13 mm2 circles were recorded by a camera installed on stereomicroscopes and then the tracks and movement distances of animals were analyzed and visualized by Tracker software. cat-1 and cat-2 strains were positive controls in this assay. Ten worms were counted per experiment, and each experiment was performed in triplicate (N = 30 animals). E–F The morphology observation of dopaminergic neurons in strains expressing α-synA53T. E Models of normal and 3 types of impaired dopaminergic neurons (degenerate, punctate, lost) in C. elegans 2 anterior deirid neurons (ADE) and 4 cephalic neurons (CEP). The fraction of animals with impaired dopaminergic neurons (F) were counted by confocal microscope. At least 30 worms were counted per experiment, and each experiment was performed in triplicate (N ≥ 90 animals). C–D, F Values in the panel are the average ± S.E.M. Differences between groups were evaluated by One-ANOVA with Tukey Post-Hoc (*, comparison with WT; #, comparison with α-synA53T; ns, not significant; *, Adjust P < 0.05; **, Adjust P < 0.01; ***, Adjust P < 0.001; #, Adjust P < 0.05; ##, Adjust P < 0.01; ###, Adjust P < 0.001; ####, Adjust P < 0.0001). Details of group comparisons are shown in Table S11-S14
In addition, we quantified the tracks and distances of locomotion of WT and transgenic animals during a period of one hour. Our observations indicated that there was a significant decrease in locomotion distance of TDP-43, α-synA53T, and α-synA53T;Aβ1-42 in comparison to WT, especially α-synA53T; Aβ1-42. This decrease was particularly prominent in the case of α-synA53T;Aβ1-42. There was no significant difference observed in the locomotion distance of the other nematodes in the control and LBD group as compared to WT (Fig. 4C,D) (Table S13). Interestingly, good motility does not necessarily indicate robust body control. WT and animals in control group mainly moved in the edge of the food based on the food density, while the positive assay controls cat-1 and cat-2 strains are aimlessly random (Fig. 4C). In LBD groups, α-synA53T, α-synA53T;TDP-43, α-synA53T;Taupro-agg, and α-synA53T;Aβ1-42;Taupro-agg demonstrated similar locomotion tracks to positive controls; however, α-synA53T;Aβ1-42 was very inactive and almost never left the starting point (Fig. 4C,D).
We also observed the deficits of dopaminergic neurons in different LBD models at day 5 adult stage. Compared with α-synA53T, α-synA53T;Aβ1-42 showed more severe damage of dopaminergic neurons, while α-synA53T;TDP-43, α-synA53T;Taupro-agg, α-synA53T;Aβ1-42;Taupro-agg showed comparatively lesser deficiency of dopaminergic neurons (Fig. 4E–F) (Table S14). The overall impairment in food sensing based upon the locomotion assays on and off food were observed in the LBD models. These were consistent with the morphological damage observed in their dopaminergic neurons.
α-Syn aggregation and expression are impacted by co-expression of other transgenes
To evaluate the impacts among α-synA53T, Taupro-agg, Aβ1-42and TDP-43 transgenes, we counted FPKM and relative expression levels of genes SNCA encoding protein α-syn, MAPT encoding protein Tau, APP encoding protein Aβ and TARDBP encoding protein TDP-43 by analyzing RNA-seq and qRT-PCR data (Fig. 5A–B) (Tables S15–S16). The expression of Aβ1-42 could increase the expression of SNCA, while the expression of Taupro-agg could reduce the expression of α-synA53T while TDP-43 and Aβ1-42;Taupro-agg had no significant effects on the expression of SNCA. The expression of α-synA53T reduced the expression of TARDBP. The agreement between RNA-seq and qRT-PCR results were good for SNCA and TARDBP but less for MAPT and APP. This could be due to the low expression levels of the later 2 proteins or differences in the technology platforms used.
Expression level of genes SNCA, MAPT, APP, TARDBP, and protein α-syn. A The normalization expression levels (FPKM) of SNCA, MAPT, APP and TARDBP in WT and transgenic strains based on the RNA-seq data obtained from Novaseq 6000 PE150 platform and aligned with GRCh38.p14. B The relative expression levels of SNCA, MAPT, APP and TARBP based on Real-Time Quantitative Reverse Transcription PCR. C–D Twenty μg protein samples were loaded and the relative proteins amount (α-synA53T/β-actin) of α-syn monomer and different sizes of α-syn oligomers were calculated. Values in the panel are the average ± S.E.M. Data were analyzed using Tukey Post-Hoc (ns not significant; *, Adjust P < 0.05; **, Adjust P < 0.01; ***, Adjust P < 0.001, ****, Adjust P < 0.0001). Values of group comparisons are shown in Tables S15-S18
To evaluate the impact of protein Aβ1-42, Taupro-agg, TDP-43, Aβ1-42;Taupro-agg on the expression of protein α-synA53T, we performed protein quantification on animals in LBD group. Our results demonstrated that the expression of Aβ1-42 could increase the expression of α-synA53T, while the expression of Taupro-agg can reduce the expression of α-synA53T considerably and TDP-43 and Aβ1-42;Taupro-agg had no significant effects on the expression of α-synA53T (Fig. 5C) (Table S17. Furthermore, the expressions of α-synA53T gene in different LBD models were found to be consistent with the expression of α-synA53T protein (Fig. 5A–B).
There are various sizes of α-syn aggregation in the brains of PD patients. We could observe distinct sizes of α-synA53T prone to aggregation in animals with α-synA53T or α-synA53T;Aβ1-42 expression. Compared to α-synA53T, α-synA53T;Aβ1-42 exhibited increased α-synA53T, while α-synA53T;TDP-43, α-synA53T;Taupro-agg and α-synA53T;Aβ1-42;Taupro-agg demonstrated less α-synA53T prone to aggregation, particularly α-synA53T;Taupro-agg (Fig. 5D) (Table S18). The highest levels of α-syn prone to aggregation was observed in α-synA53T;Aβ1-42 animals (Fig. 5D) and they had poor scores in thrashing, locomotion, and egg laying while in contrast, α-synA53T;TDP-43, α-synA53T;Taupro-agg and α-synA53T;Aβ1-42;Taupro-agg had better scores (Fig. 2). This suggested a correlation in the levels of prone to aggregation α-syn for these phenotypes.
The phenotypic severity shown by our LBD models were not absolutely correlated with the total amount of α-synA53T in nematodes (Fig. 5C) but was significantly correlated with different sizes of α-synA53T prone to aggregation (Fig. 5D).
miRNA expression levels are dysregulated in LBD models
To visualize differential miRNA expression levels in WT and transgenic animals, the hierarchical clustering analysis of differentially expressed miRNAs (DE-miRNAs) by the sva package [41] were conducted. The |Log2 (Fold change)|> 1 and adjust P value ≤ 0.05 were set as thresholds to identify DE-miRNAs. Compared to WT, expression patterns of α-synA53T;Aβ1-42;Taupro-agg, and Aβ1-42;Taupro-agg were similar; and expression patterns of WT and TDP-43 were similar (Fig. 6A). Additionally, α-synA53T and α-synA53T;Aβ1-42 displayed comparable expression patterns. The miRNA expression patterns of α-synA53T were different from α-synA53T;TDP-43, α-synA53T;Taupro-agg and α-synA53T;Aβ1-42;Taupro-agg. Compared with α-synA53T, the expression patterns of DE-miRNAs in different strains were similar with the expression patterns of DE-miRNAs compared to WT (Fig. 6B). The specific DE-miRNAs and their comparisons are shown in Tables S19-S20.
Hierarchical clustering analysis of differentially expressed miRNAs (DE-miRNAs). A miRNAs from transgenic strains containing α-synA53T compared with WT are listed (B) WT, α-synA53T;TDP-43, α-synA53T;Taupro-agg and α-synA53T;Aβ1-42; Taupro-agg compared with α-synA53T. Different colors indicate different expression levels (counts per million, CPM) of miRNAs. The Log2(Fold change) and adjusted P value of DE-miRNAs in different comparison groups are listed in Table S19 and Table S20
To further understand which miRNAs can affect the phenotypes of C. elegans in different models. We identified DE-miRNAs of α-synA53T;Aβ1-42, α-synA53T;TDP-43, α-synA53T;Taupro-agg, α-synA53T;Aβ1-42; Taupro-agg, and α-synA53T compared to WT and DE-miRNAs of α-synA53T;Aβ1-42, α-synA53T;TDP-43, α-synA53T;Taupro-agg, α-synA53T;Aβ1-42; Taupro-agg compared to α-synA53T (Fig. 7A). However, we did not find common DE-miRNAs among α-synA53T, α-synA53T;Aβ1-42, α-synA53T;TDP-43, α-synA53T;Taupro-agg and α-synA53T;Aβ1-42;Taupro-agg compared with WT. There were 5 common DE-miRNAs among α-synA53T, α-synA53T;Aβ1-42, and α-synA53T;Taupro-agg compared with WT and 3 common DE-miRNAs among α-synA53T, α-synA53T;Aβ1-42, and α-synA53T;Aβ1-42;Taupro-agg compared with WT (Fig. 7B; Table 2). Since α-synA53T;TDP-43, α-synA53T;Taupro-agg and α-synA53T;Aβ1-42;Taupro-agg displayed different phenotypes and miRNA expression patterns as compared to α-synA53T, we compared these 3 strains and WT with α-synA53T, and obtained a total of 2 DE-miRNAs (Fig. 7C; Table 3). Taken together, these results suggested a dynamic dysregulation of miRNAs due to overexpression of the transgenes.
The differential expression miRNA in wild type (WT) and transgenic strains. A Volcano plots of differential expression miRNAs (DE-miRNAs) in transgenic strains expressed α-synA53T including α-synA53T, α-synA53T;Aβ1-42, α-synA53T;TDP-43, α-synA53T;Taupro-agg, and α-synA53T;Aβ1-42;Taupro-agg compared with WT and α-synA53T;TDP-43, α-synA53T;Taupro-agg, and α-synA53T;Aβ1-42;Taupro-agg compared with α-synA53T. Log2(Fold change) > 1 and adjust P value < 0.05 were marked as upregulated DE-miRNAs and visualized as red dots; Log2(Fold change) < 1 and adjust P value < 0.05 were marked as downregulated DE-miRNAs and visualized as blue dots; miRNAs that were not DE-miRNAs were marked as stable miRNAs and visualized as grey dots. B–C Venn diagrams of different compared groups including transgenic strains that expressed α-synA53T compared with WT (B) and α-synA53T (C)
Hub dysregulated miRNAs were observed in LBD models
To discern the impact of miRNA on LBD, we performed small RNA and mRNA sequencing on different LBD models and obtained DEGs and DE-miRNAs among different groups through RNA-seq and bioinformatics analysis. The targets of DE-miRNAs which showed concordant expression levels with corresponding miRNAs and were also DEGs (|Log2(Fold change)|≥ 1.5 and adjust P value ≤ 0.01) were identified as effective targets, and the corresponding DE-miRNAs were identified as effective DE-miRNAs. The effective DE-miRNAs and effective targets were visualized by Cytoscape. Rhombuses indicated effective DE-miRNAs and circles indicated effective DEGs. Finally, we obtained 78 effective DEGs and 4 effective DE-miRNAs in α-synA53T, α-synA53T;Aβ1-42, α-synA53T;Taupro-agg compared to WT and 12 effective DEGs and 2 effective DE-miRNAs in WT, α-synA53T;TDP-43, α-synA53T;Taupro-agg and α-synA53T;Aβ1-42;Taupro-agg compared to α-synA53T (Fig. 8A, D) (Table S21–S22). The functions of the 78 effective DEGs were analyzed in DAVID GO database and they impacted the mitochondria inner membrane, mitochondria, and proton-transporting ATP synthase complex (Fig. 8G) of C. elegans. Subsequently, the gene enrichment analysis on KEGG pathway of the 78 effective DEGs showed that oxidative phosphorylation (adjust P value = 0.03), neuroactive ligand-receptor interaction (adjust P value = 0.1), and G protein-couple receptors are significantly enriched (adjust P value = 0.03) (Fig. 8H–J). The FPKM of genes corresponding to oxidative phosphorylation pathway (atp-2, cox-4, F58F12.1), neuroactive ligand-receptor interaction pathway (gar-3, gbb-1, seb-3) and G protein-couple receptors pathway (gar-3, gbb-1, seb-3) in different strains were visualized by heatmap (Fig. 8B–C). The expression levels of atp-2, cox-4 decreased in α-synA53T, α-synA53T;Aβ1-42, α-synA53T;Taupro-agg and α-synA53T;Aβ1-42;Taupro-agg compared to WT, but increased in α-synA53T;TDP-43 compared to WT. The expression of F58F12.1 decreased in LBD group compared to WT (Fig. 8B). The expression level of gar-3, gbb-1, seb-3 increased in LBD group except α-synA53T;Taupro-agg and α-synA53T;Aβ1-42;Taupro-agg compared to WT (Fig. 8C).
The hub-miRNAs and hub-targets and their functional analysis. A & D The network of differential expression miRNAs (DE-miRNAs) and the corresponding differential expression genes (DEGs) in transgenic strains expressed α-synA53T including α-synA53T, α-synA53T;Aβ1-42, α-synA53T;Taupro-agg were compared with wild type (A) and wild type, α-synA53T;TDP-43, α-synA53T;Taupro-agg, and α-synA53T;Aβ1-42;Taupro-agg compared with α-synA53T (D). B–C & E–F The hub-targets expression patterns of hub-miRNAs including cel-miR-1018 (B), cel-miR-58c (C), cel-miR-355-5p (E) and cel-miR-41-3p (F). Different colors indicated different Log2(Fold change). The GO functional analysis (GO) and Gene Set Enrichment Analysis (GSEA) based on KEGG database of C. elegans (H-J) of targets of hub-miRNAs which were DE-miRNAs in transgenic strains expressed α-synA53T including α-synA53T, α-synA53T;Aβ1-42, α-synA53T;Taupro-agg compared with WT. The Log2(Fold change) and adjusted P value of DEGs showed in A and D listed in Table S21 and S22
cel-miR-1018 and cel-miR-58c and their targets including atp-2, cox-4, F58F12.1, gar-3, gbb-1, and seb-3 were regarded as hub DE-miRNAs and hub-genes. In the α-synA53T;TDP-43, α-synA53T;Taupro-agg and α-synA53T;Aβ1-42;Taupro-agg compared to α-synA53T, cel-miR-355-5p and cel-miR-41-3p and their targets including cdc-48.3, ZK809.8, utg-38, and C30F12.2 which are involved in the regulation of mitochondria were regarded as hub DE-miRNAs and hub DEGs. The expression levels of cdc-48.3, ZK809.8, utg-38, and C30F12.2 increased in α-synA53T;TDP-43, α-synA53T;Taupro-agg and α-synA53T;Aβ1-42;Taupro-agg compared to α-synA53T (Fig. 8E–F). Taken together, these results suggested that while multiple miRNAs may be dysregulated, a few select miRNAs may act as hubs to coordinate gene expression in LBD models.
Mitochondria mass is increased in the LBD models
Mitochondria can often be an indicator of the status of cells in stress. In order to determine the status of mitochondria we stained LBD model and single transgenic animals using mitotracker green which measures mitochondria mass (Fig. 9A–H). Using WT as baseline, we observed significant increases in the area occupied by mitochondria in single transgenic Taupro-agg, α-synA53T, and double transgenic α-synA53T;Aβ1-42, with the largest increase in the latter (Fig. 9K) (Table S23). A more modest increase was observed in α-synA53T;Taupro-agg animals (Fig. 9I). The sizes of mitochondria appeared larger in α-synA53T and, α-synA53T;Aβ1-42 but not in α-synA53T;TDP-43 animals (Fig. 9E–G). The effects on mitochondria mass do not appear to be additive as the double transgenic animals did not all have more mass. For example, addition of a transgene that individually has increased mass did not automatically cause an increase. Another example is the triple transgenic animal α-synA53T;Aβ1-42;Taupro-agg which was not significantly more than WT. Thus, the mitochondria mass may depend upon the specific combination of transgenes.
The mitochondrial mass observation of wild type (WT) and transgenic strains. (K) The mitochondrial areas of worms were counted by Image J. Twenty animals were counted per experiment, and each experiment was performed in triplicate. Values in the panel are the average ± S.E.M. Differences between groups were evaluated by One-way ANOVA with Tukey Post-Hoc (*, comparison with WT; #, comparison with α-synA53T; ns, not significant; ***, Adjust P < 0.001; ****, Adjust P < 0.0001; ##, Adjust P < 0.01; ###, Adjust P < 0.001; ####, Adjust P < 0.0001). Details of group comparisons are shown in Table S23
Discussion
LBD are complex neurodegenerative disorders, and their pathogenic mechanism involve the formation of Lewy bodies mainly composed of misfolded protein α-synuclein. These Lewy bodies are often found together with other proteins such as TDP-43, Tau, and Aβ [18]. To uncover the effects of co-expression of these misfolded pathogenic proteins, we constructed 2 novel LBD models: α-synA53T; Taupro-agg and α-synA53T;Aβ1-42;Taupro-agg, based on α-synA53T [19], Taupro-agg [32] and Aβ1-42;Taupro-agg [37]. Furthermore, we have also adopted 2 other LBD models previously published, namely α-synA53T;Aβ1-42 [38] and α-synA53T;TDP-43 [39] to further investigate the pathogenesis of LBD.
In this study, LBD models including α-synA53T, α-synA53T;Aβ1-42, α-synA53T;TDP-43, α-synA53T;Taupro-agg, and α-synA53T;Aβ1-42;Taupro-agg exhibited phenotypes that corresponded to the clinical symptoms of LBD, such as significant stiffness and inflexibility in the body (Fig. 1), decreases in movement capacity (Fig. 3A), reductions in animal activity (Figs. 3B and 4D), lower body control ability (Fig. 4A–C), dopaminergic neuron deficits (Fig. 4F), α-syn prone to protein aggregation (Fig. 5D), and dementia (Fig. 3E). We also observed developmental delays (Fig. 2). While this is not a LBD phenotype, it indicates that effects of the transgenes may occur early in life and interpretation of our results should take this into account. Worms in our study may also be stressed by expression of multiple transgenes and we cannot rule out “transgenic sickness”. However, two lines of evidence suggest this is not the case. First, the phenotypes of the double transgenes differed depending upon the second transgene. Second, the triple transgenic animal appears to move better than the single transgenic. The models we studied here (α-synA53T, α-synA53T; Aβ1-42, α-synA53T;TDP-43, α-synA53T;Taupro-agg, and α-synA53T;Aβ1-42;Taupro-agg) thus recapitulate some features of LBD.
In addition, since worm bagging appeared in all LBD models (Fig. 3C), we measured the health of the nematode serotonergic and cholinergic signaling pathway. Serotonergic hermaphrodite specific neurons could deliver serotonin to G protein coupled receptors, inducing nematodes to enter an egg-laying activated state [42]. The serotonergic pathways of all LBD models were damaged and α-synA53T, α-synA53T;Aβ1-42, and α-synA53T;Aβ1-42;Taupro-agg almost had no response to serotonin (Fig. 3D), revealing impairment to the serotonergic neurons of LBD models. Cholinergic ventral C neurons are very important to help worms to lay eggs [42]. It can inhibit the egg laying of worms by releasing neurotransmitter acetylcholine. α-synA53T, α-synA53T;Aβ1-42 and α-synA53T; Taupro-agg showed little response to the agonist of acetylcholine receptors named levamisole which suggests the cholinergic neurons of these 3 LBD models were impaired. We speculated that the damage of these two neurons may be one of the reasons for the formation of worms bagging in LBD models. Interestingly, the formation of worm bagging also led to shortened lifespans of nematodes. Larvae hatch in hermaphrodites, and then eating of the internal structures of mother, resulting in the death of animals. In LBD models, the α-synA53T;Aβ1-42 individuals without worms bagging lived up to 24 days, and the α-synA53T;Aβ1-42;Taupro-agg individuals without worms bagging lived up to 35 days (Fig. 3F).
Meanwhile, the serotoninergic system and cholinergic system are associated with impaired long-term memory function and cognitive decline. The degeneration of serotoninergic neurons and cholinergic neurons were observed in PD patients [43], which were consistent with our results (Fig. 3D) and may be one of the reasons for memory ability deficits in our LBD models (Fig. 3E).
We found that the dopaminergic neurons of LBD models suffered varying degrees of damage. The most severe LBD model was α-synA53T;Aβ1-42, followed by α-synA53T with the second strongest dopaminergic neurons deficits, then α-synA53T;TDP-43, α-synA53T;Aβ1-42;Taupro-agg and α-synA53T;Taupro-agg. The dopaminergic neuron damage results were consistent with the findings of nematodes in posture, locomotor ability, activity, ability to find food, basal slowing response, and locomotion tracks. At present, it is commonly believed that the Lewy bodies formed by the misfolding of a-syn can lead to the death of dopaminergic neurons, and subsequent the clinical manifestations of LBD. The phenotypic severity shown by our LBD models were not absolutely correlated with the total amount of α-synA53T in nematodes (Fig. 5C), but was significantly correlated with different sizes of α-synA53T prone to aggregate (Fig. 5D), which was consistent with the current LBD research [44, 45]. Interestingly, the expression of human Aβ1-42, Taupro-agg and TDP-43 could affect the expression and α-synA53T prone to aggregate in C. elegans. The expression of human Aβ1-42 could induce the prone to aggregate α-synA53T protein and made worms sicker which was consistent with the hypothesis of interactions between Aβ protein and α-syn [46, 47]. We also found that the expression of human Taupro-agg could lower the expression and α-synA53T prone to aggregate and rescue the phenotypes of C. elegans. The mechanism of how Taupro-agg could lower the expression of α-synA53T was investigated. We performed outcrosses of the double transgenic animals. To our surprise, the α-synA53T animals continued to have lower expression suggesting that indeed transgene silencing occured. The nature of the silencing such as whether it is mediated by RNAi or an epigenetic phenomenon and duration in terms of number of generations remains unknown. Furthermore, the expression of Aβ1-42, Taupro-agg and α-synA53T together also could lower the aggregation of α-synA53T and made C. elegans healthier compared to α-synA53T which conflicts with current research [14]. This may be due to some unique properties of C. elegans not seen in humans as it does not express an α-syn ortholog or possibly that the interactions between the pathogenic proteins are more complex than previously known.
MicroRNAs are implicated in the regulation of neurodegenerative diseases. LBD group compared with WT, cel-miR-1018, cel-miR-58c, cel-miR-260, cel-miR-4807 were involved in the regulation of LBD diseases (Fig. 8A). Computational prediction suggests that mitochondrial function is affected by the expression of target genes and thereby induces the occurrence of LBD phenotype (Fig. 8G). cel-miR-1018 was upregulated in α-synA53T, α-synA53T;Aβ1-42, α-synA53T;Taupro-agg compared with WT. cel-miR-1018 was upregulated in dauer stage of C. elegans[48] which is a developmentally arrested and stress-resistant state which suggests α-synA53T may be involved in the developmental delay and induce some stress responses in C. elegans which were consistent with our results (Fig. 2B,F–G,J) and current research[49, 50]. The hub targets including cox-4, atp-2 and F58F12.1 of cel-miR-1018 enriched in the oxidative phosphorylation pathway was downregulated in the α-synA53T, α-synA53T;Aβ1-42, α-synA53T;Taupro-agg compared with WT (Fig. 8B–C,H) also provides further evidence that cel-miR-1018 may affect the normal function of mitochondria by silencing cox-4, atp-2 and F58F12.1 to induce the occurrence of LBD phenotype. cel-miR-58c is a member of the miR-58 family. The miR-58 family is necessary for normal development and behavior of nematodes. They are involved in the regulation of nematode body size, movement and egg laying. The knockout of miR-58 family causes the worms to stop development and be unable to respond to environmental pressure and enter the dauer stage [51]. Although miR-58 is not expressed in dopaminergic neurons [52], it is involved in all developmental stages of C. elegans [53]. cel-miR-58c was downregulated in α-synA53T, α-synA53T;Aβ1-42 but upregulated in α-synA53T;Taupro-agg compared with WT and the hub target gar-3, gbb-1, and seb-3 of cel-miR-58c are enriched in neuroactive ligand-receptor interaction and G protein-coupled receptor, indicating that cel-miR-58c is involved in the regulation of nematode development and stress resistance. It may interfere with the G protein-coupled receptor pathway by regulating the expression of gar-3, gbb-1, and seb-3. In C. elegans, cel-miR-1018 and cel-miR-58c may affect the phenotype of LBD by regulating the expression of corresponding targets, development, mitochondria-related pathways, neuroactive ligand-receptor interaction and G protein-coupled receptor. The cel-miR-1018 and cel-miR-58c may be essential in the regulation of neurodegenerative disorder in LBD C. elegans models.
Although α-synA53T;TDP-43,α-synA53T, Taupro-agg and α-synA53T;Aβ1-42;Taupro-agg are all LBD models, their PD phenotypes were much milder than α-synA53T and α-synA53T;Aβ1-42. To explore the miRNAs that may be involved in regulation, 2 hub-miRNAs were identified including cel-miR-355-5p, cel-miR-41-3p. The functions of cel-miR-355 are not fully understood. It may be involved in the regulation of innate immunity in nematodes. miR-355 in C. elegans was up-regulated, and the infection rate increased because of the knockdown of miR-355 after exposure to Pseudomonas aeruginosa [54]. miR-4, as a member of the miR-35 family, is involved in development of C. elegans. The inhibition of this miRNA family can lead to poor embryonic development, stagnation or even death [51]. Therefore, we speculated miR-355 and miR-41 may be related to the ability of C. elegans to resist stress. Notably, cdc-48.3 and C30F12.2 were the target genes of cel-miR-355-5p and cel-miR-41-3p respectively. Both genes are members of the AAA ATPase family. They are all involved in the maintenance of mitochondrial protein proteostasis. The knockout of AAA-ATPase C30F12.2 has been confirmed to impair oxidative stress tolerance and shorten the actual lifespan of nematodes. In addition, it may be involved in regulating the release and transport of neurotransmitters and thus affecting the health of nematodes [55]. When mitochondria are defective, they will try to repair themselves by fusing with functional mitochondria, while mitochondria that cannot be repaired will be degraded through autophagy. AAA-ATPase Cdc48 cooperates with the E3 ligase Parkin to regulate the degradation of mitofusin at the outer membrane of mitochondria, inhibiting the self-repair of defective mitochondria through fusion, thereby promoting the autophagy of defective mitochondria [56]. Therefore, we speculate that cel-miR-355-5p and cel-miR-41-3p may interfere with the normal function of mitochondria in LBD models by regulating the expression of cdc-48.3 and C30F12.2.
One limitation of these miRNA-seq studies is that they are done in whole animals using a bulk sequencing approach. While this is convenient, single cell sequencing is becoming more common although still not routine in C. elegans. Future studies may use this approach and find specific miRNAs in particular neurons that act as effectors in neurodegenerative processes.
Conclusion
Our results suggests that models including α-synA53T, α-synA53T;Aβ1-42, α-synA53T;TDP-43, α-synA53T; Taupro-agg, and α-synA53T;Aβ1-42; Taupro-agg demonstrate similarities to the behavior and pathogenesis of LBD such as deficits in locomotion, egg-laying, impaired serotonin and cholinergic signaling pathways, deficits in memory and body control, as well as impaired dopaminergic neurons and the formation of proteins prone to aggregate. Additionally, our study identified 4 significant miRNAs (cel-miR-1018, cel-miR-58c, cel-miR-355-5p, and cel-miR-41-3p) that are involved in LBD pathogenesis by regulating the expression of multiple genes and influencing the normal function of mitochondria, neuroactive ligand-receptor interaction and G protein-coupled receptor pathway. This study provides new tools to study Lewy body diseases as well as insights into the interactions of pathogenic proteins in age related neurodegenerative diseases.
Availability of data and materials
The datasets supporting the conclusions of this article are available in the SRA database of the NCBI repository, [PRJNA1070381; https://www.ncbi.nlm.nih.gov/sra/?term=PRJNA1070381].
Abbreviations
- α-Syn:
-
Alpha-synuclein
- Aβ:
-
Amyloid β
- APP:
-
Amyloid-beta precursor protein
- BACE-1:
-
β-Site APP cleaving enzyme
- C. elegans :
-
Caenorhabditis elegans
- CGC:
-
Caenorhaditis Genetics Center
- CPM:
-
Count Per Million
- DLB:
-
Dementia with Lewy bodies
- DEGs:
-
Differential expressed genes
- DE-miRNAs:
-
Differentially expressed miRNAs
- E. coli :
-
Escherichia coli
- FPKM:
-
Fragments per kilobase of transcript per million mapped reads
- GSEA:
-
Gene set enrichment analysis
- GO:
-
Gene ontology
- GFP:
-
Green fluorescent protein
- LBD:
-
Lewy body diseases
- NGM:
-
Nematode growth media
- 6-OHDA:
-
Oxidopamine
- PD:
-
Parkinson’s disease
- PDD:
-
Parkinson’s disease dementia
- RFP:
-
PIWI-interacting RNA (piRNA); Red fluorescent proteins
- TDP-43:
-
TAR-DNA-binding protein 43 (TDP-43)
- MPTP:
-
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- WT:
-
Wild type
- UTR:
-
Untranslated region
References
Obeso JA, Stamelou M, Goetz CG, Poewe W, Lang AE, Weintraub D, Burn D et al (2017) Past, present, and future of Parkinson’s disease: a special essay on the 200th Anniversary of the Shaking Palsy. Mov Disord 32:1264–1310. https://www.ncbi.nlm.nih.gov/pubmed/28887905
Hely MA, Reid WGJ, Adena MA, Halliday GA, Morris JGL (2008) The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Movement Disord 23:837–844. https://doi.org/10.1002/mds.21956
Outeiro TF, Koss DJ, Erskine D, Walker L, Kurzawa-Akanbi M, Burn D, Donaghy P et al (2019) Dementia with Lewy bodies: an update and outlook. Mol Neurodegener 14:5. https://www.ncbi.nlm.nih.gov/pubmed/30665447
Mehra S, Sahay S, Maji SK (2019) Alpha-Synuclein misfolding and aggregation: Implications in Parkinson’s disease pathogenesis. Biochim Biophys Acta Proteins Proteom 1867:890–908. https://www.ncbi.nlm.nih.gov/pubmed/30853581
Nuber S, Rajsombath M, Minakaki G, Winkler J, Muller CP, Ericsson M, Caldarone B et al (2018) Abrogating native alpha-synuclein tetramers in mice causes a L-DOPA-responsive motor syndrome closely resembling Parkinson’s disease. Neuron 100:75–90 e5. https://www.ncbi.nlm.nih.gov/pubmed/30308173
Killinger BA, Melki R, Brundin P, Kordower JH (2019) Endogenous alpha-synuclein monomers, oligomers and resulting pathology: let’s talk about the lipids in the room. NPJ Parkinsons Dis 5:23. https://www.nature.com/articles/s41531-019-0095-3
Shahmoradian SH, Lewis AJ, Genoud C, Hench J, Moors TE, Navarro PP, Castano-Diez D et al (2019) Lewy pathology in Parkinson’s disease consists of crowded organelles and lipid membranes. Nat Neurosci 22:1099–1109. https://www.ncbi.nlm.nih.gov/pubmed/31235907
Karanth S, Nelson PT, Katsumata Y, Kryscio RJ, Schmitt FA, Fardo DW, Cykowski MD et al (2020) Prevalence and clinical phenotype of quadruple misfolded proteins in older adults. JAMA Neurol 77:1299–1307. https://www.ncbi.nlm.nih.gov/pubmed/32568358
Wennberg AM, Whitwell JL, Tosakulwong N, Weigand SD, Murray ME, Machulda MM, Petrucelli L et al (2019) The influence of tau, amyloid, alpha-synuclein, TDP-43, and vascular pathology in clinically normal elderly individuals. Neurobiol Aging 77:26–36. https://www.ncbi.nlm.nih.gov/pubmed/30776649
Donaghy P, Thomas AJ, O’Brien JT (2015) Amyloid PET imaging in lEwy body disorders. Am J Geriatr Psychiatry 23:23–37. https://www.ncbi.nlm.nih.gov/pubmed/23831180
Skillbäck T, Farahmand BY, Rosén C, Mattsson N, Nägga K, Kilander L, Religa D et al (2015) Cerebrospinal fluid tau and amyloid-β1-42 in patients with dementia. Brain 138:2716–2731. https://doi.org/10.1093/brain/awv181
Colom-Cadena M, Gelpi E, Charif S, Belbin O, Blesa R, Marti MJ, Clarimon J, Lleo A (2013) Confluence of alpha-synuclein, tau, and beta-amyloid pathologies in dementia with Lewy bodies. J Neuropathol Exp Neurol 72:1203–1212. https://www.ncbi.nlm.nih.gov/pubmed/24226269
Tsigelny IF, Crews L, Desplats P, Shaked GM, Sharikov Y, Mizuno H, Spencer B et al (2008) Mechanisms of hybrid oligomer formation in the pathogenesis of combined Alzheimer’s and Parkinson’s diseases. PLoS One 3:e3135. https://www.ncbi.nlm.nih.gov/pubmed/18769546
Bassil F, Brown HJ, Pattabhiraman S, Iwasyk JE, Maghames CM, Meymand ES, Cox TO et al (2020) Amyloid-beta (Abeta) plaques promote seeding and spreading of alpha-synuclein and tau in a mouse model of Lewy body disorders with abeta pathology. Neuron 105:260–275 e6. https://www.ncbi.nlm.nih.gov/pubmed/31759806
McAleese KE, Walker L, Erskine D, Thomas AJ, McKeith IG, Attems J (2017) TDP-43 pathology in Alzheimer’s disease, dementia with Lewy bodies and ageing. Brain Pathol 27:472–479. https://www.ncbi.nlm.nih.gov/pubmed/27495267
Nakashima-Yasuda H, Uryu K, Robinson J, Xie SX, Hurtig H, Duda JE, Arnold SE et al (2007) Co-morbidity of TDP-43 proteinopathy in Lewy body related diseases. Acta Neuropathol 114:221–229. https://www.ncbi.nlm.nih.gov/pubmed/17653732
Yokota O, Davidson Y, Arai T, Hasegawa M, Akiyama H, Ishizu H, Terada S et al (2010) Effect of topographical distribution of alpha-synuclein pathology on TDP-43 accumulation in Lewy body disease. Acta Neuropathol 120:789–801. https://www.ncbi.nlm.nih.gov/pubmed/20669025
Walker Z, Possin KL, Boeve BF, Aarsland D (2015) Lewy body dementias. The Lancet 386:1683–1697
Lakso M, Vartiainen S, Moilanen AM, Sirvio J, Thomas JH, Nass R, Blakely RD, Wong G (2003) Dopaminergic neuronal loss and motor deficits in Caenorhabditis elegans overexpressing human alpha-synuclein. J Neurochem 86:165–172. https://www.ncbi.nlm.nih.gov/pubmed/12807436
Youssef K, Tandon A, Rezai P (2019) Studying Parkinson’s disease using Caenorhabditis elegans models in microfluidic devices. Integr Biol 11:186–207. https://doi.org/10.1093/intbio/zyz017
Harrington AJ, Hamamichi S, Caldwell GA, Caldwell KA (2010) C. elegans as a model organism to investigate molecular pathways involved with Parkinson’s disease. Dev Dynam 239:1282–1295. https://anatomypubs.onlinelibrary.wiley.com/doi/abs/https://doi.org/10.1002/dvdy.22231
Saha S, Guillily MD, Ferree A, Lanceta J, Chan D, Ghosh J, Hsu CH et al (2009) LRRK2 modulates vulnerability to mitochondrial dysfunction in Caenorhabditis elegans. J Neurosci 29:9210–9218. https://doi.org/10.1523/JNEUROSCI.2281-09.2009
Chowdhury A, Rajkumar AP (2020) Systematic review of gene expression studies in people with Lewy body dementia. Acta Neuropsychiatr 32:281–292. https://doi.org/10.1017/neu.2020.13
Giri B, Seamon M, Banerjee A, Chauhan S, Purohit S, Morgan J, Baban B, Wakade C (2022) Emerging urinary alpha-synuclein and miRNA biomarkers in Parkinson’s disease. Metab Brain Dis 37:1687–1696. https://doi.org/10.1007/s11011-021-00735-2
Rezaei O, Nateghinia S, Estiar MA, Taheri M, Ghafouri-Fard S (2021) Assessment of the role of non-coding RNAs in the pathophysiology of Parkinson’s disease. Eur J Pharmacol 896:173914. https://doi.org/10.1016/j.ejphar.2021.173914
Han LL, Tang YL, Bai XC, Liang XN, Fan Y, Shen Y, Huang F, Wang J (2020) Association of the serum microRNA-29 family with cognitive impairment in Parkinson’s disease. Aging (Albany NY) 12:13518–13528. https://doi.org/10.18632/aging.103458
Dong XY, Zheng DM, Nao JF (2020) Circulating exosome microRNAs as diagnostic biomarkers of dementia. Front Aging Neurosci 12:580199. https://doi.org/10.3389/fnagi.2020.580199
Zhang J, Zhao MY, Yan R, Liu J, Maddila S, Junn ES, Mouradian MM (2021) MicroRNA-7 protects against neurodegeneration induced by α-synuclein preformed fibrils in the mouse brain. Neurotherapeutics 18:2529–2540. https://doi.org/10.1007/s13311-021-01130-6
Esteves M, Abreu R, Fernandes H, Serra-Almeida C, Martins PAT, Barao M, Cristóvao AC et al (2022) MicroRNA-124-3p-enriched small extracellular vesicles as a therapeutic approach for Parkinson’s disease. Mol Ther 30:3176–3192. https://doi.org/10.1016/j.ymthe.2022.06.003
Recasens A, Perier C, Sue C M (2016) Role of microRNAs in the regulation of alpha-synuclein expression: a systematic review. Front Mol Neurosci 9:128. https://www.ncbi.nlm.nih.gov/pubmed/27917109
Zhao J, Yue D, Zhou Y, Jia L, Wang H, Guo M, Xu H et al (2017) The role of microRNAs in abeta deposition and tau phosphorylation in Alzheimer’s disease. Front Neurol 8:342. https://www.ncbi.nlm.nih.gov/pubmed/28769871
Fatouros C, Pir GJ, Koushika SP, Mandelkow E, Mandelkow EM, Schmidt E, Baumeister R (2012) Inhibition of tau aggregation in a novel Caenorhabditis elegans model of tauopathy mitigates proteotoxicity. Hum Mol Genet 21:3587–3603. https://www.ncbi.nlm.nih.gov/pubmed/22611162
von Bergen M, Barghorn S, Li L, Marx A, Biernat J, Mandelkow EM, Mandelkow E (2001) Mutations of tau protein in frontotemporal dementia promote aggregation of paired helical filaments by enhancing local beta-structure. J Biol Chem 276:48165–74. https://www.ncbi.nlm.nih.gov/pubmed/11606569
Wang YP, Biernat J, Pickhardt M, Mandelkow E, Mandelkow EM (2007) Stepwise proteolysis liberates tau fragments that nucleate the Alzheimer-like aggregation of full-length tau in a neuronal cell model. P Natl Acad Sci USA 104:10252–10257. https://doi.org/10.1073/pnas.0703676104
Wang YP, Martinez-Vicente M, Krüger U, Kaushik S, Wong E, Mandelkow EM, Cuervo AM, Mandelkow E (2009) Tau fragmentation, aggregation and clearance: the dual role of lysosomal processing. Hum Mol Genet 18:4153–4170. https://doi.org/10.1093/hmg/ddp367
Ash PE, Zhang YJ, Roberts CM, Saldi T, Hutter H, Buratti E, Petrucelli L, Link CD (2010) Neurotoxic effects of TDP-43 overexpression in C. elegans. Hum Mol Genet 19:3206–3218. https://www.ncbi.nlm.nih.gov/pubmed/20530643
Wang C, Saar V, Leung KL, Chen L, Wong G (2018) Human amyloid beta peptide and tau co-expression impairs behavior and causes specific gene expression changes in Caenorhabditis elegans. Neurobiol Dis 109:88–101. https://www.ncbi.nlm.nih.gov/pubmed/28982592
Huang X, Wang C, Chen L, Zhang T, Leung KL, Wong G (2021) Human amyloid beta and alpha-synuclein co-expression in neurons impair behavior and recapitulate features for Lewy body dementia in Caenorhabditis elegans. Biochim Biophys Acta Mol Basis Dis 1867:166203. https://www.ncbi.nlm.nih.gov/pubmed/34146705
Shen L, Wang C, Chen L, Leung KL, Lo E, Lakso M, Wong G (2020) TDP-1/TDP-43 potentiates human alpha-Synuclein (HASN) neurodegeneration in Caenorhabditis elegans. Biochim Biophys Acta Mol Basis Dis 1866:165876. https://www.ncbi.nlm.nih.gov/pubmed/32531261
Collins KM, Bode A, Fernandez RW, Tanis JE, Brewer JC, Creamer MS, Koelle MR (2016) Activity of the C. elegans egg-laying behavior circuit is controlled by competing activation and feedback inhibition. Elife 5:e21126. https://doi.org/10.7554/eLife.21126
Leek JT, Johnson WE, Parker HS, Jaffe AE, Storey JD (2012) The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics 28:882–883. https://www.ncbi.nlm.nih.gov/pubmed/22257669
Brewer JC, Olson AC, Collins KM, Koelle MR (2019) Serotonin and neuropeptides are both released by the HSN command neuron to initiate Caenorhabditis elegans egg laying. PLoS Genet 15:e1007896. https://doi.org/10.1371/journal.pgen.1007896
Perez-Lloret S, Barrantes FJ (2016) Deficits in cholinergic neurotransmission and their clinical correlates in Parkinson’s disease. Npj Parkinsons Dis 2:16001. https://doi.org/10.1038/npjparkd.2016.1
Bousset L, Pieri L, Ruiz-Arlandis G, Gath J, Jensen PH, Habenstein B, Madiona K et al (2013) Structural and functional characterization of two alpha-synuclein strains. Nat Commun 4:2575. https://doi.org/10.1038/ncomms3575
Sharon R, Bar-Joseph I, Frosch MP, Walsh DM, Hamilton JA, Selkoe DJ (2003) The formation of highly soluble oligomers of α-synuclein is regulated by fatty acids and enhanced in Parkinson’s disease. Neuron 37:583–595. https://doi.org/10.1016/S0896-6273(03)00024-2
Masliah E, Rockenstein E, Veinbergs I, Sagara Y, Mallory M, Hashimoto M, Mucke L (2001) beta-amyloid peptides enhance alpha-synuclein accumulation and neuronal deficits in a transgenic mouse model linking Alzheimer’s disease and Parkinson’s disease. Proc Natl Acad Sci U S A 98:12245–12250. https://doi.org/10.1073/pnas.21141239
Mandal PK, Pettegrew JW, Masliah E, Hamilton RL, Mandal R (2006) Interaction between Aβ peptide and α synuclein: molecular mechanisms in overlapping pathology of Alzheimer’s and Parkinson’s in dementia with Lewy body disease. Neurochem Res 31:1153–1162. https://doi.org/10.1007/s11064-007-9391-0
Ahmed R, Chang ZS, Younis AE, Langnick C, Li N, Chen W, Brattig N, Dieterich C (2013) Conserved miRNAs are candidate post-transcriptional regulators of developmental arrest in free-living and parasitic nematodes. Genome Biol Evol 5:1246–1260. https://doi.org/10.1093/gbe/evt086
Luth ES, Stavrovskaya IG, Bartels T, Kristal BS, Selkoe DJ (2014) Soluble, prefibrillar α-synuclein oligomers promote complex I-dependent, Ca2+-induced mitochondrial dysfunction. J Biol Chem 289:21490–21507. https://doi.org/10.1074/jbc.M113.545749
Di Maio R, Barrett PJ, Hoffman EK, Barrett CW, Zharikov A, Borah A, Hu XP et al (2016) α-synuclein binds to TOM20 and inhibits mitochondrial protein import in Parkinson’s disease. Sci Transl Med 8:342ra78. https://www.science.org/doi/https://doi.org/10.1126/scitranslmed.aaf3634
Alvarez-Saavedra E, Horvitz HR (2010) Many families of C. elegans microRNAs are not essential for development or viability. Curr Biol 20:367–373. https://doi.org/10.1016/j.cub.2009.12.051
Isik M, Korswagen HC, Berezikov E (2010) Expression patterns of intronic microRNAs in Caenorhabditis elegans. Silence 1:5. https://www.ncbi.nlm.nih.gov/pubmed/20226079
Kato M, de Lencastre A, Pincus Z, Slack FJ (2009) Dynamic expression of small non-coding RNAs, including novel microRNAs and piRNAs/21U-RNAs, during Caenorhabditis elegans development. Genome Biol 10:1–15. https://doi.org/10.1186/gb-2009-10-5-r54
Zhi LT, Yu YL, Jiang ZX, Wang DY (2017) Functions as an important link between p38 MAPK signaling and insulin signaling in the regulation of innate immunity. Sci Rep-Uk 7:14560. https://doi.org/10.1038/s41598-017-15271-2
Germany EM, Zahayko N, Huebsch ML, Fox JL, Prahlad V, Khalimonchuk O (2018) The AAA ATPase Afg1 preserves mitochondrial fidelity and cellular health by maintaining mitochondrial matrix proteostasis. J Cell Sci 131:jcs219956. https://www.ncbi.nlm.nih.gov/pubmed/30301782
Tanaka A, Cleland MM, Xu S, Narendra DP, Suen DF, Karbowski M, Youle R J (2010) Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J Cell Biol 191:1367–1380. https://www.ncbi.nlm.nih.gov/pubmed/21173115
Acknowledgements
The authors wish to thank the Genomics and Bioinformatics core of the Faculty of Health Sciences, University of Macau for their assistance with sequencing. Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). This study was supported by grants (MYRG2020-00213-FHS) from the Faculty of Health Sciences, University of Macau.
Funding
This study was funded in part by the Faculty of Health Sciences at Macau University grant MYRG2020-00213-FHS.
Author information
Authors and Affiliations
Contributions
R.L. conceptualization, produced materials and data, analyzed data, wrote the manuscript; X.H. conceptualization, produced materials and data; L.S. produced materials and data; T.Z. analyzed data; N.L. produced data; X.H. analyzed data; G.W. conceptualization, obtained funding, wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare they have no competing financial interests.
Ethics approval and consent to participate
No human subjects were included in this study.
Consent for publication
All authors read and approved the final version of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, R., Huang, X., Shen, L. et al. Novel C. elegans models of Lewy body disease reveal pathological protein interactions and widespread miRNA dysregulation. Cell. Mol. Life Sci. 81, 377 (2024). https://doi.org/10.1007/s00018-024-05383-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-024-05383-0