Abstract
Sweetpotato, Ipomoea batatas (L.) Lam. (2n = 6x = 90), is among the world’s most important food crops and is North Carolina’s most important vegetable crop. The recent introduction of Meloidogyne enterolobii poses a significant economic threat to North Carolina’s sweetpotato industry and breeding resistance into new varieties has become a high priority for the US sweetpotato industry. Previous studies have shown that ‘Tanzania’, a released African landrace, is resistant to M. enterolobii. We screened the biparental sweetpotato mapping population, ‘Tanzania’ x ‘Beauregard’, for resistance to M. enterolobii by inoculating 246 full-sibs with 10,000 eggs each under greenhouse conditions. ‘Tanzania’, the female parent, was highly resistant, while ‘Beauregard’ was highly susceptible. Our bioassays exhibited strong skewing toward resistance for three measures of resistance: reproductive factor, eggs per gram of root tissue, and root gall severity ratings. A 1:1 segregation for resistance suggested a major gene conferred M. enterolobii resistance. Using a random-effect multiple interval mapping model, we identified a single major QTL, herein designated as qIbMe-4.1, on linkage group 4 that explained 70% of variation in resistance to M. enterolobii. This study provides a new understanding of the genetic basis of M. enterolobii resistance in sweetpotato and represents a major step towards the identification of selectable markers for nematode resistance breeding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Meloidogyne spp., the root-knot nematodes (RKN), are globally distributed plant-parasitic roundworms with worldwide economic impact and a host range that includes most higher plants (Moens et al. 2009). Meloidogyne enterolobii (Yang and Eisenback 1983) is among the most damaging species in its genus and it has a host range of at least 121 documented plants, extreme pathogenicity, and the ability to develop and reproduce on crops known to carry resistance genes for other Meloidogyne species (Castagnone-Sereno 2012; Moens et al. 2009; EPPO 2011; Rutter et al. 2018). Total crop failure due to quality and yield loss has been attributed to M. enterolobii in sweetpotato (Ipomoea batatas (L.) Lam.) (Kirkpatrick et al. 2019; Ye 2018). North Carolina (NC) produced approximately 65% of the US sweetpotato crop in 2019 (USDA/NASS 2020), and in NC the two RKN of greatest concern are Meloidogyne incognita (Kofoid and White) Chitwood, the southern RKN, and M. enterolobii, the guava RKN.
Meloidogyne enterolobii was recently introduced in NC (Ye et al. 2013), and growers need resistant and locally-adapted varieties of orange-fleshed sweetpotato to stay competitive in the global marketplace. Resistance breeding is the most effective, sustainable, and economical solution to managing Meloidogyne spp. in sweetpotato (Cervantes-Flores et al. 2008a, b; Clark and Moyer 1988). While fumigant and non-fumigant nematicides are known to be effective in managing (though not eradicating) M. enterolobii, these solutions tend to be expensive, non-specific, and are increasingly being regulated out of the marketplace. For example, metam sodium, one of the most effective compounds for RKN control, is recommended for less and less frequent use (Philbrick et al. 2020). Thus, breeding for resistance as part of a systems-based, integrated pest management control strategy has become a major objective of US sweetpotato breeding programs.
Due to its complex genetics, sweetpotato lags behind many other crops in terms of genomic tools for crop improvement. However, recent advances in sweetpotato genomics provide new opportunities for genome-enabled breeding (Mollinari et al. 2020; Wadl et al. 2018; Wu et al. 2018). Plant breeders have addressed RKN by breeding resistant varieties of several crops. For example, Meloidogyne-resistant alfalfa (Hunt et al. 1972), M. incognita-resistant tomato (Bhavana et al. 2019; Singh et al. 1974), the M. incognita-resistant ‘Covington’ sweetpotato (Yencho et al. 2008), and others. Each dollar put towards research and development of nematode resistant crop varieties has been estimated to pay off several 100-fold (Boerma and Hussey 1992). However, the development of new varieties and integration of resistance genes is a time-consuming and expensive process.
Due to polyploidy and high levels of heterozygosity, the cross between two sweetpotato lines results in segregation at the F1 level (Jones et al. 1986), and a population suitable for linkage mapping and quantitative trait loci (QTL) analysis can be made in a single cross. While producing segregating sweetpotato populations is relatively simple, breeding sweetpotato is not. Many horticultural traits in sweetpotato are quantitative, including dry matter, starch, and β-carotene content (Cervantes-Flores et al. 2011). Further, the hexaploid nature of sweetpotato and allele dosage effects can lead to complex segregation ratios and trait expression levels (Jones et al. 1986). The development of new sweetpotato varieties requires many traits, such that introgression of one key trait, such as the dominant M. incognita resistance locus hypothesized by Oloka et al. (2021), does not lead to immediate variety release. Elite varieties are so classified because they excel in most traits, yet a single cross between elite lines will yield mostly progenies that do not meet stringent market demands. A further challenge for breeders is that sweetpotato generally does not tolerate backcrossing due to severe inbreeding depression and frequent self- and cross-incompatibilities (Mwanga et al. 2002). The next major variety should be high performing for most traits across multiple environments and possess the M. enterolobii resistance that does not yet exist in elite breeding germplasm.
Cervantes-Flores et al. (2008b) reported the development of the ‘Tanzania’ x ‘Beauregard’ (TB) mapping population and, using an amplified fragment length polymorphism (AFLP) based linkage map (Cervantes-Flores et al. 2008a), conducted QTL analyses for several traits including M. incognita race 3 resistance. ‘Beauregard’, a major US variety, is susceptible to M. enterolobii, M. incognita races 1, 2, 3 and 4, and M. javanica (Cervantes-Flores et al. 2002), while ‘Tanzania’ is resistant to M. enterolobii (Rutter et al. 2021; Schwarz et al. 2021), all four described races of M. incognita, and M. javanica (Cervantes-Flores et al. 2002). Using the approach proposed by Mollinari et al. (2020), Amankwaah et al. (2019) constructed an ultra-dense integrated multi-locus single nucleotide polymorphism (SNP)-based phased linkage map for the TB population using comparative SNP information from the Ipomoea trifida (Kunth.) G. Don reference genome published by Wu et al. (2018). Oloka et al. (2021) used this map for QTL analysis of the TB population for M. incognita race 3 resistance. Within the TB population, M. incognita resistance was highly skewed towards ‘Tanzania’, the resistant parent, and QTL analysis revealed one major QTL on linkage group 7 explaining 58.3% of variation for resistance (Oloka et al. 2021). Meloidogyne enterolobii had not been detected in the NC when the TB population was developed, however, M. enterolobii resistance screens by Schwarz et al. (2021) revealed ‘Tanzania’ to be resistant and ‘Beauregard’ to be susceptible. Schwarz et al. (2021) also screened eight TB progeny and found five to be resistant and three to be susceptible. This warranted a closer look at the TB population and its segregation for M. enterolobii resistance.
Here, we describe a QTL analysis for M. enterolobii resistance using biallelic SNP marker data from the TB sweetpotato mapping population, based on phenotypic data from M. enterolobii inoculation-based greenhouse bioassays. This study was made possible by new genomic tools and resources, including the high-quality genome assembly of diploid I. trifida (NCNSP306) (Wu et al. 2018), the heterozygous polyploid-optimized genotyping-by-sequencing protocol GBSpoly (Wadl et al. 2018), and R packages MAPpoly (Mollinari and Garcia 2019) and QTLpoly (Pereira et al. 2020).
Materials and methods
Plant materials
The ‘Tanzania’ x ‘Beauregard’ sweetpotato mapping population consisting of 246 full-sibling progenies was used for this experiment (Cervantes-Flores et al. 2011). TB has been maintained in a vegetative state in a virus-free greenhouse with regular watering and fertilization at 25–45 °C in 72-cell Landmark™ seedling trays (Stuewe & Sons, Corvallis, OR) in Fafard P4 soil mix (Fafard, Agawam, MA) at the North Carolina State University Method Road greenhouse complex since its inception. Two single-plant replicates were generated from vine cuttings of each genotype for a total of 516 plant samples. The following checks were included: ‘Covington’ and ‘Beauregard’ as M. enterolobii susceptible checks, and ‘Jewel’ and ‘Tanzania’ as resistant checks (Schwarz et al. 2021). Within each replicate, check lines were included as two positive (inoculated) and one negative (non-inoculated) controls.
Nematode preparation
The M. enterolobii isolate used for this trial was collected from a field under a soybean/sweetpotato rotation in Johnston County, NC in 2017 and has been maintained on ‘Rutgers’ tomato plants (Solanum lycopersicum L.) (Schwarz et al. 2021). Nematode egg extraction methods were described by Schwarz et al. (2021), which were based on protocols by Hussey and Barker (1973). Briefly, a 10% v/v water and Clorox bleach (7.4% sodium hypochlorite) solution was used to break down egg matrices on inoculated stock tomato plants, and this solution was poured through a stacked series of 20.32-cm brass sieves (Fisherbrand™ USA standard 8″ test sieves, #60, #120, #500, from top to bottom) to capture M. enterolobii eggs. The concentration of eggs in stock solution samples was estimated by counting five 1-ml aliquots at 100 × using a Nikon TMS Inverted Phase Contrast microscope, and total eggs in solution was extrapolated.
Sweetpotato planting and inoculation
Following procedures described by Schwarz et al. (2021), 12- to 15-cm sweetpotato vine cuttings were collected from the 246 TB clones and check lines. Cuttings were planted in 15-cm diameter terra cotta pots in a 1:1 mix of sand and soil (88.9% sand, 8.2% silt, 2.8% clay) (Schwarz et al. 2021). Pots were arranged in a completely randomized design of two replicates on expanded steel mesh greenhouse benches, eliminating the possibility of standing water which could contaminate adjacent plants. Greenhouse conditions were 25–28 °C with no supplemental lighting. Inoculation with M. enterolobii (10,000 eggs per plant) in 15-ml of water at a depth of 2-cm occurred 14 days post-planting. Nematode eggs were inoculated on the same day of extraction from stock culture plants. Light watering occurred as needed, with care not to overwater which might flush nematodes from the media.
Egg extractions
Egg extractions occurred 60 days post-inoculation. Sweetpotato plants were cut at crown-level and roots were rinsed of soil in wash buckets. After washing, roots were bundled in paper towels to absorb excess moisture and stored in a 4 °C refrigerator overnight. The following day the mass of each root system, including small developing storage roots, was recorded. Root gall severity ratings were performed visually by a single researcher based on the percent of total root volume with galls present (Schwarz et al. 2021): 1 galled root in 100 roots = 1% galling; 20 galled roots in 200 total = 10% galling, etc.
Egg extractions from each sample followed the same protocol described above (Hussey and Barker 1973). A 10% v/v water and Clorox bleach (7.4% sodium hypochlorite) solution was poured over individual samples in 1-L beakers and samples were soaked for 30–60 s. Wearing gloves, fine roots and storage roots were massaged in the bleach solution to dissolve the gelatinous matrix which adheres the eggs to the roots. Samples were poured through three stacked sieves (Fisherbrand™ USA standard test sieves, #60, #120, #500, from top to bottom) and carefully rinsed under running tap water to concentrate eggs in the bottom sieve. The top two sieves were then removed, and contents discarded; contents of the bottom sieve (i.e., M. enterolobii eggs) were washed to one side using tap water, and extracted eggs were poured into 50-ml Falcon tubes. Several rinses with a squeeze bottle of tap water recovered any remaining eggs. These tubes were adjusted with tap water to 50-ml total volume. Falcon tubes were stored in a 4 °C refrigerator for up to one week prior to egg counting.
Meloidogyne enterolobii quantification
Nematode eggs were quantified using four subsamples of 5-ml from each 50-ml egg extraction solution. After gentle inversion of the Falcon tube (not a swirling motion, which can concentrate eggs in the center and bias egg counts), four 5-ml aliquots from each extraction were dispensed into four separate wells of a six-well tissue culture plate. Tissue culture plates were manually etched on the underside with a 0.635-cm grid to ease counting. Egg counting was performed at 100 × using an inverted Nikon TMS Inverted Phase Contrast microscope (10 × eyepiece with 10 × objective; also 4 × and 40 × objectives as needed). The mean of the four subsamples was calculated and extrapolated to estimate the total number of eggs in the 50-ml extraction sample. Total eggs in solution represents the same value as total eggs per plant sample and final nematode egg population. As needed, samples were diluted 1:100 to facilitate counting, with care to factor this dilution in while calculating total eggs. The total number of eggs was used to calculate two measures of resistance. Reproductive factor (RF) was used to classify resistance or susceptibility by measuring the change in RKN populations as the ratio of final nematode eggs to initial inoculum (herein, 10,000 eggs) (Oostenbrink 1966; Seinhorst 1965; Trudgill and Phillips 1997). A RF greater than one suggests the nematode can proliferate on the host, classifying it as susceptible, while RF below one suggests resistance. The second measure, eggs per gram of root tissue (EGRT), was total eggs divided by total root mass (including storage roots) to standardize egg production across root systems with variable masses.
QTL analysis
Genotyping the TB population was conducted using GBSPoly (Wadl et al. 2018) as described by Amankwaah et al. (2019). GBSPoly is a genotyping-by-sequencing protocol developed for heterozygous polyploids like sweetpotato. Library preparation and sequencing were done at the North Carolina State University Genomic Sciences Laboratory as described by Oloka et al. (2021), and reads were aligned with the I. trifida (NCNSP306) reference genome (Wu et al. 2018). Dosage calling followed procedures described by Oloka et al. (2021). The R package MAPpoly (Mollinari and Garcia 2019) was used for linkage map construction of the TB population as described by Amankwaah et al. (2019). This map was 2,120 cM in length with a density of 6.9 SNPs per cM. An updated version of this map was used for the current study, with the following modifications: clones with > 23% missing marker information were removed from the linkage map, and markers which were absent in > 25% of the TB population were not considered. Clone TB119 was identified as a self of ‘Tanzania’ and was also removed. The updated map was 2084.39 cM in length, with a density of 6.9 SNPs per cM, a maximum gap of 3.86 cM, and a total of 14,588 SNPs.
QTL analysis was performed using both fixed-effect interval mapping (FEIM) and random-effect multiple interval mapping (REMIM) models for both replicates of the three traits of interest (RF, EGRT, gall severity rating), as well as the mean of both replicates for these three traits. For all analyses, REMIM window size was set at 20 cM with a forward search threshold of 0.25 and a backward elimination threshold of 0.05 based on significance thresholds established by the score-based resampling method assessing genome-wide QTL significance (Pereira et al. 2020). For all instances of the FEIM model, 1000 permutations were performed. Log base 10 of P-value (LOP) and logarithm of the odds (LOD) profiles were obtained for REMIM and FEIM models, respectively. For both models, constant d was set at 1.5 to approximate a 95% support interval by LOD-d for the FEIM model, and LOP-d for the REMIM model as described by Pereira et al. (2020).
Using the QTL positions estimated by QTLpoly, we performed the basic local alignment search tool (BLAST) within QTL support intervals using the I. trifida (NCNSP306) assembly to identify potential candidate genes involved in RKN resistance (assembly available at http://sweetpotato.uga.edu/). Graphics of the QTL analyses and for the modified linkage map were generated using the R package VIEWpoly (v. 0.1.1) (Taniguti et al. 2022) and correlograms were graphically represented using ggplot2 (v. 3.3.3) (Wickham 2016).
Results
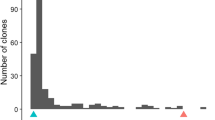
Frequency histograms for RF, gall severity ratings, and EGRT all demonstrated skewing toward resistance (Fig. 1). For mean RF, 126 TB progenies were classified as resistant (RF < 1, 51.2%), while 120 were susceptible (RF > 1, 48.8%) (Table 1), exhibiting a 1:1 segregation pattern for resistance to M. enterolobii within the TB population (χ2 = 0.146; P = 0.702). Thresholds for resistance by EGRT have not been defined, however, RF and EGRT were positively correlated (r = 0.948; P < 0.001), and a RF of 1 corresponded to approximately 158 EGRT in this population under greenhouse conditions.
Scatter plots, frequency histograms, and Pearson correlations of average root gall severity ratings, eggs per gram of root tissue, and reproductive factor (RF, or total final eggs/10,000 initial inoculated eggs) as measures of resistance to M. enterolobii within the ‘Tanzania’ x ‘Beauregard’ mapping population. Values in the top right triangle show Pearson correlation coefficient (r) between variables
Non-inoculated controls ‘Tanzania’, ‘Beauregard’, and ‘Covington’ had gall severity ratings of 0%, and no eggs were observed from extracted samples in either replicate. ‘Jewel’ (non-inoculated) had gall severity ratings of 1% in both negative control replicates, and while one replicate yielded an egg count of zero, the other replicate was contaminated with 9.0 EGRT. Positive resistant checks behaved as expected (Fig. 2). ‘Tanzania’ (inoculated) was rated as highly resistant, with average RF, EGRT and gall severity ratings of 0.026, 7.5, and 0.0%, respectively. ‘Jewel’ (inoculated) was also resistant, with RF 0.027 and 6.0 EGRT and 2.3% galling. The susceptible checks performed as expected, supporting a high degree of nematode reproduction: ‘Covington’ (inoculated) RF = 6.843 with 2190.8 EGRT and 12.5% galling, and ‘Beauregard’ (inoculated) RF = 13.691 with 3912.5 EGRT and 14.5% galling.
Images of positive control sweetpotato plants inoculated with 10,000 M. enterolobii eggs, 60 days post-inoculation. Contrast adjusted to better display galling. A ‘Covington’ (susceptible). B ‘Beauregard’ (susceptible). C ‘Tanzania’ (resistant). D ‘Jewel’ (resistant). E A particularly heavily galled replicate of ‘Beauregard’ (susceptible). Black arrow indicates galls
Root gall severity ratings were moderately correlated with EGRT within the TB population (r = 0.581, P < 0.001), and gall severity ratings were also moderately correlated with RF (r = 0.632, P < 0.001) (Fig. 1). Correlations between the two replicates of all genotypes were moderate for both RF (r = 0.534, P < 0.001) and EGRT (r = 0.529, P < 0.001). Subsamples, representing the four individual 5-ml aliquots of extracted egg solution from each plant, were strongly correlated (r = 0.99, P < 0.001).
Transgressive segregation for resistance and susceptibility was observed, where 42 lines (~ 17%) averaged a higher RF than ‘Beauregard’, and 39 lines (~ 16%) averaged a lower RF than ‘Tanzania’. These percentages were similar for average EGRT, with 42 lines measuring higher than ‘Beauregard’, and 35 lines (~ 14%) lower than ‘Tanzania’.
The 1:1 segregation pattern in the TB population for resistance to M. enterolobii about RF = 1 was strong evidence that resistance exhibited a single gene, simplex inheritance pattern in this population, motivating the QTL analyses.
QTL analysis
The score-based REMIM model in QTLpoly revealed one major QTL on LG 4 at 57.5 cM (Figs. 3 and 4) explaining 70% of variation for resistance to M. enterolobii for both RF and EGRT (Table 2). For the trait means using FEIM, RF produced a single major QTL peak at 57.5 cM (LOD = 38.51, adjusted R2 = 0.555), EGRT at 57.5 cM (LOD = 37.94, adjusted R2 = 0.550), and gall severity rating at 58.3 cM (LOD = 33.69, adjusted R2 = 0.505). The REMIM model trait means also each produced a single major QTL in approximately the same position: RF at 57.5 cM (P value < 2.22e-16, h2 = 0.7), EGRT at 57.5 cM (P value < 2.22e-16, h2 = 0.7), and gall severity rating at 59.0 cM (P value < 2.22e-16, h2 = 0.67) (Table 2).
A QTL (qIbMe-4.1) for M. enterolobii resistance was detected on chromosome 4, based on three measures of resistance. Reproductive factor (RF), eggs per gram of root tissue, and gall severity ratings were measured. Both random effects multiple interval mapping (REMIM) and fixed effect interval mapping (FEIM) models found identical peak QTL positions for RF and eggs per gram of root tissue at 57.5 cM. A Log base 10 of P-value profile for the REMIM model B Log of the odds (LOD) score profiles the FEIM model
Haplotype map and zoomed-in view of the QTL qIbMe-4.1 on chromosome 4, identifying a region of the sweetpotato genome associated with resistance to Meloidogyne enterolobii, the guava root-knot nematode. Reproductive factor (RF), eggs per gram of root tissue, and gall severity ratings were measured. Individual marker calls and predicted dosages are denoted in the haplotype map below the QTL profile, with genomic distance measured in centimorgans (cM)
The QTL peak at 57.5 cM was in the same position for RF and EGRT for both the FEIM and REMIM models in both replicates 1 and 2 and their means. The REMIM 95% support interval was 41.68–71.04 cM, spanning approximately 10.0 Mb as aligned to the I. trifida reference genome (Wu et al. 2018). The FEIM 95% support interval was 56.29–58.3 cM, spanning approximately 1.9 Mb. The QTL for mean gall severity rating peaked at 57.5 cM for REMIM and 58.3 cM for FEIM (Figs. 3 and 4). Support intervals for gall severity ratings were broader than those for RF and EGRT in both models (Table 2). Support intervals spanned from 1.9 Mb (FEIM) to 10.0 Mb (REMIM), or 0.06% to 0.3% of the 3.3 Gb sweetpotato genome.
Additive effects of parental alleles at 57.5 cM revealed that allele h in ‘Tanzania’ negatively affected the population means for RF and EGRT, while ‘Beauregard’ alleles contributed little to resistance (Fig. 5). Negative effects indicate increased resistance, while positive effects would indicate increased susceptibility. Population means (μ) for resistance traits were: μRF = 6.06, μEGRT = 1,767 eggs, and μgallrating = 3.96%. Per the trait means REMIM model, estimated effects predict that the presence of allele h in TB progeny is associated with reduced RF (-5.53), EGRT (-1,593 eggs), and gall severity ratings (-3.49%) means. Allele h at this locus provided evidence that resistance came from ‘Tanzania’, and was likely a single-dose, simplex allele. We believe this is the first QTL identified within I. batatas for resistance to M. enterolobii and we have named this locus qIbMe-4.1. The proposed naming convention describes the first (1) QTL (q) in I. batatas (Ib) for M. enterolobii (Me) on linkage group 4.
Additive allelic effects for ‘Tanzania’ and ‘Beauregard’. Allele h in ‘Tanzania’ negatively affected population means (i.e., increased resistance) for RF and EGRT at the peak QTL position 57.5 cM, while ‘Beauregard’ alleles contributed little to resistance. Population means (μ) for resistance traits were: μRF = 6.06, μEGRT = 1,767 eggs, and μgallrating = 3.96%. Allele h dropped population means by: RF (-5.53), EGRT (-1,593 eggs), and gall severity ratings (-3.49%) suggesting that resistance is derived from ‘Tanzania’ with a dosage of one
Discussion
Bioassay
Quantifying sweetpotato resistance to M. enterolobii is currently expensive and labor-intensive, and standardized resistance thresholds for M. enterolobii do not yet exist for this crop. Resistance in sweetpotato is here defined as a diminished capacity for RKN to reproduce on the host. While a RF less than one is, by this definition, resistant, maintaining nematode populations at or near the initial load is not satisfactory for growers. Ideally, a next-generation sweetpotato variety would be so highly resistant as to be considered a non-host (RF < 0.1), as defined by Hamidi and Hajihassani (2020). The TB population has demonstrated that this level of resistance is attainable in sweetpotato through a single cross with a resistant parent. ‘Tanzania’ averaged RF = 0.03 in four biological replicates, and between the two biological replicates for TB progenies, 233 out of the 246 genotypes assayed (~ 95%) were in concordance between replicates about RF = 1. Further, 35.4% (87/246) of the TB progeny could be classed as non-hosts by the RF < 0.1 definition.
Our second measure of resistance, EGRT, may be confounded by the fact that sweetpotato forms storage roots, though not all genotypes did in our bioassay. For an equal mass of storage roots and fibrous roots, the fibrous roots would have a greater surface area; M. enterolobii may interact with these root tissues differently, influencing relative EGRT. Second stage juveniles of Meloidogyne spp. establish at the zone of cell elongation behind the host plant’s root cap, which is the preferred site of invasion (Karssen et al. 2013), which could indicate that susceptible sweetpotato genotypes with heavy fibrous root production may be particularly susceptible. A third measure, root gall severity rating, is commonly used for Meloidogyne spp.; however, gall severity ratings are poorly correlated with egg counts in the case of M. enterolobii. For example, in a sweetpotato diversity bioassay, Schwarz et. al (2021) found no M. enterolobii gall severity rating above 20%, despite heavy egg production in some lines. Kiewnick et al. (2021) also observed an inconsistent relationship between gall index (a categorical rating of gall severity) and egg counts for M. enterolobii in tomato. Carmona et al. (2020) recommended that RF be used as the primary measure of resistance to M. enterolobii in sweetpotato, while gall index can facilitate the interpretation of RF.
In this experiment we found samples with high egg counts and low (or zero) gall severity ratings. For example, in replicate 1, TB210 had a gall severity rating of 0% yet 3,793 EGRT, while TB135 in replicate 1, had a gall severity rating of 50% and 1,700 EGRT. For close relative M. incognita, certain environmental conditions have been observed to favor many eggs yet few galls in sweetpotato, or many galls with few eggs. Notably, galling is the plant’s response to the nematode, while egg production is the nematode’s response to the plant (Shepherd 1979). Meloidogyne enterolobii may be more sensitive than M. incognita to these conditions. For all traits evaluated, ‘Tanzania’ was highly M. enterolobii resistant while ‘Beauregard’ was highly susceptible, which is consistent with results from Schwarz et al. (2021). Our results also agree with resistance ratings for the eight TB progeny screened by Schwarz et al. (2021): TB019, TB056, TB068, TB146, TB257 were resistant, while TB085, TB131, TB252 were susceptible.
Meloidogyne enterolobii is under internal quarantine in NC and is also subject to quarantine in several other states in the Southeastern US, and is further included on the European and Mediterranean Plant Protection Organization (EPPO) A2 pest list. These quarantines represent a barrier to resistance evaluations in field settings. The inoculation-based greenhouse bioassay provided consistent inoculation to all genotypes tested, but there were some drawbacks. Due to their small size, M. enterolobii eggs are particularly prone to being flushed from our sand-based media by an overwatering event, and great care was taken to avoid this. Contamination between samples (i.e., splashing during watering, or egg transfer on improperly rinsed sieves during extraction, etc.) may also confound results, though this would largely be an issue for negative controls, as small amounts of added inoculum should not drastically affect resistance classes of inoculated resistant plants. Pearson correlations between replicates were moderate for RF, yet highly significant (r = 0.534, P < 0.001). Eggs may have had differential hatch rates between replicates or genotypes, and root growth differences could also explain this moderate correlation.
The bioassay for M. enterolobii requires permitted and quarantined greenhouse space, specially trained staff, takes at least 74 days, and requires extensive resistance evaluations. We estimate that to screen 500 sweetpotato lines in replicated trials for M. enterolobii resistance would require between 200–500 h of labor, plus supply costs and specialized greenhouse space. It is not feasible to routinely screen large numbers of sweetpotato breeding lines for M. enterolobii resistance in this fashion, yet in a breeding program, evaluating large numbers of genotypes in a high throughput assay is increasingly necessary. The need to assay up to 500 lines annually is not uncommon, and thus designing molecular markers in this QTL region would save a tremendous amount of labor each year and would facilitate the development of a new M. enterolobii-resistant sweetpotato variety. It is not yet known how greenhouse resistance correlates with field resistance to M. enterolobii, and field trials to test greenhouse-resistant genotypes are becoming increasingly necessary.
Plant resistance to RKN is typically race-specific (Ukoskit et al. 1997). Currently it is not clear if distinct races or pathotypes exist for M. enterolobii. For example, M. enterolobii populations on a diversity of crops from sixteen nations exhibited very low genetic diversity based on several different marker types (AFLP, ISSR and RAPD), leading to the conclusion that M. enterolobii was “genetically homogeneous” (Tigano et al. 2010). A phylogenetic analysis and sequencing study of five African M. enterolobii populations found no genetic differences between groups (Onkendi and Moleleki 2013). Four populations of M. enterolobii isolated in NC were found to have no variation in virulence (Schwarz et al. 2020). It may be that diversification of this pest has yet to occur. A recent USDA report found some variation in sweetpotato cultivar response to different M. enterolobii isolates from the Carolinas (Rutter et al. 2021), but there is insufficient evidence to conclusively support the presence of multiple races or pathotypes of M. enterolobii at this time. Meloidogyne enterolobii reproduces via mitotic parthenogenesis, and extremely low diversity would be expected in this primarily asexually reproducing species, however M. incognita, which does have several races, reproduces in the same fashion (Tigano et al. 2010; Yang and Eisenback 1983). Because of the low diversity amongst M. enterolobii populations worldwide, it has been hypothesized that host resistance to one population of M. enterolobii may be effective against many (Tigano et al. 2010).
QTL analysis
Here, we report a single major QTL, qIbMe-4.1, that was consistently detected by multiple models and for multiple resistance parameters. The REMIM model detected a QTL explaining 70% of the variation in M. enterolobii RF and EGRT in the TB population. A population size of 200 individuals is sufficient for detecting QTL with large effects, especially in high-density maps (Hackett et al. 2014). For a map of this marker density, the TB population of 246 individuals was more than sufficient.
The FEIM model tests the null hypothesis that the additive allelic effects are zero using likelihood ratio tests, versus the alternate hypothesis that there is one QTL present (Pereira et al. 2020). Fixed effect models are appropriate for single-QTL scenarios; however, they offer reduced detection power compared to multiple effect models (Pereira et al. 2020). REMIM is the better model for traits explained by multiple QTL, or linked QTL, and this model produces consistently better results than FEIM (Pereira et al. 2020) by using forward–backward significance thresholds established by score-based genome-wide resampling (Zou et al. 2004).
Both FEIM and REMIM models estimated a single major QTL in the same peak position for mean RF and mean EGRT, and in a nearby position for mean gall severity ratings. We tested both replicates individually and found that both models estimated peak QTL position for RF and EGRT at 57.5 cM (Table 2). The 95% support intervals for these QTL were estimated using the peak LOP (REMIM) and LOD (FEIM) minus constant d, for which we used the widely adopted value d = 1.5 (Pereira et al. 2020; Li 2011; Wu et al. 2021). Confidence could be increased to 98% or more, but the tradeoff is a much broader support interval and therefore less precise position estimates for trait-associated markers.
In summary, we report a single major QTL, qIbMe-4.1, at 57.5 cM on LG 4 of I. batatas explaining 70% of the variation in resistance to M. enterolobii within the ‘Tanzania’ x ‘Beauregard’ mapping population. We observed a 1:1 segregation pattern for resistance based on three parameters (RF, EGRT, and gall severity ratings) and qIbMe-4.1 was detected in approximately the same position by all three measures. This segregation pattern, the presence of a single major QTL, and the allele h detected on ‘Tanzania’ at the peak QTL position all provide strong evidence that M. enterolobii may be controlled by a single locus. Detection of qIbMe-4.1 or associated markers within other populations and germplasm is necessary for validation, as it is possible that there are different genetic bases for resistance. It is possible that ‘Tanzania’ may have acquired its resistance to M. enterolobii through a different evolutionary pathway than other resistant lines, but this has not yet been explored. Validation of qIbMe-4.1 in diverse backgrounds would help to resolve this possibility.
Candidate gene search
A BLAST search within the support intervals identified above (Table 2) revealed three candidate genes in I. trifida near the peak QTL position (located at 57.5 cM; 7,039,636 bp) on chromosome 4: ift04g10180.t1, ift04g10190.t1, and ift04g10200.t1. These candidates share homology with the NBS (nucleotide binding site) class of genes, more specifically the NB-ARC domain-containing disease resistance proteins. The NB-ARC (nucleotide-binding adaptor shared by APAF-1, R proteins, and CED-4) domains are central to most R proteins, which recognize pathogens and trigger an innate immune response (Tameling et al. 2006; van Ooijen et al. 2008). Due to their proximity to the QTL peak and their homology with known R-genes, these three candidates may be appropriate targets for the design of selectable markers in breeding for M. enterolobii resistance. Future studies should identify markers within the qIbMe-4.1 locus and test prediction accuracy in diverse germplasm. These results represent major progress towards the first opportunity for marker-assisted selection in sweetpotato breeding, which supports sweetpotato research toward the release of a Southeastern US-adapted and M. enterolobii resistant sweetpotato variety in a timely, cost-effective manner.
Data availability
Phenotypic data from the described experiment and genomic data for QTL analyses are available from the corresponding author upon request.
References
Amankwaah VA (2019) Phenotyping and genetic studies of storage root chemistry traits in sweetpotato. Ph.D. Dissertation, North Carolina State University
Baselga FME, Schultheis JR, Boyette MD, Quesada-Ocampo LM, Starke KD, Monks DW (2020) Vine removal prior to harvest, and curing duration and temperature affect the incidence and severity of internal necrosis in ‘Covington’ sweetpotato. HortTechnol. 30(5): 544–551. https://doi.org/10.21273/HORTTECH04408-19
Bhavana P, Singh AK, Kumar R, Prajapati GK, Thamilarasi K, Manickam R, Maurya S, Choudhary JS (2019) Identification of resistance in tomato against root knot nematode (Meloidogyne incognita) and comparison of molecular markers for Mi gene. Australas Plant Pathol 48(2):93–100. https://doi.org/10.1007/s13313-018-0602-8
Boerma HR, Hussey RS (1992) Breeding plants for resistance to nematodes. J of Nematol 24(2):242–252
Castagnone-Sereno P (2012) Meloidogyne enterolobii (= M. mayaguensis): profile of an emerging, highly pathogenic, root-knot nematode species. Nematol. 14(2): 133–138. https://doi.org/10.1163/156854111X601650
Cervantes-Flores JC, Sosinski B, Pecota KV, Mwanga ROM, Catignani GL, Truong VD, Watkins RH, Ulmer MR, Yencho GC (2011) Identification of quantitative trait loci for dry-matter, starch, and β-carotene content in sweetpotato. Mol Breed 28(2):201–216. https://doi.org/10.1007/s11032-010-9474-5
Cervantes-Flores JC, Yencho GC, Davis EL (2002) Host reactions of sweetpotato genotypes to root-knot nematodes and variation in virulence of Meloidogyne incognita populations. HortSci. 37(7): 1112–1116. https://doi.org/10.21273/HORTSCI.37.7.1112
Cervantes-Flores JC, Yencho GC, Kriegner A, Pecota KV, Faulk MA, Mwanga ROM, Sosinski BR (2008a) Development of a genetic linkage map and identification of homologous linkage groups in sweetpotato using multiple-dose AFLP markers. Mol Breed 21(4):511–532. https://doi.org/10.1007/s11032-007-9150-6
Cervantes-Flores JC, Yencho GC, Pecota KV, Sosinski B, Mwanga ROM (2008b) Detection of quantitative trait loci and inheritance of root-knot nematode resistance in sweetpotato. J of the Am. Soc. for Hort. Sci. 133(6): 844–851. https://doi.org/10.21273/JASHS.133.6.844
Clark CA, Moyer, (1988) Compendium of sweet potato diseases. APS Press, St. Paul, Minnestoa
EPPO (2011) Meloidogyne enterolobii. EPPO Bulletin 41(3):329–339. https://doi.org/10.1111/j.1365-2338.2011.02497.x
Hackett CA, Bradshaw JE, Bryan GA (2014) QTL mapping in autotetraploids using SNP dosage information. Theoretical and Appl Genetics 127(9):1885–1904. https://doi.org/10.1007/s00122-014-2347-2
Hamidi N, Hajihassani A (2020) Differences in parasitism of root-knot nematodes (Meloidogyne spp.) on oilseed radish and oat. J. of Nematol. 52: e2020–43. https://doi.org/10.21307/jofnem-2020-043
Hunt OJ, Faulkner LR, Peaden RN (1972) Breeding for nematode resistance. Alfalfa Sci and Technol 15:355–370. https://doi.org/10.2134/agronmonogr15.c16
Hussey RS, Barker KR (1973) Comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Dis. Report.
Jones A, Dukes PD, Schalk JM (1986) Sweet potato breeding. In: Breeding Vegetable Crops. AVI Publishing Co. Westport, Connecticut.
Karssen G, Wesemael W, and Moens M (2013) Root-knot nematodes. In: Perry, R.N. and Moens, M. (eds). Plant Nematology, 2nd edition. CAB International, Wallingford, UK.
Kirkpatrick T, Lee J, Faske T (2019) The guava root-knot nematode (Meloidogyne enterolobii), a potential threat to Arkansas sweet potatoes and other crops. University of Arkansas Cooperative Extension Service Printing Services, 2.
Li H (2011) A quick method to calculate QTL confidence interval. J of Genetics 90(2):6
Moens M, Perry RN, Starr JL (2009) Meloidogyne species—a diverse group of novel and important plant parasites. In: Perry RN, Moens M, Starr JL (eds), Root-knot nematodes. CABI, Wallingford UK, pp 1–17. https://doi.org/10.1079/9781845934927.0001
Mollinari M, Olukolu BA, Pereira GS, Khan A, Gemenet D, Yencho GC, Zeng ZB (2020) Unraveling the hexaploid sweetpotato inheritance using ultra-dense multilocus mapping. G3 Genes|Genomes|Genetics 10(1): 281–292. https://doi.org/10.1534/g3.119.400620
Mollinari M, Garcia AAF (2019) Linkage analysis and haplotype phasing in experimental autopolyploid populations with high ploidy level using hidden Markov models. G3 Genes|Genomes|Genetics 9(10): 3297–3314. https://doi.org/10.1534/g3.119.400378
Mwanga ROM, Kriegner A, Cervantes-Flores JC, Zhang DP, Moyer JW, Yencho GC (2002) Resistance to sweetpotato chlorotic stunt virus and sweetpotato feathery mottle virus is mediated by two separate recessive genes in sweetpotato. J. of the Am. Soc. for Hort. Sci. 127(5): 798–806. https://doi.org/10.21273/JASHS.127.5.798
Mwanga ROM, Odongo B, p’Obwoya CO, Gibson RW, Smit NEJM., Carey EE (2001) Release of five sweetpotato cultivars in Uganda. HortSci. 36(2): 385–386. https://doi.org/10.21273/HORTSCI.36.2.385
Onkendi EM, Moleleki LN (2013) Detection of Meloidogyne enterolobii in potatoes in South Africa and phylogenetic analysis based on intergenic region and the mitochondrial DNA sequences. Eur J of Plant Pathol 136(1):1–5. https://doi.org/10.1007/s10658-012-0142-y
Oostenbrink M (1966) Major characteristics of the relation between nematodes and plants. Major characteristics of the relation between nematodes and plants. Mededlingen Landbouwhogeschool, Wageningen 66–4:1–46
Pereira GDS, Gemenet DC, Mollinari M, Olukolu BA, Wood JC, Diaz F, Mosquera V, Gruneberg WJ, Khan A, Buell CR, Yencho GC, Zeng ZB (2020) Multiple QTL mapping in autopolyploids: a random-effect model approach with application in a hexaploidy sweetpotato full-sib population. Genetics 215(3):579–595. https://doi.org/10.1534/genetics.120.303080
Philbrick AN, Adhikari TB, Louws FJ, Gorny AM (2020) Meloidogyne enterolobii, a major threat to tomato production: current status and future prospects for its management. Front Plant Sci 11:606395. https://doi.org/10.3389/fpls.2020.606395
Rutter WB, Skantar AM, Handoo ZA, Mueller JD, Aultman SP, Agudelo P (2018) Meloidogyne enterolobii found infecting root-knot nematode resistant sweetpotato in South Carolina. United States Plant Dis 103(4):775–775. https://doi.org/10.1094/PDIS-08-18-1388-PDN
Rutter WB, Wadl P, Mueller JD, Agudelo P (2021) Identification of sweetpotato germplasm resistant to pathotypically distinct isolates of Meloidogyne enterolobii from the Carolinas. Plant Dis 105(10):3147–3153. https://doi.org/10.1094/PDIS-02-20-0379-RE
Schwarz T, Li C, Ye W, Davis E (2020) Distribution of Meloidogyne enterolobii in eastern North Carolina and comparison of four isolates. Plant Health Prog 21(2):91–96. https://doi.org/10.1094/PHP-12-19-0093-RS
Schwarz TR, Li C, Yencho GC, Pecota KV, Heim CR, Davis EL (2021) Screening sweetpotato genotypes for resistance to a North Carolina isolate of Meloidogyne enterolobii. Plant Dis 105(4):1101–1107. https://doi.org/10.1094/PDIS-02-20-0389-RE
Seinhorst JW (1965) The relation between nematode density and damage to plants. Nematologica 11(1):137–154. https://doi.org/10.1163/187529265X00582
Shepherd RL (1979) A quantitative technique for evaluating cotton for root-knot nematode resistance. Phytopathol 69(4):427. https://doi.org/10.1094/Phyto-69-427
Singh B, Bhatti DS, Singh K (1974) Resistance to root-knot nematodes (Meloidogyne spp.) in vegetable crops. PANS Pest Articles & News Summaries 20(1): 58–67. https://doi.org/10.1080/09670877409412336
Tameling WIL, Vossen JH, Albrecht M, Lengauer T, Berden JA, Haring MA, Cornelissen BJC, Takken FLW (2006) Mutations in the NB-ARC domain of I-2 that impair ATP hydrolysis cause autoactivation. Plant Physiol 140(4):1233–1245. https://doi.org/10.1104/pp.105.073510
Taniguti CH, Gesteira GDS, Pereira GS, Zeng ZB, Lau J, Byrne D, Riera-Lizarazu O, Mollinari M (2022) VIEWpoly: a visualization tool to integrate and explore results of polyploid genetic analysis. J of Open Source Softw 7(74):4242
Tigano M, Siqueira KD, Castagnone-Sereno P, Mulet K, Queiroz P, Santos MD, Teixeira C, Almeida M, Silva J, Carneiro R (2010) Genetic diversity of the root-knot nematode Meloidogyne enterolobii and development of a SCAR marker for this guava-damaging species. Plant Pathol 59(6):1054–1061. https://doi.org/10.1111/j.1365-3059.2010.02350.x
Trudgill D, Phillips M (1997) Nematode population dynamics, threshold levels and estimation of crop losses. In Plant Nematode Problems and their Control in the Near East Region. FAO Plant Prod. Prot. 144(7)
Ukoskit K, Thompson PG, Watson CE, Lawrence GW (1997) Identifying a randomly amplified polymorphic DNA (RAPD) marker linked to a gene for root-knot nematode resistance in sweetpotato. J of the Am. Soc. for Hort. Sci. 122(6): 818–821. https://doi.org/10.21273/jashs.122.6.818
USDA/NASS (2020) Vegetables 2019 summary. USDA NASS.
van Ooijen G, Mayr G, Kasiem MMA, Albrecht M, Cornelissen BJC, Takken FLW (2008) Structure-function analysis of the NB-ARC domain of plant disease resistance proteins. J of Experimental Botany 59(6):1383–1397. https://doi.org/10.1093/jxb/ern045
Wadl PA, Olukolu BA, Branham SE, Jarret RL, Yencho GC, Jackson DM (2018) Genetic diversity and population structure of the USDA sweetpotato (Ipomoea batatas) germplasm collections using GBSpoly. Frontiers in Plant Sci 9:1166. https://doi.org/10.3389/fpls.2018.01166
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer-Verlag New York. https://ggplot2.tidyverse.org
Wu PY, Yang MH, Kao CH (2021) A statistical framework for QTL hotspot detection. G3 Genes|Genomes|Genetics 11(4): jkab056. https://doi.org/10.1093/g3journal/jkab056
Wu S, Lau KH, Cao Q, Hamilton JP, Sun H, Zhou C, Eserman L, Gemenet DC, Olukolu BA, Wang H, Crisovan E, Godden GT, Jiao C, Wang X, Kitavi M, Manrique-Carpintero N, Vaillancourt B, Wiegert-Rininger K, Yang X, Bao K, Schaff J, Kreuze J, Gruneberg W, Khan A, Ghislain M, Ma D, Jiang J, Mwanga ROM, Leebens-Mack J, Coin LJM, Yencho GC, Buell CR, Fei Z (2018) Genome sequences of two diploid wild relatives of cultivated sweetpotato reveal targets for genetic improvement. Nat Communications 9(1):4580. https://doi.org/10.1038/s41467-018-06983-8
Yang B, Eisenback JD (1983) Meloidogyne enterolobii n. sp. (Meloidogynidae), a root-knot nematode parasitizing pacara earpod tree in China. J. of Nematol. 15(3): 381–391
Ye W (2018) Nematodes of agricultural importance in North and South Carolina. In: Subbotin SA, Chitambar JJ (eds) Plant parasitic nematodes in sustainable agriculture of North America. Springer International Publishing, Germany, pp 247–276. https://doi.org/10.1007/978-3-319-99588-5_10
Ye WM, Koenning SR, Zhuo K, Liao JL (2013) First report of Meloidogyne enterolobii on cotton and soybean in North Carolina. United States Plant Dis 97(9):1262. https://doi.org/10.1094/PDIS-03-13-0228-PDN
Yencho GC, Pecota KV, Schultheis JR., VanEsbroeck ZP, Holmes GJ, Little BE, Thornton AC, Truong VD (2008) ‘Covington’ sweetpotato. HortSci. 43(6): 1911–1914. https://doi.org/10.21273/HORTSCI.43.6.1911
Zou F, Fine JP, Hu J, Lin DY (2004) An efficient resampling method for assessing genome-wide statistical significance in mapping quantitative trait loci. Genetics 168(4):2307–2316. https://doi.org/10.1534/genetics.104.031427
Funding
This paper is adapted from a MS thesis submitted by Simon Phillip Fraher to North Carolina State University in February 2022. Funding for this research was provided by the Bill and Melinda Gates Foundation (OPP1052983 and OPP1213329) through the Genomic Tools for Sweetpotato Improvement Project (GT4SP) and Sweetpotato Genetic Advances and Innovative Seed Systems (SweetGAINS) projects, as well as the North Carolina Agriculture Foundation Inc. and the North Carolina Crop Improvement Association (NCCIA) and North Carolina Foundation Seed Producers Inc. (NCFSP), and Specialty Crop Research Initiative Grant No. 2021–51181-35865 from the USDA National Institute of Food and Agriculture. Marcelo Mollinari was funded by a USDA NIFA-awarded AFRI grant (Project Number: 2022-67013-36269).
Author information
Authors and Affiliations
Contributions
Bioassays conducted by SF, TS, CH, AG; ZBZ, GDSG, MM, and GDSP developed bioinformatics software and conducted statistical analysis with SF; SF wrote the first draft of the manuscript; GCY, GBG, and AG funded, supported, and guided the project as SF’s MS committee. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Communicated by Sanwen Huang.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fraher, S., Schwarz, T., Heim, C. et al. Discovery of a major QTL for resistance to the guava root-knot nematode (Meloidogyne enterolobii) in ‘Tanzania’, an African landrace sweetpotato (Ipomoea batatas). Theor Appl Genet 137, 234 (2024). https://doi.org/10.1007/s00122-024-04739-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00122-024-04739-1