Abstract
Purpose
Acute hypoxaemic respiratory failure (AHRF) is a common reason for intensive care unit (ICU) admission. However, patient characteristics, outcomes, and trends over time are unclear. We describe the epidemiology and outcomes of patients with AHRF over time.
Methods
In this binational, registry-based study from 2005 to 2022, we included all adults admitted to an Australian or New Zealand ICU with an arterial blood gas within the first 24 h of ICU stay. AHRF was defined as a partial pressure of oxygen/inspired oxygen ratio (PaO2/FiO2) ≤ 300. The primary outcome was adjusted in-hospital mortality, categorised based on PaO2/FiO2 (mild: 200–300, moderate: 100–200, and severe < 100, and non-linearly). We investigated how adjusted mortality evolved based on temporal trends (by year of admission), sex, age, admission diagnosis and the receipt of mechanical ventilation.
Results
Of 1,560,221 patients, 826,106 (52.9%) were admitted with or developed AHRF within the first 24 h of ICU stay. Of these 826,106 patients, 51.4% had mild, 39.3% had moderate, and 9.3% had severe AHRF. Compared to patients without AHRF (5.3%), patients with mild (8%), moderate (14.2%) and severe (29.9%) AHRF had higher in-hospital mortality rates. As PaO2/FiO2 ratio decreased, adjusted in-hospital mortality progressively increased, particularly below an inflection point at a PaO2/FiO2 ratio of 200. The adjusted in-hospital mortality for all patients decreased over time (13.3% in 2005 to 8.2% in 2022), and this trend was similar in patients with and without AHRF.
Conclusion
The healthcare burden due to AHRF may be larger than expected, and mortality rates remain high in severe AHRF. Although mortality has decreased over time, this may reflect improvements in ICU care in general, rather than specifically in AHRF. More research is required to earlier identify AHRF and stratify these patients at risk of deterioration early, and to validate our findings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
In this large, observational, retrospective study of more than 1.5 million patients admitted to an Australian or New Zealand intensive care unit (ICU), more than 50% of patients were admitted with or developed acute hypoxemic respiratory failure (AHRF) within the first 24 h of ICU stay. Mortality increased with worsening PaO2/FiO2 ratios, particularly below a PaO2/FiO2 of 200. The disease burden of AHRF may be higher than previously expected |
Introduction
Globally, approximately one million patients develop acute hypoxaemic respiratory failure (AHRF, a partial pressure of arterial oxygen to fraction of inspired oxygen [PaO2/FiO2] ratio < 300, or an oxygen saturation to FiO2 [SpO2/FiO2] ratio < 315) annually [1,2,3,4]. Some patients with AHRF develop acute respiratory distress syndrome (ARDS) [1, 4, 5], and may require escalating respiratory supports and adjuncts, resulting in prolonged stays in the intensive care unit (ICU) and hospital. This may eventually lead to physical, psychological, and cognitive morbidity, and increased mortality [6,7,8].
Despite this, the proportion of patients admitted with mild, moderate, and severe AHRF remains unclear. There are limited data on short- and long-term outcomes of AHRF and how these have changed over time, given that most broad epidemiological studies focus on the subset of patients with ARDS [2, 5, 9, 10]. In addition, there is a paucity of data from Australia and New Zealand related to AHRF. In this multicentre retrospective registry-based observational study, we investigate the epidemiology and association between AHRF and in-hospital mortality, and trends over time for patients admitted to ICUs over the last 20 years.
Methods
Study design and participants
We received approval from the Alfred Hospital Ethics Committee (reference: 369/23) and the Australian and New Zealand Intensive Care Society Centre for Outcome and Resource Evaluation (ANZICS CORE) Management Committee to issue the data from the ANZICS adult patient database (APD) for this study. We performed a retrospective cohort study using individual patient data from 210 ICUs across Australia and New Zealand. We included all adults (age ≥ 16 years) with an index admission to ICU between 1/1/2005 and 31/12/2022 with a documented arterial blood gas (ABG) within 24 h from ICU admission, and AHRF (defined as a PaO2/FiO2 < 300). We excluded patients who did not have an ABG, a documented PaO2 or FiO2, those transferred from another ICU or a repeat ICU admission, or those admitted for palliative and/or organ donation purposes, and those where in-hospital mortality was not reported.
Data sources, definition, and collection
The ANZICS-APD is a voluntary, bi-national, clinical quality registry dataset collected by the ANZICS Centre for Outcomes and Resources Evaluation that contains information on all admissions to 98% adult ICUs in Australia and 67% of ICUs in New Zealand. Data collectors receive regular training and quality assurance reviews, and data are collected using a standardized data dictionary [11]. In addition, regular automated data checks further ensure the validity of recorded data [12]. Apart from each patient’s demographic details and chronic health parameters. The registry also captures the patient’s demographics, chronic health, and diagnostic, biochemical, and physiological information from the first 24 h of ICU admission, interventions, and outcomes at hospital discharge [11]. More information on the ANZICS-APD is found in the electronic supplementary material (ESM), Methods section.
We extracted data on patient age, sex, comorbidities, ICU admission source, admission diagnosis, acute illness severity (using the Acute Physiology and Chronic Health Evaluation [APACHE]-II, APACHE-III, or sequential organ failure assessment [SOFA] scores, or Australia New Zealand Risk of Death [ANZROD]), treatment limitation at admission to ICU, ICU organ supports (receipt of invasive mechanical ventilation [IMV] during their ICU stay and one day one of ICU, non-invasive ventilation [NIV], vasopressors, extracorporeal membrane oxygenation [ECMO], renal replacement therapy), ICU and hospital mortality, ICU and hospital length of stay, discharge destinations (home and non-home discharge [2005–2016]). With additional data reported to ANZICS-APD from 2017, non-home discharge was reported as new long-term care or rehabilitation between 2017 and 2022. We categorized patients as having no AHRF (PaO2/FiO2 > 300), mild (PaO2/FiO2 200–300), moderate (PaO2/FiO2 100–200) or severe AHRF (PaO2/FiO2 < 100).
Outcomes
The primary outcome was in-hospital mortality, stratified based on the presence and severity of AHRF. Secondary outcomes included ICU mortality, ICU and hospital length of stays, and discharge destination at hospital discharge.
Statistical analysis
We summarized baseline ICU and patient-level characteristics and unadjusted outcomes using standard descriptive statistics. For categorical data, we used counts and percentages, and for continuous data we used mean ± standard deviation (SD) or median (interquartile range, IQR) as appropriate depending on the distribution of data.
To analyze the association between AHRF and in-hospital mortality, we constructed a multivariable, hierarchical logistic regression model. We nested patients within sites, and treated sites as a random effect. We used a causal directed acyclic graph (DAG) to determine the minimum set of covariates to adjust for in the causal pathway from AHRF to in-hospital mortality (ESM, Figure S1). In the regression models, we adjusted for smoking intensity, the presence of chronic respiratory disease, chronic cardiovascular disease, and the APACHE-III score (see ESM, Methods section for definitions of covariates). We defined the severity of AHRF using PaO2/FiO2 ratios, which we modelled as a continuous non-linear variable using restricted cubic splines with four knots.
We then investigated the trends in adjusted in-hospital mortality in the overall cohort, and stratifying based on the presence and severity of AHRF (no AHRF, mild AHRF, moderate AHRF, and severe AHRF). We described these changes over time by estimating the absolute risk reduction (ARR) with its corresponding 95% confidence interval (CI). To determine whether these changes over time differed between the AHRF categories, we introduced an interaction term between time and the individual AHRF categories. Only patients with complete data for all covariates were included in the analysis. Given that there is an increased risk of Type-1 error with multiple testing, the results of the secondary objectives should be viewed as exploratory and as such, no adjustment for multiplicity was used.
We then turned our attention to predefined subgroups, stratifying the in-hospital mortality of the cohort based on the level of respiratory support (no respiratory support, non-invasive ventilation [reference group], invasive mechanical ventilation, and extracorporeal membrane oxygenation), hospital type (public tertiary [reference group], metropolitan, rural/regional, and private), biological sex (male [reference group] and female), age categories (≤ 44 years [reference group], 45–64 years, 65–84 years, and ≥ 84 years), and treatment limitations (no limitations [reference group] and limitations of care). In the analyses above, we estimated the average marginal effect, which estimates the average difference in in-hospital mortality due to a change in the predictor (i.e., between each subgroup). To better understand how the severity of AHRF affected in-hospital mortality in these subgroups, we described the association between PaO2/FiO2 ratios in each individual subgroup, again modelling PaO2/FiO2 ratio as a continuous non-linear variable using restricted cubic splines with four knots.
We performed two sensitivity analyses. First, we substituted the APACHE-III score for the APACHE-II, SOFA, and ANZROD scores. Second, we repeated the regression models in a subgroup of patients receiving IMV for more than 12 h, to reduce the chances of including patients who received IMV for non-respiratory condition reasons (for example, post-operatively).
Statistical analyses were performed using R Version 4.3.1 (R Core Team, R Foundation for Statistical Computing, Vienna, Austria) and RStudio Version 2023.06.1 (Posit Software, PBC, Boston, MA). Packages used for analysis included tidyverse [13], data.table [14], gtsummary [15], lme4 [16], survival [17], ggsurvfit [18], marginaleffects [19] and gt [20]. Reproducible code for the analysis is available online at https://github.com/BLMoran/APD_AHRF.
Post-hoc analyses
We conducted the following post-hoc analyses to better define the subgroups in our population. First, we investigated the difference in mortality between patients who were intubated within and after the first 24 h of ICU stay, patients who were admitted with and without a medical emergency team (MET) activation, and the presence of coronavirus disease 2019 (COVID-19) (see ESM, Methods section for more details) within the years 2020–2022.
Results
1,618,713 patients were admitted to an ICU in Australia and New Zealand during the study period; 1,560,221 patients were included in our analysis after applying the inclusion and exclusion criteria (ESM, Figure S2). There were 1,431,393 patients from Australia, and 128,828 patients from New Zealand. The median age was 66 years (IQR: 53–75), and 641,352 patients (41.1%) were female. Patients had relatively severe disease (median APACHE II score 15 [IQR: 11–21], median APACHE III score 52 [IQR: 39–68], median SOFA score 4 [IQR: 2–6]). Medical diagnoses were the most common reason for ICU admission (478,338, 31%). 4,451 patients (0.3%) had a diagnosis of COVID-19 pneumonitis. Table 1 presents the baseline characteristics in the overall population, and in patients without and with individual severities of AHRF, and supplemental Table S1 summarizes the characteristics between patients admitted in Australia and in New Zealand. Supplemental Table S2 summarizes the missingness in demographic and covariate data.
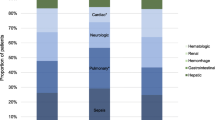
Of the study population, 826,106 (52.9%) were admitted with or developed acute hypoxaemic respiratory failure within 24 h of ICU admission; 424,382 (27.2%) had mild AHRF, 324,630 (20.8%) had moderate AHRF, and 77,094 (4.9%) had severe AHRF. The proportion of patients who had AHRF remained relatively stable over time between 50% and 60%, as did the individual severities of AHRF (Fig. 1). Respiratory diagnoses accounted for the largest proportion of patients with severe AHRF (between 23% and 32% across the study period), followed by patients receiving cardiac surgery (15–18%, Table 1, and ESM, Figure S3). The trend of ICU admission diagnoses over time, and based on AHRF severity is summarized in ESM, Fig. S3.
Patients with AHRF (103,182/826,106, 12.5%) had a higher in-hospital mortality than patients without AHRF (39,250/734,115, 5.3%); mortality correspondingly increased as AHRF became more severe (mild: 33,946/424,382, 8%, moderate: 46,214/324,630, 14.2%, severe: 23,022/77,094, 29.9%). For every decrease in PaO2/FiO2 ratio by 100, the adjusted risk of in-hospital mortality increased by 3.6% (95% CI 3.48–3.72%). Interestingly, when PaO2/FiO2 was modelled as a continuous non-linear variable, an inflection point of in-hospital mortality was noted at a PaO2/FiO2 of 200, where subsequent decreases in the PaO2/FiO2 ratio were associated with progressively larger increases in the adjusted in-hospital mortality risk (Fig. 2). This finding was consistent across several sensitivity analyses (supplemental Fig. S4). Over time, the adjusted risk of mortality decreased from 13.28% (95% CI 13.09–13.46%) in the year 2005 to 8.2% (95% CI 8.13–8.27%) in the year 2022 (absolute risk reduction: 5.079%, 95% CI: 5.077–5.081%); a similar trend is noted across mild, moderate, and severe AHRF (Fig. 3), and in all patients with and without AHRF (ESM, Fig. S5).
We then analysed data pertaining to several subgroups of interest. Patients who were intubated had a higher adjusted risk of mortality compared to patients who were never intubated (average marginal effect [AME]: 1.26%, 95% CI 1.12–1.41%). Compared to patients who were never intubated, patients who were intubated within the first 24 h of ICU stay (AME: 0.22%, 95% CI 0.01–0.34%) and after the first 24 h of ICU stay (AME: 4.73%, 95% CI 4.34–5.13%) had a higher risk of mortality. However, patients who were intubated after the first 24 h had a substantially higher risk of mortality compared to those intubated within the first 24 h (AME: 7.01%, 95% CI: 6.43–7.6%) Similarly, patients receiving non-invasive ventilation (AME: 2.6%, 95% CI 2.39–2.82%) and patients receiving ECMO (AME: 3.76%, 95% CI 2.74–4.77%) had a higher risk of mortality compared to patients who did not receive these interventions. Increasing age, admission after a MET activation, the presence of treatment limitations, COVID-19 pneumonitis and probable COVID-19 or an exacerbation of condition due to COVID-19 were also associated with higher in-hospital mortality. Table 2 and supplementary Figure S6 summarize the associations between PaO2/FiO2 ratios and adjusted in-hospital mortality within the individual subgroups.
Similar to hospital mortality, ICU mortality decreased over time (absolute risk reduction: 3.173%, 95% CI 3.171–3.174% from 2005 to 2022), and this was consistent across all severities of AHRF. There was also a reduction in adjusted length of ICU and hospital stay over the study period. As with hospital mortality, a more severe presentation of AHRF was associated with higher ICU mortality, and longer stays in the ICU and hospital. Patients with more severe AHRF were more likely have a non-home discharge, and be transferred between hospitals. Supplemental Figure S7 describes the trends in secondary outcomes over time across the entire cohort and based on AHRF severity; supplementary Table S3 summarizes the raw unadjusted primary and secondary outcomes.
Discussion
Our binational, multicentre, registry-based study found that more than 50% of patients in ICU were admitted with or developed AHRF within 24 h of ICU admission. In-hospital mortality increased with worsening PaO2/FiO2, and a substantial increase in mortality was noted below a PaO2/FiO2 of 200; nonetheless in-hospital mortality decreased across all severities over time. Patients who received respiratory supports had a higher risk of mortality; of note, patients who were intubated later during their ICU stay beyond 24 h had substantially poorer outcomes than those who were intubated within the first 24 h.
We found a non-linear association between PaO2/FiO2 and mortality, in particular a sharp increase in mortality as PaO2/FiO2 decreased below 200. These findings are based on a large multicentre registry spanning over 17 years, which increases the precision of the estimates. Furthermore, the concordance with subgroup and sensitivity analyses lent further weight to the results. Other smaller studies report similar results in patients receiving mechanical ventilation [21]. These findings raise several questions on the stratification of AHRF as “mild”, “moderate” and “severe”, and the inflection point at PaO2/FiO2 of 200 may offer an alternative categorisation of AHRF. We speculate that above PaO2/FiO2 of 200, other pathologies concomitant to respiratory failure may be the main contributor to mortality. Below a PaO2/FiO2 of 200, the contribution of respiratory failure to mortality increased. Mortality has also generally decreased with time [22], which may coincide with improvements in healthcare practices [23]. However, there was a transient increase in mortality since 2020, which may be related to the impact of COVID-19 healthcare systems [24,25,26] and management practices earlier on in the pandemic [27,28,29]. Our subgroup analysis had also found that patients with confirmed or probable COVID-19 had a higher mortality rate than those without COVID-19. Although mortality has decreased over time, this trend was noted regardless of the presence of AHRF, or an individual PaO2/FiO2. This might suggest that while management practices have improved, this may not be not specific to AHRF, and may reflect advances in management of critical illness in general. Nonetheless, without more granular data regarding the in-hospital course, it is challenging to distinguish the predictive capacity and contribution of each diagnosis and intervention to mortality. In addition, any potential explanations of the trends observed in a descriptive analysis like ours remain speculative.
The proportion of patients with AHRF is substantially higher than the prevalence of ARDS [30]. Given that up to 13.6% of all patients die, and mortality reaches in excess of 30% in severe AHRF, there may be significantly larger disease burden due to AHRF than previously expected. It is important to consider the heterogeneity in AHRF [31], and its potential trajectories. A large proportion of patients are at risk of rapid and severe deterioration with high mortality rates, particularly nearing a PaO2/FiO2 of 200 (representing 48% of the total population admitted to ICU). Moreover, trends in mortality over time were more representative of general improvements in practices in critical illness in general, rather than an improvement in AHRF management specifically. As such, earlier identification of AHRF, and appropriate risk stratification and prognostication can help improve outcomes and reduce its disease burden. Research on interventions in AHRF has mostly been limited to ARDS [32,33,34,35,36,37]. Emphasising research for interventions earlier in the disease course, particularly PaO2/FiO2 ≥ 200, may reduce progression to severe AHRF or ARDS, both of which are associated with poorer outcomes. In addition, more research is needed to better delineate the demographics and characteristics of patients with AHRF outside of Australia and New Zealand alone, and to validate our findings.
However, there are a few potential limitations. First, the analysis is based on retrospective, registry-collected data that was limited to those patients with an ABG in the first 24 h. We did not have data regarding AHRF management beyond certain respiratory supports, nor delay to intubation, progression of disease severity and organ failure beyond day 1 of ICU stay. There may be confounders which our analysis did not adjust for. Second, we defined AHRF based on the worst PaO2/FiO2 within the first 24 h of ICU admission, which underestimates the proportion of patients who develop AHRF later during their ICU stay. This may have an association with mortality, though the direction of this association is unclear. There were also 7.3% of patients who had chronic respiratory failure, and we did not have information regarding the acuity of the patient’s hypoxaemia. In addition, only a single ABG value was available for each patient, and it is unclear how more information about the oxygenation status of each patient would have affected our results (for example, in rapidly improving respiratory failure [38]). This may have concurrently led to an overestimation of AHRF, and underestimated mortality rates. Third, there are challenges in precisely estimating the FiO2 in patients who received oxygen supplementation without mechanical ventilation (i.e., conventional or high-flow oxygen therapy). Fourth, it is difficult to ascertain and whether patients were admitted due to, or with respiratory failure. While an APACHE III-J diagnostic code is tagged to each admission, some patients with AHRF may not have a code specific to acute respiratory failure or a pulmonary diagnosis. The ANZICS-APD also does not record the cause of death, which exacerbates this challenge. Fifth, there is missingness in the ANZICS-APD, particularly for interventions such as invasive mechanical ventilation (which was only made mandatory from 2019 onwards). In addition, the registry only captures 67% of patients admitted to ICUs in New Zealand, though the missing data from New Zealand would only represent at most 4% of our current cohort. This nonetheless limits the precision of our analysis and may have an unclear bias to our results. Equally important is the consideration that the ANZICS-APD was initially devised as a quality assurance registry dataset but has evolved to fit the requirements of the ICU community in Australia and New Zealand. Finally, these results may not be translatable to other countries or populations, and analyses of other regions may suggest other findings.
Conclusion
In this multicentre retrospective study, more than half of patients with critical illness were admitted with or developed AHRF within 24 h of ICU admission. As PaO2/FiO2 decreased below 200, there was a sharp increase in hospital mortality rates, which may suggest the need to reconsider how we stratify AHRF severity. Although mortality decreased over time, this was more representative of improvements in care for patients with critical illness in general, rather than AHRF specifically. The disease burden of AHRF may be higher than previously expected, and more prospective, multicentre studies are required to validate these findings.
Availability of data and materials
The ANZICS APD data dictionary and policies are available online at https://www.anzics.com.au/adult-patient-database-apd/. The participant data collected for this study are available, as a limited dataset, to member centres conditional on approval from the ANZICS Centre for Outcome and Resource Evaluation Management Committee, but it is not publicly available.
References
Villar J, Blanco J, Añón JM, Santos-Bouza A, Blanch L, Ambrós A, Gandía F, Carriedo D, Mosteiro F, Basaldúa S, Fernández RL, Kacmarek RM (2011) The ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med 37:1932–1941
Kopczynska M, Sharif B, Pugh R, Otahal I, Havalda P, Groblewski W, Lynch C, George D, Sutherland J, Pandey M, Jones P, Murdoch M et al (2020) Prevalence and outcomes of acute hypoxaemic respiratory failure in Wales: the PANDORA-WALES study. J Clin Med 9:3521
Matthay MA, Arabi Y, Arroliga AC, Bernard G, Bersten AD, Brochard LJ, Calfee CS, Combes A, Daniel BM, Ferguson ND, Gong MN, Gotts JE et al (2023) A new global definition of acute respiratory distress syndrome. Am J Respir Crit Care Med 209:37–47
Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS (2012) Acute respiratory distress syndrome: the Berlin Definition. JAMA 307:2526–2533
Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, Ranieri M, Rubenfeld G et al (2016) Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 315:788–800
Darvall JN, Bellomo R, Bailey M, Young PJ, Rockwood K, Pilcher D (2022) Impact of frailty on persistent critical illness: a population-based cohort study. Intensive Care Med 48:343–351
Damuth E, Mitchell JA, Bartock JL, Roberts BW, Trzeciak S (2015) Long-term survival of critically ill patients treated with prolonged mechanical ventilation: a systematic review and meta-analysis. Lancet Respir Med 3:544–553
Iwashyna TJ, Hodgson CL, Pilcher D, Bailey M, van Lint A, Chavan S, Bellomo R (2016) Timing of onset and burden of persistent critical illness in Australia and New Zealand: a retrospective, population-based, observational study. Lancet Respir Med 4:566–573
Villar J, Mora-Ordoñez JM, Soler JA, Mosteiro F, Vidal A, Ambrós A, Fernández L, Murcia I, Civantos B, Romera MA, Mira A, Díaz-Domínguez FJ et al (2022) The PANDORA study: prevalence and outcome of acute hypoxemic respiratory failure in the Pre-COVID-19 era. Crit Care Explor 4:e0684
Parhar KKS, Zjadewicz K, Soo A, Sutton A, Zjadewicz M, Doig L, Lam C, Ferland A, Niven DJ, Fiest KM, Stelfox HT, Doig CJ (2019) Epidemiology, mechanical power, and 3-year outcomes in acute respiratory distress syndrome patients using standardized screening. an observational cohort study. Ann Am Thorac Soc 16:1263–1272
ANZCIS Centre for Outcomes and Resource Evaluation (2022) Adult patient database data dictionary. https://www.anzics.com.au/wp-content/uploads/2021/03/ANZICS-APD-Dictionary.pdf. Accessed 1 Jan 2024
Australian and New Zealand Intensive Care Society (2021) ANZICS centre for outcomes and resource evaluation. Centre for Resource and Outcomes Evaluation 2020 Report
Wickham H, Averick M, Bryan J, Chang W, McGowan L, François R, Grolemund G, Hayes A, Henry L, Hester J, Kuhn M, Pedersen T, Miller E, Bache S, Müller K, Ooms J, Robinson D, Seidel D, Spinu V, Yutani H (2019) Welcome to Tidyverse. J Open Source Softw 4:1686
Barrett TDM, Srinivasan A, Gorecki J, Chirico M, Hocking T (2024) data.table: extension of ‘data.frame’. R package version 1.14.99. https://Rdatatable.gitlab.io/data.table, https://github.com/Rdatatable/data.table, https://r-datatable.com. Accessed 30 Jan 2024
Sjoberg DWK, Curry M, Lavery J, Larmarange J (2021) Reproducible summary tables with the gtsummary package. R J 13:570–580
Bates DMM, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
T T (2023) A package for survival analysis in R. R package version 3.5-7. https://CRAN.R-project.org/package=survival. Accessed 30 Jan 2024
Sjoberg DBM, Fruechtenicht C, Haesendonckx S, Treis T (2024) ggsurvfit: flexible time-to-event figures. R package version 1.0.0. https://www.danieldsjoberg.com/ggsurvfit. Accessed 30 Jan 2024
V A-B (2024) Marginal effects: predictions, comparisons, slopes, marginal means, and hypothesis tests. R package version 0.17.0.9003
Iannone RCJ, Schloerke B, Hughes E, Lauer A, Seo J (2024) gt: easily create presentation-ready display tables. R package version 0.10.1.9000
Ruan S, Huang C, Chien K, Kuo L, Ku S, Kuo P, Wu H (2019) PaO2/FiO2 ratio and risk of death among mechanically ventilated patients. Am J Respir Crit Care Med 199:1368–1376
Kempker JA, Abril MK, Chen Y, Kramer MR, Waller LA, Martin GS (2020) The epidemiology of respiratory failure in the United States 2002–2017: a serial cross-sectional study. Crit Care Explor 2:e0128
Esteban A, Frutos-Vivar F, Muriel A, Ferguson ND, Peñuelas O, Abraira V, Raymondos K, Rios F, Nin N, Apezteguía C, Violi DA, Thille AW et al (2013) Evolution of mortality over time in patients receiving mechanical ventilation. Am J Respir Crit Care Med 188:220–230
Begum H, Neto AS, Alliegro P, Broadley T, Trapani T, Campbell LT, Cheng AC, Cheung W, Cooper DJ, Erickson SJ, French CJ, Litton E et al (2022) People in intensive care with COVID-19: demographic and clinical features during the first, second, and third pandemic waves in Australia. Med J Aust 217:352–360
Pilcher D, Coatsworth NR, Rosenow M, McClure J (2021) A national system for monitoring intensive care unit demand and capacity: the Critical Health Resources Information System (CHRIS). Med J Aust 214:297-298 e291
The Australian and New Zealand Intensive Care Society Centre for Outcome and Resource Evaluation (2022) Report on COVID-19 admissions to intensive care in Australia
Lim ZJ, Subramaniam A, Ponnapa Reddy M, Blecher G, Kadam U, Afroz A, Billah B, Ashwin S, Kubicki M, Bilotta F, Curtis JR, Rubulotta F (2021) Case fatality rates for patients with COVID-19 requiring invasive mechanical ventilation. a meta-analysis. Am J Respir Crit Care Med 203:54–66
Cao Y, Hiyoshi A, Montgomery S (2020) COVID-19 case-fatality rate and demographic and socioeconomic influencers: worldwide spatial regression analysis based on country-level data. BMJ Open 10:e043560
Wu Z, McGoogan JM (2020) Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese center for disease control and prevention. JAMA 323:1239–1242
Hendrickson KW, Peltan ID, Brown SM (2021) The epidemiology of acute respiratory distress syndrome before and after coronavirus disease 2019. Crit Care Clin 37:703–716
Scala R, Heunks L (2018) Highlights in acute respiratory failure. Eur Respir Rev 27:180008
Briel M, Meade M, Mercat A, Brower RG, Talmor D, Walter SD, Slutsky AS, Pullenayegum E, Zhou Q, Cook D, Brochard L, Richard JC et al (2010) Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA 303:865–873
Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, Schoenfeld D, Thompson BT (2004) Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 351:327–336
Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342:1301–1308
Meade MO, Young D, Hanna S, Zhou Q, Bachman TE, Bollen C, Slutsky AS, Lamb SE, Adhikari NKJ, Mentzelopoulos SD, Cook DJ, Sud S et al (2017) Severity of hypoxemia and effect of high-frequency oscillatory ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med 196:727–733
Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, Jaber S, Arnal JM, Perez D, Seghboyan JM, Constantin JM, Courant P et al (2010) Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 363:1107–1116
Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, Hyzy R, Thompson BT, Ancukiewicz M (2006) Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med 354:1671–1684
Schenck EJ, Oromendia C, Torres LK, Berlin DA, Choi AMK, Siempos II (2019) Rapidly improving ARDS in therapeutic randomized controlled trials. Chest 155:474–482
Acknowledgements
The authors and the ANZICS CORE management committee would like to thank clinicians, data collectors and researchers at the following contributing sites: Australian Capital Territory (ACT): Calvary Bruce Private Hospital HDU, Calvary John James Hospital ICU, Canberra Hospital ICU, National Capital Private Hospital ICU, North Canberra Hospital ICU; New South Wales (NSW): Bankstown-Lidcombe Hospital ICU, Bathurst Base Hospital ICU, Blacktown Hospital ICU, Bowral Hospital HDU, Brisbane Waters Private Hospital ICU, Broken Hill Base Hospital & Health Services ICU, Calvary Mater Newcastle ICU, Campbelltown Hospital ICU, Coffs Harbour Health Campus ICU, Concord Hospital (Sydney) ICU, Dubbo Base Hospital ICU, Fairfield Hospital ICU, Figtree Private Hospital ICU, Gosford Hospital ICU, Gosford Private Hospital ICU, Goulburn Base Hospital ICU, Grafton Base Hospital ICU, Griffith Base Hospital ICU, Hornsby Ku-ring-gai Hospital ICU, Hurstville Private Hospital ICU, John Hunter Hospital ICU, Kareena Private Hospital ICU, Lingard Private Hospital ICU, Lismore Base Hospital ICU, Liverpool Hospital ICU, Macquarie University Private Hospital ICU, Maitland Hospital ICU, Maitland Private Hospital ICU, Manly Hospital & Community Health ICU, Manning Rural Referral Hospital ICU, Mater Private Hospital (Sydney) ICU, Nepean Hospital ICU, Nepean Private Hospital ICU, Newcastle Private Hospital ICU, North Shore Private Hospital ICU, Northern Beaches Hospital ICU, Norwest Private Hospital ICU, Orange Base Hospital ICU, Port Macquarie Base Hospital ICU, Prince of Wales Hospital (Sydney) ICU, Prince of Wales Private Hospital (Sydney) ICU, Royal North Shore Hospital ICU, Royal Prince Alfred Hospital ICU, Ryde Hospital and Community Health Services ICU, Shoalhaven Hospital ICU, South East Regional Hospital ICU, St George Hospital (Sydney) CICU, St George Hospital (Sydney) ICU, St George Hospital (Sydney) ICU2, St George Private Hospital (Sydney) ICU, St Vincent's Hospital (Sydney) ICU, St Vincent's Private Hospital (Sydney) ICU, Sutherland Hospital & Community Health Services ICU, Sydney Adventist Hospital ICU, Sydney Southwest Private Hospital ICU, Tamworth Base Hospital ICU, The Chris O’Brien Lifehouse ICU, Tweed Heads District Hospital ICU, Wagga Wagga Base Hospital & District Health ICU, Westmead Hospital ICU, Westmead Private Hospital ICU, Wollongong Hospital ICU, Wollongong Private Hospital ICU, Wyong Hospital ICU; Northern Territory (NT): Alice Springs Hospital ICU, Royal Darwin Hospital ICU; Queensland (Qld): Allamanda Private Hospital ICU, Brisbane Private Hospital ICU, Buderim Private Hospital ICU, Bundaberg Base Hospital ICU, Caboolture Hospital ICU, Cairns Hospital ICU, Gold Coast Private Hospital ICU, Gold Coast University Hospital ICU, Greenslopes Private Hospital ICU, Hervey Bay Hospital ICU, Ipswich Hospital ICU, John Flynn Private Hospital ICU, Logan Hospital ICU, Mackay Base Hospital ICU, Mater Adults Hospital (Brisbane) ICU, Mater Private Hospital (Brisbane) ICU, Mater Private Hospital (Townsville) ICU, Mount Isa Hospital ICU, Nambour General Hospital ICU, Noosa Hospital ICU, North West Private Hospital ICU, Pindara Private Hospital ICU, Princess Alexandra Hospital ICU, Queen Elizabeth II Jubilee Hospital ICU, Redcliffe Hospital ICU, Robina Hospital ICU, Rockhampton Hospital ICU, Royal Brisbane and Women's Hospital ICU, St Andrew's Hospital Toowoomba ICU, St Andrew's Private Hospital (Ipswich) ICU, St Andrew's War Memorial Hospital ICU, St Vincent's Hospital (Toowoomba) ICU, St Vincent’s Private Hospital Northside ICU, Sunnybank Hospital ICU, Sunshine Coast University Hospital ICU, Sunshine Coast University Private Hospital ICU, The Prince Charles Hospital ICU, The Wesley Hospital ICU, Toowoomba Hospital ICU, Townsville University Hospital ICU; South Australia (SA): Ashford Community Hospital ICU, Calvary Adelaide Hospital ICU, Calvary North Adelaide Hospital ICU, Flinders Medical Centre ICU, Flinders Private Hospital ICU, Lyell McEwin Hospital ICU, Modbury Public Hospital ICU, Repatriation General Hospital (Adelaide) ICU, Royal Adelaide Hospital ICU, St Andrew's Hospital (Adelaide) ICU, The Memorial Hospital (Adelaide) ICU, The Queen Elizabeth (Adelaide) ICU, Western Hospital (SA) ICU, Women's and Children's Hospital PICU; Tasmania (TAS): Calvary Hospital (Lenah Valley) ICU, Launceston General Hospital ICU, North West Regional Hospital (Burnie) ICU, Royal Hobart Hospital ICU; Victoria (VIC): Albury Wodonga Health ICU, Alfred Hospital ICU, Angliss Hospital ICU, Austin Hospital ICU, Ballarat Health Services ICU, Bendigo Health Care Group ICU, Box Hill Hospital ICU, Cabrini Hospital ICU, Casey Hospital ICU, Central Gippsland Health Service (Sale) ICU, Dandenong Hospital ICU, Echuca Regional Hospital HDU, Epworth Eastern Private Hospital ICU, Epworth Freemasons Hospital ICU, Epworth Geelong ICU, Epworth Hospital (Richmond) ICU, Footscray Hospital ICU, Frankston Hospital ICU, Goulburn Valley Health ICU, Grampians Health Horsham ICU, Holmesglen Private Hospital ICU, John Fawkner Hospital ICU, Knox Private Hospital ICU, Latrobe Regional Hospital ICU, Maroondah Hospital ICU, Melbourne Private Hospital ICU, Mildura Base Public Hospital ICU, Monash Medical Centre-Clayton Campus ICU, Mulgrave Private Hospital ICU, Northeast Health Wangaratta ICU, Peninsula Private Hospital ICU, Peter MacCallum Cancer Institute ICU, Royal Melbourne Hospital ICU, South West Healthcare (Warrnambool) ICU, St John Of God Hospital (Ballarat) ICU, St John of God Hospital (Bendigo) ICU, St John of God Hospital (Berwick) ICU, St John Of God Hospital (Geelong) ICU, St Vincent's Hospital (Melbourne) ICU, St Vincent's Private Hospital Fitzroy ICU, Sunshine Hospital ICU, The Bays Hospital ICU, The Northern Hospital ICU, University Hospital Geelong ICU, Warringal Private Hospital ICU, Werribee Mercy Hospital ICU, Western District Health Service (Hamilton) ICU, Western Private Hospital ICU; Western Australia (WA): Armadale Health Service ICU, Bunbury Regional Hospital ICU, Fiona Stanley Hospital ICU, Fremantle Hospital ICU, Hollywood Private Hospital ICU, Joondalup Health Campus ICU, Mount Hospital ICU, Rockingham General Hospital ICU, Royal Perth Hospital ICU, Sir Charles Gairdner Hospital ICU, St John Of God Health Care (Subiaco) ICU, St John Of God Hospital (Murdoch) ICU, St John of God Midland Public & Private ICU; New Zealand (NZ): Auckland City Hospital CV ICU, Auckland City Hospital DCCM, Braemar Hospital SCU, Christchurch Hospital ICU, Dunedin Hospital ICU, Hawkes Bay Hospital ICU, Hutt Hospital ICU, Middlemore Hospital ICU, Nelson Hospital ICU, North Shore Hospital ICU, Rotorua Hospital ICU, Southern Cross Hospital (Hamilton) ICU, Southern Cross Hospital (Wellington) ICU, Taranaki Health ICU, Tauranga Hospital ICU, Timaru Hospital ICU, Waikato Hospital ICU, Wairau Hospital ICU, Wellington Hospital ICU, Whakatane Hospital ICU, Whangarei Area Hospital—Northland Health Ltd ICU.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
RRL receives research support from the Clinician Scientist Development Unit, Yong Loo Lin School of Medicine, National University of Singapore. AB is a recipient of the National Health and Medical Research Council Emerging Leader Fellowship (201110). DP is the chairman of the ANZICS Centre for Outcomes and Resources Evaluation Clinical Quality Registry and is the vice-president and board member of ANZICS. All other authors declare no competing interests. KS acknowledges Metro Health. No other conflicts of interest for other authors.
Ethics approval and consent to participate
We received approval from the Alfred Hospital Ethics Committee (Reference 369/23) and the Australian and New Zealand Intensive Care Society (ANZICS) Centre for Outcome and Resource Evaluation Management Committee to issue the data from the ANZICS adult patient database (APD) for this study. Consent to participate was waived in view of the deidentified nature of the data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ling, R.R., Ponnapa Reddy, M., Subramaniam, A. et al. Epidemiology of acute hypoxaemic respiratory failure in Australian and New Zealand intensive care units during 2005–2022. A binational, registry-based study. Intensive Care Med (2024). https://doi.org/10.1007/s00134-024-07609-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00134-024-07609-y