Abstract
Prey naiveté has been implicated in the global decline and reintroduction failure of many threatened species. A number of tools have been developed to combat prey naiveté including in situ predator exposure using live predators. However, determining the effectiveness and persistence of these interventions can be difficult, and requires comparisons of individual responses from predator-naïve and predator-trained populations. Even when this occurs, interpreting behavioural responses can be challenging because of the constraints of experimental design in large scale population manipulations. We used a common environment experiment and placed burrowing bettongs (Bettongia lesueur) from predator-free and predator-exposed populations together in an 8 ha fenced paddock. The predator-exposed population had been previously exposed to feral cats (Felis catus) under controlled wild conditions for up to six years and initial work suggested this exposure had led to physical and behavioural changes. Within our common environment experiment we compared a range of anti-predator behaviours including trap and feeding behaviour and response to predator scent and models. We found evidence for persistent trait changes up to 7 months, the latest time behaviours were tested, after bettongs were moved to the predator-free common environment and isolated from predators. These behaviours include reduced alarm rates, preference for open areas, and responses to predator scent and model stimuli. Additionally, high risk predator cues were more likely to elicit anti-predator responses than passive measures of anti-predator behaviour. Our design shows that predator exposure leads to persistent shifts in some, but not all, anti-predator behaviours. Identifying the proximate basis of anti-predator traits is important to develop successful tools to combat prey naiveté.
Significant statement
Controlled exposure to live predators can lead to improved and persistent changes in some anti-predator responses in naïve mammals. Traits were retained for several months suggesting exposing naïve mammals to low densities of predators prior to reintroduction could assist with improving their ability to co-exist with predators in the longer term. Using high risk predator cues to test anti-predator responses was more successful that passive cues suggesting that practitioners should prioritise the use of high-risk cues when testing anti-predator responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prey naiveté is the inability to recognise or respond appropriately to predators and occurs when prey species have had evolutionary or ontogenetic isolation from predators (Carthey and Blumstein 2018). Prey naivety contributes to reintroduction failure and threatened species decline, and is a particular problem for island fauna where species have evolved in isolation from predators (Fritts and Rodda 1998). Many methods have been employed in an attempt to train naïve prey to improve their anti-predator behaviour, both in captivity and the wild (Griffin et al. 2000). Most focus on the development of traits that increase vigilance or improve the ability of prey to recognise, evade or avoid predators because these abilities are essential for the successful reintroduction of captive-bred animals or for reintroduction to areas where introduced predators are present (e.g. Miller et al. 1990; Holzer et al. 1995; McClean et al. 1996).
Understanding and addressing the issue of prey naiveté requires a measure of the baseline level of naivety present in prey populations and how it changes after experimental manipulation. Recent research has focussed on using real predators to train naïve prey with significant changes reported in physical and behavioural traits after exposure (Moseby et al. 2016; West et al. 2018a; Blumstein et al. 2019) as well as selection (Moseby et al. 2023) and improved post release survival (Moseby et al. 2016; West et al. 2018a; Blumstein et al. 2019; Ross et al. 2019). The use of in situ predator training has been considered as a possible method to improve survival of prey species by allowing them to build or improve anti-predator traits through exposure and possible selection (Moseby et al. 2016). The advantage of using real predators under wild conditions is twofold. Firstly, predator cues used by prey are not presented in isolation, and situational risk variables such as habitat and predator behaviour are incorporated into risk assessments by prey. Secondly, studying prey responses in natural settings or using real predators may provide more realistic measures of prey anti-predator behaviour than purely under captive experimental conditions. Importantly, using a variety of different approaches and quantifying a number of different variables can provide more meaningful results in predator-risk areas and insights into prey’s ability to recognise and respond appropriately. Such comprehensive evaluation may be required to both measure naivety and evaluate the success of anti-predator training.
However, such large-scale experiments have been unable to control for other factors that could influence behaviour such as the density of conspecifics or differences in habitat or environmental conditions between treatments. One way to overcome these issues is to use a common environment where predator-exposed and control animals can both be studied simultaneously under natural conditions. This reduces the potential for confounding factors and allows tighter inferences about the effect of treatment. A possibly negative effect of using a common environment experiment is that it requires removal of predator-exposed animals from their predator exposure possibly leading to loss of anti-predator behaviour. However, this allows for the study of persistence of anti-predator behaviours in the absence of predators. Such persistence may be particularly important when trained predator-exposed animals are reintroduced and need to be held for some period before release such as during transport or residency in a soft release pen.
There are a variety of ways to study the influence of in situ predator exposure. These include directly quantifying responses to predator cues such as olfactory, visual and auditory cues, responses to novel stimuli or observations of natural patterns of movements (van Heezik et al. 1999). Predator cues are used to quantify discrimination and infer predator recognition abilities, contexts and measurable responses (Carthey and Banks 2014). Presumably, because olfactory cues persist in the environment, many experiments testing for predator recognition have used scents (Carthey and Banks 2014), and because they are relatively easy to experimentally present, a number have used acoustic predator cues (Hettena et al. 2014). Fewer have used visual cues because of the difficulty in creating realistic taxidermy models (Van der Weyde et al. 2022), or photographs (Blumstein et al. 2009). Results are generally mixed, for example, experiments using models have led to variable responses (McClean et al. 1996; Blumstein et al. 2000; Atkins et al. 2016; Steindler et al. 2020). A global review by Harrison et al. (2023) found no particular predator cue was universally better at measuring the variation in anti-predator response.
Prior work has used a number of dependent variables to understand the response to predatory cues. These include comparing behaviours such as spatial avoidance, refuge use and movement (Gerard et al. 2014; Martin 2014), body posture and movement (Anson and Dickman 2013), time engaged in specific behaviours like sniffing, investigating or vigilance (Saxon-Mills et al. 2018; Steindler et al. 2018; Stryjek et al. 2018), measuring the hesitancy to feed, and comparing survival rates after release (Fey et al. 2010; Ross et al. 2019). Because prey are expected to allocate anti-predator behaviour as a function of risk (Lima and Bednekoff 1999), prey are expected to respond differently to high risk situations by adjusting the amount of time spent on particular activities such as foraging or vigilance, or activity rates. One lesson from these prior studies is the difficulty in interpreting only a single response because a high-risk situation could lead to an increase or decrease in a behaviour as part of a response that reduces exposure to threats (Parsons et al. 2018). For example, a mammal responding fearfully to predator scent at a feed tray may reduce their risk by hesitating and spending longer undergoing vigilant behaviours prior to feeding or alternatively may feed quickly and immediately before fleeing, reducing time spent in a risky situation.
Burrowing bettongs (Bettongia lesueur, hereafter bettongs) are small marsupials that became extinct on mainland Australia during the 20th century due to predation by introduced red foxes (Vulpes vulpes) and feral cats (Felis catus). Until the 1990s, wild populations of bettongs only existed on three islands where they are thought to have been isolated from mammalian predators for at least 6000 years (Short and Turner 2000; Moseby et al. 2011). Since the 1990s, bettongs have been successfully introduced to mainland Australia inside of large, fenced exclosures where introduced predators are absent. All attempts to introduce them outside of predator-proof exclosures have failed (Short and Turner 2000; Moseby et al. 2011; Bannister et al. 2016). Evolutionary naiveté is thought to have been a key contributor to the extinction of bettongs from mainland Australia and the failure of efforts to reintroduce them (Moseby et al. 2016). They are evolutionary naïve to exotic predators such as cats and foxes and have only a short ecological history of exposure to dogs and dingoes. However, when exposed to relatively low cat densities, bettongs have been shown to adjust both behavioural (e.g. increased wariness near predator cues) and physical traits (e.g. increased pes length) (West et al. 2018a; Blumstein et al. 2019; Moseby et al. 2023). Furthermore, bettongs from cat-exposed populations have been reported to show differential responses to predator scents (Saxon-Mills et al. 2018) and visual models (Steindler et al. 2020).
In this study, we used a range of predator cues and behavioural tests to determine whether exposure to wild novel predators (feral cats) led to persistent changes in anti-predator behaviour of bettongs. We did this by translocating bettongs from two populations, one that had been exposed to feral cats and one that had no exposure to predators, to a common environment and then comparing their behaviours over time to look for evidence of potential trait retention. Comparisons were primarily limited to cohort differences post translocation rather than Before/After longitudinal comparisons in individuals but prior studies have shown trait divergence in these cohorts using Before After Control Impact studies (West et al. 2018a; Moseby et al. 2018). We used a variety of cues and behavioural response measures to assess if cue type influenced cue discrimination to ensure that naiveté was tested in a biologically meaningful way (Carthey and Banks 2014).
Methods
Study area
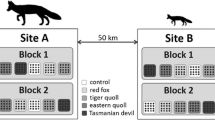
The study was conducted at the 123 km2 Arid Recovery Reserve in northern South Australia. The reserve is arid and comprised of Acacia sand dunes, sandplains, ephemeral swamps and chenopod shrubland swale habitats (Moseby and O’Donnell 2003). Rainfall is low and erratic, averaging 168 mm annually (Read 1995). Arid Recovery is a fenced reserve with six individually fenced paddocks designed to limit movement of some species (Moseby and Read 2006; Moseby et al. 2018). Species reintroduced to the area include the burrowing bettong, greater bilby (Macrotis lagotis), Shark Bay bandicoot (Perameles bougainville) and the western quoll (Dasyurus geoffroii). One paddock (control) is primarily confined as a predator-free area (electrified fence) aimed at restricting movement of all species, and was used to monitor a control population of animals. Two paddocks (cat-exposed) to the north of the reserve contained reintroduced native mammals and rabbits (Oryctolagus cuniculus) and have been exposed to variable cat density over 6 years (Fig. 1). Cats were gradually added to the pens and then controlled through shooting and trapping. Cats were known to prey on bettongs and limited their populations (Bannister et al. 2021; Moseby et al. 2019, 2023). Camera trap footage showed that the cat-exposed paddocks also had intermittent quoll incursions from mid-2019 such that prey populations may have had some low-level exposure to quoll predation in addition to cats.
Study population
Burrowing bettongs are small nocturnal marsupials that are the only macropod species to live communally in warrens (Sander et al. 1997; Short and Turner 1999). They are vocal and emit alarm calls when startled (Trenwith et al. 2023). Bettongs were reintroduced to Arid Recovery in 1999 and were maintained in predator-free paddocks for a number of years (Moseby et al. 2011). In 2014, a random selection of 352 bettongs were translocated to one of the cat-exposed paddocks to establish a population under controlled predation pressure (West et al. 2018a). Bettongs from this paddock were also subsequently moved to another cat-exposed paddock immediately north of the first one in 2017 and 2018 (Bannister et al. 2021).
Between May and early July 2020, individuals from both the control (adult 8 F, juvenile 3 F) and cat-exposed (adult 3 M 7 F, juvenile 1 F) populations were captured using cage traps and placed into a fenced holding paddock (approx. 8 ha) to conduct experiments in a common environment (Fig. 1). No males from the control group were added to preserve the genetic integrity of the cat-exposed population. Two more control females were added to this population in December 2020. Each individual was ear-tagged, microchipped (Trovan Unique ID100C) and fitted with a VHF collar (Lotek) when they exceeded the adult weight threshold of 900 g. Individuals remained in the pen for 6 to 12 months and were slowly transferred out of the common environment from December 2020 to reduce overpopulation in the pen. Animals were provided with supplementary feed and water at either two or three feeding stations throughout the period. Feeding stations were enclosed with wire mesh from August 2020 to reduce access by corvids and were also fitted with a single coil square frame antenna microchip reader (Microchips Australia) to monitor feeding activity by individual bettongs.
Behavioural experiments
We conducted a number of experiments using different cues aimed at eliciting a range of anti-predator responses (Table 1). We used the results from previously published studies to predict the expected behaviour of cat-exposed bettongs relative to control bettongs. For these predictions, we assumed that cat-exposed individuals had improved their anti-predator behaviour compared to naïve individuals in the control group. Experiments involved all individuals present in the pen at the relevant time, however, analyses used to test the retention of traits over time between treatment groups were generally restricted to just the original adult females due to the lack of adult males in the control group. However, in a few cases new recruits could be included in the analysis providing an increased sample size. This is noted below and in Supplementary Information 2. The approximate timing of experiments and bettong abundance in the Common Environment are provided in Supplementary Information 3.
Prior cat exposure - open feed tray in source paddocks
To test the hypothesis that prior cat exposure would result in enhanced vigilance, we conducted a feed tray experiment at the population level. This experiment took place in the original cat-exposed and control paddocks a few weeks prior to bettongs being captured and moved to the common environment (holding paddock). Large plastic bowls were dug into the sand, level with the ground near warrens of individually tracked bettongs. Bowls were filled with sand and two large handful of oats were mixed through the sand to attract bettongs. Camera traps (Bushnell Trophy Cam and Swift Enduro) were set 3–5 m from the bowls and set to record 60 s videos. To eliminate the effect of habituation, we aimed to use the first video from an individual for analysis to record the initial response to the feed tray. In some cases, however, other species had visited or the video length was too short for analysis, so we also recorded the video number for each video as a control variable. We recorded whether other individuals were also present in the video because this may influence behaviour.
We scored behaviours with the software BORIS (Friard and Gamba 2016) and recorded latency to feed for individuals approaching the feed tray, if individuals were already in the bowl when the video was triggered then this was recorded as zero. We also recorded the time foraging non-vigilant (head down or head mid-way up), foraging vigilant (head up), the total number of times the head was raised while foraging, time sniffing around or in the bowl and time not visible. Videos that contained < 15 s of visibility of a bettong were removed from the analysis. All videos were scored blind with respect to treatment and were conducted by the same observer.
Prior cat exposure - novel pen
We tested whether animals with prior predator-exposure would engage in more neophobic behaviour than those from predator-free environments. During the initial capture of individuals in the cat-exposed and control treatments for transfer to the holding paddock, we released captured bettongs into a small experimental pen (50 m2) and recorded their behaviour. One quarter of the pen had vegetation cover similar to the environment of the reserve, and the remaining area was bare sand.
Each bettong was placed inside an artificial burrow inside the experimental pen on the night of capture, left to settle briefly and then a cover was released allowing the bettong to emerge and move around the pen, see further description in Ross et al. (2019). Bettongs were video recorded using a hand-held night vision camera (Bushnell Equinox 72) for up to 15 min after emergence by a single observer hidden behind a hide on one side of the pen. An additional six bettongs from the control paddock were also captured and put into the experimental pen to increase sample size, but these individuals were not transferred to the holding paddock. We recorded the latency to emerge, time spent in vegetated and non-vegetated areas independent of other behaviours, and time spent engaged in moving behaviours (fast/slow/stationary), foraging, sniffing, agitated behaviours (sniffing, digging, biting or climbing at the fence) and time not visible. We also recorded wind (mild/strong) and moonlight (present/absent (new moon or cloud cover) as control variables because videos were recorded on different days.
Prior cat exposure - warren use
To test the hypothesis that prior-exposure to cats influenced bettong warren choice and movement, we monitored warren choice by each collared female (n = 16) an average of three times per week starting from the first day after bettongs were moved to the pen for three months. We also included one sub-adult female originally brought in from the control paddock which was collared and reached adulthood during this period. We formally compared the number of unique warrens used, the probability of sharing a warren with other bettongs, and the number of times individuals moved to different warrens.
Prior cat exposure - trap shyness/behaviour
We investigated whether bettongs from predator-exposed populations were more trap-shy and less docile than those from predator-free populations. We monitored the probability of being trapped and docility of the bettongs moved to the holding paddock from each treatment. We recorded all trapping events (including the number of traps used and location of traps) for the first 210 days (~ 7 months, including date of trapping) that an individual was held in the holding pen from the movement date or approximate emergence date. We initially focus here on data from all the original females moved into the pen (adults and sub-adults; n = 21), however we also tested all individuals moved and those born in the pen to increase sample size (n = 43). Trap score was recorded in the majority of cases (> 90%) by the same observer so we did not include animal handler in the analysis. We recorded whether an individual was trapped (yes/no) during a trapping event. However, since some nights were targeted to specific individuals (e.g. for collar checks) we only used trapping events where all individuals had the possibility of being trapped. Traps were baited and placed near all warrens and feeders to ensure that each individual was likely to encounter a trap. Trap docility score, a measure of reactivity including movement, noise and release behaviour, further described in West et al. (2018b), was used to record behaviour once in the trap and during removal from the trap.
Prior cat exposure - feeder stations
We tested whether cat-exposure modified bettong foraging behaviour compared with control bettongs. Feeder stations fitted with microchip readers were used to monitor individual visits to feeders for a one-month duration between 4 and 5 months after transfer to the holding paddock. The total number of visits by all microchipped animals (n = 28) was recorded for each individual per feeder for one month (we scored only a single visit in a 24 h period). Visits from both feeders were pooled for analysis and total daily visits were compared between our experimental treatments.
Prior cat exposure - vocalisation
We hypothesised that prior exposure to cats would modify bettong vocalisation rates compared to naïve bettongs. Individual bettongs from both treatments (n = 11 cat-exposed; n = 8 control) were fitted with on-board audio recorders attached to their VHF collars designed to record individual vocalisations for up to 24 h. Call types were identified using spectrograms and were manually classified from a total of four hours that included a random selection of daytime and nocturnal periods (see Trenwith et al. 2023). From five identified call types, we compared call rates from three (aggression, alarm and submissive) between the two treatments. We restricted analyses to compare call rates from females (n = 8 per treatment) during nocturnal periods only (total calls within a 2 h randomly chosen window for each bettong) because this is when bettongs were most active. For submissive call analyses, we included weight class (above or below mean female weight) as an additional predictor as it has been shown to be important in previous studies (Trenwith et al. 2023).
Olfactory response: scented feed tray
We then tested whether bettongs from cat-exposed paddocks would be better able to discriminate between predator and non-predator odours. At 24 sites spread randomly across the holding paddock (Fig. 1), a large white bowl was dug into the sand level with the ground and filled with sand and two handfuls of oats to attract bettongs. Urine collected from cats (from necropsied animals) and rabbits (urine-soaked sand from buck heaps) and a control scent of water, were mixed through sand at even quantities and randomly allocated to each site (n = 8 each scent). Observers placed an equal quantity of scented sand around the bowl edge to reduce the risk of food contamination. Placement around the bowl ensured that the scent could be detected by bettongs that approached the feed tray. At each site a motion activated camera trap (Swift Enduro or Bushnell Trophy Cam) was mounted on a stake set approximately three metres from the bowl. Cameras were set to record 60 s videos with no delay. Individuals from the cat-exposed treatment were marked with reflective tape on their radio collars to allow identification in videos. Over time we expected that individuals would likely habituate to the scent so we only used the first video from an individual from each population at each site for analysis. In some cases, there were multiple videos from individuals of the same treatment prior to the first individual from the other treatment group, so we also recorded the video number as a control variable. Using BORIS (Friard and Gamba 2016), we recorded the latency to feed, time foraging vigilant at scent tray (head up), time foraging non-vigilant (head mid-way or head down), number of vigilant looks (head up), time spent sniffing scent around or in the bowl and total time visible (Supplementary Information 1). All videos were scored blind with respect to treatment and were conducted by the same observer.
Visual response: taxidermy model
To investigate whether cat-exposed bettongs would adjust their foraging behaviour in response to the sight of a predator, we placed taxidermy models of a predator (cat), an herbivore (rabbit) and a control (bucket) outside feeding stations inside the holding paddock. Two feeding stations were already in place in the paddock to provide supplemental feed. Each night, we left one station without a model, and at the entrance to the other station we placed one of three models (cat, rabbit and a plastic bucket), alternating between the two stations over six nights. This ensured that bettongs had access to and could choose a station either with or without a model. We recorded whether each individual bettong entered a station using an automated microchip reader that was at each station. We did not record the number of times it entered because we expected that bettongs would change their behaviour towards the models over time. We restricted our data analysis to adults only, because we expected that juveniles would follow their mother. We also compared the latency to enter the station from 20:00 (a standardised time that reflected sunset) for individuals in each treatment group that entered the feeder.
Data analysis
From the scored videos from the open feed tray, novel pen and scent feed tray experiments, we calculated the proportion of time that a bettong was in sight allocated to each behaviour. We used beta regression (with a bias correction due to small sample size) to analyse the proportions of time allocated to behaviour (Ferrari and Cribari-Neto 2004; Douma et al. 2019). Since beta regression models cannot include ones or zeros (where behaviours were not recorded), we normalised our data following the equation described in Douma et al. (2019). For all models, we tested (using likelihood ratio tests LRT) specifically whether time in sight, the number of additional bettongs in video, and video number explained additional variance in the models, as well as treatment or time in sight as precision variables.
In all analyses for each experiment, we included treatment (control vs. cat-exposed) as a fixed effect as this was our primary variable of interest. For our visual or olfactory experiment, we also included model type or scent type, respectively, and an interaction between these and treatment as additional fixed factors. Similarly, for trap behaviour experiments we included treatment, time (days in pen as a continuous variable) and a treatment x time interaction as our fixed factors. Where applicable we also included Sex, Age or Status (moved or born in the common environment) as potential control predictors but these were removed when not significant. We used linear models (LM) where our response variable was continuous, generalised linear models (GLM) with a binomial error distribution for binary variables, and GLM with a Poisson error distribution for count variables or negative binomial error distributions when these models were overdispersed. Models were extended to mixed models where random effects were also included (i.e. where repeated individuals, sites or feeders were used). We used likelihood ratio tests to determine whether additional predictors or random effects were important to retain in the model.
All analyses were conducted in R (R Core Team 2021) using the packages glmmTMB (Brooks et al. 2017) and betareg (Cribari-Neto and Zeileis 2010). We used the package lmtest (Zeileis and Hothorn 2002) to determine whether control, random effect and precision variables were statistically important using LRT. Models were checked for model fit by plotting residuals (sweighted for BR models) and used the DHARMa package (Hartig 2020) for mixed models. We plotted predicted estimates of our analyses using estimated marginal means using the packages emmeans (Lenth 2020) and ggplot2 (Wickham 2016). For models where random effects were retained we used bootstrapping with 1000 iterations to calculate beta coefficients and 95% credible intervals using the package parameters (Ludecke et al. 2020). Repeatability estimates were made for trap score (docility) as this was recorded multiple times for individuals from both before and after the experiment. Repeatability was calculated using the rptR package (Stoffel et al. 2017) on the full model (i.e. fixed factors were included) with the random effect of individual used as the grouping factor and 1000 bootstrapped iterations were calculated to provide uncertainty in the estimate. We used R2 or pseudoR2 (conditional likelihood for multi-level models (Nakagawa et al. 2013) as a measure of model fit using the package performance (Ludecke et al. 2021). Other standardised effect sizes were calculated using the emeans package, and adjusting the sigma and edf (equivalent degrees of freedom) values when random effects were present (Lenth 2020). Significance was set at p < 0.05 in all statistical analyses. When bootstrapping was utilised, we report the median beta coefficient and associated 95% credible intervals. Full model results from final models are reported in Supplementary file 2.
Results
Prior cat exposure - open feed tray in source paddocks
A total of 24 videos were gathered from individuals just prior to being captured and placed in the common environment but we only analysed the 20 videos that were longer than 15 s (control = 14, cat-exposed = 6). Both treatment groups had similar latencies to approach the feed tray (β = 1.604, z = 0.198, p = 0.843), however the latency to feed was shorter when more bettongs were present (β = -12.112, z = 5.107, p = 0.018). Similarly, we found no differences between the populations in the time spent sniffing the bowl, foraging non-vigilantly, foraging vigilantly and the number of vigilant head looks (Fig. 2). The number of bettongs however, explained significant variation in time allocated to sniffing the bowl (β = -0.718, z = -6.808, p < 0.001), foraging vigilantly (β = 0.815, z = 2.311, p = 0.021), and (potentially) foraging non-vigilantly (β = 0.249, z = 1.958, p = 0.050).
a-d Response of bettongs from control and cat-exposed populations to a feed tray placed in the open. Response behaviours tested were (a) number of vigilant head looks, (b) proportion of time sniffing the bowl, (c) proportion of time feeding in vigilant manner and (d) proportion of time feeding in a non-vigilant manner. Error bars show 95% confidence intervals
Prior cat exposure - novel pen
A total of 25 bettongs (control = 15, cat-exposed = 10) were captured and placed in the novel small pen prior to their move to the common environment. Of these only 11 (control = 6, cat-exposed = 5) emerged from the artificial burrow after waiting at least 15 min, limiting sample size for analysis. Therefore, we analysed treatment and sex effects but not an interaction; we also removed sex when it was not significant. For those that emerged, the mean time the bettong was visible was 8.64 min. There was no difference in the time to emergence between cat-exposed and control bettongs (62.5 vs. 105.2 s; β = 42.7, t = 1.13, p = 0.289). However, cat-exposed bettongs spent significantly less time in the vegetated areas (β = -1.81, z = -3.48, p < 0.001) and more time sniffing, although not significant (β = 0.-0.48, z = -1.18, p = 0.237) than control bettongs (Fig. 3). We pooled fast and slow movement categories due to limited data but both treatment groups of bettongs spent similar times moving around the pen (β = -0.19, z = -0.83, p = 0.405), although females moved much more than males (β = -0.5, z = -2.31, p = 0.021). Both treatment groups were similarly engaged in agitated behaviours (β = -0.06, z = -1.55, p = 0.877). There was very limited foraging, so we did not include this behaviour for analysis.
a-d Results of behavioural comparisons between two treatment groups of bettongs when placed in a small novel pen. Behaviours include (a) latency to emerge, (b) proportion of time spent in covered vegetation, (c) proportion of time sniffing and (d) proportion of time moving. Error bars show 95% confidence intervals
Prior cat exposure - warren use
Warren use did not differ overall between the two treatment populations, which may be due to the low number of available warrens in the pen initially. However, we did not see a noticeable number of new warrens being used by the bettongs over the entire year of the study. The mean number of unique warrens per month used by all bettongs was 10.4 (SD = 3.4, range = 7 to 19). The number of unique warrens used by female bettongs (Fig. 4a) did not differ between the two treatment groups (β = 0.142, z = 0.441, p = 0.659). The probability of warren sharing by females was very high for both groups (Fig. 4b) and no treatment effect was found (median β = 0.06, CI -3.3–3.56). Females from both treatment groups moved burrows at the same low rate over the first few months (β = -0.265, z = -0.415, p = 0.678; Fig. 4c).
a-c Warren use by female bettongs from control and cat-exposed treatment groups in the first three months after being moved into a common environment. Warren use was defined as (a) the number of unique burrows used, (b) the probability of sharing a warren and (c) the mean number of times individuals moved burrows. Error bars show 95% confidence intervals for a) and c), and 95% credible intervals for b)
Prior cat exposure - trap behaviour
Females from the control treatment (n = 13) group slowly became more docile in their trap behaviour over time, compared to cat-exposed bettongs (n = 8) that did not change in their docility, although there was no significant interaction between these groups. However, when we increased our sample size to include individuals born in the pen from known populations, we found this difference over time to be more robust. Individuals from our control population became significantly more docile over time compared to cat-exposed individuals that retained their docility (median βint = -0.003, CI 0–0.01, p = 0.018). Status, sex and age were not significant predictors and removed from the models. Trap score (docility) was found to be repeatable when assessing the original females (R = 0.281, SE = 0.132, p = 0.0018, Bootstrap 95% CI: 0.024–0.539, n = 21 from 80 observations) and across all pen individuals (R = 0.215, SE = 0.081, p < 0.01, CI 0.055–0.375, n = 43 from 186 observations).
Both groups of females did not differ in their likelihood of being trapped, nor was there a significant interaction in trends over time. There was a slight increase in the trappability over time for the control group (median β = 0.007, CI -0.004–0.022), compared to the cat-exposed group (median β = -0.001, CI -0.023–0.017) which did not change in trap shyness over time. Age had no effect in trappability and was removed from the model.
Prior cat exposure only - feeder visitation
We monitored visit rate by individual bettongs from each treatment to the feeder stations for one month. A total of 850 daily visits across both feeders were recorded by 28 bettongs (control = 16, cat-exposed = 12). The number of visits ranged from 4 to 54 over the month across both feeder stations. We found no overall difference in the mean number of visits per day between the two treatments (β = -0.203, z = -1.28, p = 0.2), nor was there any effect of age or sex so these were removed from the final model.
Prior cat exposure only - vocalisations
We compared vocalisation rates for three call types identified in burrowing bettongs; aggression, alarm and submissive calls (Fig. 5). Female control bettongs produced significantly more alarm calls than females from the cat-exposed group (β = 1.504, z = 2.721, p = 0.007), however, call rates were the same between the treatments for aggression calls (β = 0.199, z = 0.317, p = 0.751) and submissive calls (β = -0.253, z = -0.307, p = 0.759). As previously noted, individuals from lower weight classes produced more submissive calls (β = 2.757, z = 3.444, p < 0.01).
Olfaction: scented feed tray
A total of 45 videos (Control = 22, CE = 23) were used for analysis with a mean visible time of 48.4 s. The number of vigilant looks (head up) while foraging was higher at cat scent in the cat-exposed treatment compared to the control group (Fig. 6a). There was a significant difference in vigilant looks between treatment groups at cat scent (median β = -1.139, credible interval − 2.661 - -0.052) with more head-up looks by the cat-exposed group. The reverse trend was found at control and rabbit scent, with a significant interaction reached between cat and rabbit scent. Latency to feed at feed trays was higher overall for the control group (Fig. 6b), with cat-exposed individuals being quicker to feed in the presence of cat scent (median β = 0.724, credible interval 0.424–1.013) and rabbit scent (median β = 0.896, CI 0.596–1.229), but not at control scent (median β = 0.154, CI -0.108–0.414). These different responses also produced significant interaction effects between the treatment groups comparing control and cat scents (median β = 0.569, CI 0.180–0.988) and control and rabbit scents (median β = 0.749, CI 0.346–1.181). We found no fixed or interaction effects for the proportion of time spent sniffing the bowl, foraging vigilant or foraging non-vigilant between treatments at the different scents (Fig. 6).
a-d Behaviour of bettongs from control and cat-exposed treatment groups at feed trays with three different scents. Behaviours show predicted (a) mean rate of vigilant looks (head up) while foraging, (b) latency to feed, (c) proportion of time sniffing scent and (d) proportion of time foraging in a vigilant manner. Error bars indicate 95% confidence or credible intervals
Visual cue: taxidermy model
A total of 200 visits were made to the feeders by both treatment groups across all models and feeder stations. Bettongs from both treatments appeared to distinguish between animal models and the control bucket as individuals entered the feeding station less often when presented with both cat and rabbit models than with the bucket or no model (Fig. 7a). Control and cat-exposed bettongs did not significantly differ in their probability of entering the feeder for any model, although cat-exposed had a lower probability of entering when the cat model was present than control bettongs. Cat-exposed individuals entered the feeder with the cat model significantly less often than when there was no model (median β = -1.39, 95% credible interval − 3.03–0) and less often with the cat model compared to the bucket (median β = -1.42, CI -3.09–0). This same trend was also seen for the control bettongs with the cat model and no model (median β = -1.14, CI -2.718–0). No significant interactions were found. There were no significant differences between treatment groups or models for latency to feed, however bettongs from both treatments took slightly longer to enter feeders when cat and rabbit models were present compared to when no model was present (Fig. 7b). Sex and age were not important and removed from the final models in both cases.
Results from the final model for all experiments with additional information is provided in Supplementary File 2. We summarise general findings and effect sizes for all analyses in Table 2. While many results do not show significant differences, likely due to low sample size, the differences between the treatment groups were nearly always in the expected direction hypothesised for the cat-exposed group i.e. a tendency to display anti-predator traits.
Discussion
Previous studies examining bettongs’ behavioural responses to predator cues have involved comparing populations of cat-exposed and cat-naïve bettongs that lived separately in different but nearby exclosures (Saxon-Mills et al. 2018; West et al. 2018a; Tay et al. 2021). The results of these prior studies provided evidence that exposure to predators can change bettongs’ escape behaviour and capacity to respond to visual and scent cues associated with predators. They suggested that cat-exposed bettongs were warier and approached feed trays more slowly, both in general, and when predator scent was present (Blumstein et al. 2019). They were also more reactive when escaping after release (Tay et al. 2021) or when approached (West et al. 2018a). However, a caveat for these previous studies is that because they compared behaviours of two populations that were physically separated, it was not possible to control for potentially confounding factors that could also influence bettong behaviour such as the density of conspecifics and differences in the animal and plant communities within the different exclosures. A key advance of the present study is that we were able to control for environmental variation by comparing the behaviour of bettong populations that had been translocated to a common environment. In line with previous work, we found differences between two populations of bettongs with varying history of cat-exposure suggesting some anti-predator behaviours persisted after being transferred to a common environment. This supports the general finding that living with cats changes bettong behaviour.
All predatory stimuli do not elicit similar responses. We detected the greatest difference between treatment groups when predator scent or fearful stimuli were used. When we added predator scent or placed animals in a risky situation (e.g. in a cage trap or in a novel environment), significant treatment effects were more likely. Conversely, behaviours measured passively when bettongs were within their natural home environment, such as general warren use or foraging at feed trays without predator stimuli, did not result in significant treatment effects. This pattern of results could reflect two things. First, other studies have found that stimuli and cues are needed to trigger anti-predator behaviour (Apfelbach et al. 2005; Carthey and Banks 2014) or to increase the strength of anti-predator responses to the point where they can be measured by human observers. This is because we forced the animal to interact with a stimulus and assess its risk. By contrast, when we followed natural behaviours, animals have already assessed the risk of engaging in various activities and we are not observing a response to an acute predator stimulus presentation. Thus, in this study, certain conditions that created a fearful situation triggered some of the strongest responses. Trap behaviour scores, where an observer approached the trap and recorded behaviour, release pen behaviour, where a bettong was placed into a novel environment on its own, and alarm call frequency, where an animal perceives there is a high risk, all varied between predator exposed and control bettongs. Previous studies using similar high risk situations such as flight initiation distance (West et al. 2018a) and escape behaviour (Tay et al. 2021) have also recorded significant differences between treatments. In addition, the persistence of anti-predator behaviours under high-risk scenarios could be due to the prolonged retention of behaviours required in high risk situations compared with low risk behaviours. Low risk behaviours may be more plastic and the first to be lost under relaxed selection; a general question that requires more study.
Predator scent at feeding trays resulted in cat-exposed bettongs being more vigilant whilst control bettongs took longer to feed at all feeding stations regardless of the type of scent present. Results could suggest that cat-exposed bettongs elected to feed quickly so they could minimise risk and were more vigilant when a predator scent was present. Such adjustment of activity in response to predator odours has been observed in a number of studies (Apfelbach et al. 2005) and is a likely trade-off between foraging effort and predation risk (Lima and Bednekoff 1999). The latency to feed has been studied previously in bettongs and other species, with some finding predator-aware individuals took longer to approach feeding stations and feed (West et al. 2018a) whilst others found it decreased latency possibly due to an attempt to reduce the time in a risky situation. Situations perceived as risky may also influence other behaviour such as vocalisations. While acoustic signalling provides important means of communication in some species, it also allows potential predators to become aware of the presence of prey and thus can increase predation threats. We found that cat-exposed individuals made fewer calls than predator-naïve individuals, particularly so for alarm calls, which is the loudest call type (Trenwith et al. 2023). While a number of studies have investigated alarm call types in response to predator detection (type, size, distance) these are usually based on predators being present at that point in time (Blumstein 2007; Dutour et al. 2021; Maiditsch and Ladich 2022). Call rates can also be a function of population density. For example, higher population density has been positively associated with increased territorial call rates (Dantzer et al. 2012). However, this is unlikely to have affected our results as population density was consistent during the study period for both treatment groups. Our study suggests that acoustic behavioural changes can be a result of predator exposure, and may be retained even when the predator is no longer present.
In comparison to scent cues, we detected no significant differences in how bettongs with different histories of cat exposure responded to taxidermy models. Interestingly, both sets of bettongs responded to the presence of taxidermy models by delaying their visit or showing lower probabilities of even entering the feeders compared with a bucket or no model, indicating that they may have recognised the models as animals but did not discriminate between a predator and a harmless herbivore. By the time they began foraging they may have assessed the risk of foraging next to the model as minimal. By contrast, an olfactory stimulus may still retain some degree of uncertainty about the true risk of predation. This likely explains why the similar feed tray experiment without scent elicited no detectable treatment differences. With no direct predator cue, it is likely that individuals did not allocate energy to anti-predator behaviour. Species that show communal behaviour, as in shared burrows in this case, may be expected to share warrens more often when under predation pressure because this would dilute predation risk and possibly enhance detection. While we found no difference between treatments in warren use, this may be due to a lack of predator stimulus, initial low availability of warrens in the holding paddock, or simply that burrow behaviour in bettongs can be highly variable (Sander et al. 1997) and may be based on individual, family or hierarchy.
Previous in situ predator exposure studies were unable to determine if the behaviours persisted after removal of predator exposure. Our design shows that predator exposure may lead to persistent shifts in some, but not all, anti-predator behaviours. Significant treatment differences were still recorded up to seven months after the last predator exposure. While we were unable to compare longitudinal differences in anti-predator traits of individuals before and after their movement to the common environment, our findings suggest these traits were retained within the population, particularly as population level studies prior to movement also showed anti-predator differences. In addition, trap docility scores were found to be repeatable within individuals providing more assurance that these traits were retained after movement to the pen. Although we found high risk predator cues were more likely to elicit anti-predator responses than passive measures of anti-predator behaviour, we only measured the change in passive anti-predator behavioural traits over time. Control bettongs became significantly more docile over time whilst cat-exposed bettongs retained their docility scores, but other passive measures like feeding and warren behaviour did not change with time in the common environment. We recommend repeating the same high risk predator cue experiments at various intervals after removal of predators to better understand the time frame over which anti-predator traits are retained or lost.
The persistence of some anti-predator responses after seven months is an exciting finding and particularly applicable to reintroduction biology where there may be delays in releasing animals due to the need to hold or transport them from an in situ predator experience prior to release. That the behavioural shifts appear to be persistent suggests that these anti-predator behaviours do not necessarily require ongoing reinforcement, at least for several months. Our results from a common environment experiment provide additional support to those from in situ predation studies in large scale exclosures where treatment and control groups are spatially separated. While our results demonstrate that predator exposure can drive shifts in behaviour they do not necessarily indicate that predator-exposed bettongs will be better able to co-exist with predators. Indeed, Bannister et al. (2016) showed that predator-exposed bettongs fared no better than predator-naïve bettongs when released into a common environment with predators. More work is needed to determine if anti-predator behaviours can improve and persist long enough to ensure survival in the wild. Additionally, although initial results suggest that there is selection for some physical traits after exposure to predators (Moseby et al. 2023), more research is necessary to determine if the mechanistic basis of behavioural changes represent ontogenetic or evolutionary changes.
Data availability
Data for this project can be accessed through OSF.
DOI https://doi.org/10.17605/OSF.IO/9H3D2. Authors request that any use of the data includes original author involvement.
References
Anson JR, Dickman CR (2013) Behavioral responses of native prey to disparate predators: naivete and predator recognition. Oecologia 171:367–377
Apfelbach R, Blanchard CD, Blanchard RJ, Hayes RA, McGregor IS (2005) The effects of predator odors in mammalian prey species: a review of field and laboratory studies. Neurosci Biobehav Rev 29:1123–1144
Atkins R, Blumstein DT, Moseby KE, West R, Hyatt M, Letnic M (2016) Deep evolutionary experience explains mammalian responses to predators. Behav Ecol Sociobiol 70:1755–1763
Bannister HL, Lynch CE, Moseby KE (2016) Predator swamping and supplementary feeding do not improve reintroduction success for a threatened Australian mammal, Bettongia lesueur. Aust Mammal 38:177–187
Bannister HL, Letnic M, Blumstein DT, Moseby KE (2021) Individual traits influence survival of a reintroduced marsupial only at low predator densities. Anim Conserv 24:904–913
Blumstein DT (2007) The evolution, function, and meaning of marmot alarm communication. Adv Stud Behav 37:371–400
Blumstein DT, Daniel JC, Griffen AS, Evans CS (2000) Insular tammar wallabies (Macropus eugenii) respond to visual but not acoustic cues from predators. Behav Ecol 11:528–535
Blumstein DT, Ferando E, Stankowich T (2009) A test of the multipredator hypothesis: yellow-bellied marmots respond fearfully to the sight of novel and extinct predators. Anim Behav 78:873–878
Blumstein DT, Letnic M, Moseby KE (2019) In situ predator conditioning of naive prey prior to reintroduction. Phil Trans R Soc B 374:20180058
Brooks MEK, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Machler M, Bolker BM (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J 9:378–400
Carthey AJ, Banks PB (2014) Naivete in novel ecological interactions: lessons from theory and experimental evidence. Biol Rev 89:932–949
Carthey AJR, Blumstein DT (2018) Predicting predator recognition in a changing world. Trends Ecol Evol 33:106–115
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org
Crane AL, Ferrari MCO (2017) Patterns of predator neophobia: a meta-analytic review. Proc R Soc B 284:20170583
Cribari-Neto F, Zeileis A (2010) Beta regression in R. J Stat Softw 34:1–24
Dantzer B, Boutin S, Humphries MM, Mc Adam AG (2012) Behavioral responses of territorial red squirrels to natural and experimental variation in population density. Behav Ecol Sociobiol 66:865–878
Douma JC, Weedon JT, Warton D (2019) Analysing continuous proportions in ecology and evolution: a practical introduction to beta and Dirichlet regression. Methods Ecol Evol 10:1412–1430
Dutour M, Walsh SL, Ridley AR (2021) Australian magpies adjust their alarm calls according to predator distance. Bioacoustics 30:458–468
Ferrari SLP, Cribari-Neto F (2004) Beta regression for modelling rates and proportions. J Appl Stat 31:799–815
Fey K, Banks PB, Korpimäki E (2010) Alien mink predation and colonisation processes of rodent prey on small islands of the Baltic Sea: does prey naïveté matter? Int J Ecol 2010:984396
Friard O, Gamba M (2016) BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol Evol 7:1325–1330
Fritts TH, Rodda GH (1998) The role of introduced species in the degradation of island ecosystems: a case history of Guam. Annu Rev Ecol Evol S 29:113–140
Gerard A, Jourdan H, Cugniere C, Millon A, Vidal E (2014) Is naivete forever? Alien predator and aggressor recognition by two endemic island reptiles. Naturwissenschaften 101:921–927
Griffin AS, Blumstein DT, Evans CS (2000) Training captive-bred or translocated animals to avoid predators. Conserv Biol 14:1317–1326
Harrison ND, Steven R, Phillips BL, Hemmi JM, Wayne AF, Mitchell NJ (2023) Identifying the most effective behavioural assays and predator cues for quantifying anti-predator responses in mammals: a systematic review. Environ Evid 12:5
Hartig F (2020) DHARMa: Residual Diagnostics for Hierarchical (Multi-Level / Mixed) Regression Models. R package version 0.3.2.0, https://CRAN.R-project.org/package=DHARMa
Hettena AM, Munoz N, Blumstein DT, Ebensperger L (2014) Prey responses to predator’s sounds: a review and empirical study. Ethology 120:427–452
Holzer C, Bergmann H, McLean I (1995) Training captive-raised, native birds to recognise their predator. In: Ganslosser U, Hodges JK, Kaumanns W (eds) Research and Captive Propagation. Filander, Furth, pp 198–206
Lenth R (2020) emmeans: Estimated Marginal Means, aka Least-Square Means. R package version 1.5.0, https://CRAN.R-project.org/package=emmeans
Lima SL (1998) Stress and decision-making under the risk of predation: recent developments from behavioral, reproductive, and ecological perspectives. Adv Stud Behav 27:215–290
Lima SL, Bednekoff PA (1999) Temporal variation in danger drives antipredator behavior: the predation risk allocation hypothesis. Am Nat 153:649–659
Ludecke D, Ben-Shachar MS, Patil I, Makowski D (2020) Extracting, computing and exploring the parameters of statistical models using R. J Open Source Softw 5:2445
Ludecke D, Ben-Shachar MS, Patil I, Waggoner P, Makowski D (2021) Performance: an R package for assessment, comparison and testing of statistical models. J Open Source Softw 6:3139
Maiditsch IP, Ladich F (2022) Acoustic and visual adaptations to predation risk: a predator affects communication in vocal female fish. Curr Zool 68:149–157
Martin CW (2014) Naive prey exhibit reduced antipredator behavior and survivorship. PeerJ 2:e665
McClean IG, Lundie-Jenkins G, Jarman PJ (1996) Teaching an endangered mammal to recognise predators. Biol Conserv 75:51–62
Miller B, Biggins D, Wemmer C, Powell R, Calvo L, Hanebury L, Wharton T (1990) Development of survival skills in captive-raised siberian polecats (Mustela eversmanni) II: predator avoidance. J Ethol 8:95–104
Moseby KE, O’Donnell E (2003) Reintroduction of the greater bilby, Macrotis lagotis (Reid) (Marsupialia:Thylacomyidae), to northern South Australia: survival, ecology and notes on reintroduction protocols. Wildl Res 30:15–27
Moseby KE, Read JL (2006) The efficacy of feral cat, fox and rabbit exclusion fence designs for threatened species protection. Biol Conserv 127:429–437
Moseby KE, Read JL, Paton DC, Copley P, Hill BM, Crisp HA (2011) Predation determines the outcome of 10 reintroduction attempts in arid South Australia. Biol Conserv 144:2863–2872
Moseby KE, Blumstein DT, Letnic M (2016) Harnessing natural selection to tackle the problem of prey naivete. Evol Appl 9:334–343
Moseby KE, Letnic M, Blumstein DT, West R (2018) Designer prey: can controlled predation accelerate selection for anti-predator traits in naïve populations? Biol Conserv 217:213–221
Moseby KE, Van der Weyde LK, Letnic M, Blumstein DT, West R, Bannister HL (2023) Addressing prey naivety in native mammals by accelerating selection for antipredator traits. Ecol Appl 33:e2780
Nakagawa S, Schielzeth H, O’Hara RB (2013) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142
Parsons MH, Apfelbach R, Banks PB, Cameron EZ, Dickman CR, Frank ASK, Jones ME, McGregor IS, McLean S, Muller-Schwarze D, Sparrow EE, Blumstein DT (2018) Biologically meaningful scents: a framework for understanding predator-prey research across disciplines. Biol Rev 93:98–114
Read JL (1995) Recruitment characteristics of the White Cypress Pine (Callitris glaucophylla) in arid South Australia. Rangel J 17:228–240
Ross AK, Letnic M, Blumstein DT, Moseby KE (2019) Reversing the effects of evolutionary prey naiveté through controlled predator exposure. J Appl Ecol 56:1761–1769
Sander U, Short J, Turner B (1997) Social organisation and warren use of the burrowing bettong, Bettongia lesueur (Macropodoidea: Potoroidae). Wildl Res 24:143–157
Saxon-Mills EC, Moseby K, Blumstein DT, Letnic M (2018) Prey naïveté and the anti-predator responses of a vulnerable marsupial prey to known and novel predators. Behav Ecol Sociobiol 72:151
Short J, Turner B (1999) Ecology of burrowing bettongs, Bettongia lesueur (Marsupialia: Potoroidae), on Dorre and Bernier Islands, Western Australia. Wildl Res 26:651–669
Short J, Turner B (2000) Reintroduction of the burrowing bettong Bettongia lesueur (Marsupialia: Potoroidae) to mainland Australia. Biol Conserv 96:185–196
Steindler LA, Blumstein DT, West R, Moseby KE, Letnic M (2018) Discrimination of introduced predators by ontogenetically naïve prey scales with duration of shared evolutionary history. Anim Behav 137:133–139
Steindler LA, Blumstein DT, West R, Moseby KE, Letnic M (2020) Exposure to a novel predator induces visual predator recognition by naïve prey. Behav Ecol Sociobiol 74:102
Stoffel MA, Nakagawa S, Schielzeth H (2017) rptR: repeatability estimation and variance decomposition by generalised linear mixed-effects models. Methods Ecol Evol. https://doi.org/10.1111/2041-210X.12797
Stryjek R, Mioduszewska B, Spaltabaka-Gedek E, Juszczak GR (2018) Wild Norway rats do not avoid predator scents when collecting food in a familiar habitat: a field study. Sci Rep 8:9475
Tay NE, Fleming PA, Warburton NM, Moseby KE (2021) Predator exposure enhances the escape behaviour of a small marsupial, the burrowing bettong. Anim Behav 175:45–56
Trenwith B, Armstrong KN, Van der Weyde LK, Moseby KE (2023) The acoustic repertoire of the burrowing bettong (Bettongia Leseur). Aust Mammal 45:264–274
Van der Weyde LK, Blumstein DT, Letnic M, Tuft K, Ryan-Schofield N, Moseby KE (2022) Can native predators be used as a stepping stone to reduce prey naivety to novel predators? Behav Ecol 34:63–75
van Heezik Y, Seddon P, Maloney RF (1999) Helping reintroduced houbra bustards avoid predation: effective anti-predator training and the predictive vlue of pre-release behaviour. Anim Conserv 2:155–163
West R, Letnic M, Blumstein DT, Moseby KE (2018a) Predator exposure improves anti-predator responses in a threatened mammal. J Appl Ecol 55:147–156
West RS, Blumstein DT, Letnic M, Moseby KE (2018b) Searching for an effective pre-release screening tool for translocations: can trap temperament predict behaviour and survival in the wild? Biodivers Conserv 28:229–243
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer-, New York. https://ggplot2.tidyverse.org
Zeileis A, Hothorn T (2002) Diagnostic checking in regression relationships. R News 2:7–10
Acknowledgements
The study was supported by Arid Recovery. The authors would like to thank the staff at Arid Recovery, B. Stepkovitch, T. Berthold, E. Reynolds, T. Manning, A. Neilson, T. Wilson, J. Read and J. Kelk for assistance with capture and monitoring of bettongs. The authors thank the reviewers for helpful comments on the manuscript. The authors also acknowledge the Kokatha people as the Traditional owners of the lands on which this research was undertaken.
Funding
This work was supported the Australian Research Council Linkage Project (LP190100291). KEM is supported by an ARC Future Fellowship (FT210100173).
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This project was conducted under ethics approval by the Animal Care and Ethics Committee of UNSW (20/109A). All applicable national and institutional guidelines for the use of animals were followed.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by A. I Schulte-Hostedde.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moseby, K.E., Blumstein, D.T., Letnic, M. et al. In situ predator exposure creates some persistent anti-predator behaviours: insights from a common environment experiment. Behav Ecol Sociobiol 78, 93 (2024). https://doi.org/10.1007/s00265-024-03491-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-024-03491-2