Abstract
Objective
To evaluate the prognostic value of peak postoperative high-sensitivity cardiac troponin I (hs-cTnI) in patients undergoing cardiac valvular (CV) or thoracic aortic (TA) surgery without concomitant coronary artery bypass grafting (CABG).
Methods
We analyzed data from a prospective, single-center registry of adult patients (≥ 18 years) who underwent elective, isolated CV or TA surgery at a tertiary hospital in Germany between January 2013 and May 2019. Hs-cTnI levels were measured preoperatively and postoperatively at regular intervals up to 48 h. The associations between peak hs-cTnI and long-term and 30-day all-cause mortality were assessed using Cox proportional hazards models with regression splines, adjusted for baseline risk via EuroSCORE II.
Results
Among 4232 patients (median age 69 years [IQR 59–76]; 38.4% female), 30-day all-cause mortality occurred in 61 patients (1.4%). Over a median follow-up of 3.1 years [IQR 1.9–5.0], 499 patients (11.8%) died. Peak hs-cTnI thresholds of 282 × the upper limit of normal (ULN) and 194 × ULN were associated with increased risk of long-term (HR 1.11, 95% CI 1.00–1.24) and 30-day mortality (hazard ratio (HR) 1.25, 95% CI 1.01–1.55), respectively. However, predictive performance was limited with area under the receiver operating curve (AUC) values of 0.56 for long-term mortality and 0.55 for 30-day mortality. Sensitivity and specificity were 41% and 71% for long-term mortality, and 54% and 55% for 30-day all-cause mortality. Negative predictive values remained high (both at 90%), but negative predictive values were low at 16% and 14%, respectively. When applying the sex-specific reference levels (16 pg/ml for women, 34 pg/ml for men), thresholds of 223 × ULN and 194 × ULN were identified for long-term and 30-day mortality, with corresponding HRs of 1.08 and 1.62 and AUCs of 0.58 and 0.57, respectively.
Conclusion
Peak hs-cTnI levels are independently associated with adverse outcomes following CV or TA surgery without CABG. However, effective risk discrimination is poor, and the identified thresholds far exceed conventional definitions of myocardial infarction. These findings support the need for surgery-specific hs-cTnI thresholds to improve perioperative risk stratification.
Graphical Abstract
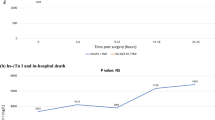
Overview of the study design, endpoints, and key findings on the prognositc value of high-sensitivity cardiac troponin I (hs-cTnI) following isolated non coronary artery bypass grafting (CABG) surgery. Patients undergoing isolated cardiac valvular (CV) or thoracic aortic (TA) procedures were included. Repeated hs-cTnI measurements were obtained for up to 48 h postoperatively. Peak hs-cTnI levels were analyzed for their associaction with long-term all-cause mortality and 30-day all-cause mortality. Results show that thresholds associated with increased mortality were substantially higher than those currently used to define perioperative myocardial injury

Similar content being viewed by others
Introduction
Cardiothoracic surgery represents a critical therapeutic approach for patients with severe structural heart or thoracic aortic disease. Advancements in surgical techniques and perioperative management have enabled cardiac surgery to be performed with relatively low risk [1]. However, the risk of substantial postoperative complications remains significant. Early identification of patients at risk for adverse outcomes remains a major clinical challenge, necessitating reliable biomarker thresholds for adequate risk stratification.
Cardiac troponins (cTn) are the standard biomarkers for detecting myocardial injury [2]. However, their interpretation following cardiothoracic surgery is challenging, as troponin elevations are expected to some extent, even without clinically significant ischemia [3]. Three definitions for postoperative myocardial infarction (PMI) have been established: The fourth universal definition of myocardial injury (UDMI) defines it as a cTn concentration exceeding 10 times the upper limit of normal (ULN) of the local testing site, accompanied by evidence of myocardial ischemia, such as new Q-waves on electrocardiography (ECG), angiographic evidence of new graft or native coronary vessel occlusion, or imaging evidence of new loss of viable myocardium within the first 48 h after coronary artery bypass grafting (CABG). The academic research consortium 2 (ARC-2) and the society for cardiovascular angiography and interventions (SCAI) define PMI by a cTn level exceeding ≥ 70 times the ULN, or a cTn level ≥ 35 times the ULN with evidence of ischemia in ECG, angiography or imaging within 48 h following CABG. All were developed and validated in the context of isolated CABG, and only the ARC-2 definition was created with high-sensitivity assays available [4,5,6,7].
The Vascular Events in Surgery Patients Cohort Evaluation (VISION) trial demonstrated an association between high-sensitivity cardiac troponin I (hs-cTnI) and increased 30-day all-cause mortality following cardiac surgery [8]. However, this association was observed at substantially higher levels than currently recommended PMI thresholds. Importantly, VISION included various types of cardiac surgery and focused solely on 30-day all-cause mortality, leaving uncertainty regarding optimal thresholds for isolated non-CABG procedures and their impact on long-term survival.
Our study aimed to evaluate the association between peak postoperative hs-cTnI levels and both short- and long-term mortality in patients undergoing isolated cardiac valvular (CV) or thoracic aortic (TA) surgery, without concomitant CABG. This investigation seeks to establish procedure-specific risk thresholds, enhancing perioperative risk stratification for this unique patient cohort.
Methods
Study design
All patients who underwent elective cardiothoracic surgery between January 1, 2013, and May 30, 2019, at our institution, a high-volume tertiary hospital in Germany (Heart and Diabetes Center NRW, Bad Oeynhausen), were enrolled in a registry including demographic, clinical, and laboratory data. We conducted an analysis on all consecutive patients who underwent CV or TA surgery without concomitant coronary revascularization.
Exclusion criteria included cardiopulmonary resuscitation within 48 h pre-operatively and cardiogenic shock within 48 h pre-operatively, pediatric (< 18 years) patients. Procedures performed without the use of cardiopulmonary bypass (CPB) were excluded to ensure consistency within the analysis. CPB is routinely employed in valvular and thoracic aortic surgery, and hs-cTnI kinetics have been shown to vary depending on CPB use [9, 10]. Patients who underwent intraoperative cryoablation for atrial fibrillation were also excluded from analysis as cryoablation is known to result in very high levels of cTn postoperatively [11]. Furthermore, we excluded patients with baseline elevations of hs-cTnI exceeding five times the ULN, which is reported as of 26 pg/ml for the assay employed at our institution.
Blood samples were collected 24 h before surgery, and then 4, 8, 12, 16, 24, 32, 40, and 48 h post-operatively as the standard practice in our institution. These samples were measured in the central laboratory of the hospital by standardized techniques. Plasma levels of hs-cTnI were measured on the Abbott ARCHITECT STAT High Sensitivity Troponin-I blood test (Abbott Laboratories, Abbott Park, IL, USA). This assay is a high-sensitivity assay that has been implemented at our institution since January 1, 2013. According to the manufacturer, this assay has a limit of detection of 1.2 ng/L and an inter-assay coefficient of variation of less than 10% at 4.7 ng/L. The reported ULN was determined by the manufacturer for both sexes in general. However, the manufacturer, backed up by guideline recommendations, promotes the use of gender-specific cutoffs with a URL of 16 ng/L for women and 34 ng/L for men. Multiples of URL (referred to as × ULN) were reported to allow better comparability to existing literature.
The primary endpoint was long-term all-cause mortality, while 30-day all-cause mortality was the secondary endpoint. Patients were followed up after discharge through routine phone calls and standardized questionnaires. Data on mortality was collected from local registry offices and by contacting general practitioners. Patients signed informed consent forms pre-operatively that allowed the collection of data and future contacts (phone calls and mail) and participation in our local registry. Sub-analyses were performed based on the specific type of surgery performed, as well as after excluding those patients with known coronary artery disease (CAD). The study was approved by the local ethics committee (Reg. Nr. 2019–501). Patients or members of the public were not involved in the design, conduct, reporting, or dissemination of this research, as it was not considered appropriate or feasible for this type of retrospective observational study.
Statistical analysis
Statistical analyses were performed using R in RStudio (Version 2023.06, RStudio Inc., Boston, MA, USA). Continuous variables were reported as mean ± standard deviation (SD) if normally distributed, and as median and interquartile range (IQR) if non-normally distributed. Normality was assessed using the Shapiro–Wilk test. Categorical variables were presented as frequencies and percentages. Group comparisons for continuous variables were conducted using the Wilcoxon rank-sum test or Student’s t-test, as appropriate. For group comparisons of sequentially measured parameters, repeated measures analysis of variance (ANOVA) was employed using the “aov” function (V 4.3.1). For binary and categorical data, the chi-square test or Fisher’s exact test was applied based on sample size and distribution characteristics. No data were imputed.
We performed unadjusted restricted cubic spline regression to explore the raw association between peak hs-cTnI levels within 48 h post-operatively and predefined endpoints. This approach allows for the visualization of non-linear relationships between hs-cTnI and clinical outcomes without adjusting for confounders. The unadjusted model describes the direct relationship between hs-cTnI and endpoints, reflecting the initial risk distribution as measured. We used 4 degrees of freedom, which corresponds to three interior knots placed at the 25th, 50th, and 75th percentiles of the hs-cTnI distribution. To account for clinical risk factors, we applied a Cox proportional hazards regression model with restricted cubic splines to evaluate the association between hs-cTnI levels and both short- and long-term mortality. This adjusted model estimates hazard ratios (HR) for adverse events while controlling for potential confounders. Specifically, adjustments were made for the European System for Cardiac Operative Risk Evaluation II score (EuroSCORE II), implemented as a continuous linear covariate. The EuroSCORE II incorporates multidimensional clinical risk factors such as age, gender, renal function, extracardiac arteriopathy, mobility status, previous cardiac surgery, chronic lung disease, active endocarditis, critical preoperative state, New York Heart Association functional class, left ventricular function, pulmonary hypertension, urgency of operation, weight of the intervention, and thoracic aortic surgery [12]. To account for perioperative and postoperative risk factors, additional analyses were performed in which the baseline risk adjustment via EuroSCORE II was extended to include duration of mechanical ventilation, length of intensive care unit (ICU) stay, postoperative need for dialysis, number of transfused red blood cell units, and time on cardiopulmonary bypass. All continuous variables were included as linear covariates in the model.
Thresholds for hs-cTnI were identified from the fitted Cox model as the minimum hs-cTnI levels at which the lower bound of the 95% confidence interval (CI) for the adjusted hazard ratio (HR) exceeds 1.0. In this spline-based Cox regression, the HR curve was centered at the median of the hs-cTnI distribution, which served as the reference point for relative risk. This approach ensures that the identified threshold reflects a statistically significant elevation in risk. Receiver Operating Characteristic (ROC) curve analyses were employed to validate these thresholds and assess sensitivity and specificity. These thresholds were used to evaluate predictive performance, including area under the receiver operating characteristic curve (AUC), sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for each outcome. To further characterize risk, we identified threshold-specific hazard ratios at these thresholds. Subgroup analyses were performed according to surgery type (especially for isolated mitral valve surgery and isolated aortic valve surgery). Sensitivity analyses were performed for sex-specific thresholds. All P-values were two-sided, and statistical significance was considered at a threshold of 0.05.
Results
Patient characteristics
Between January 1, 2013, and May 30, 2019, a total of 14,465 patients underwent cardiothoracic surgery at our institution. After applying the predefined exclusion criteria, 4232 patients were included in the final analysis. The majority of patients (n = 3530; 83.4%) underwent CV procedures, while 702 patients (16.6%) underwent TA surgery. Of the CV patients, 2382 (56.3%) underwent isolated aortic valve surgery, 636 (15.0%) isolated mitral valve surgery, and 405 (9.6%) other or combined cardiac valvular surgery (Table S1). The median age of the overall study population was 69 years [IQR 59–76], with 38.4% female sex. The median body mass index (BMI) was 27.5 kg/m2 [IQR 24.3–30.4], and the median EuroSCORE II was 1.9 [IQR 1.0–3.9]. A total of 20.2% of patients had a history of CAD. The systolic left ventricular ejection fraction was sustained at a median of 60% [IQR 55–65], and renal parameters were within the normal range with a median glomerular filtration rate (GFR) of 75 ml/min [IQR 61–88], and a median creatinine of 0.9 mg/dl [0.8–1.1].
Comparative analysis between CV and TA surgery groups revealed notable demographic and clinical differences. Patients in the CV group were, on average, older and more frequently female. The EuroSCORE II was significantly higher in the TA group at 5.2 [IQR 3.7–8.2] versus 1.5 [IQR 0.9–2.9] in the CV group. The rates of known atrial fibrillation and any type of diabetes were significantly higher in the CV group (Table 1 in the Appendix). Total time of surgery, time on aortopulmonary bypass, and aortic clamping time were significantly longer in the TA groups (Table S2).
Perioperative kinetics of hs-cTnI
The mean baseline (preoperative) hs-cTnI levels were within the normal range of the employed assay at 9 pg/ml [IQR 5–18] in the CV group and 7 [IQR 4–15] in the TA group. The perioperative kinetics of hs-cTnI exhibited a similar pattern in both groups, characterized by a rapid postoperative increase. Peak hs-cTnI levels were observed between 4 and 8 h after surgery, followed by a gradual decline over time. Repeated measures ANOVA demonstrated no significant difference in hs-cTnI kinetics between CV and TA surgery patients (P = 0.91, Fig. 1). Within 48 h post-operatively, 4194 (99.1%) of all patients exceeded hs-cTnI measurements > 35 times the ULN, and 3767 (89.0%) > 70 times the ULN.
Postoperative kinetics of high-sensitivity cardiac troponin I (hs-cTnI) Based on type of surgery performed. Measurements of hs-cTnI are presented as multiples of the upper limit of normal of 26 pg/ml (× ULN). BL baseline (measurement prior to surgery), CV cardiac valvular surgery (plotted in orange color and marked with triangular symbols), TA thoracic aortic surgery (plotted in black color and marked with rectangular symbols)
Outcome events
The median follow-up duration was 3.1 years [IQR 1.9–5.0]. During this period, long-term all-cause mortality occurred in 499 patients (11.8% of the study population). This incidence was significantly higher in the CV group, with 447 events (12.7%), compared to 52 events (7.4%) in the TA group (P < 0.001). In contrast, 30-day all-cause mortality occurred in 61 patients (1.4%), with no significant differences observed between the surgical groups (P = 0.38, Table 2 in the Appendix). An ARC-2 defined PMI occurred in 32 (0.8%) patients, and 26 (0.6%) required coronary revascularization within 48 h postoperatively. The postoperative hs-cTnI trajectory in patients requiring early revascularization is presented in Fig. S2.
Prognostic value of hs-cTnI
Figure 2 shows the unadjusted relationship between peak postoperative hs-cTnI measurements and long-term all-cause mortality (Fig. 2A) and 30-day all-cause mortality (Fig. 2B). Hazard ratio curves adjusted for EuroSCORE II as a function of peak postoperative hs-cTnI measurements are presented in Fig. 3. The lower bound of the 95% confidence interval intersected the hazard ratio of 1.0 at a peak hs-cTnI value of 282 times the ULN for long-term all-cause mortality (Fig. 3A) and at 194 times the ULN for 30-day all-cause mortality (Fig. 3B) in the overall study population. At these specific thresholds, the hazard ratios were estimated as 1.11 (95% CI 1.00–1.24) for long-term all-cause mortality and 1.24 (95% CI 1.00–1.54) for 30-day all-cause mortality. The corresponding thresholds showed an AUC of 0.56 (95% CI 0.54–0.58) and 0.55 (95% CI 0.52–0.57), respectively. Sensitivity and specificity were 41% and 71% for long-term mortality, and 54% and 55% for 30-day all-cause mortality. NPV was 90% across both endpoints, while PPV was 16% and 14%, respectively. We repeated these analyses for the subgroups of CV and TA patients only, as well as isolated aortic valve and mitral valve surgery subgroups independently, and when excluding the 855 patients with known CAD. While the calculated threshold varied between 375 and 689 times the ULN, the diagnostic metrics were comparable (Table S3). Analyses regarding 30-day all-cause mortality in these subgroups, as well as for early coronary revascularization and PMI, were not feasible due to a low number of events.
Unadjusted relationships between peak high-sensitivity cardiac troponin I (hs-cTnI) measurements 48 h post-operatively as multiples of the upper limit of normal of 26 pg/ml (× ULN) and long-term all-cause mortality (A) and 30-day all-cause mortality (B). Dashed lines represent 95% confidence intervals for the unadjusted relationship in each panel. The insets show the same data on an expanded y axis
Adjusted hazard ratio curves for long-term all-cause mortality (A) and 30-day all-cause mortality (B) as a function of peak high-sensitivity cardiac troponin I (hs-cTnI) measurements 48 h post-operatively as multiples of the upper limit of normal of 26 pg/ml (× ULN) among patients who underwent isolated cardiac valvular or thoracic aortic surgery. All hazard ratios are adjusted for the European System for Cardiac Operative Risk Evaluation Score II (EuroSCORE II). The dashed lines represent 95% confidence intervals for the adjusted hazard ratio
Analysis by time to hs-cTnI peak
Peak hs-cTnI was reached within the first 24 h postoperatively in 3702 (87.5%) patients and after 24 h in 530 (12.5%). In the early peak group, long-term all-cause mortality occurred in 387 (10.5%) patients and 30-day all-cause mortality in 38 (1.0%). For the late peak group, the numbers were 112 (21.1%) and 23 (4.3%), respectively. Among patients who reached peak hs-cTnI within 24 h, thresholds were identified at 467 times the ULN for long-term all-cause mortality (HR 1.12, 95% CI 1.00–1.25) and 357 times the ULN for 30-day all-cause mortality (HR 1.61, 95% CI 1.00–2.58). The AUCs for both thresholds were 0.55. Sensitivity and specificity were 22% and 88% for long-term all-cause mortality and 28% and 81% for 30-day mortality, respectively. In contrast, patients with later peak hs-cTnI (> 24 h postoperatively) showed thresholds of 1248 times the ULN for long-term mortality (HR 1.27, 95% CI 1.00–1.60) and 351 times the ULN for 30-day mortality (HR 2.26, 95% CI 1.00–5.12), with corresponding AUCs of 0.55 and 0.56, respectively. When analyzing the independent postoperative timepoints individually, measurements after 20 to 24 h and 40 to 48 h yielded the highest AUC of 0.64 to predict long-term all-cause mortality. For 30-day all-cause mortality, the highest yield was calculated with AUCs of 0.75 at 24 to 32 h and 32 to 48 h postoperatively, respectively (Fig. S3).
Extended models adjusted for perioperative and postoperative risk
We extended our models to adjust for the duration of ICU stay, duration on mechanical ventilation, need for postoperative dialysis, number of transfused red blood cell units, and time on cardiopulmonary bypass. In these models, peak hs-cTnI remained independently associated with both long-term and 30-day all-cause mortality. For long-term mortality, the threshold was identified at 961 times the ULN (HR 1.12, 95% CI 1.00–1.26), with an AUC of 0.53. Sensitivity and specificity were 11% and 96%, while PPV and NPV were 26% and 89%, respectively. For 30-day all-cause mortality, the threshold was 233 times the ULN (HR 1.39, 95% CI 1.00–1.93) with an AUC of 0.55. Sensitivity and specificity were 47% and 63%, and PPV and NPV were 15% and 90%.
Sex-specific upper limit of normal
When applying the sex-specific reference levels of 16 pg/mL for women and 34 pg/mL for men, the findings were consistent with the primary analysis using non-sex-specific reference levels, although slight shifts in thresholds and hazard ratios were observed. For this model, the multivariable Cox proportional hazards models adjusted for EuroSCORE II identified that the lower bound of the 95% confidence interval intersected the hazard ratio of 1.0 at a peak hs-cTnI value of 223 times the ULN for long-term all-cause mortality and 194 times the ULN for 30-day all-cause mortality. At these specific thresholds, the hazard ratios were estimated as 1.08 (95% CI 1.00–1.16) for long-term all-cause mortality and 1.62 (95% CI 1.00–2.62) for 30-day all-cause mortality. The corresponding thresholds showed an AUC of 0.58 and 0.57, respectively. Sensitivity and specificity were 62% and 54% for long-term mortality and 55% and 58% for 30-day all-cause mortality. NPVs were 91% across both endpoints, while PPVs were 16% and 15%, respectively.
Discussion
In this study, we evaluated the prognostic utility of peak hs-cTnI measurements in predicting long-term all-cause mortality and 30-day all-cause mortality in patients undergoing isolated CV or TA surgery (graphical abstract). Our main findings are that.
-
1)
Peak postoperative hs-cTnI is significantly associated with adverse outcomes across both endpoints, but diagnostic metrics were limited
-
2)
The calculated thresholds substantially exceed those in the established definitions for perioperative myocardial injury
-
3)
The application of sex-specific reference levels resulted only in modest shifts in risk thresholds and diagnostic performance
Previous studies have demonstrated that hs-cTnI thresholds associated with postoperative adverse events are substantially higher than those used in current PMI definitions. However, most of this evidence stems from CABG populations, limiting its applicability to other types of cardiac surgery [8, 13]. Isolated CV or TA surgery presents a unique challenge in assessing perioperative cardiac biomarker kinetics, particularly due to limited data available on hs-cTnI dynamics and its prognostic implications. Unlike CABG, CV and TA surgery involves non-coronary manipulations that can induce myocardial stress through different mechanisms, potentially altering biomarker release. Our study aimed to address this gap by evaluating the perioperative kinetics of hs-cTnI in patients undergoing elective CV or TA surgery with regard to its prognostic utility in predicting long-term all-cause mortality and 30-day all-cause mortality. Given the lack of established thresholds for this specific surgical population, our analysis further explored the optimal hs-cTnI cutoffs for risk prediction, both in general and sex-specific contexts.
While peak postoperative hs-cTnI measurements were significantly associated with our endpoints, the diagnostic test metrics revealed only moderate discriminatory ability. Specifically, the AUC values ranged between 0.54 and 0.58, indicating poor precision in distinguishing high-risk patients. Sensitivity remained moderate to high across both endpoints, while specificity was notably low. These findings are consistent with previous studies, suggesting that hs-cTnI alone may not serve as a highly specific discriminator of postoperative risk but remains a valuable indicator of elevated hazard [8, 14]. Similarly, previous studies, such as the VISION study, have also reported only modest discriminatory capacity for troponin-based risk prediction despite significant hazard ratios [8].
Within this collective of CV or TA surgery patients without including patients that also underwent coronary revascularization, we found a hs-cTnI threshold ≥ 282 times the ULN to be an independent predictor of long-term all-cause mortality, slightly higher than the ≥ 218 times the ULN reported in VISION [8]. This difference could be explained by the differences between study populations and procedure types. Other studies in cardiac surgery cohorts reported thresholds between 200–300 times the ULN for all-cause mortality, and even higher thresholds were reported (500 × ULN) to be associated with the need for repeat revascularization and also with increased mortality [13]. When excluding the patients with known CAD from our collective, the threshold increased to 519 times the ULN. Similarly to the findings from VISION, our thresholds were substantially higher than the values for risk prediction compared to those currently used in established PMI definitions [3, 5,6,7,8, 13]. For 30-day all-cause mortality, we calculated a threshold ≥ 193 times the ULN. Event rates and hazard ratios were higher in patients who reached peak postoperative hs-cTnI more than 24 h after surgery. These findings support the use of strategically timed measurements that consider hs-cTnI kinetics beyond the first 24 h after surgery. Importantly, time-specific AUCs peaked at 24–48 h postoperatively, suggesting this window may be more valuable for risk stratification.
Cardiac biomarkers and their release kinetics are strongly influenced by many factors, including age, sex, and various comorbidities [4, 15,16,17]. While sex-based differences in cTn assays have been reported, with female patients generally exhibiting lower concentrations, these sex-specific differences are not widely implemented in clinical practice. Although the mechanisms underlying these differences are not fully understood, variations in neurohormonal and inflammatory responses, potentially activating prothrombotic pathways, have been suggested [17]. The manufacturer of the hs-cTnI assay used in this study reports a general URL of 26 pg/mL, while acknowledging that sex-specific cutoffs of 16 pg/mL for women and 34 pg/mL for men may improve diagnostic precision. However, whether these sex-specific thresholds should be applied in clinical practice remains a matter of ongoing debate. In our analysis, the observed predictive patterns appeared to be generally comparable between the general and sex-specific thresholds, although slight shifts in cutoffs were noted. These findings suggest that while sex-specific cutoffs may fine-tune risk estimation, the overall predictive capacity remains largely consistent.
Our study has several implications for clinical practice. First, while the dynamic changes in hs-cTnI levels following CV or TA surgery highlight the potential of cardiac biomarkers in the perioperative period, frequent serial measurement may not provide substantial prognostic benefit over fewer, optimally timed measurements. In our cohort, measurements taken between 40–48 h postoperatively yielded the highest AUC for long-term all-cause mortality (0.64), and between 24–32 h for 30-day all-cause mortality (0.75). Second, although peak hs-cTnI levels were associated with adverse outcomes, their overall prognostic accuracy was limited. Third, our findings emphasize that postoperative hs-cTnI should not be interpreted in isolation. Even when adjusted for the time of ICU stay, time on mechanical ventilation, need for postoperative dialysis, and number of transfused red blood cell units, its diagnostic accuracy remained limited. Therefore, comprehensive risk assessment should integrate hs-cTnI with clinical presentation, hemodynamic monitoring, and imaging.
Limitations
Our study has several limitations that should be acknowledged. First, data were collected at a single site, and this study was conducted as a post-hoc analysis of a prospective registry, which may introduce selection bias and limit the generalizability of the findings. Second, the aim of our study was to examine postoperative hs-cTnI dynamics and its prognostic value explicitly in a non-CABG cohort. CV-TA surgery encompasses a heterogeneous group of procedures, which may contribute to variability in biomarker release and clinical outcomes. All procedures were performed at the discretion of the operators, possibly introducing differences in anesthesia and cardioplegia that might have had an impact on postoperative enzyme release. However, all procedures were performed at a single center following standardized operating protocols. Third, the endpoint for this analysis was all-cause mortality. Data on the cause of death was not available, but the predictive performance of hs-cTnI might differ depending on the cause of death (i.e., cardiovascular vs. non-cardiovascular).
Conclusion
Our findings demonstrate that peak hs-cTnI measurements are associated with adverse outcomes in patients undergoing isolated CV or TA surgery. The observed threshold-specific hazard ratios suggest that current PMI definitions may overestimate risk in this population, highlighting the need for refined hs-cTnI thresholds for more accurate perioperative risk assessment.
Abbreviations
- ANOVA:
-
Analysis of variance
- ARC-2:
-
Academic Research Consortium 2
- AUC:
-
Area under the receiver operating characteristic curve
- BMI:
-
Body mass index
- CABG:
-
Coronary artery bypass grafting
- CAD:
-
Coronary artery disease
- CI:
-
Confidence intervals
- CPB:
-
Cardiopulmonary bypass
- cTn:
-
Cardiac troponin
- CV:
-
Cardiac valvular surgery
- ECG:
-
Electrocardiography
- EuroSCORE II:
-
European System for Cardiac Operative Risk Evaluation Score II
- GFR:
-
Glomerular filtration rate
- HR:
-
Hazard ratio
- hs-cTnI:
-
High-sensitivity cardiac troponin I
- ICU:
-
Intensive care unit
- IQR:
-
Interquartile range
- NPV:
-
Negative predictive value
- PMI:
-
Postoperative myocardial infarction
- PPV:
-
Positive predictive value
- ROC:
-
Receiver operating curve
- SCAI:
-
Society of Cardiovascular Angiography and Interventions
- SD:
-
Standard deviation
- TA:
-
Thoracic aortic surgery
- UDMI:
-
Universal definition of myocardial injury
- ULN:
-
Upper limit of normal
- VISION:
-
Vascular Events in Surgery Patients Cohort Evaluation Trial
References
Chan PG, Seese L, Aranda-Michel E, Sultan I, Gleason TG, Wang Y, Thoma F, Kilic A (2021) Operative mortality in adult cardiac surgery: is the currently utilized definition justified? J Thorac Dis 13:5582–5591
Byrne RA, Rossello X, Coughlan JJ, Barbato E, Berry C, Chieffo A, Claeys MJ, Dan GA, Dweck MR, Galbraith M, Gilard M, Hinterbuchner L, Jankowska EA, Jüni P, Kimura T, Kunadian V, Leosdottir M, Lorusso R, Pedretti RFE, Rigopoulos AG, Gimenez MR, Thiele H, Vranckx P, Wassmann S, Wenger NK, Ibanez B (2023) 2023 ESC guidelines for the management of acute coronary syndromes. Eur Heart J 44:3720–3826
Devereaux PJ, Whitlock R, Lamy A (2023) Perioperative myocardial injury/infarction after cardiac surgery: the diagnostic criteria need to change. J Am Coll Cardiol 82:1313–1315
Rudolph F, Deutsch M-A, Friedrichs KP, Renner A, Scholtz W, Gerçek M, Kirchner J, Ayoub M, Rudolph TK, Schramm R, Gummert J, Rudolph V, Omran H (2025) Impact of impaired renal function on kinetics of high-sensitive cardiac troponin following cardiac surgery. Clin Res Cardiol [Internet]. Available from: https://springerlink.fh-diploma.de/10.1007/s00392-025-02595-7. Accessed 30 Jan 2025
Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD, Mickley H, Crea F, Van De Werf F, Bucciarelli-Ducci C, Katus HA, Pinto FJ, Antman EM, Hamm CW, De Caterina R, Januzzi JL, Apple FS, Garcia MAA, Underwood SR, Canty JM, Lyon AR, Devereaux PJ, Zamorano JL, Lindahl B, Weintraub WS, Newby LK, Virmani R, Vranckx P, Cutlip D, Gibbons RJ, Smith SC, Atar D, Luepker RV, Robertson RM, Bonow RO, Steg PG, O’Gara PT, Fox KAA, Hasdai D, Aboyans V, Achenbach S, Agewall S, Alexander T, Avezum A, Barbato E, Bassand JP, Bates E, Bittl JA, Breithardt G, Bueno H, Bugiardini R, Cohen MG, Dangas G, De Lemos JA, Delgado V, Filippatos G, Fry E, Granger CB, Halvorsen S, Hlatky MA, Ibanez B, James S, Kastrati A, Leclercq C, Mahaffey KW, Mehta L, Müller C, Patrono C, Piepoli MF, Piñeiro D, Roffi M, Rubboli A, Sharma S, Simpson IA, Tendera M, Valgimigli M, Van Der Wal AC, Windecker S (2019) Fourth universal definition of myocardial infarction (2018). Eur Heart J 40:237–269
Garcia-Garcia HM, McFadden EP, Farb A, Mehran R, Stone GW, Spertus J, Onuma Y, Morel MA, Van Es GA, Zuckerman B, Fearon WF, Taggart D, Kappetein AP, Krucoff MW, Vranckx P, Windecker S, Cutlip D, Serruys PW (2018) Standardized end point definitions for coronary intervention trials: the academic research consortium-2 consensus document. Circulation 137:2635–2650
Moussa ID, Klein LW, Shah B, Mehran R, Mack MJ, Brilakis ES, Reilly JP, Zoghbi G, Holper E, Stone GW (2013) Consideration of a new definition of clinically relevant myocardial infarction after coronary revascularization: an expert consensus document from the Society for Cardiovascular Angiography and Interventions (SCAI). J Am Coll Cardiol 62:1563–1570
Devereaux PJ, Lamy A, Chan MTV, Allard R, Lomivorotov V, Landoni G, Zheng H, Paparella D, McGillion MH, Belley-Côté EP, Parlow JL, Underwood MJ, Wang CY, Dvirnik N, Abubakirov M, Fominskiy E, Choi S, Fremes S, Monaco F, Urrútia G, Maestre M, Hajjar LA, Hillis GS, Mills NL, Margari V, Mills JD, Billing JS, Methangkool E, Polanczyk CA, Sant’Anna R, Shukevich D, Conen D, Kavsak PA, McQueen MJ, Brady K, Spence J, Le Manach Y, Mian R, Lee SF, Bangdiwala SI, Hussain S, Borges FK, Pettit S, Vincent J, Guyatt GH, Yusuf S, Alpert JS, White HD, Whitlock RP (2022) High-sensitivity troponin I after cardiac surgery and 30-day mortality. N Engl J Med 386:827–836
Isselbacher EM, Preventza O, Black JH, Augoustides JG, Beck AW, Bolen MA, Braverman AC, Bray BE, Brown-Zimmerman MM, Chen EP, Collins TJ, DeAnda A, Fanola CL, Girardi LN, Hicks CW, Hui DS, Jones WS, Kalahasti V, Kim KM, Milewicz DM, Oderich GS, Ogbechie L, Promes SB, Ross EG, Schermerhorn ML, Times SS, Tseng EE, Wang GJ, Joseph WY (2022) 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 146:E334–E482
Heuts S, Denessen EJS, Daemen JHT, Vroemen WHM, Sels JW, Segers P, Bekers O, van ‘t Hof AWJ, Maessen JG, van der Horst ICC, Mingels AMA (2022) Meta-analysis evaluating high-sensitivity cardiac troponin T kinetics after coronary artery bypass grafting in relation to the current definitions of myocardial infarction. Am J Cardiol 163:25–31
Martínez-Comendador J, Castaño M, Mosquera I, Plana JG, Gualis J, Martín CE, Mencía P (2011) Cryoablation of atrial fibrillation in cardiac surgery: outcomes and myocardial injury biomarkers. J Cardiothorac Vasc Anesth [Internet] 25:1030–1035. Available from: https://www.sciencedirect.com/science/article/pii/S1053077011004575. Accessed 4 Sept 2025
Nashef SAM, Roques F, Sharples LD, Nilsson J, Smith C, Goldstone AR, Lockowandt U (2012) Euroscore II. Eur J Cardiothorac Surg 41:734–745
Omran H, Deutsch MA, Groezinger E, Zittermann A, Renner A, Neumann JT, Westermann D, Myles P, Ramosaj B, Pauly M, Scholtz W, Hakim-Meibodi K, Rudolph TK, Gummert J, Rudolph V (2022) High-sensitivity cardiac troponin I after coronary artery bypass grafting for post-operative decision-making. Eur Heart J 43:2388–2403
Nam K, Shin KW, Kim TK, Kim KH, Kim KB, Jeon Y, Cho YJ (2020) Prognostic value of high-sensitivity troponin I after cardiac surgery according to preoperative renal function. Medicine (Baltimore). https://doi.org/10.1097/MD.0000000000020040
Shah ASV, Anand A, Strachan FE, Ferry AV, Lee KK, Chapman AR, Sandeman D, Stables CL, Adamson PD, Andrews JPM, Anwar MS, Hung J, Moss AJ, O’Brien R, Berry C, Findlay I, Walker S, Cruickshank A, Reid A, Gray A, Collinson PO, Apple FS, McAllister DA, Maguire D, Fox KAA, Newby DE, Tuck C, Harkess R, Parker RA, Keerie C, Weir CJ, Mills NL, Marshall L, Stewart SD, Fujisawa T, Vallejos CA, Tsanas A, Hautvast M, McPherson J, McKinlay L, Malo J, Fischbacher CM, Croal BL, Leslie SJ, Walker A, Wackett T, Armstrong R, Stirling L, MacDonald C, Sadat I, Finlay F, Charles H, Linksted P, Young S, Alexander B, Duncan C (2018) High-sensitivity troponin in the evaluation of patients with suspected acute coronary syndrome: a stepped-wedge, cluster-randomised controlled trial. The Lancet 392:919–928
Sbarouni E, Georgiadou P, Voudris V (2011) Gender-specific differences in biomarkers responses to acute coronary syndromes and revascularization procedures. Biomarkers 16:457–465. https://doi.org/10.3109/1354750X.2011.576431
Schwarzenberger JC, Sun LS, Pesce MA, Heyer EJ, Delphin E, Almeida GM, Wood M (2003) Sex-based differences in serum cardiac troponin I, a specific marker for myocardial injury, after cardiac surgery. Crit Care Med [Internet] 31. Available from: https://journals.lww.com/ccmjournal/fulltext/2003/03000/sex_based_differences_in_serum_cardiac_troponin_i,.5.aspx. Accessed 7 May 2024
Funding
Open Access funding enabled and organized by Projekt DEAL. Dr F. Rudolph has received funding from Bielefeld University (clinician scientist entry fellowship). Dr M. Gerçek has received funding from Ruhr University Bochum (advanced clinician scientist).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
ESM 1
DOCX (529 KB)
Appendix
Appendix
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rudolph, F., Deutsch, MA., Friedrichs, K.P. et al. Prognostic value of high-sensitivity cardiac troponin I after isolated non-CABG cardiac surgery. Clin Res Cardiol (2025). https://doi.org/10.1007/s00392-025-02771-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1007/s00392-025-02771-9