Abstract
Rosacea and autoimmune liver diseases (AILDs) are diseases closely associated with immune system abnormalities. AILDs primarily includes autoimmune hepatitis (AIH), primary biliary cholangitis (PBC), and primary sclerosing cholangitis (PSC). Currently, research on the association between these two conditions is limited. Therefore, this study employed the bidirectional Mendelian randomization (MR) method to investigate potential causal relationships between rosacea and AILDs based on genetic predictions. Summary data related to Rosacea, AIH, PSC, and PBC were obtained from public genome-wide association studies (GWAS). The inverse variance weighted (IVW) method was used as the primary analytical approach, supplemented by the MR-Egger, weighted mode method, weighted median, and simple mode. A series of sensitivity analyses were also conducted to identify heterogeneity and pleiotropy effects. The MR analysis results indicated a significant increase in the risk of rosacea being associated with PBC (OR = 1.09, 95% CI = 1.02–1.18, P = 0.014), but no such association was found with AIH or PSC. Furthermore, this study did not find a significant impact of rosacea on the risk of AILDs. This study represents the first in-depth exploration of the potential causal relationship between rosacea and AILDs using MR analysis. Thes findings suggest an increased risk of rosacea among PBC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rosacea is a common chronic inflammatory skin disease prevalent among individuals aged 30–50 years and is characterized primarily by persistent erythema, recurrent papules, and pustules [1]. It is typically classified into four subtypes: papulopustular, phymatous, erythematotelangiectatic, and ocular rosacea [2, 3]. The pathogenesis of rosacea is complex and not fully understood, involving intrinsic factors such as genetics, psychological factors, and obesity, as well as extrinsic factors such as smoking, alcohol consumption, ultraviolet radiation, and specific microbes such as Demodex folliculorum, Propionibacterium acnes, and Helicobacter pylori infections. These factors contribute to neurovascular dysregulation, immune system abnormalities, and meibomian and sebaceous gland dysfunction, thereby triggering the disease. It not only leads to emotional issues such as low self-esteem and depression in patients, but also negatively impacts their quality of life, posing a significant burden on societal health.
Autoimmune liver diseases (AILDs) encompasses a group of chronic liver diseases characterized by abnormal immune responses that target the liver cells or bile ducts. These diseases are typically categorized into three types [4]. Autoimmune hepatitis (AIH) is characterized by hepatocellular damage, elevated liver enzymes, serum autoantibodies, and immunoglobulins [5]. Primary biliary cholangitis (PBC) is a chronic cholestatic liver disease primarily caused by autoimmune-mediated injury to the intrahepatic bile duct epithelia, predominantly affecting middle-aged women [6]. Primary sclerosing cholangitis (PSC), on the other hand, is a relatively rare cholestatic liver disease characterized by multilayered fibrosis and bile duct obstruction both intrahepatically and extrahepatically, more commonly affecting young to middle-aged men [6]. AILDs may initially be asymptomatic, but as the disease progresses, it can lead to complications, such as cirrhosis and hepatocellular carcinoma. Currently, there is no cure, and recurrence rates after liver transplantation are high, imposing substantial economic and medical burdens on patients and society. The etiology of AILDs is complex and involves factors such as immune responses, environment, and genetics [7]. Despite the identification of some risk factors, the exact pathogenic mechanisms remain unclear. AILDs are often associated with other autoimmune diseases, further exacerbating their severity.
Therefore, to elucidate the potential causal relationship between rosacea and AILDs, this study utilized the bidirectional Mendelian randomization (MR) method. MR has gained prominence in genetic and epidemiological research, owing to its unique advantages. Compared with traditional observational studies, MR leverages genetic variants naturally occurring at birth to significantly mitigate the influence of confounding factors, ensuring the objectivity and independence of research results. This technique relies on publicly available genome-wide association study (GWAS) data and utilizes genetic variations as instrumental variables (IVs) to assess the potential causal relationships between exposures and outcomes [8]. Because genes are immutable and follow Mendelian inheritance laws, MR effectively circumvents issues commonly encountered in observational studies, such as confounding factors, reverse causation, and measurement errors [9]. Thus, in the absence of randomized controlled trials, MR is an efficient and economical method to explore causal associations. This study employed bidirectional two-sample MR analyses supplemented by comprehensive sensitivity analyses to delve deeper into the causal relationship between rosacea and AILDs.
Material and methods
Study design
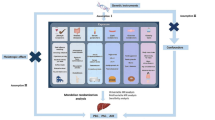
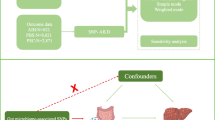
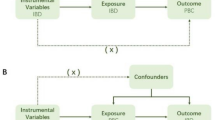
This study aimed to explore the potential causal relationship between rosacea and AILDs using bidirectional two-sample MR analysis. We utilized GWAS datasets of European ancestry, structured around three core hypotheses: first, IVs must be significantly associated with rosacea and other exposure factors; second, these IVs must be independent of confounding factors that could interfere with the exposure-outcome relationship and IVs can only affect outcomes through exposure factors, without other causal pathways. We meticulously selected single nucleotide polymorphisms (SNPs) associated with rosacea, AIH, PBC, and PSC as IVs, adhering strictly to the guidelines of the STROBE-MR (Strengthening the Reporting of Observational Studies in Epidemiology using Mendelian Randomization) statement [10]. The datasets used in this study were obtained from public databases and had prior ethical approval, thereby obviating the need for additional ethical clearance. The MR design is illustrated in Fig. 1.
Data source
The FinnGen project, initiated in 2017, aims to collect biological samples from 500,000 participants in Finland to provide scientific insights into health improvements through genetic research. By leveraging resources from the FinnGen biobank, we investigated the genetic variations associated with rosacea. This biobank comprised 16,380,452 SNPs and 212,334 European samples, including 1195 rosacea cases and 211,139 controls (GWAS ID: finn-b-L12_ROSACEA). IVs related to AIH were sourced from 220 human phenotype genetic correlation maps, encompassing 821 European patients and 484,413 European controls, involving 24,198,482 SNPs (GWAS ID: ebi-a-GCST90018785). The IVs for PBC were derived from the largest international genome-wide meta-analysis to date, including 8021 European patients and 16489 European controls, with 5,004,018 SNPs (GWAS ID: ebi-a-GCST90061440). IVs for PSC were obtained from the most comprehensive PSC GWAS conducted by the International PSC Study Group (IPSCSG), comprising 2871 European PSC patients and 12019 European controls, involving 7,891,603 SNPs (GWAS ID: ieu-a-1112). Detailed information regarding the GWAS data used in this study is presented in Table 1.
Instrumental variable selection
Before conducting the MR analysis, we rigorously screened the IVs to ensure that they were both significant and independent. First, we selected SNPs with genome-wide significance (P < 5e-8) [11]. Given the limited number of SNPs meeting the criteria for rosacea and AIH, we appropriately relaxed the P-value threshold to < 5e-6 to ensure an adequate sample size. Second, to ensure the independence of SNPs, we set a linkage disequilibrium (LD) threshold such that the LD r2 value within 10000 base pairs was less than 0.001, excluding SNPs with palindromic sequences. As we were unable to use PhenoScanner V2, we manually removed SNPs related to confounding factors using multiple online databases, such as IEU Open GWAS and GWAS Catalog. Subsequently, we used MR-polynomial residuals and outliers (MR-PRESSO) to identify and remove outlier SNPs and further corrected the results to ensure that no horizontal pleiotropy existed among selected SNPs. Finally, we calculated the F value = [beta/SE]2 for each SNP, requiring an F-value > 10 to ensure sufficient instrument strength and to minimize potential bias. These carefully selected SNPs served as IVs for subsequent MR analyses.
MR analysis
MR analysis primarily employed the inverse variance weighted (IVW) method to explore the correlations between rosacea and the risks of AIH, PBC, and PSC. We assessed the heterogeneity among the IVs using Cochran’s Q test. When the P-value of Cochran’s Q test was < 0.05, indicating heterogeneity, we used the IVW random effects model for analysis; if the P-value was ≥ 0.05, we employed the fixed effects model. To ensure the robustness of our findings, we complemented the IVW with MR-Egger, weighted median, simple mode, and weighted mode for validation. Additionally, we utilized various methods such as MR-PRESSO, MR-Egger intercept, funnel plots, and forest plots to examine potential pleiotropy and comprehensively assess the robustness of the results. Furthermore, to determine whether specific SNPs influenced the causal estimates, we conducted leave-one-out analyses. Statistical analyses were conducted using R software (version 4.3.2) with the “TwoSampleMR” (v.0.5.8) and “MR-PRESSO” (v.1.0) packages, with a significance level set at P < 0.05.
Results
Selection of IVs
Using rosacea as an exposure factor, we successfully identified and selected 13 SNPs through a rigorous screening process. After further analysis, we excluded the confounding factor rs79440702, ultimately determining 12 SNPs as IVs for the subsequent in-depth analysis. Similarly, using AIH, PBC, and PSC as exposure factors, and based on the screening method described in detail earlier, this study identified 10, 35, and 11 SNPs associated with these exposure factors, respectively. The F-values of all included SNPs were significantly greater than 10, which is a strict criterion that avoids potential bias introduced by weak IVs. Detailed information on the SNPs is provided in the Table S1–S6.
Causal effects of rosacea on AILDs
The results of the IVW estimation indicated that there was no significant association between genetically predicted rosacea and the risk of AIH (OR = 0.91, 95% CI = 0.80–1.02, P = 0.119) (Fig. 2). Additionally, the results from the MR-Egger method (OR = 0.87, 95% CI = 0.66–1.14, P = 0.330), weighted median (OR = 0.91, 95% CI = 0.77–1.08, P = 0.264), simple mode (OR = 0.95, 95% CI = 0.73–1.25, P = 0.741), and weighted mode (OR = 0.87, 95% CI = 0.69–1.09, P = 0.258) suggested no significant correlation between genetic variations in rosacea and AIH (Fig. 2 and Fig. S1). We did not detect significant heterogeneity (Cochran’s Q test: P = 0.115) or pleiotropy (MR-PRESSO global test: P = 0.162; MR-Egger regression: intercept = 0.019, P = 0.690) (Table 2). No association was found between genetically predicted rosacea (OR = 1.07, 95% CI = 0.95–1.20, P = 0.250) and the risk of PBC (Fig. 2 and Fig. S2). Although heterogeneity was observed in this analysis (P-value < 0.05 in Cochran’s Q test), it was acceptable because this study primarily employed a random-effects IVW for analysis. For PBC, no pleiotropy was detected (MR-PRESSO global test: P = 0.185; MR-Egger regression: intercept = 0.048, P = 0.369) (Table 2). Similarly, no association was found between genetically predicted rosacea (OR = 0.97, 95% CI = 0.89–1.05, P = 0.470) and the risk of PSC, and no heterogeneity was detected in this analysis (Cochran’s Q test: P = 0.823) or pleiotropy (MR-PRESSO global test: P = 0.768; MR-Egger regression: intercept = − 0.003, P = 0.916) (Fig. 2, Fig. S3 and Table 2).
MR estimates the causal effect of rosacea on AILDs. ROS rosacea; AILDs autoimmune liver diseases; AIH autoimmune hepatitis; PBC Primary biliary cholangitis; PSC Primary sclerosing cholangitis; SNPs single nucleotide-polymorphisms; MR Mendelian randomization; FE fixed effect; OR odds ratio; CI confidence interval
Causal effects of AILDs on rosacea
This study also conducted a reverse MR analysis to evaluate the potential causal effects of AILDs on rosacea. Genetic prediction indicated a causal relationship between PBC and the risk of rosacea. Using IVW estimation, we observed that having PBC might increase the risk of rosacea by 9.0% (OR = 1.09, 95% CI = 1.02–1.18, P = 0.014) (Fig. 3 and Fig. 4). In the pleiotropy analysis, we detected no significant pleiotropy (MR-PRESSO global test: P = 0.099; MR-Egger regression: intercept = 0.037, P = 0.209) and no heterogeneity (Cochran’s Q test: P = 0.084) (Table 2). There was no association between AIH (OR = 1.07, 95% CI = 0.97–1.17, P = 0.175) and the risk of rosacea, and no pleiotropy (MR-PRESSO global test: P = 0.813; MR-Egger regression: intercept = − 0.015, P = 0.727) or heterogeneity (Cochran’s Q test: P = 0.822) was detected in this analysis (Fig. 3, Fig. S4 and Table 2). Similarly, no correlation was found between PSC (OR = 0.94, 95% CI = 0.84–1.06, P = 0.308) and risk of rosacea (Fig. 3 and Fig. S5). In subsequent analyses, we detected no pleiotropy (MR-PRESSO global test: P = 0.306; MR-Egger regression: intercept = − 0.031, P = 0.477) or heterogeneity (Cochran’s Q test: P = 0.326) (Table 2).
MR estimates the causal effect of AILDs on rosacea. AILDs autoimmune liver diseases; AIH autoimmune hepatitis; PBC Primary biliary cholangitis; PSC Primary sclerosing cholangitis; ROS rosacea; SNPs single nucleotide-polymorphisms; MR Mendelian randomization; FE fixed effect; OR odds ratio; CI confidence interval
Discussion
This study employed the bidirectional two-sample MR method to investigate the causal relationship between rosacea and AILDs for the first time. The study findings demonstrated a positive association between PBC and rosacea, indicating an increased risk of rosacea with PBC, but no reverse causation was found. However, when analyzing other AILDs such as AIH and PSC, no significant statistical correlation with rosacea was observed. Similarly, rosacea did not significantly influence the prevalence of AIH or PSC.
Despite the distinct primary sites of rosacea (predominantly affecting the central facial skin) and AILDs (primarily affecting the hepatobiliary system), both conditions are closely associated with immune dysfunction. Rosacea, as an inflammatory skin disease, shares mechanistic links with immune system dysregulation, while AILDs, as autoimmune diseases, also involve immune response dysfunctions. Nevertheless, research on the association between these two diseases remains insufficient, highlighting the importance of further investigation in this field.
Whereas AIH, PBC, and PSC fall under category of AILDs, each has unique pathological characteristics. AIH is mainly characterized by hepatocellular damage, PBC by non-suppurative destructive cholangitis and chronic cholestasis, and PSC by onion-skin fibrosis of intrahepatic and extrahepatic bile ducts [5, 12, 13]. These differences are likely to stem from the distinct nature of each disease. Furthermore, patients with AILD often present with various skin symptoms, such as butterfly rash, pigmentation, and itching. Patients AIH and PBC are susceptible to conditions like alopecia areata, vitiligo, and psoriasis [14]. However, owing to the frequent association of PSC with inflammatory bowel disease, its relationship with skin diseases remains unclear.
The onset of rosacea is closely linked to keratinocytes, which produce pro-inflammatory factors that recruit and activate immune cells, especially T cells [15]. In rosacea patients, toll-like receptor 2 (TLR2) levels significantly increase, potentially leading to the enhanced expression of kallikrein 5 (KLK5) and antimicrobial peptides [16, 17]. KLK5 promotes the expression of LL-37, which triggers inflammatory responses [18]. LL-37, particularly in mast cells, activates degranulation and the release of inflammatory mediators, and plays a crucial role in leukocyte chemotaxis and inflammation-related angiogenesis. In contrast, specific antibodies, such as anti-smooth muscle antibodies or anti-mitochondrial antibodies (AMA), are often detected in AILDs. These antibodies may activate B and T cells, leading to uncontrolled immune responses to self-antigens. Notably, various HLA alleles are associated with the onset of both rosacea and AILDs, suggesting that individuals carrying predisposing autoimmune genes may be more susceptible to multiple autoimmune diseases [19].
Our results indicate a clear causal impact of PBC on rosacea; however, the influence of rosacea on PBC is not significant. This phenomenon may be due to the limited sample size of rosacea cases in GWAS, potentially biasing the accurate assessment of reverse causality individuals with PBC may be more susceptible to rosacea, but rosacea itself does not drive the progression of PBC. Furthermore, immune dysregulation associated with PBC may be a contributing factor to the onset of rosacea.
The MR method effectively mitigated the biases commonly found in observational studies, such as reverse causation and confounding factors. All SNPs included in our study had F-statistics greater than 10, thus minimizing potential biases from weak IVs. Sensitivity analyses further ensured the accuracy of the causal estimates and the robustness of our results. However, this study has several limitations. Although we focused on the causal relationships between rosacea and subtypes of AILDs, we did not delve into the specific impacts of rosacea subtypes. Additionally, our study population was primarily based on European ancestry, potentially introducing biases due to racial differences. The limited availability of IVs prompted us to set a low P-value threshold (P < 5e-6) when considering rosacea and AIH as exposure factors. Furthermore, differences between the two sample MR analyses in terms of gender, age, socioeconomic background, etc. may affect the interpretation of causal estimates and potentially weaken causal inferences. Despite efforts to identify potential confounding factors, unmeasured confounders and horizontal pleiotropy effects remain challenging to eliminate. Future research should strive to overcome these limitations to gain a deeper understanding of the complex relationship between rosacea and AILDs.
Conclusion
This study provides evidence of causal relationships between rosacea and AILDs risks. These findings provide new perspectives for the diagnosis and treatment of rosacea and AILDs. The study not only deepens our understanding of the relationship between rosacea and AILDs, but also offers new directions for future research.
Data availability
As for the data analyzed in this article are available from the IEU Open GWAS database, as follows. https://gwas.mrcieu.ac.uk/datasets/finn-b-L12_ROSACEA/ (for rosacea). https://gwas.mrcieu.ac.uk/datasets/ebi-a-GCST90018785/ (for AIH). https://gwas.mrcieu.ac.uk/datasets/ebi-a-GCST90061440/ (for PBC). https://gwas.mrcieu.ac.uk/datasets/ebi-a-ieu-a-1112 (for PSC).
References
Barakji YA, Rønnstad ATM, Christensen MO et al (2022) Assessment of frequency of rosacea subtypes in patients with rosacea: a systematic review and meta-analysis. JAMA Dermatol 158:617–625. https://doi.org/10.1001/jamadermatol.2022.0526
Wilkin J, Dahl M, Detmar M et al (2004) National rosacea society expert committee. standard grading system for rosacea: report of the national rosacea society expert committee on the classification and staging of rosacea. J Am Acad Dermatol 50:907–912. https://doi.org/10.1016/j.jaad.2004.01.048
Tan J, Almeida LM, Bewley A et al (2017) Updating the diagnosis, classification and assessment of rosacea: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol 176:431–438. https://doi.org/10.1111/bjd.15122
Horst AK, Kumashie KG, Neumann K et al (2021) Antigen presentation, autoantibody production, and therapeutic targets in autoimmune liver disease. Cell Mol Immunol 18(1):92–111. https://doi.org/10.1038/s41423-020-00568-6
Lamba M, Ngu JH, Stedman CAM (2021) Trends in incidence of autoimmune liver diseases and increasing incidence of autoimmune hepatitis. Clin Gastroenterol Hepatol Clin Pract J Am Gastroenterol Assoc 19(3):573–579. https://doi.org/10.1016/j.cgh.2020.05.061
Li Y, Wang J, Jiang H et al (2024) Causal association between autoimmune liver disease and Sjögren’s syndrome: a Mendelian randomization study. Int J Rheum Dis. https://doi.org/10.1111/1756-185x.15151
Ellinghaus D (2022) How genetic risk contributes to autoimmune liver disease. Semin Immunopathol 44(4):397–410. https://doi.org/10.1007/s00281-022-00950-8
Gupta V, Walia GK, Sachdeva MP (2017) Mendelian randomization: an approach for exploring causal relations in epidemiology. Public Health 145:113–119. https://doi.org/10.1016/j.puhe.2016.12.033
Smith GD (2010) Mendelian randomization for strengthening causal inference in observational studies. Perspect Psychol Sci 5(5):527–545. https://doi.org/10.1177/1745691610383505
Skrivankova VW, Richmond RC, Woolf BAR et al (2021) Strengthening the reporting of observational studies in epidemiology using mendelian randomization: The STROBE-MR statement. JAMA 326(16):1614–1621. https://doi.org/10.1001/jama.2021.18236
Sekula P, Del Greco MF, Pattaro C et al (2016) Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol 27(11):3253–3265. https://doi.org/10.1681/asn.2016010098
Shah RA, Kowdley KV (2020) Current and potential treatments for primary biliary cholangitis. Lancet Gastroenterol 5(3):306–315. https://doi.org/10.1016/s2468-1253(19)30343-7
Vesterhus M, Karlsen TH (2020) Emerging therapies in primary sclerosing cholangitis: pathophysiological basis and clinical opportunities. J Gastroenterol 55(6):588–614. https://doi.org/10.1007/s00535-020-01681-z
Terziroli Beretta-Piccoli B, Invernizzi P, Gershwin ME et al (2017) Skin manifestations associated with autoimmune liver diseases: a systematic review. Clin Rev Allergy Immunol 53(3):394–412. https://doi.org/10.1007/s12016-017-8649-9
Xiao W, Sha K, Wang M et al (2024) SERPINB3/B4 is increased in psoriasis and rosacea lesions and has pro-inflammatory effects in mouse models of these diseases. J Invest Dermatol. https://doi.org/10.1016/j.jid.2024.04.011
Yamasaki K, Kanada K, Macleod DT et al (2011) TLR2 expression is increased in rosacea and stimulates enhanced serine protease production by keratinocytes. J Investig Dermatol 131(3):688–697. https://doi.org/10.1038/jid.2010.351
Woo Y, Lim J, Cho D et al (2016) Rosacea: molecular mechanisms and management of a chronic cutaneous inflammatory condition. Int J Mol Sci 17(9):1562. https://doi.org/10.3390/ijms17091562
Yamasaki K, Di Nardo A, Bardan A et al (2007) Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med 13(8):975–980. https://doi.org/10.1038/nm1616
Wang CR, Tsai HW (2022) Autoimmune liver diseases in systemic rheumatic diseases. World J Gastroenterol 28(23):2527–2545. https://doi.org/10.3748/wjg.v28.i23.2527
Acknowledgements
We express our gratitude to all the GWAS researchers and volunteers who contributed to the statistical data employed in this study, and we thank the investigators for their willingness to share the data.
Funding
The Zhejiang Medical Association Clinical Medical Research special fund project: No. 2024ZYC-B10.
Author information
Authors and Affiliations
Contributions
W.W. and J.C. collaboratively designed this study, with W.W. and H.-M.T. taking charge of data collection and analysis. The initial research manuscript was authored by W.W. and Y.-S.L., while J.C. was responsible for revising the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wu, W., Tong, Hm., Li, Ys. et al. Rosacea and autoimmune liver diseases: a two-sample Mendelian randomization study. Arch Dermatol Res 316, 549 (2024). https://doi.org/10.1007/s00403-024-03331-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00403-024-03331-3