Abstract
Background
Area under expiratory flow–volume curve (AEX) is a useful spirometric tool in stratifying respiratory impairment. The AEX approximations based on isovolumic flows can be used with reasonable accuracy when AEX is unavailable. We assessed here pre- to post-bronchodilator (BD) variability of AEX4 as a functional assessment tool for lung disorders.
Methods
The BD response was assessed in 4330 subjects by changes in FEV1, FVC, and AEX4, which were derived from FVC, peak expiratory flow, and forced expiratory flow at 25%, 50%, and 75% FVC. Newly proposed BD response categories (negative, minimal, mild, moderate and marked) have been investigated in addition to standard criteria.
Results
Using standard BD criteria, 24% of subjects had a positive response. Using the new BD response categories, only 23% of subjects had a negative response; 45% minimal, 18% mild, 9% moderate, and 5% had a marked BD response. Mean percent change of the square root AEX4 was 0.3% and 14.3% in the standard BD-negative and BD-positive response groups, respectively. In the new BD response categories of negative, minimal, mild, moderate, and marked, mean percent change of square root AEX4 was − 8.2%, 2.9%, 9.2%, 15.0%, and 24.8%, respectively.
Conclusions
Mean pre- to post-BD variability of AEX4 was < 6% and stratified well between newly proposed categories of BD response (negative, minimal, mild, moderate and marked). We suggest that AEX4 (AEX) could become a useful measurement for stratifying dysfunction in obstructive lung disease and invite further investigation into indications for using bronchodilator agents or disease-modifying, anti-inflammatory therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Interpretation of Pulmonary Function Testing (PFT) relies mainly on comparing measured flows and volumes with predicted reference intervals derived on healthy subjects from similar populations [1,2,3]. In spirometry, forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), FEV1/FVC ratio, and several isovolumic flows represent the main measurements used for defining respiratory impairment. Additionally, testing before and after inhaled bronchodilator (BD) administration has been widely used, especially for diagnosis and therapeutic monitoring in asthma, chronic obstructive pulmonary disease (COPD), various overlap syndromes, and in other conditions. Various definitions of bronchodilator responsiveness have been proposed, reflecting the complexity of defining reversibility of airflow obstruction [4,5,6,7,8,9,10,11]. The 2005 joint ATS/ERS guidelines defined a significant BD response as an absolute 200 mL and a 12% change in either FEV1 or FVC [12]. Recently, Hansen et al. [13] suggested re-defining BD reactivity by using only FEV1 or percent changes, and by differentiating between negative, minimal, mild, moderate and marked responses by using the following thresholds: ≤ 0 mL/≤ 0%, ≤ 90 mL/≤ 9%, ≤ 160 mL/≤ 16%, ≤ 260 mL/≤ 26%, and > 260 mL/> 26%, respectively. Their study also correlated the new BD response categories with radiological measurements, exercise performance, dyspnea, obstructive lung disease exacerbation frequency and quality of life scores [13].
In previous publications we examined the use of a spirometric parameter, area under expiratory flow–volume curve (AEX) as an alternative metric for diagnosing and stratifying functional impairment [14,15,16]. Using this measurement also promises to lessen dependency on body plethysmography or other methods employed as the gold standard for functional assessment, which is costly, elaborate and impractical in either point-of-care setting or in large epidemiological studies. The AEX (expressed in L2 s−1) is the integral function of the variable flow by volume exhaled during a forced respiratory maneuver. While AEX can be easily computed by any modern digital spirometry acquisition system, it is currently offered by only a minority of PFT platform developers. In this context, our earlier studies assessed the utility of several AEX approximations derived from FVC and available instantaneous flows measurements (AEX1, AEX2, AEX3 and AEX4) [17]. Deriving approximated values of the area under the flow–volume loop from widely available spirometric parameters, both before and after BD administration, may mitigate the impact of AEX unavailability in some PFT programs, thereby extending the applicability of this novel measurement.
The current study assesses the pre- to post-BD variability of these AEX approximations, and correlates the association between these measurements and new, emerging, BD response criteria and categories.
Methods
The working database included 13,954 consecutive tests performed on distinct adult subjects in the Atlanta Veterans Affairs Medical Center PFT Laboratory between January 1st, 2009 and December 31st, 2015. The analyses were performed on a sub-group of subjects who underwent same-day, valid, and ATS/ERS quality-acceptable pre- and post-BD spirometry (n = 4330). The largest values among all pre- and post-BD trials have been selected.
Respiratory function assessments were performed in accord with the current ATS/ERS standards and recommendations [1, 18, 19]. Functional measurements were acquired using a Jaeger MasterLabPro system (Wurzberg, Germany), and the most recent and comprehensive reference values, the Global Lung Initiative (GLI) equations sets, were used for spirometry interpretation [2, 20]. Per ATS/ERS recommendations [12], an obstructive ventilatory defect was defined as FEV1/FVC ratio below the lower limit of normal (LLN) and a normal FVC. Restriction was diagnosed when the following three criteria were satisfied: normal FEV1/FVC ratio, FVC < FVCLLN, and Total Lung Capacity (TLC) < TLCLLN. If all three FEV1/FVC ratio, FVC and TLC were below their LLNs, then a diagnosis of mixed ventilatory defect was established. In these analyses, small airways disease was not included as a distinct category. Lung volume and DLCO reference values were those of Crapo et al. [21, 22].
As detailed elsewhere [17], we defined four spirometric parameters, AEX1 through AEX4, which were calculated as the sums of the areas of triangles and trapezoids delineated by pre-specified volumes and the respective isovolumic flows. For example, AEX4 was constructed from FVC and the following four flows: PEF, FEF25, FEF50 and FEF75, per the following formula [17]:
Similarly, AEX1–3 were derived as follows [17]:
For clarity, we limit the data presented in this article to AEX4, although the analyses pertaining to AEX1, AEX2 and AEX3 showed similar results, albeit with smaller coefficients of variation (mainly due to lower intrinsic variability of PEF, FEF25 and FEF50).
Descriptive analyses of the available variables were performed. Categorical variables were presented as frequencies or percentages. Continuous variables were described as means ± standard deviation (SD, for normally distributed variables) or as medians and 25th–75th interquartile ranges (IQR, for non-normal distributions). Student’s t test and analysis of variance were used to compare mean values, while categorical variables were compared using χ2 (likelihood ratio) test. The Tukey–Kramer HSD method was used to compare means among pairs when the variances were similar, while Wilcoxon or Kruskal–Wallis rank sum tests were performed as non-parametric methods when variances were unequal, as appropriate.
Statistical significance was defined a priori as p < 0.001. Analyses were performed using JMP Pro 15 statistical software (SAS Institute, Cary, NC, USA).
Institutional research approvals were obtained to conduct the study (Cleveland Clinic IRB EX#0504/EX#19-1129 and Emory University IRB 00049576).
Results
During the inclusion period, 4330 subjects underwent same-day, acceptable pre- and post-BD spirometry testing. Racial profiles were similar to the larger database of all subjects tested in the PFT Laboratory: 2183 (51%) were self-identified as Black or African American; 2044 (48%) were White or Caucasian; < 2% were Hispanic or Latino. Eleven per cent (n = 494) were women and 89% (n = 3836) were men. Age characteristics were also similar to the base cohort: 59 ± 12 [mean ± SD] and 60 (51–66) [median (IQR)] years. Median (IQR) height, weight and body mass index or BMI were 1.78 (1.73–1.83) m, 91 (79–107) kg, and 29 (25–33) kg/m2, respectively. Based on the standard diagnostic criteria, 30%, 57%, 9% and 5% were diagnosed with normal pattern, obstruction, restriction or mixed ventilatory defect, respectively.
During the test day and before albuterol administration, 77% of the subjects had additional lung volume determinations by body plethysmography, 7% by the helium dilution method and approximately 83% underwent DLCO measurements. Pre-BD TLC, Inspiratory Capacity (IC), IC/TLC, and DLCO are shown in Table 1. Approximately 9% of the subjects had a baseline IC/TLC less than 0.25.
Using the standard ATS/ERS bronchoresponsiveness criteria (i.e., 12% and 200 ml in FEV1 or FVC), 24% of the tested subjects had a positive response. Using the new BD response categories proposed by Hansen et al. [13], only 23% of subjects had a negative response; 45% had minimal, 18% mild, 9% moderate and 5% had a marked BD response. Figure 1 shows the mosaic plot of the newer vs. the standard BD response categories.
Notably, 3.1% of the subjects categorized as having a significant BD response by standard criteria were re-classified as a negative BD response by the new definitions. Alternatively, only 29.5% of those considered by standard criteria to be without a significant BD response maintained a negative BD response by the new categorization.
Table 1 shows the main spirometric parameters of the test set before (pre-BD) and after 2 puffs (400 mcg) of inhaled albuterol (post-BD). The largest pre- vs post-BD variability was noted in the isovolumic flows FEF25, FEF50, FEF75 and FEF25–75, i.e., 10.8%, 20.0%, 29.8% and 20.4%, respectively. Despite the fact that AEX2, AEX3 and AEX4 are computed based on the above flows, their percent pre- to post-BD changes were small overall (< 6%, Table 1). Mean sqrt AEX4% change was higher in Whites vs Blacks (4.6% vs 2.9%, p < 0.0001) and in men vs women (3.9% vs 2.4%, p = 0.0091). Weight, height, BMI, body surface area and age were not significant covariates. Interestingly, mean sqrt AEX4% change was 0.1%, 0.2%, 5.2%, 5.7% and 27.8% in restriction, normal pattern, mixed ventilatory defects, obstruction and small airway disease, respectively.
Mean percent change of the sqrt AEX4 was 0.3% and 14.3% in the negative and positive standard BD response groups, respectively (Fig. 2). In the new BD response categories of negative, minimal, mild, moderate and marked, mean % change of sqrt AEX4 was − 8.2, 2.9, 9.2, 15.0 and 24.8%, respectively (Fig. 3).
One-way analysis of % change of Sqrt AEX4 by New bronchodilator (BD) response (none, minimal, mild, moderate or marked by FEV1 absolute or percent changes ≤ 0 mL/≤ 0%, ≤ 90 mL/≤ 9%, ≤ 160 mL/≤ 16%, ≤ 260 mL/≤ 26%, and > 260 mL/> 26%, , respectively). Blue: men; red: women. Dark color (highlighted): positive response by standard BD response criteria
Given that FEV1 absolute and % changes tend to move in opposite direction, especially at extremes, we also analyzed the performance of sqrt AEX4% and absolute changes versus baseline (pre-BD) sqrt AEX4 values (Fig. 4). As noted, despite a descending trend towards higher baseline AEX4 (on X axis), the dependency was much lower than for the traditional measurements such as FEV1 and FVC.
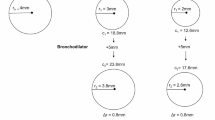
To explain the significance of the sqrt AEX (or sqrt AEX4), we illustrate in Fig. 5 the AEX-equivalent area square, which introduces two novel physiological concepts: equivalent Flow (Fequiv) and Volume (Vequiv), i.e., the flow and volume that have the following scalar relationship: Sqrt AEX = Vequiv = Fequiv. The Vequiv is highly correlated with FEV0.5 (linear fit R2 = 0.98), FEV1 (R2 = 0.96) and FEV2 (R2 = 0.89), perhaps a reflection of the fact that this portion of the flow–volume curve is generated during the first second of the forced exhalation, and that FEV1 and FEV0.5 are in close proximity, overriding the Vequiv (Fig. 5).
AEX—the integral function of flow by volume during a forced exhalation maneuver. The dotted areas delineate the AEX-equivalent squares (equal scalar sides, i.e., Vequiv = Fequiv = Sqrt σ = Sqrt AEX). Green: pre-bronchodilator; Blue : post-bronchodilator. AEX area under expiratory flow–volume curve, FVC forced vital capacity, σ sigma (square area), Sqrt square root transformation ( )
)
Discussion
The main findings in this analysis are that the pre- to post-BD variability of AEX4 (and by extension that of AEX) is low overall, i.e., less than 6% on average, and that this novel measurement, AEX4, stratifies well between newly proposed categories of BD response (differentiating between negative, minimal, mild, moderate and marked responses by using FEV1 absolute or percent changes by the following thresholds: ≤ 0 mL/≤ 0%, ≤ 90 mL/≤ 9%, ≤ 160 mL/≤ 16%, ≤ 260 mL/≤ 26%, and ≥ 260 mL/≥ 26%, respectively). These observations suggest that AEX4 (AEX) is a useful measure for stratifying dysfunction in obstructive lung disease, and invite further analysis of AEX for evaluating clinical status and indications for using bronchodilator agents or disease-modifying, anti-inflammatory therapies.
The 2005 ATS/ERS guidelines define a significant BD response as an absolute 200 mL and a 12% change in either FEV1 or FVC [12]. However, establishing the ideal definition of a significant BD response is not an easy task and not without significant limitations [4,5,6,7,8,9,10,11, 18, 23, 24]. This is likely due to several factors: (1) BD responsiveness in its current form is a conservative dichotomous nominal parameter that does not capture very well clinically relevant reversibility of thoracic overdistension, air hyperinflation or gas trapping [8, 10, 25], (2) Conventional criteria fail to unequivocally differentiate between disease categories such as asthma and COPD [26], (3) BD non-responsiveness is likely not an optimal, defining criterion for Asthma-COPD overlap syndrome [27], (4) Standard BD response criteria may constitute too blunt or too insensitive a tool, especially for extreme lung function values [10], and (5) A standard ATS/ERS BD response does not correlate well with clinical response to bronchodilators, disease control or traditional functional assessments [26, 28, 29].
The recent reassessment of BD responsiveness criteria and the proposal of BD response strata by Hansen et al. was correlated with radiologic measurements, exercise performance, dyspnea scores, obstructive lung disease exacerbation frequency, and quality of life [13]. While the observations and definitions proposed by these authors are yet to be validated in other populations and assessed against patient centric, relevant outcomes, they likely represent a significant practical advance for clinicians, e.g., in helping guide use of bronchodilator agents, etc.
In the current point-of-care PFT data set, we found that 24% of the subjects tested demonstrated a standard BD response by either FEV1 or FVC criteria. Notably, this prevalence may be overestimated in that post-BD testing was only performed when the clinician suspected obstruction or airflow limitation, and ordered pre- and post-BD spirometry. Perhaps not unexpectedly, given the reliance on only FEV1 and the less stringent criteria (OR vs. AND operator) when using the new BD response criteria, only 23% of the group had a negative response (absolute or % change of ≤ 0 mL or 0%, respectively). The term ‘negative’ BD response may be a misnomer, as it does not exclude altogether the presence of airflow limitation: a global FEV1 decrement after albuterol administration may be due to a larger closing volume in certain areas of the lungs despite an increase in ventilation due to bronchodilatation in others, or due to progressive hyperinflation and gas trapping induced by repeated forced exhalation maneuvers. As such, this specific category may require further testing for identifying specific propensities to dynamically obstruct airflow. While the largest proportion of patients with a BD response was in the minimal category (45%), only 3.4% of them had a conventional BD response, which may get us closer to what the ‘reference’ group should be. Further, 45.5% of the tests deemed to have a mild BD response by the new criteria were found to have conventional positive BD reversibility. Clearly, these associations between conventional and novel BD responsiveness criteria must be re-assessed in independent, hypothesis-testing populations.
When juxtaposing the FEV1 vs AEX4 reversibility to inhaled BD, the topic of airway-parenchymal interaction becomes highly relevant, as lung volumes and degrees of hyperinflation influence not only the airway resistance, but also the bronchial responsiveness to bronchodilator or bronchoconstrictive agents [30,31,32,33,34]. As such, the use of only one parameter (e.g., FEV1 or airway resistance) in defining BD response has one inherent limitation, i.e., that the influence of FVC or other lung volumes is not taken into consideration. Indeed, bronchial responsiveness is highly influenced by the size of the end-expiratory lung volume, TLC, FRC or FVC [30,31,32,33,34]. While Hansen et al. [13] do propose to resort to only one parameter, this drawback is possibly minimized by a multi-layered approach, which may permit better endo-phenotypic characterizations. In our case, the AEX as a physiological measurement does not overlook the effects of lung volumes, as it is influenced by both FVC size and by any flow–volume curve ‘scooping’ or ‘shrinking’. This may in fact explain its low pre- to post-BD variability in comparison to that of FEF75, FEF50 or other ‘distal’ flows.
In previous work, we showed that square root (sqrt) AEX compared favorably with traditional PFT measurements for diagnosis and severity characterization of respiratory impairment. The sqrt AEX correlated also well with several lung volumes and capacities that characterize the degree of airway hyperinflation such as IC, IC/TLC and Residual Volume/TLC ratios. Further, several other evaluations of AEX and related concepts have been published before, mostly in children or in assessment of bronchoprovocation responses, and suggested that area under the flow–volume curve or similar constructs may be useful in special populations [35,36,37,38,39]. Due to these relationships, we posit that sqrt AEX offers the promise to become a good predictor of clinical symptoms and to lessen the need for complex lung volume testing [14, 15]. We are currently investigating intrinsic variation of various AEX parameters (inter-trial variance, both pre-and post-bronchodilator use), as well its utility in various spirometric patterns and conditions, for example the use of AEX variability in small airway disease.
The strengths of this study are: (1) the large size of the PFT data set, which represents a broad population, with a wide range of diseases; (2) the use of lung volume testing by other methods such as body plethysmography, helium dilution and DLCO in a large proportion of subjects tested, which allows establishing the ‘ground truth’, and (3) introduction of a global spirometric measurement, which incorporates effects of both airway resistance (flows) and volumes (FVC).
At the same time, several limitations of the study warrant discussion, including that: (1) all data come from a single center, with significant under-representation of women, potentially limiting generalizability, (2) lung volume and BD testing was performed at clinicians’ discretion, reflecting a potential clinical bias, and (3) details were lacking regarding participants’ underlying diagnoses and symptoms, the indication for pulmonary function testing, smoking status, and long-term outcomes, thereby limiting understanding of the clinical correlates of these findings.
Conclusion
This study analyzed the variability of an approximated Area under the Expiratory flow–volume curve (AEX4) based on flows at peak expiration (PEF) and at predetermined volumes (FEF25, FEF50 and FEF75) for characterizing airway responsiveness, and using a newly proposed framework of bronchodilator responsiveness. As a functional parameter, AEX4 performs well as a surrogate marker of AEX, offering promise to help stratifying airway response patterns to inhaled bronchodilator agents and to better define clinical phenotypes and lung disease endotypes. Further studies are needed to examine the relationship of AEX4 to clinical symptoms, therapeutic impact and other patient centric outcomes, as well as best discriminating strata of AEX4.
Abbreviations
- AEX:
-
Area under expiratory flow–volume curve
- AEXk :
-
AEX approximated based on k flows
- ATS:
-
American Thoracic Society
- BD:
-
Bronchodilator
- BMI:
-
Body Mass Index
- CI:
-
Confidence interval
- COPD:
-
Chronic obstructive pulmonary disease
- DLCO:
-
Diffusing lung capacity for CO
- ERS:
-
European respiratory society
- FEFxy :
-
Forced expiratory flow at xy% of FVC
- FEF25-75 :
-
Forced expiratory flow between 25 and 75% of FVC
- FEV1 :
-
Forced expiratory volume in 1 s
- FET:
-
Forced expiratory time
- FETPEF :
-
FET at PEF
- FEVPEF :
-
Forced expiratory volume at PEF
- FEVk :
-
Forced expiratory volume at k second of expiration
- FIV1 :
-
Forced inspiratory volume in 1 s
- FIVC:
-
Forced inspiratory vital capacity
- FRC:
-
Functional residual capacity
- FVC:
-
Forced vital capacity
- GLI:
-
Global lung initiative
- HSD:
-
Honestly significant difference
- IC:
-
Inspiratory capacity
- IQR:
-
Interquartile Range
- LLN:
-
Lower limit of normal
- PEF:
-
Peak expiratory flow
- PFT:
-
Pulmonary function testing
- TLC:
-
Total lung capacity
- SD:
-
Standard deviation
- Sqrt:
-
Square root transformation (^0.5)
References
American Thoracic Society (1995) Standardization of spirometry, 1994 update. Am J Respir Crit Care Med 152(3):1107–1136. https://doi.org/10.1164/ajrccm.152.3.7663792
Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, Enright PL, Hankinson JL, Ip MS, Zheng J, Stocks J, Initiative ERSGLF (2012) Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 40(6):1324–1343. https://doi.org/10.1183/09031936.00080312
Staitieh BS, Ioachimescu OC (2017) Interpretation of pulmonary function tests: beyond the basics. J Investig Med 65(2):301–310. https://doi.org/10.1136/jim-2016-000242
Hansen JE, Casaburi R, Goldberg AS (1993) A statistical approach for assessment of bronchodilator responsiveness in pulmonary function testing. Chest 104(4):1119–1126. https://doi.org/10.1378/chest.104.4.1119
Pellegrino R, Brusasco V (2014) Point: is an increase in FEV(1) and/or FVC ≥ 12% of control and ≥ 200 mL the best way to assess positive bronchodilator response? Yes Chest 146(3):536–537. https://doi.org/10.1378/chest.14-0810
Pellegrino R, Brusasco V (2014) Rebuttal from Drs Pellegrino and Brusasco. Chest 146(3):541–542. https://doi.org/10.1378/chest.14-0811
Hansen JE, Porszasz J (2014) Rebuttal from Drs Hansen and Porszasz. Chest 146(3):542–544. https://doi.org/10.1378/chest.14-0618
Hansen JE, Porszasz J (2014) Counterpoint: is an increase in FEV(1) and/or FVC ≥ 12% of control and ≥ 200 mL the best way to assess positive bronchodilator response? No Chest 146(3):538–541. https://doi.org/10.1378/chest.14-0437
Calverley PM, Burge PS, Spencer S, Anderson JA, Jones PW (2003) Bronchodilator reversibility testing in chronic obstructive pulmonary disease. Thorax 58(8):659–664. https://doi.org/10.1136/thorax.58.8.659
Quanjer PH, Ruppel GL, Langhammer A, Krishna A, Mertens F, Johannessen A, Menezes AMB, Wehrmeister FC, Perez-Padilla R, Swanney MP, Tan WC, Bourbeau J (2017) Bronchodilator response in FVC is larger and more relevant than in FEV1 in severe airflow obstruction. Chest 151(5):1088–1098. https://doi.org/10.1016/j.chest.2016.12.017
Hansen JE, Sun XG, Adame D, Wasserman K (2008) Argument for changing criteria for bronchodilator responsiveness. Respir Med 102(12):1777–1783. https://doi.org/10.1016/j.rmed.2008.06.019
Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson DC, MacIntyre N, McKay R, Miller MR, Navajas D, Pedersen OF, Wanger J (2005) Interpretative strategies for lung function tests. Eur Respir J 26(5):948–968. https://doi.org/10.1183/09031936.05.00035205
Hansen JE, Dilektasli AG, Porszasz J, Stringer WW, Pak Y, Rossiter HB, Casaburi R (2019) A new bronchodilator response grading strategy identifies distinct patient populations. Ann Am Thorac Soc 16(12):1504–1517. https://doi.org/10.1513/AnnalsATS.201901-030OC
Ioachimescu OC, Stoller JK (2020) An alternative spirometric measurement: area under the expiratory flow–volume curve. Ann Am Thorac Soc. https://doi.org/10.1513/AnnalsATS.201908-613OC
Ioachimescu OC, McCarthy K, Stoller JK (2006) Alternative measurements to aid interpretation of spirometry: the role of area under the Expiratory flow–volume curve (AEX). Chest 130(4):119S
Ioachimescu OC, Stoller JK (2019) Assessing small airway disease in GLI versus NHANES III based spirometry using area under the expiratory flow–volume curve. BMJ Open Respir Res 6(1):e000511. https://doi.org/10.1136/bmjresp-2019-000511
Ioachimescu OC, Stoller JK (2020) Area under the expiratory flow–volume curve (AEX): actual versus approximated values. J Investig Med 68(2):403–411. https://doi.org/10.1136/jim-2019-001137
American Thoracic Society (1991) Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis 144(5):1202–1218. https://doi.org/10.1164/ajrccm/144.5.1202
Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J (2005) General considerations for lung function testing. Eur Respir J 26(1):153–161
Hankinson JL (1999) Office spirometry: does poor quality render it impractical? Chest 116(2):276–277
Crapo RO, Morris AH, Clayton PD, Nixon CR (1982) Lung volumes in healthy nonsmoking adults. Bull Eur Physiopathol Respir 18(3):419–425
Crapo RO, Morris AH (1981) Standardized single breath normal values for carbon monoxide diffusing capacity. Am Rev Respir Dis 123(2):185–189. https://doi.org/10.1164/arrd.1981.123.2.185
Freedman BJ, Meisner P, Hill GB (1968) A comparison of the actions of different bronchodilators in asthma. Thorax 23(6):590–597. https://doi.org/10.1136/thx.23.6.590
Eliasson O, Degraff AC Jr (1985) The use of criteria for reversibility and obstruction to define patient groups for bronchodilator trials. Influence of clinical diagnosis, spirometric, and anthropometric variables. Am Rev Respir Dis 132(4):858–864. https://doi.org/10.1164/arrd.1985.132.4.858
McCartney CT, Weis MN, Ruppel GL, Nayak RP (2016) Residual volume and total lung capacity to assess reversibility in obstructive lung disease. Respir Care 61(11):1505–1512. https://doi.org/10.4187/respcare.04323
Fortis S, Comellas A, Make BJ, Hersh CP, Bodduluri S, Georgopoulos D, Kim V, Criner GJ, Dransfield MT, Bhatt SP, Copdgene Investigators-Core Units: Administrative Center CI-CCAAVA (2019) Combined forced expiratory volume in 1 second and forced vital Capacity bronchodilator response, exacerbations, and mortality in chronic obstructive pulmonary disease. Ann Am Thorac Soc 16(7):826–835. https://doi.org/10.1513/AnnalsATS.201809-601OC
Diagnosis and Initial Treatment of Asthma, COPD and Asthma—COPD Overlap (a joint project of GINA and GOLD, updated April 2017). (2017). Accessed 2018
Janson C, Malinovschi A, Amaral AFS, Accordini S, Bousquet J, Buist AS, Canonica GW, Dahlen B, Garcia-Aymerich J, Gnatiuc L, Kowalski ML, Patel J, Tan W, Toren K, Zuberbier T, Burney P, Jarvis D (2019) Bronchodilator reversibility in asthma and COPD: findings from three large population studies. Eur Respir J. https://doi.org/10.1183/13993003.00561-2019
Busse WW, Holgate ST, Wenzel SW, Klekotka P, Chon Y, Feng J, Ingenito EP, Nirula A (2015) Biomarker profiles in asthma with high vs low airway reversibility and poor disease control. Chest 148(6):1489–1496. https://doi.org/10.1378/chest.14-2457
Ding DJ, Martin JG, Macklem PT (1987) Effects of lung volume on maximal methacholine-induced bronchoconstriction in normal humans. J Appl Physiol 62(3):1324–1330. https://doi.org/10.1152/jappl.1987.62.3.1324
Torchio R, Gulotta C, Ciacco C, Perboni A, Guglielmo M, Crosa F, Zerbini M, Brusasco V, Hyatt RE, Pellegrino R (2006) Effects of chest wall strapping on mechanical response to methacholine in humans. J Appl Physiol 101(2):430–438. https://doi.org/10.1152/japplphysiol.00379.2005
Meinero M, Coletta G, Dutto L, Milanese M, Nova G, Sciolla A, Pellegrino R, Brusasco V (2007) Mechanical response to methacholine and deep inspiration in supine men. J Appl Physiol 102(1):269–275. https://doi.org/10.1152/japplphysiol.00391.2006
Torchio R, Gobbi A, Gulotta C, Dellaca R, Tinivella M, Hyatt RE, Brusasco V, Pellegrino R (2009) Mechanical effects of obesity on airway responsiveness in otherwise healthy humans. J Appl Physiol 107(2):408–416. https://doi.org/10.1152/japplphysiol.00083.2009
Kaminsky DA, Chapman DG, Holbrook JT, Henderson RJ, Sugar EA, Mastronarde J, Teague WG, Busk M, Sumino K, Dixon AE, Wise RA, Irvin CG (2019) Older age and obesity are associated with increased airway closure in response to methacholine in patients with asthma. Respirology 24(7):638–645. https://doi.org/10.1111/resp.13496
Stein D, Stein K, Ingrisch S (2015) Aex: the area under the expiratory flow–volume loop. Pneumologie 69(4):199–206. https://doi.org/10.1055/s-0034-1391401
Zapletal A, Chalupova J (2003) Forced expiratory parameters in healthy preschool children (3–6 years of age). Pediatr Pulmonol 35(3):200–207. https://doi.org/10.1002/ppul.10265
Zapletal A, Hladikova M, Chalupova J, Svobodova T, Vavrova V (2008) Area under the maximum expiratory flow–volume curve: a sensitive parameter in the evaluation of airway patency. Respiration 75(1):40–47. https://doi.org/10.1159/000099615
Sovijarvi AR (1986) Flow–volume response to inhaled methacholine in asthmatics; comparison of area under the curve (AFV) with conventional parameters. Eur J Respir Dis Suppl 143:18–21
Das N, Topalovic M, Aerts JM, Janssens W (2019) Area under the forced expiratory flow–volume loop in spirometry indicates severe hyperinflation in COPD patients. Int J Chron Obstruct Pulmon Dis 14:409–418. https://doi.org/10.2147/COPD.S185931
Funding
None.
Author information
Authors and Affiliations
Contributions
OCI and JKS contributed with writing of this article; OCI contributed with statistical analyses.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ioachimescu, O.C., Stoller, J.K. Area Under the Expiratory Flow–Volume Curve (AEX): Assessing Bronchodilator Responsiveness. Lung 198, 471–480 (2020). https://doi.org/10.1007/s00408-020-00345-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-020-00345-2