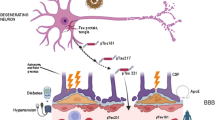
Abstract
Blood-based biomarkers (BBM) are becoming easily detectable tools to reveal pathological changes in Alzheimer’s disease (AD). A comprehensive and up-to-date overview of the association between BBM and brain MRI parameters is not available. This systematic review aimed to summarize the literature on the associations between the main BBM and MRI markers across the clinical AD continuum. A systematic literature search was carried out on PubMed and Web of Science and a total of 33 articles were included. Hippocampal volume was positively correlated with Aβ42 and Aβ42/Aβ40 and negatively with Aβ40 plasma levels. P-tau181 and p-tau217 concentrations were negatively correlated with temporal grey matter volume and cortical thickness. NfL levels were negatively correlated with white matter microstructural integrity, whereas GFAP levels were positively correlated with myo-inositol values in the posterior cingulate cortex/precuneus. These findings highlight consistent associations between various BBM and brain MRI markers even in the pre-clinical and prodromal stages of AD. This suggests a possible advantage in combining multiple AD-related markers to improve accuracy of early diagnosis, prognosis, progression monitoring and treatment response.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Timely and accurate diagnosis of Alzheimer’s disease (AD) in clinical practice is currently challenging, with misdiagnosis in the range of 20–25% when cerebrospinal fluid (CSF) or positron emission tomography (PET) biomarkers are not utilized [1, 2]. Therefore, suboptimal treatment and care, delayed or incorrect therapies, and inaccurate information about the disease and its prognosis are frequent.
Among the different biomarkers available for neurodegenerative diseases (NDDs), CSF-based biomarkers, which are predictive of brain pathological modifications, are formally integrated into the clinical diagnostic criteria for AD in their most recent formulation [3]. However, CSF sampling is invasive, expensive, and not suitable for screening purposes. Novel ultrasensitive methods, such as single-molecule array (Simoa) technology, allow the measurement of blood-based biomarkers (BBM) that show moderate to good accuracy in predicting amyloid status as assessed by either PET or CSF [4,5,6]. BBM have the potential advantage of being more accessible and cheaper than other established biomarkers, i.e. PET and CSF, and afford greater patient compliance. Moreover, BBM have been recently proposed as screening tools to detect AD in its earliest stages, before progression to AD dementia [7]. Beta-amyloid (Aβ) markers (e.g., Aβ42/40 ratio), phosphorylated tau (p-tau), neurofilament light chain (NfL), and glial fibrillary acidic protein (GFAP) are among the most advanced BBM for AD-relevant diagnostic and prognostic purposes [8], with p-tau showing high specificity and NfL having high sensitivity values. According to the biomarker classification proposed by Hampel et al. [9] and referred to as the “ATNX framework”, Aβ biomarkers belong to the “A” category, biomarkers of tau pathology (i.e., p-tau isoforms) to the “T” category, biomarkers of neurodegeneration or neuronal injury (e.g., t-tau and NfL) to the “N” category and GFAP and other BBM to the “X” category.
Not all BBM, however, have been found to perform equally well. Indeed, plasma Aβ42 peptides are highly labile and prone to aggregate, making their concentrations susceptible to variation in pre-analytical processing. The ratio of Aβ42/Aβ40 in plasma may be more useful than levels of individual Aβ peptides [10] for detecting abnormal Aβ status in both cognitively impaired [11, 12] and cognitively unimpaired people, even before Aβ positivity status can be detected by means of Amyloid PET (Aβ-PET) [13]. However, the plasma-based Aβ42/Aβ40 ratio may have lower diagnostic accuracy than the CSF-based Aβ42/Aβ40 ratio, thus representing a potential problem for its clinical application [8].
All plasma p-tau isoforms (i.e., p-tau181, p-tau217, p-tau231) have a high ability to discriminate AD from non-AD NDDs with high specificity [14,15,16,17,18,19]. Recent data have highlighted the promising performance of p-tau217 and p-tau231 in detecting AD before its clinical manifestations [17, 20,21,22].
T-tau is a biomarker of neurodegeneration that reflects the release of tau from neurons and non-specific changes in cortical thickness, but unrelated to neuronal loss [23]. T-tau levels increase in different tauopathies, such as frontotemporal dementia (FTD), corticobasal degeneration, and progressive supranuclear palsy. In the AD continuum, t-tau is often used in ratios with other biomarkers to improve its diagnostic specificity [24]. Given that blood NfL levels reflect neurodegeneration severity, but not the specific etiology, their assay is applicable to detect different NDDs, such as amyotrophic lateral sclerosis and atypical parkinsonisms. NfL levels have been suggested as a promising biomarker to discriminate cognitive decline due to AD in its prodromal or preclinical stages [25, 26], even if the validity of their discriminatory power has not been determined for levels of this biomarker obtained from blood [27]. This biomarker has also been suggested to have good prognostic value in NDDs such as Huntington's disease and Parkinson's disease [28].
GFAP levels in the blood, instead, reflect neuroinflammation and have been found to be higher in Aβ-positive than in Aβ-negative people, and in individuals with AD or mild cognitive impairment (MCI) than in healthy controls (HC) [29].
Furthermore, brain parameters obtained with multiple neuroimaging modalities, including magnetic resonance imaging (MRI) and fluorodeoxyglucose PET (FDG-PET), have provided possible markers of neurodegeneration with potential for clinical applications. Indeed, although neurodegeneration is a non-specific marker of AD in its biological definition [30], studies have shown that patients with evidence of neurodegeneration have a twofold increased risk of progression to dementia over a 5-year period than those without [31]. In this regard, structural MRI (sMRI), functional MRI (fMRI), diffusion tensor imaging (DTI) and magnetic resonance spectroscopy (MRS) can detect pre-symptomatic markers of neural alterations in cognitively unimpaired older adults and might also be used to monitor AD progression after its clinical onset [32], with more limited costs compared with other procedures [33].
The relationship between multiple biological and imaging markers has been largely studied to evaluate whether the combination of multiple biological variables improves accuracy of AD diagnosis and prediction of progression to AD dementia in cognitively unimpaired older adults and in patients with MCI.
Recently, many studies have been conducted to assess the association between CSF and MRI markers across the AD continuum, revealing a strong relationship for sMRI [34,35,36,37,38], DTI [39], fMRI [40, 41], and MRS [42].
In contrast, a comprehensive and up-to-date review on the association between BBM and brain MRI parameters is currently not available. Since the correlation between some blood- and CSF-based biomarkers is suboptimal, especially for Aβ42 [10, 43] and GFAP [44], it is possible that BBM may provide different and/or complementary insights on the neural alterations associated with AD in different disease stages. Indeed, the scientific literature on this topic is fast-growing. Simrén et al. [45] showed that plasma p-tau181 levels are increased in a subset of individuals with MCI and AD dementia, compared with HC, and are correlated with cognitive impairment and gray matter (GM) volume in temporal regions. Moreover, Verde et al. [18] provided a short review on the association between p-tau isoforms and neuroimaging features, highlighting that plasma p-tau181 levels are negatively correlated (mainly in Aβ-positive individuals) with whole brain volume as well as GM volumes of several temporo-parietal areas (i.e., hippocampus, entorhinal cortex, precuneus, and posterior cingulate cortex), cortical thickness of the temporal lobe and of an AD-signature region, and fractional anisotropy (FA) values in the genu of the corpus callosum. However, these results have been reported without details relative to the cognitive status of the participants included in the reviewed studies. Therefore, the aim of the present review was to expand the currently available knowledge on the association between the most established BBM of AD (i.e., Aβ40, Aβ42/40 ratio, Aβ42, p-tau, NfL, GFAP) and brain MRI parameters along the AD continuum.
Materials and methods
Search strategy
The review was carried out following the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines [46]. A systematic literature search was carried independently by three authors (MM, GL and RM) in May 2023 using two databases, PubMed and Web of Science; any discrepancies during the screening process were resolved through discussion. The following search terms were used: “blood biomarker” or “plasma” or “serum” combined with “Alzheimer’s disease” or “AD” or “Mild Cognitive Impairment” or “MCI” or “Subjective cognitive decline” or “SCD” and “structural MRI” or “functional MRI” or “diffusion tensor imaging” or “MRS” or “Magnetic Resonance Spectroscopy”. In addition, the reference lists of the selected original articles/reviews on similar topics were searched for additional eligible records.
Study eligibility criteria
Studies were considered eligible if they assessed the relationship between brain MRI parameters and at least one of the following main peripheral BBM, i.e., Aβ, p-tau, t-tau, NfL and GFAP, derived from patients across the AD continuum, from Subjective Cognitive Decline (SCD) to AD dementia. The following exclusion criteria were defined to identify all relevant studies: (1) no data on the selected blood-based protein biomarkers; (2) no correlation reported between blood biomarkers and MRI data; (3) meta-analysis, review articles or study protocols; (4) studies that included only cognitively unimpaired older adults; (5) studies that included patients with other neurological conditions; (6) animal studies; (7) molecular imaging studies; (8) non-peer reviewed articles; (9) articles written not in English; (10) case reports. Year of publication was not considered as inclusion/exclusion criterion, as we aimed to capture all existing research in this field.
Study selection
The initial literature search produced a total of 1683 records of which 310 were duplicate publications that were found in both the PubMed and Web of Science databases. Following removal of duplicates, 1373 records were screened by title and abstract; in addition, 48 more records were identified through other sources (i.e., the reference lists of selected original articles and papers/reviews on a similar topic) and added to the screening process. A total of 157 full-text reports were retrieved and assessed for eligibility. Based on the inclusion/exclusion criteria, 124 articles were excluded for the following reasons: 54 studies did not include data on the selected blood-based protein biomarkers; 62 did not report correlations between BBM and MRI data; 4 reported data from meta-analyses, review articles or study protocols; 3 studies included only cognitively unimpaired older adults; and one study focused on patients with other neurological conditions. Therefore, 33 unique studies were included in the systematic review. The study selection process is described in Fig. 1.
We have described all studies according to the “ATNX” framework by Hampel and colleagues [9] that suggested the addition of an “X”, inflammatory markers, to the ATN biomarker framework to reflect the whole continuum of AD.
Quality assessment
To the best of our knowledge, no standard tool to assess the quality of studies using MRI and/or blood-biomarkers for AD is available to date. Therefore, a customized scale (Table S1) was created for this study and included 15 assessment criteria to evaluate 5 areas (i.e., case definition, general methods, biomarker methods, MRI methods and reporting of findings). A total score was generated for each study as a percentage of the maximum score (i.e., 18).
Results
Details on demographic characteristics, BBM and MRI techniques used, and the main findings of each study are summarized in Table 1. All the 33 studies included in this systematic review were published between 2006 and 2023 and assessed the association between BBM (i.e., Aβ, p-tau, t-tau, NfL and GFAP) and brain parameters derived from structural MRI, DTI, and MRS.
A total of 10 studies assessed the “A” (i.e., Aβ40, Aβ42 and Aβ42/Aβ40 ratio), 7 focused on the “T” (i.e., p-tau181), 17 studies focused on the “N” (i.e., NfL, t-tau) and 5 assessed the “X” (i.e., GFAP) within the ATNX diagnostic framework of AD.
The BBM analytical procedures used in the reviewed studies were as follows: 24 studies used the Simoa technique [47] that allows quantification down to subfemtomolar concentrations (< 1 pg/mL), including Simoa with HD-1 ultrasensitive Analyzer by Quanterix, and the new flagship HD-X, i.e., the latest model fully automated bead-based immunoassay platform; three studies used the Meso Scale Discovery (MSD) platforms that are more sensitive and require less sample volume than the conventional ELISA kit [48], whereas the ELISA kit was used in 4 studies to assess Aβ isoforms; in 2 studies [49, 50] Aβ isoforms were assessed using the INNO-BIA kit (Fujirebio) based on a multiplex xMAP technique with a LABScan-200 system (Luminex), a technique with recognized good analytical performance and clinical sensitivity [49]; in 2 studies, BBM were quantified with both Simoa and MSD methods [51, 52], but only Mielke et al. [51] applied the 2 methods to assess plasma concentrations of the same biomarkers (i.e., p-tau181), thus enabling inter-method agreement assessment.
The MRI techniques used included: 27 studies used structural MRI, and the majority of these studies assessed the association between BBM and regional volumes; hippocampal volume was the MRI outcome measure most commonly investigated (15 studies), whereas 9 studies assessed the association between BBM and cortical thickness of different brain regions; 7 studies evaluated the relationship between DTI parameters of white matter (WM) microstructural integrity and BBM, and only one study assessed the relationship between MRS indices and BBM [53]; no studies investigated associations between BBM and fMRI parameters. The findings of this review are represented visually in Fig. S1.
Detailed results of “A” biomarkers
Of the 10 studies that assessed the association between Aβ biomarkers (i.e., Aβ40, Aβ42 and Aβ42/Aβ40 ratio) with brain MRI parameters, most of them used structural MRI techniques. Four of these focused their analysis on either hippocampal subregions or on the whole hippocampal volume. In older adults with SCD, higher Aβ42 levels were associated with smaller volume of the dentate gyrus and of the molecular layer [33]; the plasma Aβ42/40 ratio was negatively associated with hippocampal atrophy [54] and, in people with AD dementia, with a medial temporal atrophy (MTA) score [50]. In contrast, Hanon and colleagues [49] found no significant correlation between hippocampal volume and plasma Aβ42 levels in people with symptomatic AD (i.e., with either MCI or dementia), but a weak negative correlation with plasma Aβ40 levels in the AD dementia group only. In the Sydney Memory and Ageing Study of Poljak et al. [55], plasma Aβ42 levels and the Aβ42/Aβ40 ratio were positively associated with hippocampal volume across the AD continuum, while Aβ42 was also negatively associated with white matter hyperintensity (WMH) volume. After stratification by ApoE genotype, different patterns of association were detected for the Aβ isoforms: Aβ40 was negatively correlated with hippocampal volume only in the ApoE ε4 carriers, whereas Aβ42 and Aβ42/Aβ40 ratio were positively correlated with hippocampal volume in the ApoE ε4 carriers and negatively with WMH volume in the ApoE ε4 non-carriers. An older study, however, had found that plasma levels of Aβ40 were positively associated with larger WMH volume in a mixed sample of AD dementia, MCI and cerebral amyloid angiopathy cases [56].
Two studies investigated the mean cortical thickness (mCT) of all cortical regions in people across the AD continuum (i.e., HC, MCI and AD dementia). Fan and colleagues [57] found that plasma Aβ42 levels were negatively correlated with mCT in Aβ-PET negative people, while Aβ40 levels were positively correlated with mCT in both Aβ-PET positive and negative participants. Sotolongo-Grau et al. [58] assessed plasma Aβ markers considering both total plasma Aβ pool and the peptide associated with the cellular pellet (CP): the strongest (negative) association was detected between Aβ40 CP levels and the left hippocampal volume and the left entorhinal cortex, but also with mCT values; Aβ42 CP levels, instead, were negatively correlated with hippocampal volume only.
Moreover, 2 studies investigated the association between Aβ and DTI indices. Shahid and colleagues [59] explored the associations of diffusion microstructural metrics in the hippocampal subfields with different plasma biomarkers of AD pathology and they found no significant associations between any microstructural parameters and either Aβ40, Aβ42, or Aβ42/Aβ40 ratio in a sample comprising HC, MCI, and AD dementia groups. Instead, Wang and colleagues [60] using a Tract-Based Spatial Statistics approach found significant associations between high plasma Aβ40 levels and microstructural parameters, specifically low FA and high MD values in widespread WM tracts in a group of people with SCD.
Detailed results of “T” biomarkers
A total of 7 studies assessed the associations between p-tau isoforms, especially p-tau181, and brain MRI parameters. Two studies from the same research group on different cohorts found that higher plasma p-tau181 levels were negatively correlated with hippocampal volume only in AD dementia patients [61]. The second study found that, across the AD continuum, there was a negative correlation with total brain volume and a positive correlation with ventricular volume [62]. In the latter study, after stratifying by diagnostic group and accounting for age and sex differences, higher p-tau181 levels were associated with lower hippocampal volume in the HC and MCI groups only, but not in patients at the dementia stage. In contrast, Krebs et al. [54] found no significant associations between p-tau181 levels and brain MRI parameters in a group of people with SCD. Moreover, one study that investigated WM microstructural parameters found that plasma p-tau181 levels were positively correlated with mean diffusivity (MD), radial diffusivity (RD), axial diffusivity (AxD), but negatively correlated with FA values in different WM tracts across diagnostic groups [63]. In detail, p-tau181 levels were primarily associated with FA and AxD values in left-sided limbic WM connections (i.e., hippocampal cingulum, fornix and lemniscus) in the AD dementia group, and with all DTI indices in right-sided associative and projection tracts (i.e., tapetum, posterior corona radiata and the retrolenticular part of the right internal capsule).
Only a few studies assessed multiple p-tau isoforms. Ossenkoppele et al. [52] found associations between MRI parameters and p-tau217 and p-tau181 plasma levels: both p-tau measures were negatively correlated with cortical thickness values of an AD-signature region of interest comprising bilateral entorhinal, inferior and middle temporal and fusiform cortex in a mixed group of HC, MCI and AD dementia. Similarly, both p-tau217 and p-tau181 plasma levels were found to be associated with lower temporo-parietal GM volume in Aβ-PET positive patients with MCI [64]. In addition, Mielke et al. [51] showed that, in a group comprehensive of HC and MCI patients, increasing levels of Simoa p-tau181, MSD p-tau181, and MSD p-tau217, but not Simoa p-tau231, were significantly associated with higher WMH volume and lower WM microstructural integrity in 2 WM tracts used as regions of interest (i.e., lower FA values in the genus of corpus callosum and in the hippocampal cingulum bundle). In a sensitivity analysis conducted on 164 participants with all 4 p-tau biomarkers, also Simoa p-tau231 levels were significantly associated with higher WMH volume and lower FA values in the same WM regions of interest.
Detailed results of “N” biomarkers
A total of 17 studies evaluated the associations between either NfL or t-tau with brain MRI parameters. Studies that used structural MRI techniques showed that NfL levels were negatively correlated with hippocampal volume [26, 65, 66], parietal GM volume [67], ratio of hippocampal volume/TIV [68], right lateral temporal lobe, right inferior parietal, and left superior frontal lobe volumes [69]. In these studies, the associations were mainly found among patients with AD dementia, while no associations were found in potentially pre-symptomatic stages, such as SCD [70]. In the study by Pereira et al. [71], instead, a negative correlation was found between plasma NfL values and hippocampal and nucleus accumbens volumes in MCI patients, but not in either the HC or the AD dementia subsamples, independently of Aβ positivity status. Moreover, Benedet et al. [72] found that increases in plasma NfL levels were associated with reduced GM and WM volumes in both HC and cognitively impaired (CI) older adults. However, more widespread associations were observed in the CI group, with a much larger involvement of frontal and lateral temporal cortices additionally to the medial temporal lobe, when compared with the HC group. Additionally, a study that focused on GM microstructural parameters (by using neurite orientation dispersion and density imaging) found that NfL levels were negatively associated with microstructural integrity of one hippocampal subfield (i.e., the CA4-dentate gyrus) across the AD continuum [59]. In the AD dementia subgroup, instead, negative associations were found between t-tau levels and microstructural integrity of the subiculum and of the CA4-DG.
Associations between BBM of neurodegeneration and WM integrity parameters were also extensively observed. In MCI patients, plasma NfL levels were found to be associated negatively with FA and positively with RD, AxD, and MD values in WM tracts that differed between ApoE ε4 carriers (i.e., widespread across anterior corona radiata, internal capsule and genu of the corpus callosum) and non-carriers (i.e., primarily in fornix, cingulum and uncinate fasciculi) [73]. A positive association between NfL levels and MD values was also found by another study on a sample including patients with MCI and AD dementia in areas of the temporal lobes and the cingulate cortex [74]. Moreover, in a sample of autosomal dominant mutation carriers, a strong association between higher NfL levels and lower FA and higher MD, AxD and RD values across all WM tracts was found, whereas no association were observed in HC [75]. Similarly, other authors who evaluated autosomal dominant mutation carriers described a negative correlation between plasma NfL values and whole brain volume and hippocampal volume, while a positive correlation was found with ventricular volume [76]. In a sample comprehensive of HC, MCI and AD dementia cases, Pereira et al. [71] found a negative correlation between higher plasma NfL and lower left precuneus and right middle temporal gyrus cortical thickness values. After stratifying the sample by diagnosis, no correlation emerged in the HC groups, whereas negative correlations were found in the precuneus in the MCI and AD dementia groups with evidence of Aβ positivity from CSF analysis. In contrast, Elahi et al. [77] found an association between NfL and WMH volume only.
Plasma t-tau was found negatively correlated with mCT in both Aβ-PET positive and Aβ-PET negative participants in a mixed group comprehensive of HC, MCI and AD dementia [57]. This finding was confirmed by Marks and colleagues [66] who observed an association between elevated plasma t-tau levels and lower thickness of temporal cortices in both HC and MCI patients. However, no associations were found between plasma t-tau and FA values in the corpus callosum. In contrast, Illan-Gala et al. [69] found no correlation between plasma t-tau levels and cortical thickness in an AD dementia group. One study found that higher t-tau levels were associated with higher ventricular volume, but not with hippocampal volume in the adjusted analysis [26].
Detailed results of “X” biomarkers
Of the 5 studies that focused on inflammation markers (i.e. GFAP), 4 assessed the association with structural MRI parameters and one measured the association with metabolites by means of MRS. Ebenau et al. [70] found that higher GFAP levels correlated with lower hippocampal volume and higher MTA in a group of SCD. However, this association was weak and did not survive statistical adjustment for age and sex. Elahi et al. [77], instead, found a significant association with higher WMH volume, but not with global GM volume across the AD continuum. Moreover, other authors showed associations between higher plasma GFAP levels and lower WM volumes in temporal and parietal areas in a mixed group [67], lower temporal cortical thickness and greater WMH volume in Aβ-PET positive HC and MCI patients [78]. One MRS study found that plasma GFAP levels were significantly associated with myo-inositol (mIns) values in the posterior cingulate cortex of HC and MCI patients [53]. Moreover, after stratifying the sample according to ApoE genotype, plasma GFAP levels were significantly associated with the ratio between mIns and total creatine in ApoE ε4 carriers only.
Quality assessment results
Although variable levels of quality were observed across studies, the overall level appeared to be good, with only 3 out of 33 studies achieving a total score below 50% (Table S2). All studies reported enough details on BBM analysis methods and all but one study reported comprehensive demographic characteristics of the included participant groups. However, only in 13 studies the underlying hypotheses were stated explicitly. No significant difference in quality scores (t(31) = − 0.10, p = 0.992) was observed between studies that focused on a single blood biomarker only (n = 25, 61.1% ± 11.1%) and those that investigated 2 or more biomarkers (n = 8, 61.6% ± 4.9%).
Discussion
This systematic review includes 33 studies that assessed the association between the main BBM and MRI markers in the AD continuum, highlighting consistent associations between these 2 types of marker, in some instances even in the earliest stages of disease (i.e. SCD and MCI). Most of the studies (n = 17) focused on markers of neurodegeneration, showing that high levels of NfL are associated with temporal (e.g. hippocampus), frontal and parietal atrophy [67,68,69]. Moreover, high levels of NfL, as well as t-tau, also correlated with white matter microstructural alterations across different WM tracts [59, 73,74,75,76]. Together, these findings suggest that plasma NfL is a promising biomarker that detects neuronal injury in AD, and may have potential for prognosis and monitoring of disease progression. However, in most studies, BBM–MRI associations were only found in a more advanced stage of disease (i.e. in patients with dementia due to AD), and only few of these findings were confirmed in earlier preclinical or prodromal disease stages, i.e. in older adults who were either cognitively unimpaired, had SCD or were experiencing MCI [71, 72]. Since high plasma NfL concentrations are also found in other NDDs [28], further evidence is needed to demonstrate whether plasma NfL concentrations increase already in preclinical and prodromal stages of AD and whether such alterations may be indicative of early neural changes specific for AD. The findings of two studies involving carriers of AD mutations in amyloid precursor protein and in presenilin 1 and 2 genes seem to support this hypothesis [75, 76].
T-tau plasma level increases are also indicative of neurodegeneration across the AD continuum, although this biomarker has been assessed only by a few studies. Negative associations have been found primarily between higher t-tau values and general indices of brain parenchymal loss, e.g. lower mCT and higher ventricular volume, but not with hippocampal volume [79]. These associations were found consistently across studies but one that investigated a sample of people with AD dementia [69]. However, Marks et al. [66] have also found that higher t-tau was associated with reduced temporal cortical thickness in HC and MCI group, but only in one of the two cohorts investigated. More robust evidence is therefore needed to establish how useful plasma t-tau might be in clinical application.
Although the majority of the reviewed studies focused on biomarkers of neurodegeneration, different indices of plasma Aβ were also found associated with MRI metrics. Plasma Aβ42/Aβ40 ratio was negatively associated with MTA in AD dementia patients [50], but also with an index of hippocampal atrophy (i.e., 1 − hippocampal volume/TIV) in older adults with SCD [54]. These findings are in line with previous evidence suggesting that the Aβ42/Aβ40 ratio of these markers derived from plasma may be more useful than individual Aβ peptide concentrations in detecting abnormal Aβ status in both cognitively impaired and cognitively unimpaired participants [10,11,12]. Moreover, research with older adults with SCD found that higher levels of Aβ42 were associated with lower volume of the left dentate gyrus [33], while higher plasma Aβ40 levels were associated with reduced WM integrity [60]. These findings suggest that the detection of such BBM-MRI associations may signal incipient AD-related pathology and that they may be equally informative in both Aβ positive and negative individuals [57] and across the clinical AD continuum [58].
When assessing the associations between p-tau isoforms and brain MRI parameters, a range of different results was found. While the study by Krebs et al. [54] found no association between plasma levels of p-tau181 and brain MRI outcome measures in SCD, other investigations found that increased levels of this BBM were associated with several indices of GM loss, such as hippocampal volume, reduced total brain and temporo-parietal volumes and temporal cortical thickness, even in HC and MCI groups [51, 52, 62, 64]. Similarly, plasma levels of p-tau isoforms were also associated with WM alterations, i.e., higher WMH volume [51] and decreased microstructural integrity across different WM tracts in various disease stages [51, 63]. Among all these studies, the most consistent association in HC and MCI groups was that between p-tau isoforms (p-tau181 and p-tau217) and temporal grey matter volumes and cortical thickness. This pattern of findings is interesting and, if validated in future studies, alterations in plasma p-tau levels might be a reliable marker of neural alterations due to AD even in preclinical stages.
Increasing evidence suggests that blood GFAP levels can be used to detect early-stage AD [29]. The majority of the studies that focused on this inflammation marker found associations between high levels of GFAP and lower WM in temporal and parietal areas [67], lower temporal cortical thickness and WMH only in Aβ-PET positive cases [78], and altered levels of mIns, a marker of astrocytic function, in a brain area particularly affected by AD, i.e., the PCC/precuneus [53]. Interestingly, these studies describe associations between GFAP and imaging markers in similar brain areas (i.e. temporal and parietal regions) in HC and MCI groups, even when different MRI techniques are used (i.e. structural MRI, DTI or MRS). However, further multimodal imaging studies with different MRI sequences, applied in populations ranging from cognitively unimpaired older adults to the AD spectrum, are needed to confirm these encouraging findings.
Interesting results emerged when associations between BBM and MRI markers were assessed while accounting for Aβ status. In general, associations were detected primarily in Aβ positive older adults only: (1) NfL levels were negatively correlated with cortical thickness in the precuneus of CSF Aβ positive patients with either MCI or AD dementia [71]; (2) higher GFAP levels were associated with lower temporal cortical thickness and greater WMH volume in Aβ-PET positive cases [78]; and (3) higher p-tau 217 and p-tau 181 values were associated with lower temporo-parietal GM volume in Aβ-PET positive cases [64]. However, one study also found that Aβ42 levels were negatively associated with the mCT in Aβ-PET negative cases only [57]. All together, these results suggest a relevant impact of Aβ status, i.e., BBM-MRI associations may be detected primarily in people showing signs of AD pathological changes.
Similarly, stratifying samples by ApoE genotype revealed that several significant associations were detectable in ε4 carriers only: (1) increases in plasma NfL associated with reduced GM volume in HC [72]; (2) Aβ40 level negatively associated with hippocampal volume [55]; and (3) higher GFAP levels positively correlated with mIns/tCr concentration [53]. ApoE genotype, therefore, is confirmed as a strong determinant of AD-related neural alterations and genetic profiling may enhance the detection of clinically relevant BBM–MRI associations, especially in individuals at higher risk of AD.
In some studies, the association between BBM e MRI characteristics was assessed by stratifying by diagnostic group [62, 71]. These investigations highlighted that, in HC and MCI groups, higher p-tau181 levels are associated with lower hippocampal volume and higher NfL values with reduced cortical thickness, hippocampal and accumbens volumes.
Only Pereira et al. [71] investigated how both plasma and CSF NfL concentrations were associated with MRI markers in the same sample: while negative correlations were found between plasma NfL and cortical thickness starting from the MCI stage, negative correlations emerged between CSF NfL and cortical thickness already in HC. These findings suggest that CSF NfL analysis may be more sensitive than blood analysis in detecting AD-related brain atrophy in pre-symptomatic stages. However, these associations were observed for patients with and without amyloid pathology, confirming that NfL is a non-specific marker of AD.
The literature currently available is not exempt from limitations. First, many of the papers included used data obtained from the same datasets: 9 studies used ADNI [26, 52, 58, 62, 63, 71,72,73, 79]; 4 used BIOFINDER [52, 53, 61, 79]; 3 used the Mayo Clinic Study of Aging cohort [51, 66, 78]; and 2 used the Translational Biomarkers in Aging and Dementia dataset [61, 72]. Although it is not possible to determine the extent of sample overlaps across studies, it is highly likely that the same data from the same participants have been re-used in multiple investigations. As a consequence, this might have introduced a bias in this modestly sized literature, especially for tau and NfL markers. Second, none of the studies had carried out an a priori power calculation and 6 studies included small samples of participants (i.e. n < 20). This may be primarily explained by the fact that investigations of BBM for AD have only started in recent years. Indeed, most studies included in this review were exploratory and presented no research hypotheses (third limitation). Fourth, a high degree of heterogeneity was observed in the range of neuroimaging outcome measures investigated, as these were primarily volumes of specific regions of interest. Although focusing on specific brain areas, e.g. the hippocampus, is justified by established knowledge of the typical AD pattern of GM atrophy, this approach might miss clinically relevant associations beyond the medio-temporal lobe and across networks of interconnected brain areas. Fifth, studies of associations of BBM with functional and multimodal MRI investigations are lacking, thus preventing any possible speculations on the potential association between BBM and alterations in brain activity, rather than just structural damage, that may be more sensitive to AD pathology in both preclinical (i.e. cognitively unimpaired older adults) and prodromal (i.e. MCI) stages [80, 81]. Sixth, although the majority of studies (n = 24) used Simoa to measure BBM, other analytical methods (e.g., ELISA, MSD, and Luminex) were also used across studies, thus limiting comparability of findings. In this regard, Mielke et al. [51] measured plasma p-tau181 with both Simoa and MSD techniques, reporting a Spearman correlation coefficient of 0.66 indicative of a moderately strong correlation between the two measures. Finally, the different statistical approaches applied in each study could also be a source of heterogeneity in the summarized findings. Indeed, some studies used simple (either parametric or non-parametric) tests to investigate linear correlations between continuous BBM and MRI variables of interest (e.g., [65]), while others applied more refined methods, such as either partial correlation or regression analysis adjusted for multiple factors [51].
Conclusions
The findings of this systematic review highlight a high degree of association between BBM and a variety of brain MRI outcome measures. Variance in plasma levels of Aβ42 e Aβ42/Aβ40 and higher levels of the other biomarkers (i.e., p-tau, t-tau, NfL and GFAP) were consistently associated with more severe neural alterations. A number of relationships appear early in the course of the disease (even in preclinical stages), suggesting that BBM may represent complementary screening tools for AD. However, given the mild degree of heterogeneity observed in findings in the early preclinical and prodromal stages of the AD continuum, further studies are needed to elucidate how different BBM may be optimally informative of neural alterations in preclinical AD (e.g. HC and SCD with and without evidence of amyloid pathological changes). Moreover, multiple factors can interact or modify the association between BBM and MRI findings, such as age, gender, education, creatinine level, ApoE genotype, Aβ status, thus highlighting the need to consider these variables when assessing BBM-MRI marker relationships but, more in general, when using BBM for clinical purposes. Among the assessed BBM, p-tau isoforms (representative of “T” in the ATN system) are known to be predictive of Aβ status (indicative of “A”) [82] and, according to our results, are consistently associated, from an early clinical stage (i.e. MCI), with temporal grey matter volumes and alterations in cortical thickness (representative of “N”). For this reason, they may be more useful than other BBM in supporting the diagnostic process. The results from this review are encouraging and supportive of further investigations into the combination of MRI and BBM for improving accuracy of early diagnosis, prognosis, and monitoring of disease progression or response to treatment. Future investigations of multimodal neuroimaging outcome measures by means of advanced statistical modelling approaches would be needed to confirm if and to what extent BBM could be indicative of the status of brain alterations across different disease stages.
References
Bradford A, Kunik ME, Schulz P, Williams SP, Singh H (2009) Missed and delayed diagnosis of dementia in primary care: prevalence and contributing factors. Alzheimer Dis Assoc Disord 23(4):306–314
Beach TG, Monsell SE, Phillips LE, Kukull W (2012) Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005–2010. J Neuropathol Exp Neurol 71(4):266–273
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH et al (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7(3):263–269
Vergallo A, Mégret L, Lista S, Cavedo E, Zetterberg H, Blennow K et al (2019) Plasma amyloid β 40/42 ratio predicts cerebral amyloidosis in cognitively normal individuals at risk for Alzheimer’s disease. Alzheimers Dement 15(6):764–775
Verberk IMW, Slot RE, Verfaillie SCJ, Heijst H, Prins ND, van Berckel BNM et al (2018) Plasma amyloid as prescreener for the earliest Alzheimer pathological changes. Ann Neurol 84(5):648–658
Chatterjee P, Elmi M, Goozee K, Shah T, Sohrabi HR, Dias CB et al (2019) Ultrasensitive detection of plasma amyloid-β as a biomarker for cognitively normal elderly individuals at risk of Alzheimer’s disease. J Alzheimers Dis 71(3):775–783
Georgakas JE, Howe MD, Thompson LI, Riera NM, Riddle MC (2023) Biomarkers of Alzheimer’s disease: Past, present and future clinical use. Biomark Neuropsychiatry 8:100063
Angioni D, Delrieu J, Hansson O, Fillit H, Aisen P, Cummings J et al (2022) Blood biomarkers from research use to clinical practice: what must be done? A report from the EU/US CTAD task force. J Prev Alzheimers Dis 9(4):569–579
Hampel H, Cummings J, Blennow K, Gao P, Jack CR, Vergallo A (2021) Developing the ATX(N) classification for use across the Alzheimer disease continuum. Nat Rev Neurol 17(9):580–589
Hardy-Sosa A, León-Arcia K, Llibre-Guerra JJ, Berlanga-Acosta J, Baez SDLC, Guillen-Nieto G et al (2022) Diagnostic accuracy of blood-based biomarker panels: a systematic review. Front Aging Neurosci 14:683689
Hu Y, Kirmess KM, Meyer MR, Rabinovici GD, Gatsonis C, Siegel BA et al (2022) Assessment of a plasma amyloid probability score to estimate amyloid positron emission tomography findings among adults with cognitive impairment. JAMA Netw Open 5(4):e228392
West T, Kirmess KM, Meyer MR, Holubasch MS, Knapik SS, Hu Y et al (2021) A blood-based diagnostic test incorporating plasma Aβ42/40 ratio, ApoE proteotype, and age accurately identifies brain amyloid status: findings from a multi cohort validity analysis. Mol Neurodegener 16(1):30
Schindler SE, Bollinger JG, Ovod V, Mawuenyega KG, Li Y, Gordon BA et al (2019) High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology 93(17):e1647–e1659
Ashton NJ, Pascoal TA, Karikari TK, Benedet AL, Lantero-Rodriguez J, Brinkmalm G et al (2021) Plasma p-tau231: a new biomarker for incipient Alzheimer’s disease pathology. Acta Neuropathol 141(5):709–724
Balogun WG, Zetterberg H, Blennow K, Karikari TK (2023) Plasma biomarkers for neurodegenerative disorders: ready for prime time? Curr Opin Psychiatry 36(2):112–118
Ding X, Zhang S, Jiang L, Wang L, Li T, Lei P (2021) Ultrasensitive assays for detection of plasma tau and phosphorylated tau 181 in Alzheimer’s disease: a systematic review and meta-analysis. Transl Neurodegener 10(1):10
Gonzalez-Ortiz F, Kac PR, Brum WS, Zetterberg H, Blennow K, Karikari TK (2023) Plasma phospho-tau in Alzheimer’s disease: towards diagnostic and therapeutic trial applications. Mol Neurodegener 18(1):18
Verde F (2022) Tau proteins in blood as biomarkers of Alzheimer’s disease and other proteinopathies. J Neural Transm (Vienna) 129(2):239–259
Palmqvist S, Janelidze S, Quiroz YT, Zetterberg H, Lopera F, Stomrud E et al (2020) Discriminative accuracy of plasma phospho-tau217 for Alzheimer Disease vs other neurodegenerative disorders. JAMA 324(8):772–781
Ashton NJ, Brum WS, Di Molfetta G, Benedet AL, Arslan B, Jonatis E et al (2023) Diagnostic accuracy of the plasma ALZpath pTau217 immunoassay to identify Alzheimer's disease pathology. medRxiv 2023.07.11.23292493
Ferreira PCL, Therriault J, Tissot C, Ferrari-Souza JP, Benedet AL, Povala G et al (2023) Plasma p-tau231 and p-tau217 inform on tau tangles aggregation in cognitively impaired individuals. Alzheimers Dement 19(10):4463–4474
Jonaitis EM, Janelidze S, Cody KA, Langhough R, Du L, Chin NA et al (2023) Plasma phosphorylated tau 217 in preclinical Alzheimer’s disease. Brain Commun 5(2):fcad057
Zetterberg H (2017) Review: Tau in biofluids - relation to pathology, imaging and clinical features. Neuropathol Appl Neurobiol 43(3):194–199
Fink HA, Linskens EJ, Silverman PC, McCarten JR, Hemmy LS, Ouellette JM et al (2020) Accuracy of biomarker testing for neuropathologically defined Alzheimer disease in older adults with dementia. Ann Intern Med 172(10):669–677
Hu H, Chen K, Ou Y, Cao X, Chen S, Cui M et al (2019) Neurofilament light chain plasma concentration predicts neurodegeneration and clinical progression in nondemented elderly adults. Aging (Albany NY) 11(17):6904–6914
Mattsson N, Andreasson U, Zetterberg H, Blennow K (2017) Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA Neurol 74(5):557–566
Zhang J, Cheng H, Liu W, Li H, Song Y, Jia L (2022) Neurofilament light chain in cerebrospinal fluid or blood as a biomarker for mild cognitive impairment: a systematic review and meta-analysis. Medicine (Baltimore) 101(9):e28932
Palermo G, Mazzucchi S, Della Vecchia A, Siciliano G, Bonuccelli U, Azuar C et al (2020) Different clinical contexts of use of blood neurofilament light chain protein in the spectrum of neurodegenerative diseases. Mol Neurobiol 57(11):4667–4691
Kim KY, Shin KY, Chang K (2023) GFAP as a potential biomarker for Alzheimer’s disease: a systematic review and meta-analysis. Cells 12(9):1309
Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB et al (2018) NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14(4):535–562
Yu J, Li J, Suckling J, Feng L, Pan A, Wang Y et al (2019) Frequency and longitudinal clinical outcomes of Alzheimer’s AT(N) biomarker profiles: a longitudinal study. Alzheimers Dement 15(9):1208–1217
Dang C, Wang Y, Li Q, Lu Y (2023) Neuroimaging modalities in the detection of Alzheimer’s disease-associated biomarkers. Psychoradiology 3:1–17
Cantero JL, Iglesias JE, Van Leemput K, Atienza M (2016) Regional hippocampal atrophy and higher levels of plasma amyloid-beta are associated with subjective memory complaints in nondemented elderly subjects. J Gerontol A Biol Sci Med Sci 71(9):1210–1215
Mao C, Sha L, Li J, Huang X, Chu S, Lei D et al (2021) Relationship between general cognition, visual assessed cortical atrophy, and cerebrospinal fluid biomarkers in Alzheimer’s Disease: A cross-sectional study from a Chinese PUMCH cohort. J Alzheimers Dis 82(1):205–214
Meeker KL, Butt OH, Gordon BA, Fagan AM, Schindler SE, Morris JC et al (2022) Cerebrospinal fluid neurofilament light chain is a marker of aging and white matter damage. Neurobiol Dis 166:105662
Müller-Ehrenberg L, Riphagen JM, Verhey FRJ, Sack AT, Jacobs HIL (2018) Alzheimer’s Disease biomarkers have distinct associations with specific hippocampal subfield volumes. J Alzheimers Dis 66(2):811–823
Nathan PJ, Lim YY, Abbott R, Galluzzi S, Marizzoni M, Babiloni C et al (2017) Association between CSF biomarkers, hippocampal volume and cognitive function in patients with amnestic mild cognitive impairment (MCI). Neurobiol Aging 53:1–10
Wan M, Liu H, Liu X, Zhang W, Xiao X, Zhang S et al (2022) Associations of multiple visual rating scales based on structural magnetic resonance imaging with disease severity and cerebrospinal fluid biomarkers in patients with Alzheimer’s disease. Front Aging Neurosci 14:906519
Alm KH, Bakker A (2019) Relationships between diffusion tensor imaging and cerebrospinal fluid metrics in early stages of the Alzheimer’s disease continuum. J Alzheimers Dis 70(4):965–981
Qiu T, Zeng Q, Zhang Y, Luo X, Xu X, Li X et al (2022) Altered functional connectivity pattern of hippocampal subfields in individuals with objectively-defined subtle cognitive decline and its association with cognition and cerebrospinal fluid biomarkers. Eur J Neurosci 56(12):6227–6238
Weiler M, de Campos BM, Teixeira CVDL, Casseb RF, Carletti-Cassani AFMK, Vicentini JE et al (2017) Intranetwork and internetwork connectivity in patients with Alzheimer disease and the association with cerebrospinal fluid biomarker levels. J Psychiatry Neurosci 42(6):366–377
Piersson AD, Mohamad M, Rajab F, Suppiah S (2021) Cerebrospinal fluid amyloid beta, tau levels, apolipoprotein, and 1H-MRS brain metabolites in Alzheimer’s disease: a systematic review. Acad Radiol 28(10):1447–1463
Huang S, Wang Y, Guo J (2022) Biofluid biomarkers of Alzheimer’s Disease: Progress, problems, and perspectives. Neurosci Bull 38(6):677–691
Benedet AL, Milà-Alomà M, Vrillon A, Ashton NJ, Pascoal TA, Lussier F et al (2021) Differences between plasma and cerebrospinal fluid glial fibrillary acidic protein levels across the Alzheimer Disease continuum. JAMA Neurol 78(12):1471–1483
Simrén J, Leuzy A, Karikari TK, Hye A, Benedet AL, Lantero-Rodriguez J et al (2021) The diagnostic and prognostic capabilities of plasma biomarkers in Alzheimer’s disease. Alzheimers Dement 17(7):1145–1156
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71
Rissin DM, Kan CW, Campbell TG, Howes SC, Fournier DR, Song L et al (2010) Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat Biotechnol 28(6):595–599
O’Bryant SE, Xiao G, Zhang F, Edwards M, German DC, Yin X et al (2014) Validation of a serum screen for Alzheimer’s disease across assay platforms, species, and tissues. J Alzheimers Dis 42(4):1325–1335
Hanon O, Vidal J, Lehmann S, Bombois S, Allinquant B, Tréluyer J et al (2018) Plasma amyloid levels within the Alzheimer’s process and correlations with central biomarkers. Alzheimers Dement 14(7):858–868
Hsu J, Lee W, Liao Y, Lirng J, Wang S, Fuh J (2017) Plasma biomarkers are associated with agitation and regional brain atrophy in Alzheimer’s disease. Sci Rep 7(1):5035
Mielke MM, Frank RD, Dage JL, Jeromin A, Ashton NJ, Blennow K et al (2021) Comparison of plasma phosphorylated tau species with amyloid and tau positron emission tomography, neurodegeneration, vascular pathology, and cognitive outcomes. JAMA Neurol 78(9):1108–1117
Ossenkoppele R, Reimand J, Smith R, Leuzy A, Strandberg O, Palmqvist S et al (2021) Tau PET correlates with different Alzheimer’s disease-related features compared to CSF and plasma p-tau biomarkers. EMBO Mol Med 13(8):e14398
Spotorno N, Najac C, Stomrud E, Mattsson-Carlgren N, Palmqvist S, van Westen D et al (2022) Astrocytic function is associated with both amyloid-β and tau pathology in non-demented APOE ϵ4 carriers. Brain Commun 4(3):fcac135
Krebs C, Brill E, Minkova L, Federspiel A, Kellner-Weldon F, Wyss P et al (2023) Investigating compensatory brain activity in older adults with subjective cognitive decline. J Alzheimers Dis 93(1):107–124
Poljak A, Crawford JD, Smythe GA, Brodaty H, Slavin MJ, Kochan NA et al (2016) The relationship between plasma Aβ levels, cognitive function and brain volumetrics: sydney memory and ageing study. Curr Alzheimer Res 13(3):243–255
Gurol ME, Irizarry MC, Smith EE, Raju S, Diaz-Arrastia R, Bottiglieri T et al (2006) Plasma beta-amyloid and white matter lesions in AD, MCI, and cerebral amyloid angiopathy. Neurology 66(1):23–29
Fan L, Tzen K, Chen Y, Chen T, Lai Y, Yen R et al (2018) The relation between brain amyloid deposition, cortical atrophy, and plasma biomarkers in amnesic mild cognitive impairment and Alzheimer’s Disease. Front Aging Neurosci 10:175
Sotolongo-Grau O, Pesini P, Valero S, Lafuente A, Buendía M, Pérez-Grijalba V et al (2014) Association between cell-bound blood amyloid-β(1–40) levels and hippocampus volume. Alzheimers Res Ther 6(5–8):56
Shahid SS, Wen Q, Risacher SL, Farlow MR, Unverzagt FW, Apostolova LG et al (2022) Hippocampal-subfield microstructures and their relation to plasma biomarkers in Alzheimer’s disease. Brain 145(6):2149–2160
Wang X, Zhao M, Lin L, Han Y (2021) Plasma β-amyloid levels associated with structural integrity based on diffusion tensor imaging in subjective cognitive decline: the SILCODE study. Front Aging Neurosci 12:592024
Karikari TK, Pascoal TA, Ashton NJ, Janelidze S, Benedet AL, Rodriguez JL et al (2020) Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol 19(5):422–433
Karikari TK, Benedet AL, Ashton NJ, Lantero Rodriguez J, Snellman A, Suárez-Calvet M et al (2021) Diagnostic performance and prediction of clinical progression of plasma phospho-tau181 in the Alzheimer’s Disease Neuroimaging Initiative. Mol Psychiatry 26(2):429–442
Nabizadeh F, Pourhamzeh M, Khani S, Rezaei A, Ranjbaran F, Deravi N (2022) Plasma phosphorylated-tau181 levels reflect white matter microstructural changes across Alzheimer’s disease progression. Metab Brain Dis 37(3):761–771
Thijssen EH, La Joie R, Strom A, Fonseca C, Iaccarino L, Wolf A et al (2021) Association of plasma p-tau217 and p-tau181 with clinical phenotype, neuropathology, and imaging markers in Alzheimer’s disease and frontotemporal lobar degeneration: a retrospective diagnostic performance study. Lancet Neurol 20(9):739–752
Altomare D, Stampacchia S, Ribaldi F, Tomczyk S, Chevalier C, Poulain G et al (2023) Plasma biomarkers for Alzheimer’s disease: a field-test in a memory clinic. J Neurol Neurosurg Psychiatry 94(6):420–427
Marks JD, Syrjanen JA, Graff-Radford J, Petersen RC, Machulda MM, Campbell MR et al (2021) Comparison of plasma neurofilament light and total tau as neurodegeneration markers: associations with cognitive and neuroimaging outcomes. Alzheimers Res Ther 13(1):199
Asken BM, VandeVrede L, Rojas JC, Fonseca C, Staffaroni AM, Elahi FM et al (2022) Lower white matter volume and worse executive functioning reflected in higher levels of plasma GFAP among older adults with and without cognitive impairment. J Int Neuropsychol Soc 28(6):588–599
Barker W, Quinonez C, Greig MT, Behar R, Chirinos C, Rodriguez RA et al (2021) Utility of plasma neurofilament light in the 1Florida Alzheimer’s Disease Research Center (ADRC). J Alzheimers Dis 79(1):59–70
Illán-Gala I, Lleo A, Karydas A, Staffaroni AM, Zetterberg H, Sivasankaran R et al (2021) Plasma tau and neurofilament light in frontotemporal lobar degeneration and Alzheimer Disease. Neurology 96(5):e671–e683
Ebenau JL, Pelkmans W, Verberk IMW, Verfaillie SCJ, van den Bosch KA, van Leeuwenstijn M et al (2022) Association of CSF, plasma, and imaging markers of neurodegeneration with clinical progression in people with subjective cognitive decline. Neurology 98(13):e1315–e1326
Pereira JB, Westman E, Hansson O (2017) Association between cerebrospinal fluid and plasma neurodegeneration biomarkers with brain atrophy in Alzheimer’s disease. Neurobiol Aging 58:14–29
Benedet AL, Leuzy A, Pascoal TA, Ashton NJ, Mathotaarachchi S, Savard M et al (2020) Stage-specific links between plasma neurofilament light and imaging biomarkers of Alzheimer’s disease. Brain 143(12):3793–3804
Nabizadeh F, Balabandian M, Rostami MR, Kankam SB, Ranjbaran F, Pourhamzeh M (2022) Plasma neurofilament light levels correlate with white matter damage prior to Alzheimer’s disease: results from ADNI. Aging Clin Exp Res 34(10):2363–2372
Parbo P, Madsen LS, Ismail R, Zetterberg H, Blennow K, Eskildsen SF et al (2020) Low plasma neurofilament light levels associated with raised cortical microglial activation suggest inflammation acts to protect prodromal Alzheimer’s disease. Alzheimers Res Ther 12(1):3
Schultz SA, Strain JF, Adedokun A, Wang Q, Preische O, Kuhle J et al (2020) Serum neurofilament light chain levels are associated with white matter integrity in autosomal dominant Alzheimer’s disease. Neurobiol Dis 142:104960
Weston PSJ, Poole T, Ryan NS, Nair A, Liang Y, Macpherson K et al (2017) Serum neurofilament light in familial Alzheimer disease: a marker of early neurodegeneration. Neurology 89(21):2167–2175
Elahi FM, Casaletto KB, La Joie R, Walters SM, Harvey D, Wolf A et al (2020) Plasma biomarkers of astrocytic and neuronal dysfunction in early- and late-onset Alzheimer’s disease. Alzheimers Dement 16(4):681–695
Shir D, Graff-Radford J, Hofrenning EI, Lesnick TG, Przybelski SA, Lowe VJ et al (2022) Association of plasma glial fibrillary acidic protein (GFAP) with neuroimaging of Alzheimer’s disease and vascular pathology. Alzheimers Dement (Amst) 14(1):e12291
Mattsson N, Zetterberg H, Janelidze S, Insel PS, Andreasson U, Stomrud E et al (2016) Plasma tau in Alzheimer disease. Neurology 87(17):1827–1835
De Marco M, Ourselin S, Venneri A (2019) Age and hippocampal volume predict distinct parts of default mode network activity. Sci Rep 9(1):16075
Koch W, Teipel S, Mueller S, Benninghoff J, Wagner M, Bokde ALW et al (2012) Diagnostic power of default mode network resting state fMRI in the detection of Alzheimer’s disease. Neurobiol Aging 33(3):466–478
Gao F, Dai L, Wang Q, Liu C, Deng K, Cheng Z et al (2023) Blood-based biomarkers for Alzheimer’s disease: a multicenter-based cross-sectional and longitudinal study in China. Sci Bull (Beijing) 68(16):1800–1808
Funding
This research was supported by funding obtained under the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.3—Call for tender No. 341 of 15/03/2022 of the Italian Ministry of University and Research funded by the European Union—NextGenerationEU, Project code PE0000006, Concession Decree No. 1553 of 11/10/2022 adopted by the Italian Ministry of University and Research, CUP D93C22000930002, “A multiscale integrated approach to the study of the nervous system in health and disease” (MNESYS).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Literature search, selection and quality assessments were carried out by GL, MM and RM. The first draft of the manuscript was written by GL, MM and RM. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mitolo, M., Lombardi, G., Manca, R. et al. Association between blood-based protein biomarkers and brain MRI in the Alzheimer’s disease continuum: a systematic review. J Neurol (2024). https://doi.org/10.1007/s00415-024-12674-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00415-024-12674-w