Abstract
The Americas hold the greatest bird diversity worldwide. Likewise, ectoparasite diversity is remarkable, including ticks of the Argasidae and Ixodidae families – commonly associated with birds. Considering that ticks have potential health implications for humans, animals, and ecosystems, we conducted a systematic review to evaluate the effects of bioclimatic, geographic variables, and bird species richness on tick infestation on wild birds across the Americas. We identified 72 articles that met our inclusion criteria and provided data on tick prevalence in wild birds. Using Generalized Additive Models, we assessed the effect of environmental factors, such as habitat type, climatic conditions, bird species richness, and geographic location, on tick infestation. Our findings show that most bird infestation case studies involved immature ticks, such as larvae or nymphs, while adult ticks represented only 13% of case studies. We found birds infested by ticks of the genera Amblyomma (68%), Ixodes (22%), Haemaphysalis (5%), Dermacentor (1%), and Rhipicephalus (0.8%) in twelve countries across the Americas. Our findings revealed that temperature variation and bird species richness were negatively associated with tick infestation, which also varied with geographic location, increasing in mid-latitudes but declining in extreme latitudes. Our results highlight the importance of understanding how environmental and bird community factors influence tick infestation in wild birds across the Americas and the dynamics of tick-borne diseases and their impact on biodiversity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Americas have the greatest bird diversity worldwide (Orme et al. 2006), which explains the large diversity of ectoparasites associated with wild birds. Among the ectoparasites related to birds, ticks of the families Argasidae (⁓ 108 spp.) and Ixodidae (⁓ 137 spp.) are also diverse in terms of number of species (Nava et al. 2017; Dantas-Torres et al. 2019; Guglielmone et al. 2021; Instituto Nacional de Tecnología Agropecuaria [INTA] 2022; Guglielmone et al. 2023). In the Americas, wild birds are hosts and dispersers of larvae, nymph, and adult ticks of different genera of Argasidae (e.g., Argas and Ornithodoros) and Ixodidae (e.g., Ixodes, Haemaphysalis, Amblyomma, Dermacentor, and Rhipicephalus) (Morshed et al. 2005; Guglielmone et al. 2014; Nava et al. 2017; Gomez-Puerta et al. 2020). These avian hosts may have multiple interactions with different tick species, highlighting the complex ecological interactions between birds and ticks.
Tick host specificity can greatly vary among definitive and intermediate hosts (Nava and Guglielmone 2013; Esser et al. 2016). Immature ticks (larvae and nymphs) exhibit a generalist and opportunistic feeding behavior, parasitizing a wide range of vertebrate hosts, including many avian species. In contrast, most adult ticks are specialized to specific mammalian hosts (Nava and Guglielmone 2013; Esser et al. 2016; Fecchio et al. 2020a). Moreover, some nidicolous ticks, such as Argas persicus (Oken, 1818), Ixodes auritulus (Neumann, 1904), Ixodes brunneus (Koch, 1844), or Ixodes uriae (White, 1852), have a high degree of host specificity, relying on birds for their entire life cycle and live predominantly in the nests or burrows of their avian hosts (Sonenshine and Roe 2013; Guglielmone et al. 2014, 2023; Nava et al. 2017). In contrast, non-nidicolous tick species, such as Amblyomma longirostre (Koch, 1844) or Amblyomma nodosum (Neumann, 1899), wait for their avian hosts in emergent vegetation and only parasitize birds in their immature stage (Sonenshine and Roe 2013; Guglielmone et al. 2014, 2023). Additionally, generalist tick species like Amblyomma calcaratum (Neumann, 1899) or Ixodes pacificus (Cooley and Kohls 1943) can parasitize several orders of birds and mammals during their immature stages (Guglielmone et al. 2014, 2023). On the other hand, in adult stages, ticks like A. longirostre, A. nodosum, or A. calcaratum typically display a certain degree of specialization, depending on mammal hosts of the families Erethizontidae (New World porcupines) or Myrmecophagidae (anteaters) (e.g., Coendou prehensilis (Linnaeus, 1758), Coendou quichua (Thomas, 1899), Coendou spinosus (F. Cuvier, 1823), Tamandua tetradactyla (Linnaeus, 1758) or Tamandua mexicana (Saussure, 1860)) (Guglielmone et al. 2014). This biological diversity in feeding habits and host specificity underscores the complexity of tick interactions. Understanding this is crucial, as some tick species are vectors of pathogenic organisms such as bacteria (e.g., Rickettsia, Anaplasma, or Borrelia), protozoa (e.g., Babesia or Theileria), nematodes (e.g., Ackertia or Monanema), and viruses (e.g., Crimean-Congo hemorrhagic fever virus or tick-borne encephalitis virus) that negatively affect human welfare, domestic and wild animals health, and the economy (Jongejan and Uilenberg 2004; Estrada-Peña et al. 2012; Boulanger et al. 2019; Tokarz and Lipkin 2020; Erkyihun and Alemayehu 2022).
Several studies have examined tick infestation in wild birds, but these have been relatively few and scattered across the Americas. Wild birds in the Americas display varying levels of tick infestation. For example, in Neotropics, tick infestation rates range from 8 to 28%, while in temperate regions, values range from 4 to 70% (Klich et al. 1996; Morshed et al. 2005; Miller et al. 2016; Domínguez et al. 2019). It has been broadly suggested that tick infestation risk in birds is mediated by environmental factors such as geographic location (e.g., latitude), climatic conditions (e.g., temperature and precipitation), and ecological factors such as habitat type and disturbance (e.g., fragmentation), and bird species richness (Lindgren et al. 2000; Ogrzewalska et al. 2011; Jore et al. 2014; Fecchio et al. 2021a; Lilly et al. 2022). In this context, a comprehensive knowledge of the environmental and ecological factors influencing tick infestation in wild birds across the Americas is crucial for understanding the dynamics of tick-borne parasite transmission in the context of potential climate change scenarios, habitat disturbance, and biodiversity loss.
We conducted a systematic review of the available literature to determine the factors (e.g., weather or habitat degradation) and bird richness that determine the infestation of wild birds in the Americas. Given that the prevalence of tick-infested birds changes with temperature and humidity, habitat type, and host diversity (LoGiudice et al. 2003; Oorebeek and Kleindorfer 2008; Ogrzewalska et al. 2011; Fecchio et al. 2021a), we hypothesized that tick infestation in wild birds will be positively correlated with habitat degradation, temperature, and precipitation because these conditions seem to be needed for establishment, development, and host-seeking of ticks.
Methods
Literature survey and data inclusion criteria
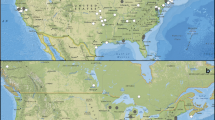
We conducted a literature search using the Web of Science and Scopus databases (January 1960 to December 2022), using the search terms “bird*” OR “avian*” AND “tick*” in the title, abstract, and keywords. To ensure a standard of quality of results and study replicability, we limited our search to peer-reviewed articles in English, excluding reviews or other documents (e.g., books, theses, technical reports, or institutional dossiers) that may contain duplicate information from articles (Bohada-Murillo et al. 2021). The literature review followed the methodology proposed in the PRISMA statement (Page et al. 2021). The initial search yielded 3205 articles, which were reduced to 2890 after eliminating duplicates. Then, we reduced them to 167 articles after discarding those that did not contain information about tick-infested wild bird communities. The selected articles were thoroughly reviewed to determine if they met the following inclusion criteria: (1) reported prevalence of tick infestation in wild birds or information allowing its calculation, (2) georeferenced location or detailed description of the sample area that allows its location. Inclusion criteria assessment was performed by the same person (AB) to avoid a potential inter-observer bias. We identified 72 articles that met our inclusion criteria and provided data on tick prevalence in wild birds. Nineteen articles present prevalence data for multiple localities, resulting in 149 case studies (i.e., reports of tick infestations from unique study locations), each one representing an observation of tick prevalence in wild birds within specific localities (Fig. 1). Of the 72 included articles, 261 case studies focused on ticks identified at the genus level, infesting wild birds: 179 of Amblyomma, 57 of Ixodes, 14 of Haemaphysalis, and 11 of other genera (Table 1). These case studies represent a subset categorized by tick genus out of the total observations, each corresponding to a specific observation of tick prevalence within a particular tick genus and locality.
Data extraction
From each article, we extracted tick infestation prevalence information (including ticks in any life stage) distinguishing larva, nymph, immature (i.e., larva and nymph), or adult stages along with tick genera. Prevalence represents the proportion of infested birds to the total birds examined (i.e., prevalence = number of infested birds/number of examined birds × 100) (e.g., Cardona-Romero et al. 2020; Dumas et al. 2022). In addition, we extracted locality information (country and habitat type), geographic location (i.e., latitude and longitude coordinates and elevation), and bird species richness (i.e., the number of bird species examined at each locality, de Angeli et al. 2021). When the articles did not explicitly provide the coordinates, we employed a systematic approach to estimate them. If the articles included specific details such as landmarks, geographic features, and mentions of towns or forest reserves, we utilized Google Earth Pro 7.3.4 software to estimate the coordinates. In addition, if the habitat information was not detailed in the article, we used the 'historical imagery' feature to complete the habitat description (Google LLC 2021). This feature allowed us to access images from specific periods corresponding to each article's description. In articles where a single prevalence value was reported for multiple localities within the same region or in proximity, we established one coordinate using ecological criteria to ensure unit homogeneity (Strnad et al. 2017). We obtained bioclimatic information for each locality using the dataset WorldClim 2.1 with a resolution of 2.5 min (Fick and Hijmans 2017). The bioclimatic variables included in our analysis represented temperature and precipitation metrics. Temperature variables included annual mean temperature (BIO1), maximum temperature of the warmest month (BIO5), and minimum temperature of the coldest month (BIO6). Precipitation variables included annual precipitation (BIO12), precipitation of the wettest month (BIO13), and precipitation of the driest month (BIO14). Additionally, we considered the differences between the maximum temperature of the warmest month and the minimum temperature of the coldest month (Delta temperature hereafter) and between the precipitation of the wettest month and the precipitation of the driest month (Delta precipitation hereafter). These variables were selected because they are known to influence the activity and phenology of ticks (Estrada-Peña et al. 2014; Nava et al. 2017). We classified habitat types based on the intensity of agricultural land use as natural (habitats covered by native vegetation with no agricultural use), semi-natural (habitats dominated mainly by natural vegetation but indirectly modified for agricultural activities), or agricultural-semiurban (habitats directly managed for agriculture or located directly adjacent to or within an urban environment) (Flynn et al. 2009).
Statistical analyses
We evaluated the effects of bioclimatic variables, geographic location, habitat type, and bird richness on the proportion of infested birds (infestation hereafter). Independent variables, such as bioclimatic factors and elevation, were centered to facilitate interpretation in our models (Schielzeth 2010). To avoid multicollinearity, we initially used a correlation matrix to identify and remove highly correlated variables (e.g., r >|0.75|). Subsequently, we applied the Variance Inflation Factor (VIF) to the remaining variables, refining our model selection (VIF > 5 indicates high multicollinearity) (James et al. 2021). We fitted Generalized Additive Models (GAM) with a quasi-binomial error distribution and a logit link function, using the proportion of infested birds as the response variable (Wood 2017). We included bird richness, elevation, and the bioclimatic variables (BIO6 and BIO13 or Delta temperature and Delta precipitation, depending on model configuration) as linear predictor variables. Habitat type was treated as a categorical variable (natural, semi-natural, or agricultural-semiurban), which was included as a fixed factor in our models to compare tick infestation across the habitat categories explicitly. To account for the spatial effects on bird infestation, coordinates were added as smooth terms in each model, using both latitude and longitude (spatial location hereafter), or only latitude, to determine the most appropriate spatial model representation (Hunsicker et al. 2016; Wood 2017). For detailed model specifications and retained variables in the best models ranked using the Akaike Information Criterion (AIC), see Table S1. We assessed the degree of non-linearity of the smooth terms in our models by calculating the effective degrees of freedom (edf); an edf value of 1 suggests that the relationship between the predictor and the response is almost linear, while edf value between 1 and 2 suggests a weakly non-linear relationship, an edf value greater than 2 indicates a non-linear relationship (Wood 2017). Separate models were fitted for the wild bird infestation by ticks according to their life stages: Model 1 (included adult and immature ticks), Model 2 (adult ticks), Model 3 (immature ticks), Model 4 (nymphs ticks), and Model 5 (larvae ticks). Additionally, we fitted four models for Amblyomma tick infestation according to life stages: Model 6 (adult and immature Amblyomma ticks), Model 7 (immatures Amblyomma ticks), Model 8 (nymphs Amblyomma ticks), and Model 9 (larvae Amblyomma ticks). We only develop models for the genus Amblyomma (68% of the case studies in the dataset) due to the small number of case studies for the other genera (Table 1). After model selection procedures, we conducted a 'least square means' analysis to perform pairwise comparisons among habitat types (natural, semi-natural, or agricultural-semiurban) regarding bird infestation proportion. All analyses were performed using “mgcv” (Wood 2011) and “lsmeans” (Lenth 2016) packages in R version 4.3.3 (R Core Team 2024).
Results
Tick infestation of bird communities in the Americas
Of the 149 case studies selected in our review, 81% (121) reported tick infestations, and 69% came from studies conducted in tropical regions of the Americas (mainly in Brazil, 32%) between 2000 and 2022. Out of all the case studies of bird infestation, 35% documented larvae and 34% nymphs. Immature ticks were reported in 33% of the case studies (i.e., those in which the larval and nymphal stages could not separated). Adult ticks were only present in 13% of the case studies. Likewise, 57% of the infestation case studies were recorded in natural habitats, followed by seminatural habitats (22%) and agriculture-semiurban habitats (21%). The studies identified birds infested by ticks of the genera Ixodes, Haemaphysalis, Amblyomma, Dermacentor, and Rhipicephalus in twelve American countries. Of the 261 case studies of tick genera recorded, Amblyomma was the most common (68%), followed by Ixodes (22%) and Haemaphysalis (5%) (Table 1). Of the 179 case studies involving bird infestations by Amblyomma ticks, 85% occurred in tropical regions, and 57% occurred in natural habitats. The Amblyomma tick species most associated with birds were A. longirostre (21%), A. nodosum (15%), and A. calcaratum (8%).
Across the reviewed studies, a total of 2253 bird individuals, representing 570 species, 58 families, and 18 orders, were reported to be infested by ticks. Among these, the families Thraupidae (12%), Turdidae (11%), and Tyrannidae (11%) were most prevalent. Specifically, the species most frequently infested by ticks included Trichothraupis melanops (2%) and Tachyphonus coronatus (1.5%) within Thraupidae, Troglodytes aedon (1.4%, Troglodytidae), and Catharus ustulatus (1.3%, Turdidae). The tick genus Amblyomma was dominant, accounting for 68% (1530) of the infestations reported, followed by Ixodes with 23% (519), and Haemaphysalis with 7% (159). The most common tick species identified were A. longirostre 18% (406), A. nodosum 10% (219), Ixodes scapularis 6% (145), and Haemaphysalis leporispalustris 6% (138).
Effects of bioclimatic, geographic variables and bird richness on infestation
Tick infestation in wild birds was associated with climatic conditions, bird species richness, and geographic location. Adult tick infestation was positively correlated with elevation and negatively correlated with temperature variation, precipitation, and richness (Model 2, Table 2). Infestation by nymphal ticks was negatively influenced by temperature variation and elevation (Model 4, Table 2). Moreover, bird species richness negatively affected bird infestation by the genus Amblyomma (except for the Amblyomma nymph stage) (Model 6 to Model 9, Table 2). A subsequent pairwise comparison in the models revealed significant differences in tick infestation between natural and semi-natural habitats, with the natural habitat showing lower infestation rates across Models 2, 4, and 5 (Table S3, Figure S1). For an overview of each model's predictive variables, please refer to supplementary Tables S1 and S2.
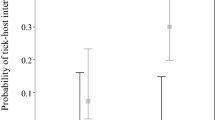
We found that spatial location had a significant influence on tick infestation in wild birds across various life stages, including adults, immatures, and both (Model 1 to 3, Table 2, Fig. 2a, b). Furthermore, latitude significantly affected nymph ticks and Amblyomma nymph ticks (Model 4 and 8, Table 2). High tick infestations in wild birds have been observed in localities from 15° to 28° south latitude and 28° to 45° north latitude, as reported in studies by Beldomenico et al. (2003), Sonenshine and Clifford (1973), and Teel et al. (1998). Lower infestations were found in localities above these latitudes, as reported in studies by Klich et al. (1996), Gonzalez-Acuña et al. (2004), and Cicuttin et al. (2019) (Fig. 2a, b). In the Northern Hemisphere, the USA temperate broadleaf and mixed forests recorded the highest infestation levels. Likewise, the Brazilian Atlantic Forest and Cerrado ecoregions showed the highest infestation levels in the Southern Hemisphere. However, our analysis revealed no significant spatial relationship for larval infestation (Models 5 and 9, Table 2), suggesting that spatial factors were not the determinants of infestation patterns in the tick life stage. We found that bird species richness exhibited a trend across the Amblyomma models, except for the Amblyomma nymph stage (Model 8, Table 2). Bird infestation shows spatial variability patterns across different localities on the continent in almost half of the models (Model 1 to Model 4, and Model 8, Table 2).
Discussion
In this study, we found that tick infestation in wild birds in the Americas was related to climatic conditions, bird species richness, and geographic location. Our results showed that tick infestation, especially by Amblyomma ticks, in wild birds was negatively associated with bird species richness. This relationship between parasite infestation and community diversity is similar to the 'dilution effect' hypothesis proposed by Keesing and Ostfeld (2021). According to this hypothesis, communities with greater diversity and equity of host species decrease the probability of encounters between ticks and highest-quality hosts (Ostfeld and Keesing 2000; LoGiudice et al. 2008; Civitello et al. 2015). Our estimation of bird species richness served as a proxy of the true richness of bird communities, but it may be influenced by different bird capture methods used in each study (de Angeli et al. 2021). In the case studies reviewed, we observed that lower bird infestation by Amblyomma ticks occurred in localities with the highest bird species richness (Ogrzewalska et al. 2008; Maturano et al. 2015; Martinez-Sanchez et al. 2020). Consequently, bird species richness appears to be a determinant of bird infestation in the Americas, which can be affected by habitat disturbance and can lead to changes in infestation patterns (Estades and Temple 1999; Ehlers Smith et al. 2015).
Another factor influencing tick infestation in wild birds in the Americas is the temperature, particularly during the adult and nymphal stages. Climatic variables, especially temperature fluctuations, significantly affect tick distribution, survival, and questing behavior (Cumming 2002; Vail and Smith 2002; Ogden et al. 2004; Berger et al. 2014; Estrada-Peña et al. 2014). Being poikilothermic, ticks exhibit non-linear increases in inter-stadial development rates with rising ambient temperatures (Randolph 2004; Faccini et al. 2021). Under favorable conditions, ticks can remain in questing positions in vegetation for several days. However, they often descend due to increased saturation deficits or atmospheric dryness (Vail and Smith 2002; Randolph 2004; Berger et al. 2014). For example, Oorebeek and Kleindorfer (2008) reported that tick abundance on passerines fluctuates with host availability and climatic conditions, with higher tick populations during months characterized by high humidity, rainfall, and lower temperatures. Additionally, it has been documented that ticks, especially in their immature stages, tend to quest at lower vegetation heights when temperatures are high and relative humidity is low, reducing contact with vertebrate hosts (Lefcort and Durden 1996; Vail and Smith 2002; Randolph 2004; Prusinski et al. 2006; Berger et al. 2014; Portugal et al. 2020). Therefore, temperature variations may be an important determinant of tick infestation patterns in wild birds, affecting their distribution, behavior, and interactions with their hosts.
Tick infestations in wild birds vary with location, suggesting complex ecological interactions in the different localities that influence the bird infestation. We found that bird communities in mid-latitude regions generally have higher proportions of tick infestation, while locations at latitudinal extremes exhibit lower proportions. This result partially supports the notion that the intensity of the parasite-host association increases with latitude (Fecchio et al. 2021b; Zvereva and Kozlov 2021). Environmental changes, especially those related to climate, can alter the ecological niches of ticks and their interaction with host species (Ostfeld and Keesing 2000). It has been proposed that the intensity of association between host and parasites increases at high latitudes due to a variety of factors, such as the presence of a diverse number of parasites or environmental conditions that make parasites more dependent on their hosts (Hawkins 1994; Kamiya et al. 2014; Fecchio et al. 2021b). However, the decrease in infestation at the latitudinal extremes of the Americas could be due to a combination of factors that negatively affect tick density (e.g., mean temperature and saturation deficit) (Diuk-Wasser et al. 2006). Additionally, global climate change effects on biodiversity may impact parasite-host dynamics, suggesting that current infestation patterns may change as ecological conditions change (Lafferty 2009).
Finally, some bird families known to forage in the lower forest strata vegetation (e.g., Thamnophilidae, Furnariidae, Tyrannidae, Pipridae, Troglodytidae, Turdidae, Parulidae, or Thraupidae) seem to be more susceptible to tick infestation (Labruna et al. 2007; Oorebeek and Kleindorfer 2009; Guglielmone et al. 2014; Martinez-Sanchez et al. 2020). This observation may be due to a capture bias, as birds that forage in the lower forest strata vegetation are the ones that are typically captured using traditional methods. Our review is consistent with these findings and shows that families Thraupidae, Turdidae, and Tyrannidae represent the majority of tick-infested birds. Specifically, species such as T. melanops and T. coronatus within Thraupidae, T. aedon within Troglodytidae, and C. ustulatus within Turdidae were commonly infested. This suggests a possible correlation between bird phylogeny and susceptibility to tick infestation, possibly due to shared ecological traits or host phylogenetic conservation, increasing exposure to ticks (Poulin 2007; Barrow et al. 2019; Fecchio et al. 2021b).
Our research identified the environmental and ecological factors influencing tick infestation in wild birds across the Americas. Understanding these factors is critical for assessing the risks associated with tick-borne pathogen transmission (Moller et al. 2013; Fecchio et al. 2020b). We found that climatic conditions are key determinants of tick infestation patterns, as reported in some studies (Cumming 2002; Ogden et al. 2008; Oorebeek and Kleindorfer 2008; Pfaffle et al. 2013; Estrada-Peña and de la Fuente 2014). Furthermore, the inverse relationship between tick infestation and bird species richness highlights the potential role of biodiversity in mitigating disease transmission (LoGiudice et al. 2003; Keesing and Ostfeld 2021). The general patterns described here have implications for disease transmission dynamics, highlighting that environmental and ecological factors modulate the intensity of parasite-host associations and disease risk across different geographic regions.
Conclusion
Our results show that the prevalence of tick infestation in wild birds in the Americas is related to climatic conditions, bird species richness, and geographic location. Changes in biodiversity resulting from habitat degradation due to climate change could modify the dynamics of tick infestation. In this sense, our results highlight the value of biodiversity as a buffer for parasite infestation in bird communities. Tick infestation in wild birds exhibits complex geographic patterns across different latitudes in the Americas, increasing in mid-latitudes and declining at the extreme latitudes of the continent. Identifying how environmental and wild bird community factors determine tick infestation is crucial to understanding tick-borne disease dynamics and its effects on biodiversity.
Data availability
The data was deposited in Figshare under the reference number https://doi.org/10.6084/m9.figshare.24452050.
Code availability
Not applicable.
References
Barrow LN et al (2019) Deeply conserved susceptibility in a multi-host, multi-parasite system. Ecol Lett 22:987–998. https://doi.org/10.1111/ele.13263
Beldomenico P et al (2003) Ixodid ticks (Acari: Ixodidae) present at Parque Nacional El Rey. Argentina Neotrop Entomol 32:273–277. https://doi.org/10.1590/S1519-566X2003000200012
Berger KA, Ginsberg HS, Dugas KD, Hamel LH, Mather TN (2014) Adverse moisture events predict seasonal abundance of Lyme disease vector ticks (Ixodes scapularis). Parasit Vectors 7:181. https://doi.org/10.1186/1756-3305-7-181
Bohada-Murillo M, Castaño-Villa GJ, Fontúrbel FE (2021) Effects of Dams on Vertebrate Diversity: A Global Analysis. Diversity 13:528. https://doi.org/10.3390/d13110528
Boulanger N, Boyer P, Talagrand-Reboul E, Hansmann Y (2019) Ticks and tick-borne diseases. Med Mal Infect 49:87–97. https://doi.org/10.1016/j.medmal.2019.01.007
Cardona-Romero MM-S, ET., et al (2020) Rickettsia parkeri strain Atlantic rainforest in ticks (Acari: Ixodidae) of wild birds in Arauca, Orinoquia region of Colombia. Int J Parasitol Parasites Wildl 13:106–113. https://doi.org/10.1016/j.ijppaw.2020.09.001
Cicuttin GL, De Salvo MN, Venzal JM, Nava S (2019) Borrelia spp. in ticks and birds from a protected urban area in Buenos Aires city, Argentina. Ticks Tick Borne Dis 10:101282. https://doi.org/10.1016/j.ttbdis.2019.101282
Civitello DJ et al (2015) Biodiversity inhibits parasites: broad evidence for the dilution effect. PLoS ONE 112:8667–8671. https://doi.org/10.1073/pnas.1506279112
Cumming GS (2002) Comparing Climate and Vegetation as Limiting Factors for Species Ranges of African Ticks. Ecology 83:255–268. https://doi.org/10.1890/0012-9658(2002)083[0255:Ccaval]2.0.Co;2
Dantas-Torres F, Martins TF, Muñoz-Leal S, Onofrio VC, Barros-Battesti DM (2019) Ticks (Ixodida: Argasidae, Ixodidae) of Brazil: Updated species checklist and taxonomic keys. Ticks Tick Borne Dis 10:101252. https://doi.org/10.1016/j.ttbdis.2019.06.012
de Angeli DD, Filion A, Fecchio A, Braga ÉM, Poulin R (2021) Migrant birds disperse haemosporidian parasites and affect their transmission in avian communities. Oikos 130:979–988. https://doi.org/10.1111/oik.08199
Diuk-Wasser MA et al (2006) Spatiotemporal patterns of host-seeking Ixodes scapularis nymphs (Acari: Ixodidae) in the United States. J Med Entomol 43:166–176. https://doi.org/10.1603/0022-2585(2006)043[0166:spohis]2.0.co;2
Domínguez L, Miranda RJ, Torres S, Moreno R, Ortega J, Bermúdez SE (2019) Hard tick (Acari: Ixodidae) survey of Oleoducto trail, Soberania National Park, Panama. Ticks Tick Borne Dis 10:830–837. https://doi.org/10.1016/j.ttbdis.2019.04.001
Dumas A et al (2022) Transmission patterns of tick-borne pathogens among birds and rodents in a forested park in southeastern Canada. PLoS ONE 17:e0266527. https://doi.org/10.1371/journal.pone.0266527
Ehlers Smith YC, Ehlers Smith DA, Seymour CL, Thébault E, van Veen FJF (2015) Response of avian diversity to habitat modification can be predicted from life-history traits and ecological attributes. Landscape Ecol 30:1225–1239. https://doi.org/10.1007/s10980-015-0172-x
Erkyihun GA, Alemayehu MB (2022) One Health Approach for the Control of Zoonotic Diseases. Zoonoses 2. https://doi.org/10.15212/zoonoses-2022-0037
Esser HJ, Herre EA, Blüthgen N, Loaiza JR, Bermúdez SE, Jansen PA (2016) Host specificity in a diverse Neotropical tick community: an assessment using quantitative network analysis and host phylogeny. Parasites Vectors 9:372. https://doi.org/10.1186/s13071-016-1655-6
Estades CF, Temple SA (1999) Deciduous-Forest Bird Communities in a Fragmented Landscape Dominated by Exotic Pine Plantations. Ecol Appl 9:573–585. https://doi.org/10.2307/2641145
Estrada-Peña A, de la Fuente J (2014) The ecology of ticks and epidemiology of tick-borne viral diseases. Antiviral Res 108:104–128. https://doi.org/10.1016/j.antiviral.2014.05.016
Estrada-Peña A, Ayllón N, de la Fuente J (2012) Impact of climate trends on tick-borne pathogen transmission. Front Physiol 3:64. https://doi.org/10.3389/fphys.2012.00064
Estrada-Peña A et al (2014) Divergent environmental preferences and areas of sympatry of tick species in the Amblyomma cajennense complex (Ixodidae). Int J Parasitol 44:1081–1089. https://doi.org/10.1016/j.ijpara.2014.08.007
Faccini JLH, De Almeida TK, Sousa IC, Junior LMC, Luz HR (2021) Temperature effects on the non-parasitic phase of Amblyomma parvum (Acari: Ixodidae). Systematic and Applied Acarology 26:1168–1176. https://doi.org/10.11158/saa.26.6.12
Fecchio A et al (2020) Low host specificity and lack of parasite avoidance by immature ticks in Brazilian birds. Parasitol Res 119:2039–2045. https://doi.org/10.1007/s00436-020-06698-0
Fecchio A et al (2020) Host movement and time of year influence tick parasitism in Pantanal birds. Exp Appl Acarol 82:125–135. https://doi.org/10.1007/s10493-020-00530-1
Fecchio A et al (2021) Migration and season explain tick prevalence in Brazilian birds. Med Vet Entomol 35:547–555. https://doi.org/10.1111/mve.12532
Fecchio A, Martins TF, Ogrzewalska M, Schunck F, Weckstein JD, Dias RI (2021) Higher probability of tick infestation reveals a hidden cost of army ant‐following in Amazonian birds. J Avian Biol 52. https://doi.org/10.1111/jav.02759
Fick SE, Hijmans RJ (2017) WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int J Climatol 37:4302–4315. https://doi.org/10.1002/joc.5086
Flynn DF et al (2009) Loss of functional diversity under land use intensification across multiple taxa. Ecol Lett 12:22–33. https://doi.org/10.1111/j.1461-0248.2008.01255.x
Gomez-Puerta LA, Muñoz-Leal S, Labruna MB, Venzal JM (2020) Confirmación de Argas neghmei (Ixodida: Argasidae) en Perú y reporte del carpintero andino (Colaptes rupicola) como nuevo hospedero. Rev Peru Biol 27:533–536. https://doi.org/10.15381/rpb.v27i4.19202
Gonzalez-Acuña D, Venzal J, Skewes-Ramm O, Rubilar-Contreras L, Daugschies A, Guglielmone AA (2004) First record of immature stages of Amblyomma tigrinum (Acari: Ixodidae) on wild birds in Chile. Exp Appl Acarol 33:153–156. https://doi.org/10.1023/b:appa.0000030015.18088.e6
Google LLC (2021) Google Earth Pro. 7.3.4. edn. Google LLC, Mountain View
Guglielmone AA, Robbins RG, Apanaskevich DA, Petney TN, Estrada-Peña A, Horak IG (2014) The hard ticks of the world, vol 10. Springer, Dordrecht
Guglielmone AA, Nava S, Robbins RG (2021) Neotropical Hard Ticks (Acari: Ixodida: Ixodidae): A Critical Analysis of Their Taxonomy, Distribution, and Host Relationships. Springer International Publishing, Berlin/Heidelberg, Germany
Guglielmone AA, Nava S, Robbins RG (2023) Geographic distribution of the hard ticks (Acari: Ixodida: Ixodidae) of the world by countries and territories. Zootaxa 5251:1–274. https://doi.org/10.11646/zootaxa.5251.1.1
Hawkins BA (1994) Pattern and process in host-parasitoid interactions. Cambridge University Press
Hunsicker ME et al (2016) Characterizing driver–response relationships in marine pelagic ecosystems for improved ocean management. Ecol Appl 26:651–663. https://doi.org/10.1890/14-2200
Instituto Nacional de Tecnología Agropecuaria [INTA] (2022) Listado nombres de especies válidas de Garrapatas duras. http://rafaela.inta.gob.ar/nombresgarrapatas/ Accessed
James GW, Daniela, Hastie T, Tibshirani R (2021) An introduction to statistical learning : with applications in R. Springer, New York
Jongejan F, Uilenberg G (2004) The global importance of ticks. Parasitology 129(Suppl):S3-14. https://doi.org/10.1017/s0031182004005967
Jore S et al (2014) Climate and environmental change drives Ixodes ricinus geographical expansion at the northern range margin. Parasites Vectors 7:1–14. https://doi.org/10.1186/1756-3305-7-11
Kamiya T, O’Dwyer K, Nakagawa S, Poulin R (2014) What determines species richness of parasitic organisms? A meta-analysis across animal, plant and fungal hosts. Biol Rev Camb Philos Soc 89:123–134. https://doi.org/10.1111/brv.12046
Keesing F, Ostfeld RS (2021) Dilution effects in disease ecology. Ecol Lett 24:2490–2505. https://doi.org/10.1111/ele.13875
Klich M, Lankester MW, Wu KW (1996) Spring migratory birds (Aves) extend the northern occurrence of blacklegged tick (Acari: Ixodidae). J Med Entomol 33:581–585. https://doi.org/10.1093/jmedent/33.4.581
Labruna MB et al (2007) Ticks collected on birds in the state of Sao Paulo, Brazil. Exp Appl Acarol 43:147–160. https://doi.org/10.1007/s10493-007-9106-x
Lafferty KD (2009) The ecology of climate change and infectious diseases. Ecology 90:888–900. https://doi.org/10.1890/08-0079.1
Lefcort H, Durden LA (1996) The effect of infection with Lyme disease spirochetes (Borrelia burgdorferi) on the phototaxis, activity, and questing height of the tick vector Ixodes scapularis. Parasitology 113:97–103. https://doi.org/10.1017/S0031182000066336
Lenth RV (2016) Least-Squares Means: The R Package lsmeans. J Stat Softw 69:1–33. https://doi.org/10.18637/jss.v069.i01
Lilly M et al (2022) Local Community Composition Drives Avian Borrelia burgdorferi Infection and Tick Infestation. Vet Sci 9. https://doi.org/10.3390/vetsci9020055
Lindgren E, Tälleklint L, Polfeldt T (2000) Impact of climatic change on the northern latitude limit and population density of the disease-transmitting European tick Ixodes ricinus. Environ Health Perspect 108:119–123. https://doi.org/10.1289/ehp.00108119
LoGiudice K, Ostfeld RS, Schmidt KA, Keesing F (2003) The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. PLoS ONE 100:567–571. https://doi.org/10.1073/pnas.0233733100
LoGiudice K, Duerr ST, Newhouse MJ, Schmidt KA, Killilea ME, Ostfeld RS (2008) Impact of host community composition on Lyme disease risk. Ecology 89:2841–2849. https://doi.org/10.1890/07-1047.1
Martinez-Sanchez ET et al (2020) Associations between wild birds and hard ticks (Acari: Ixodidae) in Colombia. Ticks Tick Borne Dis 11:101534. https://doi.org/10.1016/j.ttbdis.2020.101534
Maturano R, Faccini JLH, Daemon E, Fazza POC, Bastos RR (2015) Additional information about tick parasitism in Passeriformes birds in an Atlantic Forest in southeastern Brazil. Parasitol Res 114:4181–4193. https://doi.org/10.1007/s00436-015-4651-4
Miller MJ et al (2016) Molecular Ecological Insights into Neotropical Bird-Tick Interactions. PLoS ONE 11:e0155989. https://doi.org/10.1371/journal.pone.0155989
Moller AP et al (2013) Assessing the effects of climate on host-parasite interactions: a comparative study of European birds and their parasites. PLoS ONE 8:e82886. https://doi.org/10.1371/journal.pone.0082886
Morshed MG et al (2005) Migratory songbirds disperse ticks across Canada, and first isolation of the Lyme disease spirochete, Borrelia burgdorferi, from the avian tick, Ixodes auritulus. J Parasitol 91:780–790. https://doi.org/10.1645/GE-3437.1
Nava S, Guglielmone AA (2013) A meta-analysis of host specificity in Neotropical hard ticks (Acari: Ixodidae). Bull Entomol Res 103:216–224. https://doi.org/10.1017/S0007485312000557
Nava S, Venzal JM, Acuña DG, Martins TF, Guglielmone AA (2017) Ticks of the Southern Cone of America: diagnosis, distribution, and hosts with taxonomy, ecology and sanitary importance. Academic Press, London
Ogden NH et al (2004) Investigation of Relationships Between Temperature and Developmental Rates of Tick Ixodes scapularis (Acari: Ixodidae) in the Laboratory and Field. J Med Entomol 41:622–633. https://doi.org/10.1603/0022-2585-41.4.622%JJournalofMedicalEntomology
Ogden NH, Bigras-Poulin M, Hanincová K, Maarouf A, O’Callaghan CJ, Kurtenbach K (2008) Projected effects of climate change on tick phenology and fitness of pathogens transmitted by the North American tick Ixodes scapularis. J Theor Biol 254:621–632. https://doi.org/10.1016/j.jtbi.2008.06.020
Ogrzewalska M, Pacheco RC, Uezu A, Ferreira F, Labruna MB (2008) Ticks (Acari: Ixodidae) infesting wild birds in an Atlantic Forest area in the State of São Paulo, Brazil, with isolation of Rickettsia from the tick Amblyomma longirostre. J Med Entomol 45:770–774. https://doi.org/10.1603/0022-2585(2008)45[770:TAIIWB]2.0.CO;2
Ogrzewalska M, Uezu A, Jenkins CN, Labruna MB (2011) Effect of forest fragmentation on tick infestations of birds and tick infection rates by Rickettsia in the Atlantic forest of Brazil. EcoHealth 8:320–331. https://doi.org/10.1007/s10393-011-0726-6
Oorebeek M, Kleindorfer S (2008) Climate or host availability: what determines the seasonal abundance of ticks? Parasitol Res 103:871–875. https://doi.org/10.1007/s00436-008-1071-8
Oorebeek M, Kleindorfer S (2009) The prevalence and intensity of tick infestation in passerines from south Australia. Emu 109:121–125. https://doi.org/10.1071/MU08052
Orme CDL et al (2006) Global Patterns of Geographic Range Size in Birds. PLoS Biol 4:e208. https://doi.org/10.1371/journal.pbio.0040208
Ostfeld RS, Keesing F (2000) Biodiversity series: The function of biodiversity in the ecology of vector-borne zoonotic diseases. Can J Zool 78:2061–2078. https://doi.org/10.1139/z00-172
Page MJ et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev 10:89. https://doi.org/10.1186/s13643-021-01626-4
Pfaffle M, Littwin N, Muders SV, Petney TN (2013) The ecology of tick-borne diseases. Int J Parasitol 43:1059–1077. https://doi.org/10.1016/j.ijpara.2013.06.009
Portugal JS, Wills R, Goddard J (2020) Laboratory Studies of Questing Behavior in Colonized Nymphal Amblyomma maculatum Ticks (Acari: Ixodidae). J Med Entomol 57:1480–1487. https://doi.org/10.1093/jme/tjaa077
Poulin R (2007) Evolutionary Ecology of Parasites, 2nd edn, New Jersey
Prusinski M et al (2006) Habitat Structure Associated with Borrelia burgdorferi Prevalence in Small Mammals in New York State. Environ Entomol 35:308–319. https://doi.org/10.1603/0046-225X-35.2.308
R Core Team (2024) R: A Language and Environment for Statistical Computing. 4.3.3.3 edn. R Foundation for Statistical Computin, Vienna, Austria
Randolph SE (2004) Tick ecology: processes and patterns behind the epidemiological risk posed by ixodid ticks as vectors. Parasitology 129(Suppl):S37-65. https://doi.org/10.1017/s0031182004004925
Schielzeth H (2010) Simple means to improve the interpretability of regression coefficients. Methods Ecol Evol 1:103–113. https://doi.org/10.1111/j.2041-210X.2010.00012.x
Sonenshine DE, Clifford CM (1973) Contrasting incidence of Rocky Mountain spotted fever in ticks infesting wild birds in eastern U.S. Piedmont and coastal areas, with notes on the ecology of these ticks. J Med Entomol 10:497–502. https://doi.org/10.1093/jmedent/10.5.497
Sonenshine DE, Roe RM (2013) Biology of Ticks, vol 2, 2nd edn. Oxford University Press, New York
Strnad M, Hönig V, Růžek D, Grubhoffer L, Rego ROM (2017) Europe-Wide Meta-Analysis of Borrelia burgdorferi Sensu Lato Prevalence in Questing Ixodes ricinus Ticks. Appl Environ Microbiol 83 https://doi.org/10.1128/aem.00609-17
Teel PD, Hopkins SW, Donahue WA, Strey OF (1998) Population Dynamics of Immature Amblyomma maculatum (Acari: Ixodidae) and Other Ectoparasites on Meadowlarks and Northern Bobwhite Quail Resident to the Coastal Prairie of Texas. J Med Entomol 35:483–488. https://doi.org/10.1093/jmedent/35.4.483
Tokarz R, Lipkin WI (2020) Discovery and Surveillance of Tick-Borne Pathogens. J Med Entomol 58:1525–1535. https://doi.org/10.1093/jme/tjaa269
Vail SC, Smith G (2002) Vertical movement and posture of blacklegged tick (Acari: Ixodidae) nymphs as a function of temperature and relative humidity in laboratory experiments. J Med Entomol 39:842–846. https://doi.org/10.1603/0022-2585-39.6.842
Wood SN (2011) Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J R Stat Soc Series B Stat Methodol 73:3–36. https://doi.org/10.1111/j.1467-9868.2010.00749.x
Wood SN (2017) Generalized Additive Models: An Introduction with R, 2nd edn. Chapman and Hall/CRC, Boca Raton
Zvereva EL, Kozlov MV (2021) Latitudinal gradient in the intensity of biotic interactions in terrestrial ecosystems: Sources of variation and differences from the diversity gradient revealed by meta-analysis. Ecol Lett 24:2506–2520. https://doi.org/10.1111/ele.13851
Acknowledgements
We are grateful to the research groups Genética, Biodiversidad y Manejo de Ecosistemas—GEBIOME, and Ecosistemas Tropicales (Universidad de Caldas). AB acknowledges the support from the Sistema General de Regalías, the Universidad de Caldas, and the Doctorado en Ciencias Agrarias, as well as the Laboratorio de Ecología de la Pontificia Universidad Católica de Valparaíso, where this manuscript was developed during a doctoral stay in Chile. We appreciate the comments of K. Wells and two anonymous reviewers who helped us to improve an earlier version of the manuscript.
Funding
Open Access funding provided by Colombia Consortium. This research was funded by the Vicerrectoría de Investigaciones y Posgrados—Universidad de Caldas (grant: 0180617). AB acknowledges the support of Minciencias – Colombia (Convocatoria del Fondo de Ciencia, Tecnología e Innovación del Sistema General de Regalías para la conformación de una lista de proyectos elegibles para ser viabilizados, priorizados y aprobados por el OCAD dentro del Programa de Becas de Excelencia cohorte 1–2019, project “Formación de Capital Humano de Alto Nivel Universidad de Caldas”, BPIN 2019000100035).
Author information
Authors and Affiliations
Contributions
AB and GJCV originally formulated the idea. AB and ETMS performed the systematic review. AB, GJCV, and FEF analyzed the data. AB, GJCV, ETMS, FEF, and JAL wrote the manuscript; HERC and FARP review & editing. GJCV founding acquisition, supervision, project administration.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Section Editor: Konstans Wells.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Busi, A., Martínez-Sánchez, E.T., Alvarez-Londoño, J. et al. Environmental and ecological factors affecting tick infestation in wild birds of the Americas. Parasitol Res 123, 254 (2024). https://doi.org/10.1007/s00436-024-08246-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00436-024-08246-6