Abstract
Mechanosensing of fibroblasts plays a key role in the development of fibrosis. So far, no effective treatments are available to treat this devastating disorder. Spectrins regulate cell morphology and are potential mechanosensors in a variety of non-erythroid cells, but little is known about the role of spectrins in fibroblasts. We investigate whether αII- and βII-spectrin are required for the phenotypic properties of adult human dermal (myo)fibroblasts. Knockdown of αII- or βII-spectrin in fibroblasts did not affect cell adhesion, cell size and YAP nuclear/cytosolic localization. We further investigated whether αII- and βII-spectrin play a role in the phenotypical switch from fibroblasts to myofibroblasts under the influence of the pro-fibrotic cytokine TGFβ1. Knockdown of spectrins did not affect myofibroblast formation, nor did we observe changes in the organization of αSMA stress fibers. Focal adhesion assembly was unaffected by spectrin deficiency, as was collagen type I mRNA expression and protein deposition. Wound closure was unaffected as well, showing that important functional properties of myofibroblasts are unchanged without αII- or βII-spectrin. In fact, fibroblasts stimulated with TGFβ1 demonstrated significantly lower endogenous mRNA levels of αII- and βII-spectrin. Taken together, despite the diverse roles of spectrins in a variety of other cells, αII- and βII-spectrin do not regulate cell adhesion, cell size and YAP localization in human dermal fibroblasts and are not required for the dermal myofibroblast phenotypical switch.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic organ injury often results in the development of fibrosis: an excessive production, post-translational modification and stiffening of extracellular matrix (ECM) components (Rockey et al. 2015). Pathological stiffening of the ECM creates a pro-fibrotic feedback loop (Parker et al. 2014) but how mechanical cues are transduced to change cell function and fate remains incompletely understood. Driving the fibrotic response are activated fibroblasts or pericytes that acquire the myofibroblast phenotype, which is characterized by a well-developed endoplasmic reticulum and an extensive contractile actomyosin cytoskeleton (Klingberg et al. 2013). Decades of research have been devoted to the contractile apparatus in the regulation of the myofibroblast phenotype. More recently, structural proteins belonging to the spectrin family were found to act as functional adaptors between the actomyosin cytoskeleton and the plasma membrane and are thought to regulate transduction of mechanical signals (Liem 2016; Stankewich et al. 2011).
Spectrins form a major component of the cytoskeleton at the membrane-cytoskeleton interface (Bennett 1990a; Sormunen 1993) and play an important role in maintaining cellular integrity (Bennett and Baines 2001). Spectrins form tetrameric flexible heterodimers, which contain two alpha and two beta subunits (Dubreuil et al. 1989; MacDonald and Cummings 2004) and have been evolutionary conserved in species as different as echinoderms (Fishkind et al. 1987), Sophophora (Bennett 1990a; Deng et al. 1995; Dubreuil et al. 1987, 1990), birds (Wasenius et al. 1989) and humans (Bennett 1990b; Leto et al. 1988; Sevinc and Fung 2011). They were first discovered in metazoan erythrocytes where they support the membrane cytoskeleton (Bennett 1990a, 1990b; Bennett and Baines 2001). In erythrocytes, two different spectrin genes are found, SPTA1 (αI-spectrin) and SPTB1 (βI-spectrin). Both subtypes are uniquely expressed in erythrocytes and thus not found in other cell types (Wasenius et al. 1989). More recently, other spectrin proteins were identified in non-erythrocyte cells (Bennett 1990a; Dubreuil et al. 1990; Moon and McMahon 1990). SPTAN1 encodes several isoforms of the non-erythrocyte αII-spectrin polypeptide that are generated through alternative splicing. In addition, non-erythrocyte β-spectrins are encoded by four similar genes: SPTBN1 (βII-spectrin), SPTBN2 (βIII-spectrin), SPTBN4 (βIV-spectrin) and SPTBN5 (bV-spectrin (βHeavy)). Here, we focus on αII-spectrin and βII-spectrin, since they have been reported to provide mechanical stability and maintaining cell integrity, plasma membrane stability and morphology—key features of cellular mechanosensing (Bialkowska 2005; Machnicka et al. 2012; Metral et al. 2009; Stankewich et al. 2011). Furthermore, αII-spectrin and βII-spectrin regulate cell adhesion (Metral et al. 2009) and cell spreading (Bialkowska 2005; Meriläinen et al. 1993; Stankewich et al. 2011) and contain domains that function in protein sorting, vesicle trafficking and endocytosis (Bialkowska 2005; Devarajan et al. 1997; Kamal et al. 1998).
The functional domain in the αII-spectrin subunit is the highly conserved Src Homology 3 (SH3) domain (Musacchio et al. 1992), which initiates Rac activation during initial cell adhesion (Bialkowska 2005). In addition, αII-spectrin contains a calmodulin binding site (Bennett 1990a; Dubreuil et al. 1987), which might be involved in cell contraction and migration. Furthermore, αII-spectrin is reported to be involved in regulation of actin dynamics (Bialkowska 2005) and βII-spectrin is involved in TGFβ1 signaling, where it functions as a SMAD adaptor protein (Baek et al. 2011; Kitisin et al. 2007; Tang et al. 2003). Additionally, spectrins associate with, as well as regulate, Yes-associated protein 1 (YAP) (Fletcher et al. 2015; Wong et al. 2015). YAP is a mechanosensitive transcriptional co-factor of genes involved in proliferation and suppression of apoptotic genes (Calvo et al. 2013; Dupont et al. 2011; Janmey et al. 2013) and is regulated by both Hippo and TGFβ1 signaling (Liu et al. 2015; Piersma et al. 2015a, b). Whether spectrins play a role in the myofibroblast phenotypical switch remains unknown. Here, we study the role of αII-spectrin and βII-spectrin in stiffness-induced cell spreading and adhesion, YAP translocation and wound closure in human dermal fibroblasts. Furthermore, we examine the role of αII-spectrin and βII-spectrin in TGFβ1-induced myofibroblast differentiation.
Materials and methods
Reagents and antibodies
Reagents were as follows: human plasma fibronectin (20 μg/mL, F1056; Sigma-Aldrich, Munich, Germany), human recombinant TGFβ1 (10 ng/mL, 100-21C; Peprotech, London, UK), αII-spectrin siRNA (25 ng/cm2, EHU093741; Sigma-Aldrich), βII-spectrin siRNA (25 ng/cm2, EHU081451; Sigma-Aldrich), Renilla luciferase siRNA (25 ng/cm2, EHURLUC; Sigma-Aldrich), Alexa647-labeled streptavidin (8 μg/mL, S32357; Thermo Fisher Scientific, Landsmeer, The Netherlands), TRITC labeled-Phalloidin (100 nM, P1951; Sigma-Aldrich). Antibodies used: mouse anti-αII-spectrin (2 μg/mL, sc-376849; Santa Cruz, Dallas, USA), mouse anti-βII-spectrin (2 μg/mL, sc-376487; Santa Cruz), mouse anti-αSMA (0.28 μg/mL, M0851; DAKO; Glostrup, Denmark), mouse anti-collagen type I (1 μg/mL, ab90395; Abcam, Cambridge, UK), mouse anti-vinculin (9.3 μg/mL, V9131; Sigma-Aldrich).
Cell manipulations
Before the onset of experiments, normal adult human dermal fibroblasts (CC-2511, nHDF-Ad-Der; Lonza, Basel, Switzerland) were propagated in DMEM (12-604F; Lonza) supplemented with 2 mM l-glutamine, 50 U/L penicillin/streptomycin and 10% FCS. For protein knockdown experiments, cells were seeded at 15.000 cells/cm2 and transfected with siRNA using Lipofectamine RNAiMax reagent (Thermo Fischer Scientific) and incubated for 72 h in DMEM supplemented with 1.5 mM l-glutamine, 38 U/L penicillin/streptomycin and 7.5% FCS. siRNA targeting Renilla luciferase was used as negative control. After the transfection period, cells were cultured for an additional 96 h in DMEM containing 0.5% FCS supplemented with 2 mM l-glutamine and 50 U/L penicillin/streptomycin to ensure elimination of the spectrin proteins, as they are relatively long-lived proteins. Efficiency of knockdown was subsequently determined by means of qPCR and immunofluorescence. For cell adhesion, cell spreading and YAP translocation studies, cells were reseeded on fibronectin-functionalized polyacrylamide gels for 24 h. Cell spreading was determined by measuring cell surface area with Nuance FX software (Perkin Elmer, Groningen, The Netherlands). Cell adherence was determined by quantifying the number of cells in 25 FOVs. YAP translocation was measured by means of immunofluorescence.
For myofibroblast differentiation experiments and the wound healing assay, the trypsinized cells were reseeded on polystyrene culture wells (for mRNA measurements or wound healing) or slides (for immunostaining); cultured in DMEM containing 0.5% FCS, 2 mM l-glutamine, 50 U/L penicillin/streptomycin and 0.17 mmol/L ascorbic acid (A8960; Sigma-Aldrich); and supplemented with or without TGFβ1 (10 ng/mL) for 72 h. For the wound healing assay, IBIDI inserts were removed after 48 h, leaving another 24 h for the cells to repopulate the wound area.
Fibronectin-functionalized polyacrylamide hydrogels
To determine the role of spectrins in cell adhesion and spreading, cells were seeded on fibronectin-functionalized polyacrylamide hydrogels with an elastic modulus of either 2 or 50 kPa. Polyacrylamide hydrogels were prepared as described previously (Wouters et al. 2016). In brief, gels were prepared between a chemically modified glass plate and coverslip. The glass plate was cleaned by immersion in 99.9% ethanol for 15 min and treated with dichlorodimethylsilane to avoid polyacrylamide interactions. Glass coverslips were treated with 0.5% trimethoxypropylmethacrylate in 99.1% ethanol, which was activated using 0.3% glacial acetic acid to facilitate covalent adhesion of polyacrylamide hydrogels. Differences in stiffness (elastic modulus) were obtained by varying the ratio between acrylamide and bisacrylamide and the Young’s modulus was validated by means of Atomic Force Microscopy (AFM). Hydrogel polymerization was initiated with TEMED and APS. To functionalize the surface of the hydrogels, they were overlaid with 2 mg/ml l-DOPA (in 10 mM Tris) and incubated for 30 min. Next, l-DOPA was washed off and hydrogels were functionalized with 20 μg/mL plasma fibronectin for 2 h at 37 °C.
RNA isolation, cDNA synthesis and qRT-PCR
To obtain total RNA, the FavorPrep Tissue Total RNA Purification Mini Kit (FATRK; Favorgen Biotech Corp., Taiwan) was used in accordance with the manufacturer’s protocol. RNA concentration and purity were determined by UV spectrophotometry (NanoDrop Technologies, Wilmington, NC). To assess gene expression, the RNA was reverse transcribed using the First Strand cDNA synthesis kit (Thermo Fisher Scientific) using random hexamer primers in accordance with the manufacturer’s instructions. Gene expression quantification was performed using qRT-PCR analysis and SYBR Green Supermix (Roche, Basel, Switzerland). The thermal cycling conditions were 2 min at 95 °C (enzyme activation), followed by 15 s at 95 °C, 30 s at 60 °C, and 30 s at 72 °C (40 cycles). All qPCRs were performed with a ViiA™ 7 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Melting curve analysis was performed to verify the absence of primer dimers. Analysis of the data was performed using ViiA7™ Real-Time PCR System Software v1.2.4 (Applied Biosystems). Primer sequences are provided in Table 1.
Immunofluorescence
For spectrin immunofluorescence, cells cultured for 7 days were fixed in 4% PFA and incubated with 10% goat serum in PBS for 1 h. Primary antibodies were incubated in PBS + 2.2% BSA at room temperature (RT) for 2 h. For YAP immunofluorescence, cells were permeabilized with 0.5% Triton X-100 and subsequently incubated with PBS 10% goat serum RT for 1 h. Primary antibodies were incubated in PBS + 0.1% Triton X-100 and 2.2% BSA at 4 °C for 16 h. For α-smooth muscle actin and collagen immunofluorescence, methanol/acetone (1:1) fixed cells were incubated with 10% goat serum for 1 h and primary antibodies were incubated in PBS + 2.2% BSA at RT for 2 h. For all immunofluorescence, secondary antibodies were diluted in PBS + 2.2% BSA at RT for 1 h and subsequently incubated with Alexa647-labeled streptavidin in PBS containing 4′,6-diamidino-2-phenylindole (DAPI, 1:5000, 10236276001; Roche) for 30 min. Actin was visualized by incubation with TRITC labeled-Phalloidin in PBS for 30 min. Between incubations, cells were washed thrice with PBS containing 0.5% Tween-20. Slides were mounted in Citifluor (Agar Scientific, Stansted, UK) and used for immunofluorescence microscopy.
Statistics
All data are represented as means ± SD of at least three independent experiments and were analyzed by GraphPad Prism Version 7.01 for Windows (GraphPad Software, Inc., La Jolla, CA, USA) by either one-way or two-way ANOVA followed by Bonferroni post hoc analysis.
Results
αII- and βII-spectrin do not influence cell adhesion
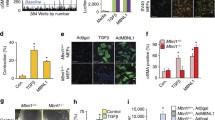
In order to elucidate the role of spectrins on fibroblast behavior, we performed siRNA-mediated knockdown. We found that both αII- and βII-spectrin have a long half-life; we only observed > 90% gene expression knockdown 168 h (7 days) after transfection (Fig. 1a, b) and also observed knockdown at the protein level (Fig. 1c–h). Interestingly, βII-spectrin siRNA knockdown also decreased the expression of αII-spectrin about twofold. Next, we investigated whether αII- and βII-spectrin have an effect on cell adhesion (Fig. 2a) by seeding cells on either soft (2 kPa) or stiff (50 kPa) fibronectin-coated substrates. Cell adhesion did not differ between 2 and 50 kPa in either the control cells or the αII-spectrin- and βII-spectrin-deficient cells.
αII-spectrin and βII-spectrin knockdown with esiRNA. (a, b) mRNA expression of αII-spectrin (SPTAN1) and βII-spectrin (SPTBN1) 7 days after esiRNA transfection. One-way ANOVA; **p < 0.01, ****p < 0.0001. (c–h) Representative immunofluorescent images of αII-spectrin and βII-spectrin 7 days after esiRNA transfection. Original magnification × 200
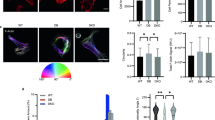
αII- and βII-spectrin do not mediate fibroblast spreading and adhesion. (a) Cell adhesion on 2 and 50 kPa polyacrylamide hydrogels. (b) Effect of hydrogel stiffness on cell spreading. Two-way ANOVA; ***p < 0.001, ****p < 0.0001. (c–h) F-actin (phalloidin) and nuclear (DAPI) staining to visualize cell size and cell adhesion. Original magnification × 400. DAPI, 4′,6-diamidino-2-phenylindole; kPa, Kilo Pascal; PAAM, polyacrylamide
Cell spreading on soft and stiff substrates is independent of αII- and βII-spectrin
The morphological and cytoskeletal changes of fibroblasts are well documented for cells cultured on fibronectin-coated surfaces with stiffness ranging from 2 to 50 kPa. When grown in sparse culture with no cell-cell contacts, fibroblasts show an abrupt change in spread area that occurs at a stiffness range above 3 kPa (Yeung et al. 2005). We indeed observed major differences in cell size (spreading) between 2 and 50 kPa gels: cells cultured on 2 kPa were markedly smaller than cells cultured on 50 kPa (Fig. 2b–h). This was the case both for control cells as for αII-spectrin- or βII-spectrin-deficient cells but we observed no significant differences in cell size between the spectrin-deficient cells and the control group. These data suggest that αII- and βII-spectrin do not affect the stiffness-dependent changes in cell size of dermal fibroblasts.
αII- and βII-spectrin do not regulate YAP localization
YAP is a mechanosensitive transcriptional co-activator that has been shown to govern the phenotypical switch to myofibroblasts and accumulates in the nucleus on increased stiffness of the ECM (Piersma et al. 2015a, b; Szeto et al. 2016). One of the mechanisms of YAP nuclear accumulation involves polymerization of actin monomers into stress fibers (Aragona et al. 2013; Das et al. 2016; Dupont et al. 2011). Recently, αII- and βII-spectrin were shown to regulate cytoplasmic retention of YAP in stretched epithelial cells, by interacting with and activating Hippo signaling at the plasma membrane (Fletcher et al. 2015). Because fibroblasts and myofibroblasts rely heavily on their contractile cytoskeleton and are known for their ability to spread over great distances, we investigated the effects of substrate stiffness and the presence of αII- and βII-spectrin on YAP localization. We observed major differences in YAP localization between 2 and 50 kPa hydrogels (Fig. 3): on 2 kPa, almost all cells displayed cytoplasmic retention of YAP (Fig. 3a–c), while on 50 kPa, the majority of cells showed both nuclear and cytoplasmic localization of YAP (Fig. 3d–f). However, we observed no differences in YAP localization between spectrin-deficient cells and the control cells, suggesting that spectrins do not regulate YAP localization in dermal fibroblasts.
YAP nuclear accumulation is independent from αII- and βII-spectrin. (a–f) Yes-associated protein 1 (YAP; green) localization in spectrin KD fibroblasts cultured on either 2 or 50 kPa polyacrylamide hydrogels. Nuclei are stained with DAPI. Original magnification × 400. DAPI, 4′,6-diamidino-2-phenylindole; kPa, Kilo Pascal
αII- and βII-spectrin do not regulate fibroblast migration and wound healing
Others showed βH-spectrin to be involved in epithelial cell migration in Sophophora (Urwyler et al. 2012). Therefore, we asked whether spectrins are necessary for fibroblasts wound closure in vitro. We mimicked wound closure by means of IBIDI inserts and found no differences in wound repopulation in fibroblasts stimulated with or without TGFβ1 (Fig. 4a–l). Moreover, knockdown of spectrins did not affect the population rate of the wound area (Fig. 4(m)).
αII- and βII-spectrin do not affect the myofibroblast phenotype
Myofibroblasts play an important role in both regular wound healing as well as dysregulated wound healing, the latter resulting in fibrosis. αSMA stress fiber formation is an important hallmark of the myofibroblast phenotype, which can be induced by TGFβ1. We indeed observed an increase in ACTA2 mRNA levels (Fig. 5a) and formation of αSMA stress fibers on TGFβ1 exposure (Fig. 5b–g). However, we observed no differences in the percentage of αSMA-positive cells between spectrin-deficient and control cells, suggesting that spectrins are not required for TGFβ1-induced formation of αSMA stress fibers. This was reflected in the mRNA levels of ACTA2 between spectrin-deficient and control cells, although knockdown of βII-spectrin resulted in slightly lower ACTA2 mRNA levels in TGFβ1-stimulated cells. Interestingly, knockdown of αII-spectrin had a significant effect on ACTA2 expression in non-stimulated cells: the mRNA level was markedly lower (Fig. 5a).
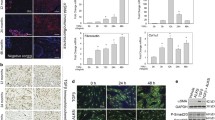
Spectrins do not regulate αSMA stress fiber formation. (a) mRNA expression of ACTA2 (αSMA) after 4 days of stimulation with TGFβ1 on αII-spectrin (SPTAN1) and βII-spectrin (SPTBN1) KD cells. Two-way ANOVA; **p < 0.01. (b–g) Representative immunofluorescent images of α-smooth muscle actin stress fiber formation in αII-spectrin (SPTAN1) and βII-spectrin (SPTBN1) KD cells. Nuclei are stained with DAPI. Original magnification × 200. αSMA, α smooth muscle actin; DAPI, 4′,6-diamidino-2-phenylindole; TGF, transforming growth factor
Collagen deposition is spectrin independent
To determine if another hallmark function of myofibroblasts, namely the increased synthesis of collagen type I, is regulated by spectrins, we determined mRNA levels and collagen deposition. We indeed observed large differences between cells stimulated with or without TGFβ1. The increase in COL1A1 mRNA levels (Fig. 6a) was accompanied by an increase in collagen deposition (Fig. 6(b–g). However, αII- and βII-spectrin knockdown did not affect mRNA levels of COL1A1 in TGFβ1-stimulated cells (Fig. 6a) or the deposition of collagen type I (Fig. 6b–g). Interestingly, knockdown of βII-spectrin had a major effect on COL1A1 expression in non-stimulated cells: the mRNA level was markedly lower (Fig. 6a).
Spectrins do not regulate collagen type I synthesis. (a) mRNA expression of COL1A1 after 4 days of stimulation with TGFβ1 on αII-spectrin (SPTAN1) and βII-spectrin (SPTBN1) KD cells. Two-way ANOVA; *p < 0.05, ****p < 0.001. (b–g) Representative immunofluorescent images of collagen type I deposition in αII-spectrin (SPTAN1) and βII-spectrin (SPTBN1) KD cells. Nuclei are stained with DAPI. Original magnification × 200. ANOVA, analysis of variance; DAPI, 4′,6-diamidino-2-phenylindole; TGF, transforming growth factor
αII- and βII-spectrin are not necessary for focal adhesion assembly
We determined the formation of focal adhesions by means of vinculin staining as a function of TGFβ1. As expected, a major increase in focal adhesions was observed under the influence of TGFβ1 (Fig. 7a–f). However, we did not observe any differences in focal adhesion formation between spectrin-deficient and control cells.
αII- and βII-spectrin do not control vinculin adhesions. (a–f) Representative immunofluorescent images of vinculin after 4 days of stimulation with TGFβ1 in αII-spectrin (SPTAN1) and βII-spectrin (SPTBN1) KD cells. Original magnification × 200. DAPI, 4′,6-diamidino-2-phenylindole; TGF, transforming growth factor
TGFβ1 attenuates SPTAN1 and SPTBN1 expression
Since we did not observe differences in myofibroblast parameters between control and cells KD for spectrins, we wondered what happens with endogenous SPTAN1 and SPTBN1 mRNA levels when cells are stimulated with TGFβ1. Interestingly, TGFβ1 stimulation had a direct negative effect on SPTAN1 and SPTBN1 gene expression, as incubation with TGFβ1 resulted in significantly lower mRNA levels of SPTAN1 and SPTBN1 (Fig. 8a, b). The effect of TGFβ1 on SPTBN1 was more pronounced than for SPTAN1. To evaluate any possible compensatory mechanisms of αII- and βII-spectrin knock down, we interrogated the expression of three alternative spectrins, SPTBN2, SPTBN4, and SPTBN5. Interestingly, we found that knock down of αII-spectrin and βII-spectrin reduced the expression of SPTBN2/SPTBN4 and SPTBN4, respectively (Supplemental Fig. 1). Additionally, TGFβ1 exposure further decreased expression of all three alternative spectrins, which is in line with the expression pattern of SPTAN1 and SPTBN1.
Discussion
Although much is known about the function of spectrins in erythrocytes, less detailed information is available regarding the function of spectrins in non-erythroid cells, including fibroblasts. Since spectrins regulate cell morphology and are potential mechanosensors, we investigated whether αII- and βII-spectrin are required for the phenotypic properties of adult human dermal (myo)fibroblasts.
We first determined the effect of αII- and βII-spectrin on cell adhesion and cell spreading on 2 and 50 kPa gels and noticed that αII- and βII-spectrin do not regulate the adhesion or spreading of adult dermal fibroblasts, nor did we find morphological differences. This is of interest, as knockdown of spectrins results in major changes in shell shape in a variety of cell types. Mouse embryonic fibroblasts devoid of SPTBN1 obtained at E14.5 showed an impaired cell spreading and had a more rounded and spiky appearance. In addition, a reduction in cell proliferation was observed (Stankewich et al. 2011). Unfortunately, SPTBN1 null mice are embryonic lethal, so the functions of βII-spectrin in adult fibroblasts are not known. The discrepancy between our data obtained with adult cells compared with the above mentioned embryonic cells suggests that there could be age-related differences regarding the role of spectrins in cell shape of a specific cell type. This is substantiated by the observation that no changes in cell shape or morphology were observed in embryonic epithelial cells of SPTBN1 knockdown mice (Stankewich et al. 2011), whereas major cell shape differences were observed in adult epithelial cells of humans (Kizhatil et al. 2007).
Next, we determined whether αII- and βII-spectrin have an effect on the translocation of YAP as a function of stiffness and cell spreading. We mimicked cell spreading by sparsely culturing the fibroblasts on 2 and 50 kPa gels. As expected, YAP was largely localized in the cytoplasm in cells cultured at 2 kPa and was abundantly localized in the nucleus in cells cultured at 50 kPa. Deficiency of αII- or βII-spectrin did not change the translocation pattern of YAP. It has been shown that αII-spectrin and βII-spectrin have a mechanosensory function in the Hippo pathway in epithelial cells (Fletcher et al. 2015). This pathway is activated in densely confluent epithelial cell cultures and inactivated when cell density is sparse, allowing cells to spread across the substrate. In these situations, the transcriptional activator YAP is mainly located in the cytoplasm or nucleus, respectively (Aragona et al. 2013; Zhao et al. 2007). Knockdown of αII-spectrin and βII-spectrin prevents retention of YAP in the cytoplasm in high density cultures (Fletcher et al. 2015). Since knockdown of αII- and βII-spectrin did not have an effect on the localization of YAP under our conditions (sparse cell density on a soft or stiff substrate), we postulate that under these conditions, the localization of YAP is mainly regulated by Hippo-independent mechanisms, including actin polymerization and Smad shuttling (Dupont et al. 2011; Zhao et al. 2007). Our data suggest that spectrins are, in contrast to their crucial role in the Hippo pathway to regulate YAP, not required to regulate fibroblast YAP in the mechanotransduction pathway that acts parallel to the Hippo pathway.
Fibroblasts and more specifically myofibroblasts, are at the heart of fibrosis (Hinz 2010). Fibroblasts undergo major morphological changes when are they activated into myofibroblasts by, e.g., TGFβ1 and changes occur in tissue stiffening during the fibrotic process (Chia et al. 2012; Hinz 2009; Huang et al. 2012). We therefore questioned whether spectrins play a role in the myofibroblast phenotypical switch. We found that knockdown of spectrins did not affect myofibroblast formation, nor did we observe changes in the organization of αSMA stress fibers. Additionally, we found that focal adhesion assembly was unaffected by spectrin deficiency. The finding that the function of myofibroblasts without αII- and βII-spectrin seems unchanged is illustrated by the observation that collagen type I mRNA expression and protein deposition are unaffected, together with unaffected wound closure. These results were unexpected, because it has been shown that knockdown of βII-spectrin leads to the disruption of TGFβ1 signaling as mediated by SMAD proteins (Kitisin et al. 2007; Lim et al. 2014; Munoz et al. 2014; Thenappan et al. 2011). TGFβ1 signaling via SMAD proteins is key for the induction of the myofibroblast phenotypical shift and collagen production (Piersma et al. 2015a). However, these studies primarily focused on the epithelial lineage and mainly in the context of embryonic mouse development (Kitisin et al. 2007; Lim et al. 2014; Munoz et al. 2014; Thenappan et al. 2011). Few studies have focused on the role of spectrins in fibrosis (Mishra et al. 2004; Wang et al. 2012); however, in a model of CCl4-induced hepatic fibrosis, βII-spectrin was found to be upregulated (Wang et al. 2012) in contrast to our findings. Fibroblasts derived from different anatomical sites arise from distinct developmental origins (Lynch et al. 2018), and even within an organ, multiple sub lineages of fibroblasts exist and may explain the differences in spectrin function. Along these lines, it is unknown whether hepatic stellate cells are derived from the embryonic endoderm or mesoderm lineage (Yin et al. 2013).
Since we were intrigued by our observations, we also investigated endogenous gene expression of SPTBN1 when fibroblasts were stimulated with TGFβ1 and noted a fourfold reduction in SPBTN1 mRNA levels. This suggests that in adult human dermal myofibroblasts, βII-spectrin does not interfere with SMAD-mediated gene expression, which is confirmed by our siRNA data, where SPTBN1 levels are reduced more than 20-fold in combination with TGFβ1 without seeing an effect on collagen production or αSMA formation. The latter suggests that in fibroblasts, downregulation of αII- and βII-spectrin is a pre-requisite for the myofibroblast phenotype switch. Additionally, we studied possible compensatory mechanisms after spectrin knock down but found that loss of αII- and βII-spectrin decreased expression of alternative spectrins. These data suggest that either the expression of various spectrins is tightly linked, or that the siRNA used in this study may have off-target effects. In either case, we demonstrated that loss of spectrins is not required for the acquisition of a dermal myofibroblast phenotype.
In conclusion, αII- and βII-spectrin do not regulate cell adhesion, cell size and YAP localization in human dermal fibroblasts and are not required for the dermal myofibroblast phenotypical switch.
References
Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, Dupont S, Piccolo S (2013) A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 154:1047–1059
Baek HJ, Pishvaian MJ, Tang Y, Kim TH, Yang S, El ZM, Mendelson J, Shetty K, Kallakury B, Berry DL, Shin KH, Mishra B, Reddy EP, Kim SS, Mishra L (2011) Transforming growth factor-β adaptor, β2-spectrin, modulates cyclin dependent kinase 4 to reduce development of hepatocellular cancer. Hepatology 53:1676–1684
Bennett V (1990a) Spectrin: a structural mediator between diverse plasma membrane proteins and the cytoplasm. Curr Opin Cell Biol 2:51–56
Bennett V (1990b) Spectrin-based membrane skeleton: a multipotential adaptor between plasma membrane and cytoplasm. Physiol Rev 70:1029–1065
Bennett V, Baines AJ (2001) Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev 81:1353–1392
Bialkowska K (2005) SH3 domain of spectrin participates in the activation of Rac in specialized calpain-induced integrin signaling complexes. J Cell Sci 118:381–395
Calvo F, Ege N, Grande-Garcia A, Hooper S, Jenkins RP, Chaudhry SI, Harrington K, Williamson P, Moeendarbary E, Charras G, Sahai E (2013) Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol 15:637–646
Chia HN, Vigen M, Kasko AM (2012) Effect of substrate stiffness on pulmonary fibroblast activation by TGF-β. Acta Biomater 8:2602–2611
Das A, Fischer RS, Pan D, Waterman CM (2016) YAP nuclear localization in the absence of cell-cell contact is mediated by a filamentous actin-dependent, myosin II and phospho-YAP-independent pathway during extracellular matrix mechanosensing. J Biol Chem 291:6096–6110
Deng H, Lee JK, Goldstein LSB, Branton D (1995) Drosophila development requires spectrin network formation. J Cell Biol 128:71–79
Devarajan P, Stabach PR, De Matteis MA, Morrow JS (1997) Na,K-ATPase transport from endoplasmic reticulum to Golgi requires the Golgi spectrin-ankyrin G119 skeleton in Madin Darby canine kidney cells. Proc Natl Acad Sci U S A 94:10711–10716
Dubreuil RR, Byers TJ, Branton D, Goldstein LSB, Kiehart DP (1987) Drosophilia spectrin. I. Characterization of the purified protein. J Cell Biol 105:2095–2102
Dubreuil RR, Byers TJ, Sillman AL, Bar-Zvi D, Goldstein LSB, Branton D (1989) The complete sequence of Drosophila alpha-spectrin: conservation of structural domains between alpha-spectrins and alpha-actinin. J Cell Biol 109:2197–2205
Dubreuil RR, Byers TJ, Stewart CT, Kiehart DP (1990) A β-spectrin isoform from Drosophila (βH) is similar in size to vertebrate dystrophin. J Cell Biol 111:1849–1858
Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S (2011) Role of YAP/TAZ in mechanotransduction. Nature 474:179–183
Fishkind DJ, Bonder EM, Begg DA (1987) Isolation and characterization of sea urchin egg spectrin: calcium modulation of the spectrin-actin interaction. Cell Motil Cytoskeleton 7:304–314
Fletcher GC, Elbediwy A, Khanal I, Ribeiro PS, Tapon N, Thompson BJ (2015) The spectrin cytoskeleton regulates the Hippo signalling pathway. EMBO J 34:940–954
Hinz B (2010) The myofibroblast: paradigm for a mechanically active cell. J Biomech 43:146–155
Hinz B (2009) Tissue stiffness, latent TGF-beta1 activation, and mechanical signal transduction: implications for the pathogenesis and treatment of fibrosis. Curr Rheumatol Rep 11:120–126
Huang X, Yang N, Fiore VF, Barker TH, Sun Y, Morris SW, Ding Q, Thannickal VJ, Zhou Y (2012) Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am J Respir Cell Mol Biol 47:340–348
Janmey PA, Wells RG, Assoian RK, McCulloch CA (2013) From tissue mechanics to transcription factors. Differentiation 86:112–120
Kamal A, Ying YS, Anderson RGW (1998) Annexin VI-mediated loss of spectrin during coated pit budding is coupled to delivery of LDL to lysosomes. J Cell Biol 142:937–947
Kitisin K, Ganesan N, Tang Y, Jogunoori W, Volpe EA, Kim SS, Katuri V, Kallakury B, Pishvaian M, Albanese C, Mendelson J, Zasloff M, Rashid A, Fishbein T, Evans SR, Sidawy A, Reddy EP, Mishra B, Johnson LB, Shetty K, Mishra L (2007) Disruption of transforming growth factor-beta signaling through beta-spectrin ELF leads to hepatocellular cancer through cyclin D1 activation. Oncogene 26:7103–7110
Kizhatil K, Davis JQ, Davis L, Hoffman J, Hogan BLM, Bennett V (2007) Ankyrin-G is a molecular partner of E-cadherin in epithelial cells and early embryos. J Biol Chem 282:26552–26561
Klingberg F, Hinz B, White ES (2013) The myofibroblast matrix: implications for tissue repair and fibrosis. J Pathol 229:298–309
Leto TL, Fortugno-Erikson D, Barton D, Yang-Feng TL, Francke U, Harris AS, Morrow JS, Marchesi VT, Benz EJ (1988) Comparison of nonerythroid alpha-spectrin genes reveals strict homology among diverse species. Mol Cell Biol 8:1–9
Liem RKH (2016) Cytoskeletal integrators: the spectrin superfamily. Cold Spring Harb Perspect Biol. https://doi.org/10.1101/cshperspect.a018259
Lim JA, Baek HJ, Jang MS, Choi EK, Lee YM, Lee SJ, Lim SC, Kim JY, Kim TH, Kim HS, Mishra L, Kim SS (2014) Loss of b2-spectrin prevents cardiomyocyte differentiation and heart development. Cardiovasc Res 101:39–47
Liu F, Lagares D, Choi KM, Stopfer L, Marinković A, Vrbanac V, Probst CK, Hiemer SE, Sisson TH, Horowitz JC, Rosas IO, Fredenburgh LE, Feghali-Bostwick C, Varelas X, Tager AM, Tschumperlin DJ (2015) Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am J Physiol - Lung Cell Mol Physiol 308:L344–L357
Lynch MD, Watt FM (2018) Fibroblast heterogeneity: implications for human disease. J Clin Invest 128(1):26–35
MacDonald RI, Cummings JA (2004) Stabilities of folding of clustered, two-repeat fragments of spectrin reveal a potential hinge in the human erythroid spectrin tetramer. Proc Natl Acad Sci U S A 101:1502–7150
Machnicka B, Grochowalska R, Bogusławska DM, Sikorski AF, Lecomte MC (2012) Spectrin-based skeleton as an actor in cell signaling. Cell Mol Life Sci 69:191–201
Meriläinen J, Palovuori R, Sormunen R, Wasenius VM, Lehto VP (1993) Binding of the alpha-fodrin SH3 domain to the leading lamellae of locomoting chicken fibroblasts. J Cell Sci 105:647–654
Metral S, Machnicka B, Bigot S, Colin Y, Dhermy D, Lecomte MC (2009) aII-spectrin is critical for cell adhesion and cell cycle. J Biol Chem 284:2409–2418
Mishra B, Tang Y, Katuri V, Fleury T, Said AH, Rashid A, Jogunoori W, Mishra L (2004) Loss of cooperative function of transforming growth factor-beta signaling proteins, smad3 with embryonic liver fodrin, a beta-spectrin, in primary biliary cirrhosis. Liver Int 24:637–645
Moon RT, McMahon AP (1990) Generation of diversity in non-erythroid spectrins. J Biol Chem 265:4427–4433
Munoz NM, Katz LH, Shina JH, Gi YJ, Menon VK, Gagea M, Rashid A, Chen J, Mishra L (2014) Generation of a mouse model of T-cell lymphoma based on chronic LPS challenge and TGF-beta signaling disruption. Genes Cancer 5:348–352
Musacchio A, Gibson T, Lehto VP, Saraste M (1992) SH3—an abundant protein domain in search of a function. FEBS Lett 307:55–61
Parker MW, Rossi D, Peterson M, Smith K, Sikstrom̈ K, White ES, Connett JE, Henke CA, Larsson O, Bitterman PB (2014) Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J Clin Invest 124:1622–1635
Piersma B, De Rond S, Werker PMN, Boo S, Hinz B, Van Beuge MM, Bank RA (2015b) YAP1 is a driver of myofibroblast differentiation in normal and diseased fibroblasts. Am J Pathol 185:3326–3337
Piersma B, Bank RA, Boersema M (2015a) Signaling in fibrosis: TGF-β, WNT, and YAP/TAZ converge. Front Med 2
Rockey DC, Bell PD, Hill JA (2015) Fibrosis—a common pathway to organ injury and failure. N Engl J Med 372:1138–1149
Sevinc A, Fung LWM (2011) Non-erythroid beta spectrin interacting proteins and their effects on spectrin tetramerization. Cell Mol Biol Lett 16:596–609
Sormunen R (1993) Alpha-spectrin in detergent-extracted whole-mount cytoskeletons of chicken embryo heart fibroblasts. Histochem J 25:678–686
Stankewich MC, Cianci CD, Stabach PR, Ji L, Nath A, Morrow JS (2011) Cell organization, growth, and neural and cardiac development require alpha II-spectrin. J Cell Sci 124:3956–3966
Szeto SG, Narimatsu M, Lu M, He X, Sidiqi AM, Tolosa MF, Chan L, De Freitas K, Bialik JF, Majumder S, Boo S, Hinz B, Dan Q, Advani A, John R, Wrana JL, Kapus A, Yuen DA (2016) YAP/TAZ are mechanoregulators of TGF-β-Smad signaling and renal fibrogenesis. J Am Soc Nephrol doi:https://doi.org/10.1681/ASN.2015050499
Tang Y, Katuri V, Dillner A, Mishra B, Deng CX, Mishra L (2003) Disruption of transforming growth factor-beta signaling in ELF beta-spectrin-deficient mice. Science 229:574–577
Thenappan A, Shukla V, Khalek FJA, Li Y, Shetty K, Liu P, Li L, Johnson RL, Johnson L, Mishra L (2011) Loss of transforming growth factor beta adaptor protein b-2 spectrin leads to delayed liver regeneration in mice. Hepatology 53:1641–1650
Urwyler O, Cortinas-Elizondo F, Suter B (2012) Drosophila sosie functions with H-spectrin and actin organizers in cell migration, epithelial morphogenesis and cortical stability. Biol Open 1:994–1005
Wang Z, Z1 W, Liu F, Tu W, Chang Y, Yao J, Wu W, Jiang X, He X, Lin J, Song Y (2012) Embryonic liver fodrin involved in hepatic stellate cell activation and formation of regenerative nodule in liver cirrhosis. J Cell Mol Med 16:118–128
Wasenius VM, Saraste M, Lehto VP (1989) From the spectrin gene to the assembly of the membrane skeleton. Int J Dev Biol 33:49–54
Wong KKL, Li W, An Y, Duan Y, Li Z, Kang Y, Yan Y (2015) β-Spectrin regulates the hippo signaling pathway and modulates the basal actin network. J Biol Chem 290:6397–6407
Wouters OY, Ploeger DT, van Putten SM, Bank RA (2016) 3,4-Dihydroxy-L-phenylalanine as a novel covalent linker of extracellular matrix proteins to polyacrylamide hydrogels with a tunable stiffness. Tissue Eng Part C Methods 22:91–101
Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, Zahir N, Ming W, Weaver V, Janmey PA (2005) Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton 60:24–34
Yin C, Evason KJ, Asahina K, Stainier DYR (2013) Hepatic stellate cells in liver development, regeneration, and cancer. J Clin Invest 123:1902–1910
Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, Zheng P, Ye K, Chinnaiyan A, Halder G, Lai Z, Guan KL (2007) Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev 21:2747–2761
Acknowledgments
We thank Theo Borghuis for excellent technical assistance.
Funding
This work was supported by the Netherlands Institute of Regenerative Medicine (NIRM, FES0908) and the Dutch Kidney Foundation. Part of the work has been performed at the University Medical Center Groningen Microscopy and Imaging Center (UMIC), which is sponsored by NWO-grant 40-00506-98-9021 (TissueFaxs).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Supplemental Fig 1
Knock down of αII- and βII-spectrin affects expression of SPTBN2 and SPTBN4. Relative mRNA expression of SPTBN2, SPTBN4 and SPTBN5 in response to siRNA-mediated knockdown of SPTAN2 (αII-spectrin) and SPTBN2 (βII-spectrin). Two-way ANOVA; * p < 0.05, ** p < 0.01, *** p < 0.001. ANOVA, analysis of variance; TGF, transforming growth factor. (GIF 56 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
About this article
Cite this article
Piersma, B., Wouters, O.Y. & Bank, R.A. αII-spectrin and βII-spectrin do not affect TGFβ1-induced myofibroblast differentiation. Cell Tissue Res 374, 165–175 (2018). https://doi.org/10.1007/s00441-018-2842-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-018-2842-x