Abstract
The majority of taxa with peltate leaves are perennial herbs native to swampy or aquatic habitats or to mesic shaded understorey habitats. These large peltate leaves are formed by a meristematic bridge at the lamina–petiole junction. However, there are also several strong-light exposed, small-leaved, xero- and scleromorphic Myrtaceae with leaf peltation which is formed without a meristem fusion/bridge. Here, abaxial laminar tissue at the insertion point of the petiole forms a basal extension, so that a weak peltation occurs. This shifts the petiole onto the adaxial laminar surface. The formation of micropeltation in Myrtaceae leads to erect leaves that are strongly appressed to the shoot axis and the entire foliate, vertical shoots appear as “green columns”, a result that is also the case in taxa with reflexed minute leaves. It seems that micropeltation achieves the same goal as leaf reflexion in small-leaved taxa—reduction of heat-load and transpiration during the hottest phases of the day by a lower light interception at midday compared to the morning and evening. Thus, physiologically micropeltation and reflexion of minute leaves seem to be the result of convergent evolution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In most cormophytes, leaves are the organs that are responsible for most of the photosynthesis. Many plant functional traits can be correlated with environmental conditions (Towers et al. 2024), so over evolutionary time particular plant morphological forms become associated with particular environments. Plants growing in mesic conditions usually have large leaves or at least have high specific leaf area (e.g., Wright et al. 2017; Campetella et al. 2020). A global survey of environmental constraints on leaf size determined that temperature and moisture predicted smaller leaves in arid and very cold environments. However, for plants growing in deep shade, such as under a dense forest canopy, lower energy inputs would allow for larger leaf sizes than predicted by modeling (Wright et al. 2017). Larger leaf surfaces in shaded habitats increase energy gain efficiency in the peltate Centella asiatica (L.) Urban (Apiaceae; Borges et al. 2024). Plants growing in dry and hot environments (xerophytes) usually have minute leaves as is also the case for plants growing on infertile soils (sclerophytes) (Beadle 1966; Hill 1998; De Micco and Aronne 2012). Thus, the most important evolutionary and environmental forces leading to leaf reduction are: 1.) water deficit (e.g., Thoday 1931; Blum and Arkin 1984; Blum 1996; Bosabalidis and Kofidis 2002; Körner 2003; Parsons 2010; Seidling et al. 2012); 2.) low temperatures (e.g., Parsons 2010; Wright et al. 2017); and 3.) infertile soils (e.g., Loveless 1961, 1962; Beadle 1966; Seddon 1974; Hill and Merrifield 1993; Hill 1998; Salleo and Nardini 2000; Düll and Kutzelnigg 2011; Dörken and Jagel 2014; Dörken and Parsons 2016, 2017; Dörken et al. 2017). The latter two can be in some cases combined with water deficit.
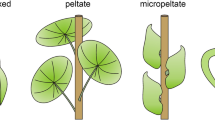
Given the leaf morphology/environment relationship above, leaf orientation plays an important role, as does the leaf shape (Wright et al. 2017; Dörken et al. 2023). A striking example of a functional leaf adaptation is peltation—the formation of leaves with a large, in most cases shield-like lamina and a petiole attached on the abaxial surface. Species with peltate leaves are, however, relatively uncommon. While some species are from rather xeric habitats, most are perennial herbs native to swampy or aquatic habitats or in the shaded understorey of mesic habitats (Ebel 1998; Wunnenberg et al. 2021), but also in some carnivorous plants (e.g., Drosera, Troll 1932). Ebel (1998) showed that the distribution of taxa with peltate leaves is influenced by the degree of oceanicity, so that the majority of peltate perennials are native in habitats associated with the conditions typical for oceanicity degrees between 1 and 3. There is a dramatic decline of taxa with peltate leaves in subtropical, meridional and temperate steppes, deserts, and semi-deserts, and leaf peltation is absent in the arctic zone (Ebel 1998).
Peltate leaves of species growing in wet or aquatic conditions can reach giant dimensions, e.g., the floating leaves of the Giant Waterlilies, Victoria spp. (Nymphaeaceae) with a long petiole and a lamina up 2 m in diameter (Kaul 1976; Verhage 2022). These giant leaves seem to be the result of selection pressure to produce large surface areas at an economical material cost, a feature which might help to outcompete other plants in fast-drying ephemeral pools (Box et al. 2022). Even within a taxon, the leaf size and structure vary markedly depending on the microclimate. Borges et al. (2024) showed for Centella asiatica that individuals from shaded environments develop longer petioles, larger leaf surfaces, and higher leaf fresh and dry masses in comparison to individuals from light exposed habitats.
All these peltate leaves undergo more or less the same ontogeny, which is characterized by the presence of a marginal meristem and the formation of a meristematic fusion at the junction of lamina and petiole (Franck 1976; Gleissberg et al. 2005). This meristematic bridge is formed by fused adaxial leaf tissue of the leaf margin (Wei-Pei and Si-Mei 1986; Gleissberg et al. 2005).
Micropeltation is still understudied and mentioned only in a few mostly taxonomic reports on sclerophyllous Myrtaceae (Carrick and Chorney 1979; Craven 1980, 1987; Byrnes 1984; Brophy et al. 2013), but see also Dörken and Parsons (2018); Dörken et al. (2023). In this present study, we examine the ontogeny, morphology, anatomy, and also the distribution of species with micropeltate leaves with a special emphasis on the Myrtaceae.
Materials and methods
Origin of research material
Material of Melaleuca micromera Schauer was collected in the Botanic Garden Konstanz (Germany) where the plant is cultivated in a temperate house. Melaleuca minutifolia F. Muell. was collected in the Darwin Botanic Gardens (Australia), Melaleuca thyoides Turcz. from the field west of Lake Grace, Western Australia, Regelia inops (Schauer) Schauer from the field in Perth (Western Australia), Calytrix arborescens (F. Muell.) Benth. in the Litchfield National Park near Florence Falls, Beaufortia micrantha Schauer from the field in Fitzgerald River National Park, Western Australia. All species are in the Myrtaceae.
Methods
Fixation of plant material
Freshly collected material was photographed and then fixed in FAA (100 ml FAA = 90 ml ethanol 70% + 5 ml acetic acid 96% + 5 ml formaldehyde solution 37%) before being stored in 70% ethanol.
Microtome technique
The anatomy was studied from serial sections using the classical paraffin technique and subsequent astrablue/safranin staining (Gerlach 1984, detailed methods in Dörken et al. 2023).
Scanning electron microscopy (SEM) analysis
For SEM-analysis, the FAA-material was dehydrated in formaldehyde dimethyl acetal (FDA) for 24 h (Gerstberger and Leins 1978) and later critical point dried. Specimens were then mounted on stubs and coated with 6 nm of platinum using a Sputter Coater SCD 50 Bal-tec (Balzers). The specimens were examined with an Auriga Zeiss TM.
Photodocumentation
Macrophotography was performed with a digital camera (Canon PowerShot IS2) and microphotography with a digital microscope (Keyence VHX 500F) equipped with a high-precision VH mounting stand with X–Y stage and bright-field illumination (Keyence VH-S5).
Special terms
Macropeltation: large leaves with a well-developed or long petiole inserted on the abaxial leaf surface in the center or toward the leaf margin. It is a result of a meristematic fusion/bridge at the junction of the lamina and the petiole.
Micropeltation: tiny leaves with a short or almost entirely reduced petiole inserted on the adaxial leaf surface and with some mesophyll-tissue extensions behind the insertion point, not just some slightly thickened epidermis. These extensions are formed in the absence of a meristematic fusion/bridge. Micropeltate leaves are usually strongly appressed to the stem. In this study, tiny leaves with slight laminar extensions at the lamina–petiole junction caused by thickened epidermal cells are not accepted as micropeltate.
Results
Some Myrtaceae have micropeltate leaves with a strongly reduced leaf size and a petiole inserted in lower parts on the adaxial surface (Figs. 1, 2). We are certain that at least eight species or subspecies of Melaleuca (Table 1) have such leaves, along with a few species of the myrtaceous genera Regelia (Fig. 2a–e), Calytrix (Fig. 2f–h), and Beaufortia (Fig. 2i–l) (see Table 1 later in the text). They are formed in the absence of a meristematic fusion/bridge. The primordia and the earliest juvenile leaves are bifacial and non-peltate with a petiole inserted at the marginal base of the lamina (Fig. 1e, f). The adaxial surface of primordia and juvenile leaves is turned toward the shoot apex (Fig. 1c, d). The abaxial surface of the leaves is light exposed throughout its entire development (Figs. 1a, b, g, j, 2a). At maturity, the adaxial surface is located toward the shoot axis, but often the tip is not strongly appressed to it. Thus, the distal parts of the adaxial surface also have some light exposure, which leads to a shift from primary bifacial to isobilateral at maturity, with palisade parenchyma located toward all light exposed surfaces. Even by the earliest ontogenetic stages, the petiole ceases development (Fig. 2c, d) and due to the extremely short petiole, the lower parts of mature leaves are strongly appressed to the shoot axis (Figs. 1a, b, c, g, i, j, l, 2a, d, e, f, I, j). As the leaf expands the abaxial laminar tissues at the petiole start forming a basal extension, which extends basally as the leaf expands (Figs. 1c, d, 2c, h, l), so that finally a peltate morphology is formed at the most basal parts of the leaf (Figs. 1 f, h, k, m, 2b, g, k). A full ontogenetic study of such peltation in minute leaves (SEM and microtome sections) is presented in Dörken and Parsons (2018).
Micropeltation in Melaleuca (Myrtaceae); A, G, J fresh material; B, E, F, H, K, L, M SEM images; C, D, I light microscope sections; A–I M. micromera; A–B Shoot axis with numerous appressed, erect peltate leaves; C Longitudinal section showing the shoot apex and non-peltate juvenile leaves in distal parts and mature peltate leaves in lower parts of the shoot; D Longitudinal section of the shoot apex; primordia and juvenile leaves non-peltate and bifacial; E Adaxial view of a young, non-peltate, bifacial leaf; F Adaxial view of a young leaf in earliest stages of the peltation process already leading to the shift of the petiole on the adaxial surface; G Shoot axis with numerous appressed, erect, mature peltate leaves; H Mature peltate leaf in adaxial view; I Longitudinal section of a mature, peltate leaf; J–K M. thyoides; J: Shoot axis with numerous appressed, erect mature peltate leaves; K Mature peltate leaf showing the ledge of tissue at the base of the leaf below the petiole point of attachment; L–M M. minutifolia; L Shoot axis with numerous appressed, erect, mature peltate leaves, note the basal part of the leaf is slightly reflexed from the stem; M Mature peltate leaf in adaxial view showing the ledge of tissue at the base of the leaf below the petiole point of attachment
Micropeltation in mature leaves of Regelia, Calytrix, and Beaufortia (Myrtaceae); A fresh material; B, C, F–L SEM images; D, E light microscope sections; A–E Regelia inops; A Shoot axis with numerous appressed, erect peltate leaves; B-C Peltate leaf in adaxial view, with a petiole inserted in basal part on the adaxial surface; B Entire leaf (SEM image); C Basal part showing the insertion point of the petiole; D-E Longitudinal section of a shoot axis with a peltate leaf; D Entire leaf; E Basal part showing the short petiole inserted on the adaxial surface and basal extension of the lamina; F–H Calytrix arborescens; F Shoot axis with numerous appressed, erect peltate leaves; G Entire leaf showing petiole location; H Peltate leaf in adaxial view, with a petiole inserted in lower third on the adaxial surface; I-L Beaufortia micrantha; I Shoot axis with numerous appressed, erect peltate leaves; J Detail of the micropeltate foliage; K–L Peltate leaf in adaxial view, with a petiole inserted in basal parts on the adaxial surface; K Entire leaf; lamina with numerous trichomes; L Basal part showing the insertion point of the petiole and extension of the lower leaf lamina
Discussion
Peltation is a peculiar foliar feature. It is not restricted to a distinct group and occurs in numerous unrelated taxa—357 species from 99 genera, 40 families, and 25 orders (Wunnenberg et al. 2021). In some species a facultative leaf peltation can occur, when, in addition to the non-peltate majority, occasionally peltate leaves are also formed. This can occur between individuals of the same species or even within an individual, as is found in some woody species, e.g., Corylus (Betulaceae) or Tilia (Malvaceae) or some herbaceous ones such as Geranium and Pelargonium (Geraniaceae) (Natho et al.1990). In addition, they also occur in some large trees such as Macaranga (Troll 1933; Ebel 1998; Wunnenberg et al. 2021) exposed to drier and sun exposed canopy conditions. Almost unknown is the fact that peltation also occurs in some small-leaved (Figs. 1, 2), strong-light exposed woody species, growing on dry or infertile soils. Leaf reduction in these taxa represents either a xero- or scleromorphic adaptation or a combination of both (Dörken and Parsons 2018; Dörken et al. 2023).
The first description of micropeltation is that of six Melaleuca species comprising ´Series VII. Peltatae´ in the first flora of Australia (Bentham 1866) along with a single species of Regelia. Subsequently, it is not mentioned anywhere until in the four Australian taxonomic papers from 1979 to 1984 listed at the end of our Introduction above. There has been nothing taxonomic, since the 22 known peltate Melaleuca species were treated among the 290 known Melaleuca species in the monograph of Brophy et al. (2013).
As is the case for the genus Melaleuca as a whole, Western Australia is by far the most species-rich state for species listed with micropeltate leaves (16 species) mostly in the south-west (11 species) (listed in Brophy et al. 2013). After careful examination of specimens of the Western Australian species at PERTH herbarium, our determination is that seven species are truly micropeltate (Table 1). The precursor of a micropeltate leaf is a sessile leaf and some species with sessile leaves do not have the basal extension of the lamina due to extended mesophyll so cannot be classified as truly micropeltate. Plant form of the 22 species has been recorded as shrubs-only (11 species) and the rest as being either shrubs or trees, including four species reaching as high as 10–15 m (Table 1). For leaf length, using the measure for short as < 30 mm long and very short as < 10 mm long (Brophy et al. 2013), all 22 species have short leaves. Of these, 15 are very short. (M. micromera, < 1.5 mm long, Fig. 1a–i). Melaleuca minutifolia is clearly one of the species with peltate leaves (Fig. 1l–m) as was specified by Brophy et al. in 2013. However, we found the peltation to be only weakly developed in our 2023 material and only found fully expressed on older leaves. Our earlier paper on M. minutifolia (Dörken and Parsons 2018) provides a full description with figures of the species without any mention of peltation at all. We believe that the material used in that paper was still too immature to have developed any leaf peltation. However, the reinvestigation of mature leaves collected from old trees (Fig. 1l–m) clearly shows a weak basal peltation.
The structure and the formation of the investigated micropeltate leaves of Melaleuca, Regelia, Beaufortia, and Calytrix were similar throughout. Phylogenetically, the first three genera are closely related (Edwards et al. 2010). However, the species that we believe are reliably micropeltate are distributed across three recognized clades of the Melaleuceae with the majority (of the species that were included in the Edwards et al. 2010 analysis) as members of clade C. Regelia and Beaufortia are members of clade A along with Melaleuca punicea (formerly Petraeomyrtus punicea). The only other species not part of that tribe is Calytrix arborescens in the more derived Chamelaucieae. (Thornhill et al. 2015). Examination of many Calytrix specimens in PERTH herbarium indicates that this seems to be the only species in the genus in the Northern Territory and Western Australia to have micropeltation.
The micropeltate leaves differ only marginally in size and shape and in the dimension of the basal laminar extension leading to the peltate shape. Thus, foliate vertical shoots represent more or less green “columns”, with abaxial light exposed leaf surfaces (Figs. 1a, g, j, 2a). The photosynthetic tissues are arranged, so that light (and heat) interception at midday is lower than in the morning and evening. This probably leads to a lower heat-load during the hottest phases of the day (Falster and Westoby 2003; Wright et al. 2017; Dörken et al. 2023). Consequently, this ameliorates heat-induced water loss, which is an important environmental stress in xeric habitats (Dörken et al. 2023). Interestingly, this effect is also achieved by the formation of strongly reflexed small-sized leaves, showing a markedly recurved petiole and/or lamina, so that the abaxial leaf surface is strongly appressed to the shoot axis and the adaxial surface is light exposed. A detailed study dealing with the comparison between micropeltation and reflexion of minute leaves is available in Dörken et al. (2023). The species with micropeltate leaves are predominantly distributed in south western Australia and across northern Australia. All species occur in areas that have the lowest modeled rainfall of the driest quarter below 75 mm and for northern species much lower at below 10 mm (Table 2). For northern species, this is in the winter when the highest modeled temperatures are still above 20 degrees for the coldest quarter. In contrast, for the southern species the driest quarter is summer when the lowest modeled temperatures where the species occur are over 18 degrees. Therefore, both northern and southern species endure moderately high temperatures when water deficit is most severe. Nevertheless these species occur with many other species with very small leaves that are not peltate, so there seems no obvious environmental explanation for the development of leaf peltation. From a physiological point of view erect micropeltate leaves are functionally similar to minute, reflexed leaves, despite the different ontogenetic origin. Thus, they are a good example of physiologically convergent evolution in sometimes distantly related taxa. However, within a clade, they seem likely to be phylogenetically related (Dörken et al. 2023).
Forming peltate organs without the fusion of marginal leaf tissues or a meristematic bridge at the lamina–petiole junction also occurs in seed cones of numerous cupressoid Cupressaceae. As in our investigated Melaleuca species, it is caused by an excessive ad- and abaxial swelling of the cone scales in distal parts. This closes the seed cones, and protects the fertilized ovules (Jagel and Dörken 2015).
However, not all structures that seem to be (micro-)peltate at first glance such as radial coniferous microsporangiophores are necessarily true peltate leaves. Troll (1932, pp. 291–292) compared the peltate shape of Equisetum sporophylls and the male reproductive structures of Taxus and regarded both as peltate leaves in the sense of sporophylls. However, the radial (= perisporangiate) microsporangiophores of Taxus and other Taxaceae do not correspond to a single peltate leaf, but to a radial synangium which is formed by several fused dorsiventral (= hyposporangiate) microsporangiophores (Mundry and Mundry 2001; Dörken et al. 2011; Schulz et al. 2014; Dörken and Nimsch 2016, 2023; Dörken 2023). This brief discussion illustrates well that peltate structures can be formed in different ways and that not all peltate structures have to be actually a leaf.
Concluding discussion
In developmental terms, the expansion of the epidermis and mesophyll on the abaxial leaf surface of micropeltate leaves is similar to the initial development of the Tropaeolum leaf (Gleissberg et al. 2005) but without the “querzone”. Thus, the abaxial surface expands more than the adaxial, but this is limited, perhaps due to the very small leaf size, and the leaf does not develop the circularity as seen in Tropaeolum. All of our investigated species with micropeltate leaves occur in environments with a severe dry season when very low precipitation coincides with high temperatures. Micropeltation leads to erect leaves that are strongly appressed to the shoot axis and most are to some extent stem clasping so on the adaxial side of the leaf a more humid microenvironment would be retained compared to the exposed abaxial surface. Thus, the foliate vertical shoots represent “green columns”. On such shoots, light interception at midday is lower than in the morning and evening, which reduces heat-load and transpiration as is also the case in small-leaved species with strongly reflexed leaves (Falster and Westoby 2003). However, much work is needed before we know the extent to which micropeltation occurs in other Myrtaceous tribes or in small-leaved taxa of other families and if micropeltation and reflexion in small-sized leaves lead to a similar physiology.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
References
Beadle NCW (1966) Soil phosphate and its role in molding segments of the Australian flora and vegetation with special reference to xeromorphy and sclerophylly. Ecology 47:992–1007. https://doi.org/10.2307/1935647
Bentham G (1866) Flora Australiensis: a description of the plants of the Australian territory, vol 3. Reeve & Co, London
Blum A (1996) Crop responses to drought and the interpretation of adaptation. Plant Growth Regul 20:135–148. https://doi.org/10.1007/978-94-017-1299-6_8
Blum A, Arkin GF (1984) Sorghum root growth and water use as affected by water supply and growth duration. Field Crop Res 9:131–142. https://doi.org/10.1016/0378-4290(84)90019-4
Borges C, Lusa MG, Baldessar A, Garcia Rodrigue GA, Rodrigues AC (2024) Morpho-anatomical adaptations of Centella asiatica (Apiaceae) in different coastal sand microenvironments of Restinga and in an urbanized area. Flora (Journal Pre-proof, published 26072024). https://doi.org/10.1016/j.flora.2024.152572
Bosabalidis AM, Kofidis G (2002) Comparative effects of drought stress on leaf anatomy of two olive cultivars. Plant Sci 163:375–379. https://doi.org/10.1016/S0168-9452(02)00135-8
Box F, Erlich A, Guan JH, Thorogood C (2022) Gigantic floating leaves occupy a large surface area at an economical material cost. Sci Adv. https://doi.org/10.1126/sciadv.abg3790
Brophy JJ, Craven LA, Doran JC (2013) Melaleucas, their botany, essential oils and uses. ACIAR, Canberra
Burbidge AA (2016) A taxonomic revision of Beaufortia (Myrtaceae, Melaleuceae). Nuytsia 27:165–202
Byrnes NB (1984) A revision of Melaleuca L. (Myrtaceae) in northern and eastern Australia 1. Austrobaileya 2:65–76
Campetella G, Chelli S, Simonetti E, Damiani C, Bartha S, Wellstein C, Giorgini D, Puletti N, Mucina L, Cervellini M, Canullo R (2020) Plant functional traits are correlated with species persistence in the herb layer of old-growth beech forests. Sci Rep 10:19253. https://doi.org/10.1038/s41598-020-76289-7
Carrick J, Chorney K (1979) A review of Melaleuca L. (Myrtaceae) in South Australia. J Adel Bot Gard 1:281–319
Craven LA (1980) A review of the genus Calytrix Labill. (Myrtaceae) in Northern Australia. Brunonia 3(2):217–246
Craven LA (1987) A taxonomic revision of Calytrix Labill. (Myrtaceae). Brunonia 10(1):1–138
De Micco V, Aronne G (2012) Morpho-anatomical traits for plant adaptation to drought. In: Aroca R (ed) Plant responses to drought stress. Springer-Verlag, Berlin, pp 37–61
Dörken VM (2023) The structure and vasculature in pollen cones of Taxus (Taxaceae, Gymnospermae) and its evolutionary significance. Feddes Rep 135(2):112–123
Dörken VM, Jagel A (2014) Pinus sylvestris – Wald-Kiefer (Pinaceae), Baum des Jahres 2007. Jahrb Bochumer Bot Ver 5:246–254
Dörken VM, Nimsch H (2016) Some new aspects about the evolution of pollen cones and perisporangiate microsporangiophores in Taxaceae. Bull Cupressus Conservation Proj 5:3–21
Dörken VM, Nimsch H (2023) Anomalous pollen cones in Pseudotaxus chienii (Taxaceae): a further support for the pseudanthial origin of the Taxus pollen cone. Feddes Rep 134(3):149–156. https://doi.org/10.1002/fedr.202300002
Dörken VM, Parsons R (2016) Morpho-anatomical studies on the change in the foliage of two imbricate-leaved New Zealand podocarps: Dacrycarpus dacrydioides and Dacrydium cupressinum. Plant Syst Evol 302:41–54. https://doi.org/10.1007/s00606-015-1239-5
Dörken VM, Parsons RF (2017) Morpho-anatomical studies on the leaf reduction in Casuarina (Casuarinaceae): the ecology of xeromorphy. Trees 31:1165–1177. https://doi.org/10.1007/s00468-017-1535-5
Dörken VM, Parsons RF (2018) The foliar change in two species of Melaleuca (Myrtaceae): a morpho-anatomic and ontogenetic approach. Trees 32:1013–1028. https://doi.org/10.1007/s00468-018-1692-1
Dörken VM, Zhang ZX, Mundry IB, Stützel Th (2011) Morphology and anatomy of male reproductive structures in Pseudotaxus chienii (W.C. Cheng) W.C. Cheng (Taxaceae). Flora 206:444–450. https://doi.org/10.1016/j.flora.2010.08.006
Dörken VM, Parsons RF, Marshall AT (2017) Studies on the foliage of Myricaria germanica (Tamaricaceae) and their evolutionary and ecological implication. Trees 31:997–1013. https://doi.org/10.1007/s00468-017-1523-9
Dörken VM, Ladd PG, Parsons RF (2023) Convergent morphology and anatomy in the microphyllous leaves of selected heathland Myrtaceae and Asteraceae. Trees 37:1225–1247. https://doi.org/10.1007/s00468-023-02422-4
Düll R, Kutzelnigg H (2011) Taschenlexikon der Pflanzen Deutschlands und angrenzender Länder, 7th edn. Quelle and Meyer, Wiebelsheim
Ebel F (1998) Die Schildblättrigkeit krautiger Angiospermensippen in ihrer Beziehung zu Standort und Verbreitung. Flora 193:203–224. https://doi.org/10.1016/S0367-2530(17)30841-1
Edwards RD, Craven LA, Crisp MD, Cook LG (2010) Melaleuca revisited: cpDNA and morphological data confirm that Melaleuca L. (Myrtaceae) is not monophyletic. Taxon 59:744–754. https://doi.org/10.1002/tax.593007
Falster DS, Westoby M (2003) Leaf size and angle vary widely across species: what consequences for light interception? New Phytol 158:509–525. https://doi.org/10.1046/j.1469-8137.2003.00765.x
Franck DH (1976) The morphological interpretation of epiascidiate leaves - An historical perspective. Bot Rev 42:345–388
Gerlach D (1984) Botanische Mikrotomtechnik, eine Einführung, 2nd edn. Thieme, Stuttgart
Gerstberger P, Leins P (1978) Rasterelektronenmikroskopische Untersuchungen an Blütenknospen von Physalis philadelphia (Solanaceae). Ber Dtsch Bot Ges 91:381–387. https://doi.org/10.1111/j.1438-8677.1978.tb03660.x
Gleissberg S, Groot EP, Schmalz M, Eichert M, Kölsch A, Hutter S (2005) Developmental events leading to peltate leaf structure in Tropaeolum majus (Tropaeolaceae) are associated with expression domain changes of a YABBY gene. Dev Genes Evol 215:313–319. https://doi.org/10.1007/s00427-005-0479-8
Hill RS (1998) Fossil evidence for the onset of xeromorphy and scleromorphy in Australian Proteaceae. Aust Syst Bot 11:391–400. https://doi.org/10.1071/SB97016
Hill RS, Merrifield HE (1993) An Early Tertiary macroflora from West Dale, southwestern Australia. Alcheringa 17:285–326. https://doi.org/10.1080/03115519308619596
Jagel A, Dörken VM (2015) Morphology and morphogenesis of the seed cones of the Cupressaceae - part II: Cupressoideae. Bull Cupressus Conservation Proj 4:51–78
Kaul RB (1976) Anatomical observations on floating leaves. Aquat Bot 2:215–234
Körner C (2003) Alpine plant life, 2nd edn. Springer, Berlin
Loveless AR (1961) A nutritional interpretation of sclerophylly based on differences in the chemical composition of sclerophyllous and mesophytic leaves. Ann Bot (Oxford) 25:168–184. https://doi.org/10.1093/oxfordjournals.aob.a083740
Loveless AR (1962) Further evidence to support a nutritional interpretation of sclerophylly. Ann Bot (Oxford) 26:551–561. https://doi.org/10.1093/oxfordjournals.aob.a083814
Mundry IB, Mundry M (2001) Male cones in Taxaceae s.l.—an example of Wettstein´s pseudanthium concept. Plant Biol 3:405–416. https://doi.org/10.1055/s-2001-16466
Natho G, Müller C, Schmidt H (1990) Morphologie und Systematik der Pflanzen, Teil 2 (L-Z). Fischer, Stuttgart., p 675
Parsons RF (2010) Whipcord plants: a comparison of south-eastern Australia with New Zealand. Cunninghamia 11:277–281
Salleo S, Nardini A (2000) Sclerophylly: evolutionary advantage or mere epiphenomenon? Plant Biosyst 134:247–259. https://doi.org/10.1080/11263500012331350435
Schulz C, Klaus KV, Knopf P, Mundry M, Dörken VM, Stützel Th (2014) Male cone evolution in conifers: not all that simple. Am J Plant Sci 5(18):2842–2857. https://doi.org/10.4236/ajps.2014.518300
Seddon G (1974) Xerophytes, xeromorphs and sclerophylls: the history of some concepts in ecology. Biol J Linn Soc 6:65–87. https://doi.org/10.1111/j.1095-8312.1974.tb00714.x
Seidling W, Ziche D, Beck W (2012) Climate responses and interrelations of stem increment and crown transparency in Norway Spruce, Scots Pine and Common Beech. For Ecol Manage 284:196–204. https://doi.org/10.1016/j.foreco.2012.07.015
Thoday D (1931) The significance of reduction in the size of leaves. J Ecol 19:297–303
Thornhill AH, Ho SYW, Külheim C, Crisp MD (2015) Interpreting the modern distribution of Myrtaceae using a dated molecular phylogeny. Mol Phylogenet Evol 93:29–43. https://doi.org/10.1016/j.ympev.2015.07.007
Towers IR, Vesk PA, Wenk EH, Gallagher RV, Windecker SM, Wright IJ, Falster DS (2024) Revisiting the role of mean annual precipitation in shaping functional trait distributions at a continental scale. New Phytol 241:1900–1909
Troll W (1932) Morphologie der schildförmigen Blätter. Planta 17:231–314
Troll W (1933) Verzeichnis der mit Schild- bzw. Schlauchblättern Versehenen Angiospermen Bot Jb 65:559–596
Verhage L (2022) Once, twice, three times a genome–how the three sub-genomes of a water lily control leaf development. Plant J 110(3):625–626. https://doi.org/10.1111/tpj.15777
Wei-Pei C, Si-Mei Z (1986) Peltate leaf development in Brasenia schreberi Gruel. J Integr Plant Biol 28(4):341–348
Wright IJ, Dong N, Maire V, Prentice IC, Westoby M, Diaz S, Gallagher RV, Jacobs BF, Kooyman R, Law EA, Leishman MR, Niinemets U, Reich PB, Sack L, Villar R, Wang H, Wilf P (2017) Global climatic drivers of leaf size. Science 357:917–921. https://doi.org/10.1126/science.aal4760
Wunnenberg J, Rjosk A, Neinhuis C, Lautenschläger T (2021) Strengthening structures in the petiole–lamina junction of peltate leaves. Biomimetics 6(2):25. https://doi.org/10.3390/biomimetics6020025
Acknowledgements
The authors are grateful to the Botanic Garden Konstanz (Germany), the Darwin Botanic Gardens (Australia) and the Australian National Herbarium, Canberra Australia for generously providing research material. The herbarium of Western Australia provided access to examine some specimens. Furthermore, the authors thank Dr. Michael Laumann and Dr. Paavo Bergmann (Electron Microscopy Center, Department of Biology, University of Konstanz, Germany) for technical support (paraffin technique and SEM) and Prof. Michael D. Crisp (Australian National University Canberra, Australia), Dr. Phillip Kodela (Royal Botanic Gardens, Sydney, Australia), and Brendan Lepschi (Australian National Herbarium, Canberra) for advice and access to data on the family Myrtaceae.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Writing— preparation of the original draft: VMD. Revision and editing: VMD, RP, and PL. Conceptualization and planning the project: VMD, RP, and PL. Data analysis: VMD, RP, and PL. Investigation and enquiry: VMD. Methods—sectioning and photography: VMD. Material— collecting: VMD and PL.
Corresponding author
Ethics declarations
Conflict of interest The authors declare no conflict of interest.
Additional information
Communicated by Beck.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dörken, V.M., Ladd, P.G. & Parsons, R.F. Micropeltation in Myrtaceae: a neglected subject. Trees (2024). https://doi.org/10.1007/s00468-024-02565-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00468-024-02565-y