Summary
Introduction
The role of the family history in the development and prognosis of gastroesophageal cancer is a controversially discussed topic as appropriate data from western cohorts are lacking. This study aims to explore its associations with disease and outcome parameters in a large European cohort.
Methods
We retrospectively analyzed self-reported family history in patients with gastroesophageal cancer treated between 1 January 1990 and 31 December 2021 at the Medical University of Vienna. Association analyses with patient characteristics, tumor characteristics, symptoms and overall survival (OS) were performed.
Results
In our cohort of 1762 gastroesophageal cancer patients, 592 (34%) reported a positive family history of cancer (159, 9%, gastroesophageal cancer). No associations were found with histopathological parameters or initial symptoms; however, a positive family history correlated with female gender (cancer in general: p = 0.011; gastroesophageal cancer: p = 0.015). Family history of cancer in general was associated with earlier cancer stages (p = 0.04), higher BMI (p = 0.005), and alcohol consumption (p = 0.010), while a positive history for gastroesophageal cancer was associated with higher age at diagnosis (p = 0.002) and stomach cancer (p = 0.002). There was no statistically significant association of positive family history with OS (p = 0.1, p = 0.45), also not in subgroups for histology (adeno and squamous cell), number of family members and degree of relative.
Conclusion
Our results emphasize that a positive family history is neither statistically significantly associated with prognosis nor with specific histopathological features in patients with gastroesophageal cancer. Yet, associations with distinct patient characteristics and positive family history indicate that specific subgroups might profit from endoscopic surveillance. Prospective studies are warranted to investigate these findings further.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastroesophageal cancer is a devastating disease with high mortality rates worldwide particularly in younger patients [1]. Moreover, the longstanding belief that the prevalence is highest in Asian patients due to lifestyle and environmental factors has to be challenged as Asian expats in northern America still show higher rates than Caucasian whites (sic) [2, 3].
Thus, it is surmised that genetic factors might play an important role in gastroesophageal cancer development; however, so far only one distinguished hereditary cancer syndrome could be associated with particularly high frequency of gastroesophageal malignancies. Hereditary diffuse gastric cancer (HDGC) is an autosomal dominant syndrome widely caused by mutations in the tumor suppressor gene CDH1 [4]. Other cancer syndromes, such as Lynch syndrome which is characterized by mismatch repair deficiency and is most commonly associated with colorectal cancer, might also lead to increased rates of upper gastrointestinal malignancies [5,6,7]. Yet, only a minority of gastroesophageal cancer patients are diagnosed with genetic cancer syndromes while familial accumulation is frequently observed and often believed to be associated with Helicobacter pylori infection, younger age at cancer onset and reduced survival time [8, 9].
Limited data exist on the impact of family history on the characteristics and prognosis of these patients. Thus, knowledge and awareness about familial gastroesophageal cancer, especially in Caucasian patients, have to be enhanced to optimize patient care and identify relatives who might profit from surveillance strategies [10, 11]. Our study focuses on examining gastroesophageal cancer patients with a self-reported family history in a western cohort, aiming to improve understanding of familial disease patterns and enhance care through potential genetic screening and increased surveillance.
Patients, material and methods
Patient and data recruitment
Patients aged ≥ 18 years, who were diagnosed and/or treated for pathologically confirmed gastroesophageal cancer between 1 January 1990 and 31 December 2021 at the Medical University of Vienna were included in this analysis. Programmed cell death ligand 1 (PD-L1) positivity was defined as combined positive score (CPS) ≥ 1 and/or tumor proportion score (TPS) ≥ 1 and is routinely performed at our hospital since 2020. Human epidermal growth factor receptor 2 (HER2) positivity was defined as immunohistochemical staining +++ or ++ with HER‑2 gene amplification and has been routinely performed since 2012. Mismatch repair deficiency (dMMR) was evaluated by immunohistochemical staining and although not routinely performed is available in a subset of patients. Treatment strategies were reviewed by an interdisciplinary tumor board and patients received the best available treatment according to current treatment guidelines at the time of diagnosis.
Clinical data, including patient characteristics, tumor characteristics, symptoms, treatment strategies as well as data on family history, were retrospectively retrieved by structured electronical chart review and then collected and stored in a FilemakerPro®-based (Claris International, Santa Clara, California, USA) database. The quality of retrospective data was ensured by standardized history taking. Information on patients’ genetics was not available due to regulatory restrictions in Austria.
Family history was considered positive when a first, second or third-degree relative suffered from a patient-reported oncological disease. First-degree was classified as parents, siblings and children. Second-degree was classified as grandparents, grandchildren, aunts/uncles and nieces/nephews. Third-degree was classified as great-grandparents, great-aunts/great-uncles and first cousins [12]. If multiple relatives had a history of (gastroesophageal) cancer, the relative with the closest degree was taken into account for our analysis.
Alcohol consumption was classified as “none” in patients without any alcohol intake, “moderate” in patients with < 2 alcoholic beverages a day and “abuse” in patients with ≥ 2 alcoholic beverages a day according to international guidelines. Nicotine abuse was defined as positive when either active smoking was still ongoing and/or the patient had a history of ≥ 10 pack years.
Death was registered according to hospital chart data and/or public data provided by Statistik Austria. Patients without a registered date of death at the time of data cut-off were censored at the date that they were last known to be alive. Overall survival (OS) was defined as the time from initial cancer diagnosis to the patient’s death or last follow-up date.
All procedures were performed following the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1964 and later versions. The study was approved by the ethics committee of the Medical University of Vienna (reference number: 1600/2021).
Statistical analysis
Descriptive analyses of clinical data were conducted using measures such as the median, range and interquartile range (IQR) for continuous data. For categorical variables absolute numbers, percentages and number of missing values were reported. Differences between groups were assessed using the χ2-test for categorical data or Wilcoxon rank sum test for continuous data. Missing values are stated in the respective tables.
Comparisons of OS were performed using log-rank tests and Kaplan Meier estimator, which was used to estimate median OS and visualize survival curves. For testing the effect size of the parameters on OS we additionally performed Cox regression analysis. For possible confounding factors that were significant within the univariable analysis as well as in association with family history, an interaction coefficient in the Cox regression model was added.
Multivariable analysis included possible confounding factors and the interaction term if statistically significant in the bivariable model. Additionally, clinically relevant factors such as stage, tumor location and histological subtype were included. Two multivariable Cox regression models were built, one with family history in general and one with family history in the upper gastrointestinal (GI) tract.
Two-tailed p-values < 0.05 were considered to indicate statistical significance. Due to the hypothesis-generating design of the studies, no correction for multiple testing was applied unless specified otherwise [8]. All statistical analyses were performed using R Studio software (version 4.2.3; R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient and tumor characteristics
Our cohort of gastroesophageal cancer patients consists of 1762 patients, who were treated at the Medical University of Vienna between 1 January 1990 and 31 December 2021. In this western cohort, 21 (1%) patients were of Asian ethnicity (3 Japan, 10 China, 2 India, 1 Kirgizstan, 1 Thailand, 1 Mongolia, 1 Vietnam, 2 Korea). Median age at first diagnosis of gastroesophageal cancer was 64 years (range 23–92 years, IQR: 55–72 years) in the overall cohort and 1235 (70%) patients were men. At the time of data cut-off (1 November 2022) 1353 (77%) patients were already deceased.
Median overall survival (OS) from cancer diagnosis to death was 21.5 months in the overall cohort (95% CI 20.6–23.1 months).
Other patient and tumor characteristics as well as symptoms at first cancer diagnosis are available in Supplementary tables 1–3. Therapeutic strategies applied in this cohort are summarized in Supplementary table 4.
Family history in gastroesophageal cancer patients
In our cohort of 1762 gastroesophageal cancer patients 826 (58%) reported no history of malignant diseases in their family and 592 (42%) had a close relative with cancer diagnosis. Of those with positive family history in the overall cohort, 159 (27%) had known gastroesophageal cancer in their family. In patients with a positive family history in general, 496 (84%) were first-degree relatives, 63 (11%) were second-degree relatives and 3 (1%) were third-degree relatives, 387 (65%) had only one family member and 176 (30%) multiple family members with cancer diagnoses. In patients with a positive family history of gastroesophageal cancer 113 (71%) were first-degree, 38 (24%) second-degree and 3 (2%) third-degree relatives. In 30 patients the information about the degree of the relative was missing, 131 (82%) patients with a positive family history of gastroesophageal cancer had only one recorded family member and 24 (15%) had multiple family members with gastroesophageal cancer.
Of the patients with Asian ethnicity 6 (29%) patients had a positive family history of cancer in general, with 5 (83%) of them for gastroesophageal cancer.
Age analysis in patients with positive family history
The median age for patients with positive family history in general was 64 years (range 27–92 years, IQR: 55–71 years) compared to 63 years (range: 23–92 years, IQR: 54–72 years) in patients without a family history of cancer. The median age for patients with positive family history of gastroesophageal cancer was 66 years (range: 30–91, IQR: 57–75) compared to 63 years (range: 23–92, IQR: 54–71) in patients without family history of gastroesophageal cancer. Positive family history and age groups are shown in Supplementary figure 1. The median age for patients with a positive family history and Asian ethnicity was 47 years (range: 30–71 years), while the median age for Asian patients without positive family history was 65 years (range: 40–78 years).
Association with patient and tumor characteristics
Concerning the association of a positive family history with patient and tumor characteristics, the family history in general was associated with sex (p = 0.011), BMI (p = 0.040), alcohol consumption (p = 0.010) and stage (p = 0.043). A family history of gastroesophageal cancer was associated with sex (p = 0.015), age (p = 0.031) and tumor location (p = 0.002). Other patient and tumor parameters are listed in Table 1.
Symptoms at first diagnosis were neither associated with family history in general nor with gastroesophageal cancer and are shown in Table 2.
Overall survival (OS) analysis
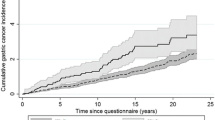
Family history in general on its own was not associated with OS (negative (n = 826): median 20.6 months (95% CI 17.6–22.2 months); positive (n = 592): median 23.3 months (95% CI 20.9–26.7 months); p = 0.10) and neither was family history of gastroesophageal cancer (negative (n = 1259): median OS 21.6 months (95% CI 20.1–23.2 months); positive (n = 159): median 21.0 months (95% CI 16.7–32.3 months); p = 0.45) (see Fig. 1).
Moreover, we also analyzed whether the number of family members with a positive family history as well as the degree of relatives was associated with the OS. Both analyses showed that there is no statistically significant association, neither in patients with positive family history in general nor in patients with a positive family history of gastroesophageal cancer (negative family history vs. one family member vs. multiple family members: p = 0.17 and p = 0.68; negative family history versus positive in first-degree relatives versus positive in second or third-degree relatives: p = 0.27 and p = 0.52; respectively) (see Fig. 2).
Subgroup analysis for histological subtypes
In addition, subgroup analysis for histological subtypes did not show a statistically significant association with OS (family history in general: adenocarcinoma: p = 0.18; squamous cell carcinoma: p = 0.35; family history gastroesophageal cancer: adenocarcinoma: p = 0.78; squamous cell carcinoma: p = 0.97) (see Supplementary Fig. 2).
Subgroup analysis for age categories
When only looking at young patients with age lower or equal to 45 years at first diagnosis, neither family history in general (p = 0.85) nor family history of gastroesophageal cancer (p = 0.08) was associated with the OS either; however, for older patients aged 65 years and above, those with a family history in general showed longer OS (HR = 0.78, 95% CI 0.66–0.93; p = 0.005). A significant interaction between family history of gastroesophageal cancer and age on OS was observed. When family history is negative the relationship between age and OS is particularly pronounced, with longer survival for younger patients (≤ 45 vs. > 45&< 65 years: HR = 0.77, 95% CI 0.6–1, p = 0.047; ≥ 65 vs. > 45&< 65 years: HR = 1.14, 95% CI 1.00–1.30, p = 0.048); however, when the family history of gastroesophageal cancer is positive, the effect of age on OS is reversed, such that patients at age 45 years and below, had a higher risk compared to patients with age above 45 years and below 65 years (HR = 2.15, 95% CI 1.02–4.49, p = 0.044). Family history in general and age showed a similar interaction, where patients aged 65 years or older had better survival rates than younger patients when they had a positive family history (HR = 0.77, 95% CI 0.6–0.99, p = 0.042). No other significant interactions between family history and other parameters on OS were observed.
Subgroup analysis for stage categories
Also, family history in general and family history of gastroesophageal cancer with respect to separate tumor stages were not significantly associated with OS (stage 1: p = 0.64, p = 0.17; stage 2: p = 0.48, p = 0.93; stage 3: p = 0.46, p = 0.49; stage 4: p = 0.99, p = 0.54, respectively).
Multivariate survival analysis
In multivariate analysis stage (HR = 2.174, 95% CI 1.7015–2.778, p < 0.0001), age and tumor location stayed statistically significantly associated with OS in both models. Family history in general was significant in the multivariable model (HR = 1.37, 95% CI 1.11–1.69; p = 0.004), while family history for upper GI malignancies stayed insignificant (HR = 0.93, 95% CI 0.63–1.37; p = 0.7). Additionally, the interaction effect between family history in general and age stayed significant, while the family history of tumors in the upper GI tract and age did not. Detailed results are available in Supplementary tables 5 and 6.
Discussion
Family history is known to be a major risk factor for diverse cancer entities [13, 14]. Public interest in hereditary cancers, such as the sustained increase in genetic testing following Angelina Jolie’s public surgery disclosure (also known as the “Angelina Jolie effect”), contrasts with the low awareness and data scarcity regarding gastroesophageal cancer familial risks [1, 15,16,17,18]. Thus, our analysis aimed to provide further insight into this subject in a large European cohort.
Although the occurrence of gastric cancer in first-degree relatives is known to be associated with a twofold to tenfold increase in the individual risk of gastric cancer development [13, 18], molecular mechanisms for this familial aggregation are unclear and it is surmised that shared environmental factors like diet and Helicobacter pylori infections might play key roles. Recently, a group of researchers from South Korea found that eradication of Helicobacter pylori in first-degree relatives of gastric cancer patients can reduce the cancer risk (1.2% vs. 2.7% in the treatment and placebo groups, respectively) [19]; however, the prevalence of Helicobacter pylori is low in most high-income countries [20], which is also reflected in our cohort with 29% positivity in the overall cohort versus 41% in the subgroup of Asian descent. Thus, this risk factor in western cohorts might not be of the same importance for higher incidence rates. In our analysis, potential environmental factors including Helicobacter infection, nicotine abuse, alcohol consumption, and higher BMI were not associated with a family history of gastroesophageal cancer. Thus, in western cohorts, genetic syndromes might be a more important player than environmental factors.
Consequently, genetic testing, although not recommended for the general gastroesophageal cancer population, might improve patient management [6, 7]; however, genetic counselling is often accompanied by reluctance from patients and healthcare professionals [4].
On one hand, skepticism about genetic testing is founded in historical events and fuelled by populist ideologies [21, 22]. On the other hand, uncertainty about legislation as well as incomprehensible laws about genetic testing, might complicate the process of genetic counselling [23]. In consequence, patients as well as treating physicians are often unwilling to talk about genetic testing. Thus, it is of utmost importance to identify subgroups who benefit most from genetic counselling.
In order to put more emphasis on preventative and early diagnostic procedures for high-risk individuals, concrete data from large cohorts such as ours are essential. Our analysis provides the insight that a positive family history is frequent in patients with gastroesophageal cancer. Especially in female patients, who experience gastroesophageal cancer less often than males, high percentages of a positive family history of cancer in general as well as gastroesophageal cancer were notable. This is in line with previous findings that female patients with a positive family history are more susceptible to developing gastric cancer, which was in part associated with genetic factors having a stronger influence on the female gender [24, 25].
Furthermore, it has been reported that the number and the degree of relatives affected by gastric cancer are associated with higher risk of developing the disease [26]; however, we could not observe a significant impact on survival concerning the degree and the number of relatives.
Yet, patients with a positive family history in general were statistically significantly diagnosed in earlier stages in our cohort. Contrary to data from Asian cohorts, we could not show that family history was associated with better prognosis in more advanced tumor stages [27], but that the stage itself remains the most prognostic factor in gastroesophageal tumors in our analysis. Thus, it is of utmost importance that patients are diagnosed early. Especially in older patients, who are known to have worse prognosis, a positive family history was associated with prolonged OS [28], while in patients with a negative family history of gastroesophageal cancer, younger patients had favorable outcomes. Thus, more efficient surveillance of patients with a positive family history might lead to beneficial outcomes independent of patient age; however, prospective data in western cohorts are missing and retrospective analyses like ours pose some disadvantages. Therefore, some limitations need to be addressed. Although reporting of family history was gathered in a structured manner, missing data due to imprecise self-reporting poses a potential bias. Furthermore, information on genetic testing is not centrally stored due to strict documentation policies, which do not allow the evaluation of any associations between hereditary and familial cancer. Thus, large-scal prospective studies with modern cohorts are warranted to evaluate the cost-effectiveness of genetic testing and regular endoscopy in patients with a positive family history [16].
In conclusion, our analysis offers a valuable contribution to the research of family history in western gastroesophageal cancer cohorts. Based on our results, we demonstrate that patients with a positive family history have distinct clinical features and support that specific screening and surveillance strategies including genetic testing and regular endoscopy should be evaluated in individuals with a positive family history in large prospective trials.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- AC:
-
Adenocarcinoma
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- CPS:
-
Combined positive score
- dMMR:
-
Mismatch repair deficiency
- GEJ:
-
Gastroesophageal junction
- HER2:
-
Human epidermal growth factor receptor 2
- HR:
-
Hazard ratio
- OS:
-
Overall survival
- PD-L1:
-
Programmed cell death ligand 1
- SCC:
-
Squamous cell carcinoma
- TPS:
-
Tumor proportion score
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. https://doi.org/10.3322/caac.21660.
Gill S, Shah A, Le N, Cook EF, Yoshida EM. Asian ethnicity-related differences in gastric cancer presentation and outcome among patients treated at a Canadian cancer center. J Clin Oncol. 2003;21(11):2070–6. https://doi.org/10.1200/JCO.2003.11.054.
Jin H, Pinheiro PS, Callahan KE, Altekruse SF. Examining the gastric cancer survival gap between Asians and whites in the United States. Gastric Cancer. 2017;20(4):573–82. https://doi.org/10.1007/s10120-016-0667-4.
Blair VR, McLeod M, Carneiro F, Coit DG, D’Addario JL, van Dieren JM, et al. Hereditary diffuse gastric cancer: updated clinical practice guidelines. Lancet Oncol. 2020;21(8):e386–e97. https://doi.org/10.1016/s1470-2045(20)30219-9.
Kumar S, Dudzik CM, Reed M, Long JM, Wangensteen KJ, Katona BW. Upper endoscopic surveillance in lynch syndrome detects gastric and duodenal adenocarcinomas. Cancer Prev Res. 2020;13(12):1047–54. https://doi.org/10.1158/1940-6207.CAPR-20-0269.
Lordick F, Carneiro F, Cascinu S, Fleitas T, Haustermans K, Piessen G, et al. Gastric cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(10):1005–20. https://doi.org/10.1016/j.annonc.2022.07.004.
Obermannová R, Alsina M, Cervantes A, Leong T, Lordick F, Nilsson M, et al. Oesophageal cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(10):992–1004. https://doi.org/10.1016/j.annonc.2022.07.003.
Song M, Camargo MC, Weinstein SJ, Best AF, Männistö S, Albanes D, et al. Family history of cancer in first-degree relatives and risk of gastric cancer and its precursors in a Western population. Gastric Cancer. 2018;21(5):729–37. https://doi.org/10.1007/s10120-018-0807-0.
Boland CR, Yurgelun MB. Historical perspective on familial gastric cancer. Cell Mol Gastroenterol Hepatol. 2017;3(2):192–200. https://doi.org/10.1016/j.jcmgh.2016.12.003.
Lachter J. Screening first-degree relatives for gastric cancer prevention. Gastrointest Endosc. 2016;83(1):274. https://doi.org/10.1016/j.gie.2015.08.010.
Raymond VM, Stoffel EM. Familial gastric and pancreatic cancers: diagnosis and screening. Am Soc Clin Oncol Educ Book. 2013;33:e44–e8. https://doi.org/10.14694/EdBook_AM.2013.33.e44.
National Cancer Institute. NCI dictionary of genetics terms. https://www.cancer.gov/publications/dictionaries/genetics-dictionary. Accessed 30 Nov 2023.
Jacobs MF. Predicting cancer risk based on family history. Elife. 2021; https://doi.org/10.7554/eLife.73380.
Turati F, Edefonti V, Bosetti C, Ferraroni M, Malvezzi M, Franceschi S, et al. Family history of cancer and the risk of cancer: a network of case-control studies. Ann Oncol. 2013;24(10):2651–6. https://doi.org/10.1093/annonc/mdt280.
Liede A, Cai M, Crouter TF, Niepel D, Callaghan F, Evans DG. Risk-reducing mastectomy rates in the US: a closer examination of the Angelina Jolie effect. Breast Cancer Res Treat. 2018;171(2):435–42. https://doi.org/10.1007/s10549-018-4824-9.
Choi YJ, Kim N. Gastric cancer and family history. Korean J Intern Med. 2016;31(6):1042–53. https://doi.org/10.3904/kjim.2016.147.
Luu MN, Quach DT, Hiyama T. Screening and surveillance for gastric cancer: does family history play an important role in shaping our strategy? Asia-Pac J Clncl Oncology. 2022;18(4):353–62. https://doi.org/10.1111/ajco.13704.
Yaghoobi M, Bijarchi R, Narod SA. Family history and the risk of gastric cancer. Br J Cancer. 2010;102(2):237–42. https://doi.org/10.1038/sj.bjc.6605380.
Choi IJ, Kim CG, Lee JY, Kim Y‑I, Kook M‑C, Park B, et al. Family history of gastric cancer and Helicobacter pylori treatment. N Engl J Med. 2020;382(5):427–36. https://doi.org/10.1056/NEJMoa1909666.
Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, et al. Global prevalence of helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017;153(2):420–9. https://doi.org/10.1053/j.gastro.2017.04.022.
Bíró K, Dombrádi V, Fekete Z, Bányai G, Boruzs K, Nagy A, et al. Investigating the knowledge of and public attitudes towards genetic testing within the Visegrad countries: a cross-sectional study. BMC Public Health. 2020;20(1):1380. https://doi.org/10.1186/s12889-020-09473-z.
Burn J, Duff G, Holtzman N. Three views of genetics: the enthusiast, the visionary, and the sceptic. Interview by Tessa Richards. BMJ. 2001;322(7293):1016. https://doi.org/10.1136/bmj.322.7293.1016.
Paneque M, Guimarães L, Bengoa J, Pasalodos S, Cordier C, Esteban I, et al. An European overview of genetic counselling supervision provision. Eur J Med Genet. 2023;66(4):104710. https://doi.org/10.1016/j.ejmg.2023.104710.
Nagase H, Ogino K, Yoshida I, Matsuda H, Yoshida M, Nakamura H, et al. Family history-related risk of gastric cancer in Japan: a hospital-based case-control study. Jpn J Cancer Res. 1996;87(10):1025–8. https://doi.org/10.1111/j.1349-7006.1996.tb03104.x.
Palli D, Galli M, Caporaso NE, Cipriani F, Decarli A, Saieva C, et al. Family history and risk of stomach cancer in Italy. Cancer Epidemiol Biomarkers Prev. 1994;3(1):15–8.
La Vecchia C, Negri E, Franceschi S, Gentile A. Family history and the risk of stomach and colorectal cancer. Cancer. 1992;70(1):50–5. https://doi.org/10.1002/1097-0142(19920701)70:1〈50::aid-cncr2820700109〉3.0.co;2‑i.
Han MA, Oh MG, Choi IJ, Park SR, Ryu KW, Nam BH, et al. Association of family history with cancer recurrence and survival in patients with gastric cancer. J Clin Oncol. 2012;30(7):701–8. https://doi.org/10.1200/jco.2011.35.3078.
Choi Y, Kim N, Kim KW, Jo HH, Park J, Yoon H, et al. Gastric cancer in older patients: a retrospective study and literature review. Ann Geriatr Med Res. 2022;26(1):33–41. https://doi.org/10.4235/agmr.21.0144.
Funding
Open access funding provided by Medical University of Vienna.
Author information
Authors and Affiliations
Contributions
The manuscript has been read and approved by all authors. All authors significantly contributed to the preparation of the manuscript and are in agreement with the content of the manuscript. All authors were involved in creating the study design and concept. All authors confirm that the manuscript is original and has not been submitted elsewhere.
Corresponding author
Ethics declarations
Conflict of interest
A. Ilhan-Mutlu: participation in advisory boards organized by MSD, Servier, Daiichi Sankyo and BMS, lecture honoraria from Eli Lilly, Servier, BMS, MSD, Astellas and Daiichi Sankyo, local principal investigator for clinical trials sponsored by BMS and Roche, consulting for Astellas and MSD, travel support from BMS, Roche, Eli Lilly and Daiichi Sankyo. H.C. Puhr has received travel support from Eli Lilly, MSD, Novartis, Pfizer, Pierre Fabre and Roche and received lecture honoraria from Eli Lilly. G.W. Prager has the following to declare: advisory role/speaker fees: Merck Serono, Roche, Amgen, Sanofi, Lilly, Servier, Taiho, Bayer, Halozyme, BMS, MSD, Celgene, Pierre Fabre, Shire, CECOG, Astra Zeneca. A.S. Berghoff has received research support from Daiichi Sankyo and Roche, honoraria for lectures, consultation or advisory board participation from Roche, Bristol-Meyers Squibb, Merck, Daiichi Sankyo as well as travel support from Roche, Amgen, Daiichi Sankyo, and AbbVie. M. Preusser has received honoraria for lectures, consultation or advisory board participation from the following for-profit companies: Bayer, Bristol-Myers Squibb, Novartis, Gerson Lehrman Group (GLG), CMC Contrast, GlaxoSmithKline, Mundipharma, Roche, BMJ Journals, MedMedia, Astra Zeneca, AbbVie, Lilly, Medahead, Daiichi Sankyo, Sanofi, Merck Sharp & Dome, Tocagen, Adastra, Gan & Lee Pharmaceuticals, Servier. E.S. Bergen, L. Berchtold, L. Zingerle, M. Felfernig, L. Weissenbacher, G. Jomrich, R. Asari and S.F. Schoppmann declare that they have no competing interests.
Ethical standards
All procedures performed in studies involving human participants or on human tissue were in accordance with the ethical standards of the institutional and with the 1975 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the ethics committee of the Medical University of Vienna (reference number: 1600/2021). The risk of publication of sensitive patient data was minimized by restrictions of data accession and pseudonymized statistical analysis. Due to the retrospective design no separate informed consent was necessary in the scope of this study. This is in line with local ethical standards and was approved by the ethics committee of the Medical University of Vienna (reference number: 1600/2021).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
508_2024_2432_MOESM1_ESM.pdf
Supplementary figure 1: Distribution of positive family history of cancer in general (A) and for gastroesophageal cancer (B) in different age groups.
508_2024_2432_MOESM2_ESM.pdf
Supplementary figure 2: Overall survival in adenocarcinoma patients with positive family history in general (A) and gastroesophageal cancer (B) as well as in squamous cell carcinoma patients with positive family history in general (C) and gastroesophageal cancer (D)
508_2024_2432_MOESM6_ESM.docx
Supplementary table 4: Therapeutic strategies. neoadjuvant regimen given to oligometastatic patients in order to achieve surgical respectability. *560 (79%) of patients received first line chemotherapy without prior systemic therapy for gastroesophageal cancer, **102 (38%) received definitive chemoradiotherapy.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ilhan-Mutlu, A., Puhr, H.C., Berchtold, L. et al. Association of family history with patient characteristics and prognosis in a large European gastroesophageal cancer cohort. Wien Klin Wochenschr (2024). https://doi.org/10.1007/s00508-024-02432-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00508-024-02432-3