Abstract
Purpose
The Palliative Care Outcomes Collaboration (PCOC) aims to enhance patient outcomes systematically. However, identifying crucial items and accurately determining PCOC phases remain challenging. This study aims to identify essential PCOC data items and construct a prediction model to accurately classify PCOC phases in terminal patients.
Methods
A retrospective cohort study assessed PCOC data items across four PCOC phases: stable, unstable, deteriorating, and terminal. From July 2020 to March 2023, terminal patients were enrolled. A multinomial mixed-effect regression model was used for the analysis of multivariate PCOC repeated measurement data.
Results
The dataset comprised 1933 terminally ill patients from 4 different hospice service settings. A total of 13,219 phases of care were analyzed. There were significant differences in the symptom assessment scale, palliative care problem severity score, Australia-modified Karnofsky performance status, and resource utilization groups-activities of daily living among the four PCOC phases of care. Clinical needs, including pain and other symptoms, declined from unstable to terminal phases, while psychological/spiritual and functional status for bed mobility, eating, and transfers increased. A robust prediction model achieved areas under the curves (AUCs) of 0.94, 0.94, 0.920, and 0.96 for stable, unstable, deteriorating, and terminal phases, respectively.
Conclusions
Critical PCOC items distinguishing between PCOC phases were identified, enabling the development of an accurate prediction model. This model enhances hospice care quality by facilitating timely interventions and adjustments based on patients' PCOC phases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Australian Palliative Care Outcomes Collaboration (PCOC) [1], established in 2005, is a national palliative care outcome and benchmarking program. The primary objective of PCOC is to systematically improve patient outcomes (including pain and symptom control). PCOC measures timeliness of palliative care, response to urgent needs, anticipatory care, and responsive care [1]. The PCOC has been shown to be feasible, desirable, and useful in improving outcomes in patients receiving palliative care [2]. The PCOC collects data items, including the symptom assessment scale (SAS) [3], palliative care problem severity score (PCPSS) [4, 5], Australia-modified Karnofsky performance status (AKPS) [6], and resource utilization groups-activities of daily living (RUG-ADL) [4, 5], and applies them in determining PCOC palliative care phases (hereafter referred to as PCOC phases). These phases, including the stable, unstable, deteriorating, and terminal phases, allow the palliative care team to adjust the care plan and rapidly respond to symptoms according to the PCOC phases. The phase of care is a clinically relevant category of care that describes the palliative care trajectory, which is a measure of relative resource utilization linked directly to clinical needs, irrespective of diagnosis or prognosis [7]. They reflect the patient’s quality of life and his or her family’s needs. A new phase is assigned whenever a clinical change requires patient/family reassessment and modification of the care plan [7].
Taiwan began implementing the PCOC program in 2019 and systematically incorporated measurable PCOC outcomes into routine clinical practice. There are some challenges in identifying the discriminative critical items between phases and quickly determining the PCOC phases; this is especially important for patients classified as in the unstable phase of care. When phase is assessed correctly, clinicians and members discuss the corresponding care planning, especially in unstable patients. Unfortunately, there is a lack of currently available prediction models to accurately classify the PCOC palliative phases. The ability to detect changes in care needs has thus become an urgent yet challenging necessity. Therefore, this study endeavors to address these challenges by identifying symptom and functional items from PCOC data and constructing a prediction model to accurately classify PCOC phases in hospice patients. We hypothesize significant differences in the characteristics of SAS, PCPSS, AKPS, and RUG-ADL among the four phases of care. Leveraging insights into the distinct features of PCOC phases and palliative care needs, we aim to enhance daily care quality, thereby improving the care intervention and reflect the resource implications of the care provided.
Methods
Study design
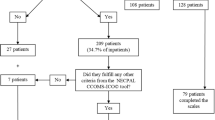
A retrospective cohort study with secondary analysis of routinely collected patient and phase-level data was conducted to assess PCOC data items across four PCOC phases. Figure 1 illustrates how PCOC measures work together for phase classification. In this study, we use a multinomial mixed-effect regression model for analyzing multivariate data in the repeated‐measurement setting. We treat the SAS, PCPSS, AKPS, and RUG-ADL data items as variables and PCOC phases (stable, unstable, deteriorating, and terminal) as outcomes. The PCOC information of terminally ill patients enrolled in the one of four hospice services between July 2020 and March 2023 was collected for analysis.
Integration of PCOC assessments and ratings for phase classification flowchart. This flowchart illustrates how the integration of functional status and symptom assessments aids in determining the appropriate phase classification. In PCOC, functional assessment is conducted using the RUG-ADL and AKPS scales, while pain and other symptoms are assessed using the PCPSS (Palliative Care Problem Severity Score) and SAS (Symptom Assessment Scale). The combined use of these rated tools guides clinical judgment in determining the probable PCOC phases (stable, unstable, deteriorating, or terminal). Among these tools, SAS and PCPSS are prioritized for assessment. Symptom assessments are classified as follows: Absent (PCPSS = 0, SAS = 0) indicates a stable or terminal phase; mild (PCPSS = 1, SAS = 1–3) corresponds to a stable or terminal phase; moderate (PCPSS = 2, SAS = 4–7) is associated with a deteriorating or terminal phase; and severe (PCPSS = 3, SAS = 8–10) denotes an unstable or terminal phase. Terminal phase patients are likely to die in a matter of days. Functional assessment is classified as follows: RUG-ADL 4–5 and AKPS 90–100 indicate a stable phase; RUG-ADL 6–13 and AKPS 70–80 correspond to an unstable or deteriorating phase; RUG-ADL 14–17 (or a rapid drop of 20) and AKPS 50–60 also signify an unstable or deteriorating phase; and RUG-ADL 18 and AKPS ≦ 40 indicate a terminal phase
Setting and population
The study setting was a 1193-bed medical center located in an urban area, with 1.8 million people. This institute had a palliative care program during the study period. There are four hospice services, including hospice inpatient care (HIC), hospice share care (HSC), hospice home care (HHC), and hospice nursing facility care (HFC). Terminally ill patients referred to the one of four hospice services were analyzed. The inclusion criteria were as follows: (1) patients with life-limiting illnesses not responsive to curative treatment; (2) limited disease prognosis, including those with cancer and other terminal illnesses; and (3) patients who willingly accepted hospice care initially. The exclusion criteria were as follows: (1) the patient’s rejection of ongoing palliative care and (2) patients or their family/caregiver refused to be assessed by PCOC instruments.
Operational definitions of the PCOC phase
The PCOC phase is determined by a holistic clinical assessment that considers the needs of the patients and their families and carers, and patients may move between phases in any sequence [7,8,9]. The definitions used in this study are as follows [8]:
-
1.
Stable phase: Patient problems and symptoms are adequately controlled by an established plan of care.
-
2.
Unstable phase: An urgent change in the plan of care to manage new unexpected symptoms is required.
-
3.
Deteriorating phase: The patient experiences a gradual worsening of existing symptoms. The care plan addresses anticipated needs but requires periodic review.
-
4.
Terminal phase: Death is likely within days. The PCOC phase is a reliable and acceptable measure that can be used for care planning and quality improvement [8].
Data collection
The study was approved by the Institutional Review Board (B-ER-109-05) (anonymous)with which the first author was affiliated. Data that could be used to identify individuals were not collected. Routine clinical data were collected, and patient consent was not needed. The palliative care team completed a 6-month education program, including complex case studies for PCOC evaluation, to establish interrater consistency. All nurses (20 ward, 16 home care, and 4 shared care) received this training, launched by the Health Promotion Administration, Ministry of Health and Welfare, Taiwan. Following this, we incorporated PCOC into our daily care and implemented a monthly audit mechanism to ensure data accuracy. The PCOC data were collected for the Hospice Databank. We longitudinally measured the PCOC data items in everyday clinical practice for patients receiving HIC and every weekly encounter with patients receiving HSC, HHC, and HFC. A databank is a web-based, electronic quality monitoring system, point-of-care assessment, and reporting tool for collecting clinical and PCOC data in palliative care.
The PCOC is initially implemented at XXX (anonymous) University Hospital in July 2020. The nursing professionals of palliative care were well trained to measure clinical information and five acceptable and reliable PCOC assessment tools [3,4,5,6], including the SAS, PCPSS, AKPS, RUG-ADL, and PCOC phases. The specialized hospice nurses administered 17 standardized response options. In this study, rating refers to the assessment of various measures. Serial data collected throughout hospice care for each patient track ongoing changes. These ratings inform PCOC data, revealing changes over time within the same subjects [8]. In PCOC, symptoms are measured using two key measures. The SAS data items, which included difficulty sleeping, appetite, nausea, bowels, breathing, fatigue, and pain, are rated by patients or proxies when patients are unable to self-report due to severe illness, debilitation, cognitive impairment, communication barriers, or unconsciousness. The SAS was evaluated on a patient self-rating scale from 0 to 10 (0: none; 10: worst possible pain). The four domains of clinician-rated PCPSS items included pain, other symptoms, psychological/spiritual, and family/carers. The PCPSS was assessed on a scale from 0 to 3 (0: none; 3: worst possible symptoms). Functional assessment utilizing the RUG-ADL and AKPS scales. The AKPS, the description of performance status, was assessed on a scale from 0 to 100 (0: death; 10: comatose; 20: total bedfast; 100: normal). The RUG-ADL data items included bed mobility, toileting, eating, and transfers. The RUG-ADL was assessed with scores of 1, 3, 4, and 5 for bed mobility, toileting, and eating and scores of 1, 2, and 3 for transfers. The four PCOC phases included stable, unstable, deteriorating, and terminal.
Statistical analysis
The Center for Quantitative Sciences performed the data analysis. Descriptive statistics were tabulated for characteristics of PCOC phases. To analyze the characteristics of hospice patients across different PCOC phases, the Kruskal‒Wallis rank-sum test was used for continuous variables, and the chi-squared test/Fisher’s exact test was used for categorical variables. We treated SAS, PCPSS, AKS, and RUG-ADL scores as continuous variables for statistical analysis. All statistical tests were two-sided, and statistical significance was indicated by a p-value of 0.05. R statistical software (V.4.0.2) for Windows was used for all analyses.
Prediction model
An analysis of the effects of a single variable on the PCOC phases is conducted using a multinomial mixed-effect regression model. In this study, repeated measurements were taken in patients. We used a mixed-effects model for analysis and prediction, including fixed and random effects.
Results
Characteristics of four PCOC phases
A total of 13,219 assessments from 1933 patients were analyzed in this study. The number of assessments per patient ranged from a minimum of one to a maximum of 54. The characteristics and measured outcomes of the patients by PCOC phases of care are presented in Table 1. Of these assessments, the stable phase comprised 7508 ratings (56.8%), the unstable phase comprised 1252 ratings (9.5%), the deteriorating phase comprised 2781 ratings (21%), and the terminal phase comprised 1678 ratings (12.7%). The mean age of these patients with ratings was 68–71 years old, with over 80% of the ratings occurring in patients with cancer.
There were significant differences in the hospice service settings, SAS, PCPSS, AKPS, and RUG-ADL in the PCOC phases. Since the collection protocol is different by setting (daily vs weekly), of these assessments, HIC consisted of 6792 (51.4%) ratings, HSC consisted of 3085 (23.3%) ratings, HHC consisted of 3220 (24.4%) ratings, and HFC consisted of 122 (0.9%) ratings. In the stable phase, 4070 (54.21%) ratings occurred in HIC, 1567 (20.87%) ratings occurred in HSC, 1787 (23.80%) ratings occurred in HHC, and 84 (1.12%) ratings occurred in HFC. For the unstable phase, 465 (37.14%) ratings occurred in HIC and 439 (35.06%) in HSC. The distribution of PCOC phases is different in the hospice service settings. Patients receiving HIC and HFC were more likely to be in the stable phase (59.92% and 68.85%). The proportion of HSC patients in the unstable and deteriorating phases was higher (14.23% and 23.76%). In hospice home care patients, the proportion of patients in the terminal phase was higher (15.34%).
There were significant differences in the SAS scores among the four PCOC phases. The SAS also increased between stable and unstable phases. Fatigue was the most severe symptom in the phases of unstable, deterioration, and terminality (scores 3.53, 3.93, and 3.68, respectively). In the stable phase, fatigue, bowels, and pain are the most common symptoms. A person in an unstable phase is most likely to experience fatigue, pain, and breathing difficulties. In the deteriorating and terminal phases, fatigue, breathing, and appetite are the most common symptoms.
Among the four types of PCOC phases, there were significant differences in PCPSS. The PCPSS in the unstable phase is significantly higher than that in the stable phase. In the deteriorating and terminal phases, all PCPSS items except psychological/spiritual problems decrease. In hospice care, AKPS and RUG-ADL were the major issues, with significant decreases in AKPS and increases in RUG-ADL, especially in terminal phases (Table 1).
The significance of PCOC data items among different PCOC phases
We used the multinomial mixed-effect regression model to evaluate the effect of a single PCOC data item among the unstable, deteriorating, terminal, and stable phases during palliative care. When mixed-effect regression is used, missing values will be deleted. The final analysis included 10,030 assessments and 1646 patients.
Unstable versus stable phase
The SAS showed less difference from the unstable phase to the stable phase; in contrast, patients with higher PCPSS scores, including pain, other symptoms, and family/caregivers, were more likely to be in the unstable phase (odds ratio 3.18, 6.85 and 1.79, p < 0.001). AKPS and most RUG-ADL scores were not significantly different between these two phases, but a higher score of bed mobility was observed in the unstable phase (odds ratio 1.36, p = 0.0411). In different hospice care models, HSC and HHC had a higher probability of being in the unstable phase than HIC (odds ratio 7.02 and 4.24, p < 0.001) (Fig. 2A; Supplementary Table S1).
The PCOC data items among different PCOC phases. The Y-axis shows the PCOC data items. The X-axis shows the odds ratio of fixed effects. A For the unstable phase, the patients had higher PCPSS scores, including pain, other symptoms, and family/caregivers (p < 0.001). B For the deteriorating phase, the patients have higher PCPSS scores, including pain, other symptoms, and family/caregivers (p < 0.001), and higher scores of functional statuses for bed mobility and eating (p < 0.001). C For the terminal phase, the patients have higher PCPSS scores, including pain, other symptoms, psychological/spiritual, and family/caregivers (p < 0.001) and higher scores of functional statuses for bed mobility, eating, and transfers
Deteriorating versus stable phase
The SAS was similar among the deteriorating and stable phases. However, pain, other symptoms, and family/carers in PCPSS were more predominant in the deteriorating phase (odds ratio 2.13, 5.13, and 1.63, all p < 0.001). Higher scores of bed mobility and eating were more possible in the deteriorating phase (odds ratio 1.39 and 1.54, p = 0.065, < 0.001). Among hospice care models, HSC and HHC are more likely to be in a deteriorating phase compared to HIC (odds ratio 3.29 and 2.04, p < 0.001). Noncancer patients had a lower odds ratio (odds ratio 0.62, p = 0.0408) than cancer patients in the deteriorating phase (Fig. 2B; Supplementary Table S2).
Terminal versus stable phase
Increased SAS of breathing was more often in the terminal phase (odds ratio 1.26, p < 0.001), and high PCPSS, including pain, other symptoms, psychological/spiritual, and family/carers, was regarded as the terminal phase (odds ratio 1.60, 2.05, 1.31, and 2.13, p < 0.001, < 0.001, = 0.013, < 0.001). The odds ratio for the terminal phase was significantly elevated in a higher score of bed mobility, eating, and transfers (odds ratio 1.99, 1.60, 2.11, p < 0.001, = 0.013, = 0.026). HHC had a higher odds ratio (odds ratio 4.72, p < 0.001) than HIC of being in the terminal phase. Noncancer patients had a low odds ratio (odds ratio 0.36, p < 0.001) in the terminal phase compared to cancer patients (Fig. 2C; Supplementary Table S3).
Terminal versus deteriorating phase
Increased SAS of breathing was more often in the terminal phase (odds ratio 1.15, p < 0.001), and high family/carers were regarded as the terminal phase (odds ratio 1.35, p < 0.001). The odds ratio for the terminal phase was significantly elevated in a higher score of transfers (odds ratio 2.40, p < 0.001). HHC had a higher odds ratio (odds ratio 2.52, p < 0.001) in the terminal phase compared to the deteriorating phase. (Supplementary Fig. 1 and Supplementary Table S4).
Prediction model for PCOC palliative care
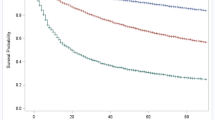
We created a prediction model using the PCOC data items to predict the palliative phase of the patient. The formulas for the prediction model of this study are provided in the Supplementary Fig. 2. In the stable, unstable, deteriorating, and terminal phases, the sensitivity was 0.94, 0.46, 0.64, and 0.69, respectively. Specificities were 0.74, 0.98, 0.93, and 0.98 in the stable, unstable, deteriorating, and terminal phases, respectively. The F1 score was 0.87 in the stable phase, 0.56 in the unstable phase, 0.68 in the deteriorating phase, and 0.75 in the terminal phase (Table 2; Supplementary Table S5). The AUCs for prediction were 0.93, 0.93, 0.92, and 0.96 in the stable, unstable, deteriorating, and terminal phases, respectively (Fig. 3). With this model, the PCOC measures work together to predict the phase of care.
The performance of the prediction model for PCOC palliative care. The receiver operating characteristic curve (ROC) and average area under the ROC curve (AUC) of the multinomial mixed-effect regression model. The AUCs for the prediction models were 0.93, 0.93, 0.92, and 0.96 in the stable, unstable, deteriorating, and terminal phases, respectively
Discussion
Main findings/results of the study
We employed a multinomial mixed-effect regression model for the analysis of PCOC phase-level data from a cohort study with repeated measurements in routine clinical practice. The main conclusions of our study are as follows: (i) There are significant differences in SAS, PCPSS, AKPS, and RUG-ADL scores among the four PCOC phases. (ii) The SAS exhibited less difference between stable and unstable phases. (iii) From the unstable to the terminal phase, the PCPSS for pain and other symptoms decreased, while the PCPSS for psychological/spiritual, bed mobility, eating, and transfers increased. (iv) We developed a robust model based on PCOC items to predict PCOC phases. These findings shed light on the distinct characteristics and significant differences observed across the four PCOC phases of care, highlighting the multifaceted nature of patient care needs in palliative settings [1]. The model provided valuable insights into the relative importance of SAS, PCPSS, AKPS, and RUG-ADL in distinguishing between PCOC phases. Factors such as symptom severity, functional status, and hospice care model emerged as significant predictors of phase transition. Understanding these variations is crucial for tailoring interventions and improving the quality of care provided to terminal patients.
PCOC phase is a measure of relative resource utilization linked directly to clinical needs [9]. In accordance with previous research regarding symptom burden in cancer patients [9,10,11], this study found that fatigue emerged as a prevalent and severe symptom across all phases; other symptoms such as pain, breathing difficulties, and appetite disturbances exhibited distinct patterns across the phases. The clinical needs for psychological and spiritual care and functional activities increase from the unstable to the terminal phase. These findings underscore the importance of comprehensive symptom management strategies tailored to the evolving needs and physical/psychological/spiritual burdens experienced by patients as they progress through different phases of palliative care [10]. Moreover, the high AUC values further validate the efficacy of the prediction model in distinguishing between phases, underscoring its potential utility in clinical practice. Health care providers can proactively identify changes in PCOC phase and tailor interventions accordingly to optimize patient outcomes. For example, the odds ratio for the terminal phase was significantly elevated in a higher score of bed mobility, eating, and transfers. From the patient characteristics and distribution of PCOC phases, we could timely and dynamically optimize resource allocation, respite care for caregivers and multidisciplinary referrals, as well as an adequate medical intervention for patients based on their clinical profiles and phase-specific needs in the hospice care [8, 10]. These results indicated that the combined suite of PCOC measures were found to be suitable for routine clinical care of patients requiring hospice care [2, 9]. The authors found the unstable and terminal phases more difficult to predict, whereas previously deteriorating phase has been reported to be more challenging to distinguish [2]. Different machine learning models to identify patterns in patient responses signifying different phases of illness might be used in the future to enhance performance [14].
The symptom assessment scale (SAS) exhibited less difference between stable and unstable phases. In contrast, PCPSS pain, other symptoms, and family/caregiver problems have a multifactorial impact on patients’ well-being and may interact synergistically to exacerbate symptom burden and distress. These factors play a pivotal role in driving the transition to unstable phases compared to the SAS, addressing the complex symptom dynamics and care needs of palliative care patients. For both patient- and clinician-reported outcomes, there was a poor relationship between the SAS pain score and the PCPSS pain score. We expected those with higher SAS pain scores would have higher PCPSS pain scores. The study found that hospice patients and their proxies tend to express less symptom distress from pain. The poor correlation between SAS pain score and the PCPSS pain score may be affected by many factors. This study is inconsistent with previous research, which has shown that while proxy reports are generally accurate regarding symptom distress, clinicians tend to underestimate symptoms, and unpaid caregivers often overestimate symptoms compared to patient reports [15,16,17]. In terms of the concept of symptom distress, Asian patients often exhibit stoicism when in pain, which is directly related to our cultural values [13]. As a result, this may translate to lower scores in the SAS of pain distress compared to Western studies. Furthermore, Taiwanese opioid consumption for pain management has mildly increased from 2008 to 2018 [18]. As their illness progressed, the patients may have redefined their concept of pain [19]. Developing a cultural sensitivity of rapid assessment and reassessment after adapting local models of clinical care delivery is a fertile development for future research in palliative care.
The study categorized hospice service into four different care settings: HIC, HSC, HHC, and HFC. When providing hospice care, we should consider the type of care required, the patient's condition, and the resources of the healthcare facility. For example, when patients have severe symptoms (SAS scores) and complex psychological or spiritual needs, hospice inpatient care (HIC) could provide more comprehensive care. On the other hand, hospice home care (HHC) may be suitable for clinically stable patients with poor performance status [20, 21]. To build a statistical model, all variables were included to accurately represent relationships and interactions. According to our formula in Supplementary Fig. 2, the hospice service setting is a significant predictor of phase classification. For the HFC category, where data entries per cell were less than 10, Fisher’s exact test was used instead of the chi-squared test because it can handle small sample sizes problems. We also assess the adequacy of the data volume for different PCOC phase statistical tests for SAS, PCPSS, RUG, and AKPS, treating these variables as continuous. Thus, the Kruskal–Wallis rank sum test was appropriately used to determine significant differences in median scores across the four PCOC phases. We did not evaluate under the HFC subgroups, so actually this sample size is sufficient.
Strengths and limitations
One of the major strengths of our study was the pioneering use of a predictive model for enhancing care delivery by facilitating timely interventions and resource allocation tailored to the specific needs of patients in each phase. In routine clinical practice, repeated data collection allows for a more comprehensive understanding of a patient's condition over time. It can help detect trends and heterogeneity among each patient that may not be apparent from a single data point, thereby reducing variability and increasing statistical power. For the analysis of longitudinal data, a multinomial mixed-effect regression model performed well [22]. We find that AUCs are good at classifying PCOC phases in our model. This prediction model facilitates the provision of precision palliative care, a pragmatic approach that captures individual, time-varying, and complex patient/caregiver needs while considering limited human and system resources. By using phase and a measure of function, PCOC has also embedded a new common language for rapidly describing the position on the care trajectory of individual patients [7]. Future research might focus on validating and refining predictive models to further enhance their clinical utility and inform evidence-based practices in palliative care settings.
This study has room for further improvement, which is left for future work. First, this is a single-center and retrospective nature, which necessitates caution in generalizing the results. Future large-scale multicenter studies are warranted. Second, the percentage of patient/proxy scores has not been further examined. There is an unquantifiable level of proxies making clinical ratings for patients, which likely leads to an underestimation of the magnitude of improvement reported. Quantifying the discrepancy between patient and proxy ratings will be essential for future research. Third, patients with relatively stable conditions not requiring intensive assessment are transferred to HHC, HSC, or HFC, with collection protocol is different by setting (daily vs weekly). However, we acknowledge that varying data collection protocols across care settings might impact the identification of phase transitions. Finally, given the observational nature of the study, confounding by indication might affect the outcome. Also, unbalanced group size might lead to possible bias and affect estimates. Therefore, multivariable analyses were used to allow adjustment for all available confounders.
Conclusions
This study contributes to the refinement of palliative care practice by providing insights into the dynamic nature of PCOC phases and offering a reliable prediction tool for phase classification. Clinical features identified between PCOC phases enable early detection, intervention, and potentially improved patient outcomes. Based on the comprehensive clinical assessments and phase characteristics, individualized care targeting improvement in end-of-life care can be formulated.
Data Availability
No datasets were generated or analysed during the current study.
References
Eagar K, Watters P, Currow DC et al (2010) The Australian Palliative Care Outcomes Collaboration (PCOC)–measuring the quality and outcomes of palliative care on a routine basis. Aust Health Rev 34:186–192
Daveson BA, Allingham SF, Clapham S et al (2021) The PCOC Symptom Assessment Scale (SAS): a valid measure for daily use at the point of care and in palliative care programs. PLoS ONE 16:e0247250
Toye C, Walker H, Kristjanson LJ et al (2005) Measuring symptom distress among frail elders capable of providing self reports. Nurs Health Sci 7:184–191
Eagar K, Green J, Gordon R (2004) An Australian casemix classification for palliative care: technical development and results. Palliat Med 18:217–226
Eagar K, Gordon R, Green J et al (2004) An Australian casemix classification for palliative care: lessons and policy implications of a national study. Palliat Med 18:227–233
Abernethy AP, Shelby-James TM, Fazekas BS et al (2005) The Australian-modified Karnofsky Performance Status (AKPS) scale: a revised scale for contemporary palliative care clinical practice. BMC Palliat Care 4:7
Currow DC, Allingham S, Yates P et al (2015) Improving national hospice/palliative care service symptom outcomes systematically through point-of-care data collection, structured feedback and benchmarking. Support Care Cancer 23:307–315
Masso M, Allingham SF, Banfield M et al (2015) PCOC phase: inter-rater reliability and acceptability in a national study. Palliat Med 29:22–30
Chang PJ, Lin CF, Juang YH, Chiu JY, Lee LC, Lin SY, Huang YH (2023) Death place and palliative outcome indicators in patients under palliative home care service: an observational study. BMC Palliat Care 19:44
Cuthbert CA, Boyne DJ, Yuan X, Hemmelgarn BR, Cheun WY (2020) Patient-reported symptom burden and supportive care needs at cancer diagnosis: a retrospective cohort study. Support Care Cancer 28:5889–5899
Reilly CM, Bruner DW, Mitchell SA, Minasian LM, Basch E, Dueck AC, Cella D, Reeve BB (2013) A literature synthesis of symptom prevalence and severity in persons receiving active cancer treatment. Support Care Cancer 21:1525–1550
Barbera L, Seow H, Howell D et al (2011) Symptom burden and performance status in a population-based cohort of ambulatory cancer patients. Cancer 116:5767–5776
Anderson KO, Green CR, Payne R (2009) Racial and ethnic disparities in pain: causes and consequences of unequal care. J Pain 10:1187–1204
Sandham MH, Hedgecock EA, Siegert RJ et al (2022) Intelligent palliative care based on patient-reported outcome measures. J Pain Symptom Manage 63:747–757
Clapham S, Daveson BA, Allingham SF, Morris D, Blackburn P, Johnson CE, Eagar K (2021) Patient-reported outcome measurement of symptom distress is feasible in most clinical scenarios in palliative care: an observational study involving routinely collected data. Int J Qual Health Care 19:mzab075
Sebring K, Shattuck J, Berk J et al (2018) Assessing the validity of proxy caregiver reporting for potential palliative care outcome measures in Parkinson’s disease. Palliat Med 32:1522–1528
Laugsand EA, Sprangers MAG, Bjordal K et al (2010) Health care providers underestimate symptom intensities of cancer patients: a multicenter European study. Health Qual Life Outcomes 8:104
Wang JJ, Teng SF, Chu YR et al (2022) Evaluation of opioid consumption trends for pain in Taiwan and comparison with neighboring Asian countries. J Food Drug Anal 30:104–110
Sprangers MA, Schwartz CE (1999) Integrating response shift into health-related quality of life research: a theoretical model. Soc Sci Med 48:1507–1515
Hui D, Bruera E (2020) Models of palliative care delivery for patients with cancer. J Clin Oncol 38:852–865
Lai WS, Liu IT, Tsai JH et al (2021) Hospice delivery models and survival differences in the terminally ill: a large cohort study. BMJ Support Palliat Car
Hedeker D (2003) A mixed-effects multinomial logistic regression model. Stat Med 22:1433–1446
Acknowledgements
We are grateful to the staff and management of the Center for Hospice Palliative Shared Care and the Center for Quantitative Sciences, Clinical Medicine Research Center of National Cheng Kung University Hospital.
Funding
This work was supported in part by Higher Education Sprout Project, Ministry of Education to the Headquarters of University Advancement at National Cheng Kung University (NCKU), the Ministry of Science and Technology (MOST; Taiwan) under Research Grants of MOST 111-2634-F-006-002 and MOST 111-2634-F-006-007, the Ministry of Health and Welfare (MOHW111-TDU-B-221-114005), and the National Cheng Kung University Hospital (NCKUH-11102061).
Author information
Authors and Affiliations
Contributions
ITL, WSL, and PCL had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis; Concept and design: ITL, PCL, WSL, JHT, YTH; Acquisition, analysis, or interpretation of data: ITL, PCL, GLC, YYC; Drafting of the manuscript: ITL, WSL, and PCL; Critical revision of the manuscript for important intellectual content: All authors; Statistical analysis: PFS and YCL; Supervision: PCL.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the institutional review board of NCKUH (B-ER-109–05) and conducted in accordance with the Declaration of Helsinki.
Consent for publication
All authors agree for publication.
Competing interests
The authors declare no competing interests.
Disclaimer
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, IT., Tsai, JH., Lin, PC. et al. The multinomial mixed-effect regression model for predicting PCOC phases in hospice patients. Support Care Cancer 32, 624 (2024). https://doi.org/10.1007/s00520-024-08832-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-024-08832-5