Abstract
Clozapine is a second-generation antipsychotic drug that offers superior treatment results in patients with schizophrenia but is also associated with significant risks. This study analyzes data on pharmacotherapy with clozapine and the associated adverse drug reactions (ADRs) in an inpatient setting including 38,349 patients. Data about the use of clozapine and reports of severe ADRs within the period 1993–2016 were obtained from the multicentered observational pharmacovigilance program “Arzneimittelsicherheit in der Psychiatrie” (AMSP). In total, 586 severe clozapine-associated ADRs were documented (1.53% of all patients exposed). Patients aged ≥65 years had a higher risk of ADRs than patients aged <65 years (1.96 vs. 1.48%; p = 0.021). Significantly more ADRs were attributed to clozapine alone (396; 67.6% of all 586 ADRs) than to a combination with other drugs. The most frequent ADRs were grand mal seizures (0.183% of all 38,349 patients exposed), delirium (0.180%), increased liver enzymes (0.120%), and agranulocytosis (0.107%). We detected 24 cases (0.063%) of clozapine-induced extrapyramidal symptoms, of which 8 (0.021%) were attributed to clozapine alone. Five ADRs resulted in death (0.013%): 2 due to agranulocytosis (41 cases total) (mortality = 4.88%) and 3 due to paralytic (sub)ileus (16 cases) (mortality = 18.75%). The median dose of clozapine in all patients treated was 300 mg/day, in patients who developed ADRs 250 mg/day. The main risk factor for an ADR was pre-existing damage of the affected organ system. Overall, the results of this study highlight the importance of alertness—especially of frequently overlooked symptoms—and appropriate monitoring during treatment with clozapine, even at low doses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schizophrenia is a severe psychiatric disorder characterized by positive and negative symptoms and cognitive impairments. It is one of the 25 most common causes of disability worldwide (Global Burden of Disease Study 2013 Collaborators 2015). This demonstrates the importance of an appropriate treatment of this disease. Antipsychotics are primarily used to treat schizophrenia, mania, and psychotic symptoms due to other illnesses. Antipsychotics are commonly categorized into “first-generation antipsychotics” (FGAs) and “second-generation antipsychotics” (SGAs). FGAs are effective in the treatment of the so called “positive symptoms” such as hallucinations and delusions due to their high antagonistic affinity for dopamine receptors of the D2 subtype (D2-receptors) (Farde et al. 1988; Kapur et al. 2006; Moschny et al. 2021). The possible occurrence of ADRs attributed to this D2-receptor blockade such as extrapyramidal symptoms (EPS) must be considered during prescription. The high risk of EPS and tardive dyskinesia associated with FGAs have contributed to their declining utilization over the years (Leslie and Rosenheck 2002; Toto et al. 2019). SGAs, on the other hand, have a lesser risk of causing EPS and tardive dyskinesia. This has been associated with their comparatively lower affinity for D2 but higher affinities for D1, D4 and D5 receptors as well as for numerous serotonergic (especially the 5-HT2A receptor), adrenergic, histaminergic, and muscarinic receptors (Meltzer et al. 1989; Jann et al. 1993; Moschny et al. 2021). However, the considerable risks of treatment with SGAs should not be neglected, as these drugs can also be associated with increased mortality and sometimes life-threatening adverse drug reactions (ADRs).
Clozapine was the first SGA and following its discovery, numerous other SGAs such as quetiapine, olanzapine, and risperidone followed (Leucht et al. 2013; Moschny et al. 2021). Over the last 20 to 25 years, SGAs have gradually replaced FGAs in everyday clinical practice (Leslie and Rosenheck 2002; Gill et al. 2005). Clozapine launched in the 1970s to the pharmacological market and shaped the term “atypical antipsychotic” or “SGA”. Despite the remarkable chemical, pharmacological and clinical heterogeneity, this term has since been generally applied to other antipsychotics (Möller 2000). Clozapine currently is the “gold standard” in treatment of treatment-resistant schizophrenia and is approved almost exclusively for this area of application (Conley and Kelly 2001; Moore et al. 2007; McIlwain et al. 2011), an example for an exception is the possible treatment of dyskinesia in Parkinson’s disease with clozapine (Fox et al. 2018) and beneficial efficacy outcomes in Parkinson’s disease psychosis (Wagner et al. 2021). Clozapine has a high affinity for D1, D4, serotonin, and α receptors and a comparatively low affinity for D2-receptors. Its high antipsychotic efficacy is primarily due to its affinity for the D4 receptor, whereas the much rarer occurrence of EPS (Schmauss et al. 1989) is attributed to the only mild D2-receptor blockade (Farde et al. 1988; Meltzer et al. 1989; Kapur et al. 2006). Further advantages of clozapine-treatment are a reduction in negative symptoms, comorbid substance use, aggression, and suicidality (Meltzer et al. 2003; Frogley et al. 2012; van der Zalm et al. 2021), as well as improved treatment adherence and patient satisfaction (McEvoy et al. 2006; Stroup et al. 2016).
Since the frequent occurrence of deaths associated with agranulocytosis shortly after the market launch in 1972 (Amsler et al. 1977), the prescription of clozapine has been subject to special conditions. However, agranulocytosis is by far not the only concerning ADR under clozapine with potentially life-threatening sequelae. Although rare, ADRs such as epileptic seizures, delirium, elevated liver enzymes, myocarditis and paralytic ileus are also relevant (Nielsen et al. 2013). Due to the severe ADRs and the more complicated handling of clozapine with obligatory blood cell monitoring, the use of clozapine in clinical practice is often conservative and cautious and often used much later than indicated despite its outstanding efficacy (Wheeler 2008). Early and consistent ADR management and pharmacovigilance are mandatory in the clinical treatment. Therefore, this study analyses the clozapine associated ADRs in an inpatient setting, which are highly relevant for its everyday clinical use. The inpatient data consists of a total of 38,349 patients treated with clozapine during the study period, with 586 documented ADRs attributed to clozapine. This study, therefore, encompasses a much larger and, to our knowledge, unprecedented sample compared to previously published studies. This should provide a more realistic and naturalistic picture of severe clozapine-associated ADRs, of which some are often overlooked in everyday clinical practice. Our aim is to bridge this knowledge gap, clarifying the use of clozapine and the necessity for monitoring of specific ADRs to enhance patients’ safety.
Methods
The AMSP program
Data on the prescription of clozapine and reports of severe ADRs within the period 1993–2016 were obtained from the database of the Drug Safety Program in Psychiatry (German: “Arzneimittelsicherheit in der Psychiatrie”; AMSP) which is a multicentered observational pharmacovigilance program that has been collecting data about psychotropic drug use in psychiatric inpatients since 1993. More than 100 hospitals from Germany, Austria, and Switzerland have participated in the ongoing AMSP-project (Grohmann et al. 2014). In our study-period, the AMSP program gathered reports from 107 hospitals, 67 of which reported clozapine associated ADRs.
The AMSP program records an ADR if it’s “severe”, newly occurring, unusual, occurring during discontinuation of the drug, or in the context of pharmacokinetic interactions. According to AMSP guidelines, an ADR is considered severe if one of the following reasons is met: (1) immediate threat of the event, (2) relevant impairment of the patient’s everyday functions, (3) creating the need for special measures (transfer, surgery), or (4) (for some ADRs) exceeding a quantitative limit (e.g. a drop in neutrophil granulocytes to below 1.500/µl). For each organ system the AMSP protocol provides additional guidelines to further coordinate ADRs and standardize classification (Grohmann et al. 2014).
Assessment and recording of ADRs
To record ADRs, physicians in participating hospitals act as “drug monitors” who document ADRs on standardized questionnaires where the symptoms and classification as “severe” are described in detail. The questionnaires also include age, sex, psychiatric and somatic diagnosis, dosage of the medication, a probability grading (see below), alternative hypotheses, patient-related risk factors, measures taken to treat the ADR, course, and possible previous exposures to the drug as well as supplementary data on laboratory, technical examinations, etc. After the patient’s written consent, the documented cases are re-examined for plausibility by a senior physician of the hospital. In addition to the ADRs, the number of inpatients and patients treated with the respective medication is also documented in the AMSP program. The diagnoses are documented in AMSP as assessed clinically by the treating physicians according to the International Classification of Disease, 10th Version (ICD-10).
The cases are then discussed in regional case conferences, where the documentation forms are completed with further relevant information and sent to the data collection center. There, every case is checked for completeness and plausibility by an experienced psychiatrist of the AMSP program. Twice a year, a selection of cases (e.g., of high clinical interest, severe or unusual cases) is discussed in detail at central case conferences. The cases are critically analyzed in a demographic and data-based context by experts.
Probability grading and categorization of ADRs
The AMSP-program assesses the probability with which an ADR can be attributed to a specific drug. Therefore, the ADRs are differentiated according to the so-called “probability grades” (“W grades”) defined by the AMSP program (Grohmann et al. 2004, 2014):
-
W grade 1 = possible: The ADR is unusual for the drug or its dose, has an unusual time course or the probability of another cause for the ADR is greater than 50%.
-
W grade 2 = probable: The probability of another cause is less than 50%, the ADR matches the time course and is known for the drug.
-
W grade 3 = definite: fulfillment of the W2 criteria plus re-occurrence of the ADR on re-exposure with the drug.
-
W grade 4 = questionable or not sufficiently documented: Causal relationship between the ADR and the drug are not assessable or unclear. The ADR needs to be recorded due to clinical importance, e.g. deaths.
The ADRs analyzed in this study are severe ADRs which have been rated as grade 2 or 3 in the context of clozapine probability.
In this study, the ADRs in clozapine users were further subdivided according to the affected organ system and clinical presentation.
Often, patients are treated with more than one drug. In these cases, multiple drugs can be imputed and be held as responsible for having caused an ADR. Each individual drug then goes through the probability grading process described above. In this study therefore, the ADRs are divided into three groups of imputations: If clozapine alone is held to be responsible for the occurrence of a certain ADR, this is referred to as “imputed alone”. If another drug or several other drugs are causally linked to the ADR in addition to clozapine, the term “imputed in combination” is used. In addition to these two, the term “all cases” refers to both, imputed alone and in combination.
Statistical analysis
The main part of the statistical analysis was carried out with Excel©. ADRs attributed to clozapine were reported as absolute numbers and percentages of the total quantity of ADRs of the respective form of imputation as well as of the total number of patients treated with clozapine. The most frequent ADRs (ADRs which accounted for more than 10% of all 586 documented ADRs) were subject to further statistical tests in the means of chi-square tests and calculations of odds ratios (ORs) and 95% confidence intervals (CIs). The tests were restricted to comparison of the ADR frequency related to age, and sex. The significance level was set to 5% (p > 0.05).
Results
Data on patients treated with clozapine
Between 1993 and 2016, a total of 495,615 psychiatric inpatients were observed as part of the AMSP project. 333,175 of these patients (67.2%) were treated with antipsychotics and 38,349 (7.7%) with clozapine. In 33,573 (87.6% of 38,349) of the patients treated with clozapine, schizophrenia was the patient’s primary diagnosis. Other primary diagnoses as a reason for the current hospitalization such as depression (4.9%), organic disorders (2.9%), neuroses or personality disorders (1.8%), mania (1.6%), and addiction (0.4%) were less frequent.
A majority of patients treated with clozapine were younger than 65 years (34,471 patients; 89.9%) and 3878 patients (10.1%) were 65 years old or older. Therefore, significantly more younger than older patients were treated with clozapine (p < 0.001). 20,939 (54.6%) of the clozapine-treated patients were male, 17,410 (45.4%) were female. Thus, significantly more men than women were treated with clozapine (p < 0.001).
Demographic data of patients with clozapine-associated ADRs
In total 586 ADRs (1.53% of all patients treated with clozapine) were documented. 396 cases (67.6%) were attributed to clozapine alone (“single imputations”) and 190 (32.4%) were attributed to a combination of drugs including clozapine ("multiple imputations”).
Table 1 shows the age and sex of patients who developed an ADR (all cases and single imputations. A significant sex difference in the occurrence of both all clozapine-associated ADRs and those with multiple imputations could not be observed. In 510 of the 586 ADRs cases, the patients were between 18 and 64 years old (87.0%). Overall, patients aged ≥65 years had a significantly higher risk of clozapine-associated ADRs in general than younger patients (p = 0.021) and when clozapine was imputed in combination (p = 0.018). However, there was no significant difference between the two age groups when clozapine was imputed alone (p > 0.05).
Clozapine-associated ADRs
Type and clinical presentation of clozapine-associated ADRs
Table 2 lists all the 586 ADRs that were causally linked to clozapine subdivided by the affected organ system. Regarding all cases, neurological disorders excluding EPS occurred most frequently (17.9% of all ADRs) followed by blood count changes (15.2%). Other common ADRs were delirium and confusion (12.1%), gastrointestinal disorders (11.4%), and cardiovascular disorders (10.2%). A total of 396 ADRs imputed only clozapine, i.e., 67.6% of all clozapine-associated ADRs. The most common ADRs in which clozapine was imputed alone were blood count changes (20.5% of the 396 ADRs imputed alone), cardiovascular disorders (12.9%), and gastrointestinal disorders (12.4%). Figure 1 shows a comparison of the most common ADRs (all cases, single imputations, and multiple imputations).
The ADRs were further specified according to the specific clinical presentation of the ADR (Table 3; Fig. 2). By far the most common ADRs associated with clozapine were grand mal seizures (12.0% of all ADRs) and delirium (11.8%). Liver enzyme elevations (7.9%) and agranulocytosis (7.0%) were also common. The most common ADRs with clozapine in single imputation were delirium (10.35% of “imputed alone” cases), agranulocytosis (8.8%), and elevated liver enzymes (8.6%).
Drugs imputed in clozapine-associated ADRs with multiple imputations
The most frequently used specific drug combinations imputed in the 190 ADRs in multiple imputations are briefly mentioned here. 26 ADRs were documented (13.7% of the 190 ADRs) in which both clozapine and haloperidol were imputed. 25 ADR cases (13.2%) occurred in combination with lithium, 19 (10.0%) in combination with valproic acid. In 16 cases (8.4%), the combination of clozapine and drugs for the treatment of gastrointestinal complaints was imputed. More than 10 ADR cases occurred under combination with each amisulpride (6.8%), chlorprothixene (6.3%), risperidone (5.8%), and levomepromazine (5.8%). In 6 ADR cases, 5 of which presented as epileptic seizures and as ileus in one case, a triple combination of clozapine, haloperidol, and biperiden was imputed. Overall, most ADRs with multiple imputations occurred under combination of clozapine with other SGAs (46 cases, 24.2%) or with butyrophenones (48 cases, 25.3%).
Comparison of the ADRs regarding the different imputations
The most common ADRs were subject to further analyses, particularly in comparison of the different imputations. The most important results are briefly described below and presented in Fig. 1 and Fig. 2. In general (considering all cases), ADRs were significantly more often attributed to clozapine alone than to a combination with other drugs (p < 0.001).
When looking at the ADR frequencies subdivided by the affected organ system (Fig. 1), neurological disorders without EMPS occurred significantly more frequently when clozapine was imputed in combination (33.1% of “imputed in combination” cases) than when imputed alone (10.6% of “imputed alone” cases; p < 0.001). EPS also occurred significantly more frequently under combination imputation (8.4%) than sole imputation of clozapine (2.0%; p < 0.001). In contrast, there were significantly more cases of blood count changes with clozapine being imputed alone (20.5%) than imputed in combination with other drugs (4.2%; p < 0.001). Cardiovascular disturbances also were significantly more frequently observed when clozapine was imputed alone (12.9%) than in combination (4.7%; p = 0.002).
Regarding the specific ADRs (Fig. 2) grand mal seizures appeared significantly more often with clozapine being imputed in combination (25.8%) than alone (5.3%; p < 0.001). (Sub-)ileus also occurred significantly more often when clozapine was imputed in combination (6.3%) than alone (1.0%; p < 0.001). In contrast, agranulocytosis occurred significantly more frequently with clozapine alone being imputed (8.8%) than in combination (3.2%; p = 0.012). Neutropenia (6.3%) occurred exclusively when clozapine was imputed alone, and therefore significantly more often than under combination imputation (p < 0.001). Same applies to eosinophilia (5.1%; p = 0.002).
Analyses of the most common ADRs in relation to sex and age
Table 4 lists the most common ADRs according to sex and age of the affected patients.
Sex—Women were significantly more frequently affected by blood count changes than men (p = 0.021; OR = 0.59; CI: 0.37–0.93). In contrast, significantly more cardiovascular disorders occurred in men than in women (p = 0.011; OR = 2.12; CI: 1.18–3.82).
Age—Differences related to age were only found for neurological disorders and for delirium and confusion. Regarding differences related to age, neurological disorders affected significantly more often younger patients (<65 years) than older patients (≥65 years; p = 0.034; OR = 2.34; CI: 1.05–5.26). Delirium and confusion affected older patients significantly more often than younger patients (p = 0.011; OR = 0.45; CI: 0.24–0.84).
Dosage of clozapine
The median dose in all patients treated with clozapine was 300 mg/day. The median dose in patients who developed a clozapine-related ADR (imputed alone) was 250 mg/day. The median dose in all the most common types of clozapine-associated ADRs (listed in Table 5) was less than 450 mg/day. The highest median doses were found in Pisa syndromes (412.5 mg/day) and grand mal seizures (400 mg/day). The exact median doses for the most frequent ADRs associated with clozapine are listed in Table 5.
Risk factors for the development of ADRs under clozapine treatment
In slightly more than half of the ADR-patients (326, 55.6% of all ADR cases) no risk factors were documented. At least one risk factor was documented in 260 patients (44.4%), with the most common risk factor being previous damage to the affected organ system in 114 patients (19.5%), most often in patients with a toxic delirium (N = 22), grand mal seizures (N = 18), or a Pisa syndrome (N = 9) including cerebral atrophy, cerebrovascular lesions, dementia, hypoxic perinatal, or traumatic cerebral damage as the most common pre-existing impairments. Other risk factors, all of which accounted for less than 10% of the ADRs, were ADR sensitivity, rapid dose titration, reduction of dose or discontinuation, infection, change in smoking habits, high starting dose, previous treatment with other antipsychotics, and harmful use/addiction. Risk factors were by far most often documented in ADR cases of delirium: Risk factors were present in 50 of 69 cases of delirium (72.5% of all delirium cases).
Measures taken to counteract the ADRs and course of the ADRs
Countermeasures—Clozapine was discontinued in 393 cases (67.1% of all ADR cases). The dose of clozapine was reduced in 153 patients (26.1%). In only 13 cases (2.2%), clozapine treatment was continued at the same dosage. In 136 patients with ADRs (23.2%), other specialists were consulted, 107 patients (18.3%) were transferred to another specialty department. Drugs to counteract the ADR were used in 214 of the ADR cases (36.5%), in 119 patients (20.3%) the ADR was counteracted by non-pharmacological measures. In 17 ADR cases (2.9%) no specific measures were taken or documented.
Course of the ADR—In 447 cases (76.3%), the clozapine-associated ADR subsided completely. In 92 patients (15.7%), the ADR was reported to be in remission at the end of the observation period, whereas in 40 cases, the ADR remained stable/unchanged. In 2 cases (0.3%) the further course was unknown. In 5 cases, the affected patient (0.9%; 5 out of 586) died because of the ADR.
Clozapine-associated ADRs resulting in death
Agranulocytosis—Among the 5 deaths (0.013% of all 38,349 patients treated with clozapine), 2 were the result of agranulocytosis. With a total of 41 cases of clozapine-induced agranulocytosis, the mortality of this ADR was 4.9%. In both cases, clozapine was imputed in combination with other psychotropic drugs, in one case with mirtazapine, in the other with quetiapine.
(Sub-)Ileus—In 3 patients, a paralytic (sub-)ileus resulted in the affected patient’s death. With a total of 16 cases of clozapine-induced (sub-)ileus, this implies a mortality of 18.8%. In all cases of fatal (sub-)ileus, the patients had pre-existing damage to the intestine and concomitantly used drugs with additive anticholinergic and/or constipating effects (i.e., pirenzepine, mebeverine, amitriptyline, perazine, biperiden, levomepromazine, chlorprothixene, haloperidol).
Comparison of clozapine-associated ADR frequencies to ADR frequencies of other antipsychotics
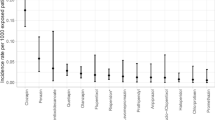
Figure 3 shows the ADR rate of different antipsychotics in relation to the number of patients treated with the respective drug. A table with the exact data can be found in the supplementary material of this study.
A total of 333,175 patients were treated with antipsychotics in general of which 3591 (1.08%) developed an ADR. A majority of the reported ADRs were associated with the use of SGAs with 1.23% of all patients treated with SGAs developing an ADR.
Amisulpride was the antipsychotic with the highest ADR rate (1.57% of patients treated with amisulpride) closely followed by clozapine (1.53% of patients treated with clozapine). Overall, clozapine was therefore significantly more often imputed in ADRs than e.g., haloperidol (p < 0.001), olanzapine (p = 0.007), and risperidone (p < 0.001). There was no significant difference between the ADR frequency of clozapine and amisulpride (p = 0.705).
When considering “single imputation” ADRs, clozapine was most commonly imputed (1.03%) followed by amisulpride (0.97%) and olanzapine (0.77%). Clozapine-associated ADRs were therefore significantly more often observed than olanzapine-associated ones (p < 0.001). In comparison to amisulpride, no significant difference was found (p = 0.552).
Discussion
Of the 38,349 patients receiving clozapine, 586 developed an ADR. Neurological disorders (excluding EPS) occurred most frequently followed by blood count changes. Other common ADRs were delirium and confusion, gastrointestinal disorders, and cardiovascular disorders. A total of 396 ADRs imputed only clozapine, 190 ADRs imputed multiple drugs including clozapine. In general (considering all cases), ADRs were significantly more often attributed to clozapine alone than to a combination with other drugs. Women were significantly more frequently affected by blood count changes than men. In contrast, significantly more cardiovascular disorders occurred in men than in women. Regarding differences related to age, neurological disorders affected younger patients (<65 years) than older patients (≥65 years) significantly more often. Delirium and confusion, however, affected older patients significantly more often than younger patients. Five deaths due to clozapine treatment were documented, 2 were the result of agranulocytosis and 3 of a paralytic (sub-)ileus. Therefore, we found the (sub-)ileus to be the most lethal ADR of clozapine.
When comparing the ADR rate of individual antipsychotics with one another, clozapine was the antipsychotic with the second highest occurrence of ADRs, only exceeded by amisulpride (although in a statistically insignificant manner). This is most likely due to the large number of reports of hyperprolactinemia and galactorrhea under treatment with amisulpride (Glocker et al. 2021), which is a fairly easily detectable and also less dangerous ADR than common severe ADRs due to clozapine such as a toxic delirium, grand mal seizures, or agranulocytosis/neutropenia.
Clozapine-induced ADRs according to age and sex
Older patients may be more susceptible to ADRs, as has also been described for clozapine-associated ADRs (Ismail et al. 2012; Bishara and Taylor 2014). A frequently stated reason for this phenomenon is the increasing tendency towards polypharmacy in older patients (Spina and de Leon 2007). We found older patients to be significantly more often affected by an ADR when a combination of drugs was imputed and in contrast no significant age-difference when clozapine was imputed alone. Van der Horst et al. (2023), on the other hand, concluded that neither monotherapy nor polytherapy with clozapine increased the risk of ADRs. The same study found women to be significantly more often affected by ADRs than men (van der Horst et al. 2023), a finding which could not be replicated in our study, perhaps due to the different study designs. The study by van der Horst et al. (2023) had a cross-sectional design which limited the authors ability to establish causality between drugs and ADRs a limitation, which is not shared by the present study in which causality is meticulously assessed by the AMSP team members.
Association of clozapine dosage and the occurrence of ADRs
In our study, patients with clozapine-associated ADRs were treated with a lower median dose of clozapine than what was used for all 38,349 patients (250 vs. 300 mg/day). Our results suggest that many clozapine-associated ADRs tend to occur at lower doses, such as during the titration phase, which is often performed during inpatient stay. The dose-dependency of clozapine-induced ADRs is not fully clarified yet (Chandru and Gunja 2023) and further studies are needed, as the data appears to be very limited (Siafis et al. 2023). However, in general, the individual plasma concentration of clozapine seems to be more relevant than the actual dose (Wohkittel et al. 2016; Siskind et al. 2021). A systematic review from 2022 found an association between ADRs and clozapine plasma levels for EEG abnormalities, heart rate variability, obsessive–compulsive symptoms, hyperinsulinemia, metabolic syndrome, and constipation. Therefore, they stated that therapeutic drug monitoring (TDM) of plasma clozapine concentrations has a significant benefit in clinical practice, as it improves drug safety and patient satisfaction (Skokou et al. 2022).
Clozapine-associated ADRs
Epileptic seizures
In general, clozapine has a higher risk of seizures than other antipsychotics (Druschky et al. 2019). In the present study, grand mal seizures were found to be the most frequent clozapine-associated ADR. The risk for clozapine-induced seizures was significantly higher when other drugs that potentially lower the seizure threshold were concomitantly administered. A recent Japanese study on clozapine-induced epileptic seizures associated clozapine with an increased risk of seizures, especially at high doses (>400 mg/day) (Hatano et al. 2023). Of 1874 clozapine-associated ADRs, 11.04% were seizures (Hatano et al. 2023), very similar to our results. High doses, younger age, antipsychotic polypharmacy, and combination with lithium were also significantly associated with the occurrence of clozapine-induced seizures (Hatano et al. 2023). However, unlike Hatano et al. 2023, we were unable to confirm an age-dependent effect of clozapine-associated seizures. On the other hand, other studies found clozapine-induced electroencephalography (EEG) abnormalities to be more common in younger than in older patients (Kikuchi et al. 2014). It has been suggested that clozapine-induced seizures are not necessarily precipitated by nonspecific EEG abnormalities (Treves and Neufeld 1996; Chung et al. 2002). In general, minor EEG abnormalities in patients treated with clozapine are very common (Günther et al. 1993) and appear to correlate with the plasma clozapine concentration (Varma et al. 2011). Because these findings only rarely reveal evidence of seizure activity, routine EEG monitoring is not generally recommended (Kar et al. 2016). Moreover, seizures in patients treated with clozapine with or without a known seizure disorder can generally be efficiently managed with antiepileptics, therefore, they should not be considered a reason for clozapine discontinuation (Nielsen et al. 2013).
Cardiovascular disorders
In general, the use of clozapine cannot be causally linked to an increased mortality or morbidity due to cardiovascular complications in patients treated with clozapine (Joy et al. 2017). However, clozapine is one of the antipsychotics with the highest risk of cardiovascular ADRs (Friedrich et al. 2020) and potentially fatal cardiovascular clozapine-associated ADRs, such as clozapine-induced myocarditis and cardiomyopathy, though both rare, are well known. A meta-analysis from 2016 analyzed studies on cardiac ADRs due to clozapine and observed an incidence of myocarditis of <0.1–1% and of cardiomyopathy of <0.01–0.1% with an average mortality rate of 25% (Curto et al. 2016). Our study found the cardiovascular system to be the fifth most common affected organ system by clozapine-induced ADRs, however, no fatal outcomes were observed. Whether long-term consequences of the ADRs and associated mortality occurred after discharge could not be accurately assessed due to the exclusive clinical setting of our study. While we found that significantly more men than women were affected by clozapine-associated cardiovascular complications, data in the current literature on sex-differences in relation to these ADRs is lacking.
Neutropenia and agranulocytosis
The perhaps most prominent and most feared clozapine-induced ADR is agranulocytosis. Indeed, the risk of agranulocytosis of clozapine by far exceeds that of other antipsychotics and is highest within the first 3 months of treatment (Glocker et al. 2023). In 2018, a meta-analysis including 108 studies on the epidemiology of clozapine-associated neutropenia (<1500 neutrophils/μl) found that the longitudinal incidence per 100 person-years of exposure was 3.8% and of severe neutropenia it was 0.9% (defined as <500/μl ≙ agranulocytosis according to AMSP criteria). The incidence of clozapine-induced neutropenia-related deaths was 0.013% (Myles et al. 2018). In our study, the incidence of agranulocytosis-related deaths amongst all patients treated with clozapine was 0.005% and the lethality of clozapine-induced agranulocytosis was 4.9%. No deaths were associated with neutropenia, so the lethality of both ADRs together was 3.0%. Agranulocytosis and neutropenia occurred significantly more frequently with single imputation of clozapine than in combination with other drugs. Other studies also did not attribute polypharmacy including clozapine to a higher ADR risk (van der Horst et al. 2023). Our results support the general consensus that agranulocytosis can occur at low doses (Choudhury et al. 2021), for example during the titration phase. Unlike many other ADRs, higher age does not appear to be a risk factor for clozapine-induced neutropenia and agranulocytosis in our study. This result is contrary to findings of other authors who found higher age to be associated with higher risk for neutropenia and agranulocytosis (Alvir et al. 1993; Balda et al. 2015; Lorenzo-Villalba et al. 2020). While we found that women were significantly more frequently affected by blood count changes than men, a more recent study from 2016 challenges this notion suggesting women had a lower risk of clozapine-associated dyscrasias (Demler et al. 2016). Due to these inconsistent findings, altered monitoring intervals according to age or sex are currently not available.
Severe weight gain
The propensity of SGAs to cause weight gain is perhaps one of their most worrisome ADRs, especially because of the long-term health implications (Doane et al. 2022). While clozapine’s risk of weight gain is exceeded for example by olanzapine, it still remains an antipsychotic with one of the highest risks of this ADR (Schneider et al. 2020). The current study found a frequency of clozapine-induced severe weight gain of 0.089%, which is considerably lower than in comparative studies: In a retrospective study on weight gain under clozapine treatment the cumulative incidence of patients who became significantly overweight during clozapine treatment was over 50% (Umbricht et al. 1994). A study from 2010 found a mean increase in weight of 2.62 kg/week in 72.41% of Asian patients treated with clozapine (Mahendran et al. 2010). The much lower incidence of weight gain in our study may be the result of the inpatient setting of our study: While antipsychotic-induced weight gain begins early during treatment, it may continue over a longer period of time (i.e., after discharge). Moreover, patients treated with clozapine may already be overweight or have gained weight due to previous treatment with antipsychotics (Doane et al. 2022), perhaps making weight gain less apparent or more difficult to attribute to clozapine. This fact and, as will be discussed in more detail below, the underreporting of ADRs should also be considered.
Gastrointestinal disorders
Perhaps overshadowed by agranulocytosis, clozapine’s propensity to impair gastrointestinal motility is often overlooked (Shirazi et al. 2016; Cohen 2017). This effect is most likely due to clozapine’s anticholinergic, antihistaminergic, and anti-serotonergic properties resulting in the slowdown of intestinal peristalsis (Xu et al. 2021). An observational study including 188 patients found a significantly higher incidence of 2.12% of constipation and ileus during treatment with clozapine (Ingimarsson et al. 2018) compared to our study (0.05%). Three of the 5 documented deaths under clozapine treatment were attributable to this ADR, the (sub-)ileus therefore was the most lethal ADR of clozapine in our study, even more lethal than agranulocytosis. Cohen compared studies on the occurrence of clozapine-induced gastrointestinal hypomobility and agranulocytosis. He also found a higher mortality rate for gastrointestinal hypomobility (15–27.5%) than for agranulocytosis (2.2–4.2%) (Cohen 2017). This alarming finding calls for new standards in monitoring for the (sub-)ileus in patients treated with clozapine. Moreover, it is imperative to avoid the concomitant use of other drugs with strong anticholinergic properties in patients treated with clozapine whenever possible as this can further increase the risk of ileus: In both cases of fatal clozapine-induced ileus in our study, an array of other anticholinergic drugs such as pirenzepine were used. High clozapine dose is known to be a risk factor for the development (Palmer et al. 2008) and mortality (West et al. 2017) of clozapine-induced ileus. Although anticholinergic properties appear to have dose-dependent effects (Lavrador et al. 2021), the results of our study emphasize monitoring patients even with lower dose, as the median dose in patients with an ileus was “only” 262 mg/day and therefore lower than in other ADRs such as seizures.
Delirium
Delirium was the second most commonly observed clinical presentation of clozapine-induced ADRs in the present study. A study on 139 psychiatric inpatients from 2003 suggested that delirium occurs in up to 10% of inpatients treated with clozapine and particularly affects older patients (Centorrino et al. 2003). While our incidence of delirium was extensively lower, our study also confirms the aforementioned age-dependent effect. Moreover, we found that the risk of delirium was higher in patients treated with multiple drugs, confirming the frequent assumption that polypharmacy increases the risk of delirium (Kurisu et al. 2020). In our study, delirium was particularly often associated with risk factors (72.5% of all delirious patients). This correlation has also been reported by other authors (Pisani et al. 2002). One reason for the high number of risk factors could be the comparatively higher age of the patients with clozapine-associated delirium, pre-existing brain damage (such as dementia), and a more complex medical history including a longer medication list.
Extrapyramidal symptoms
Upon its discovery, clozapine was initially considered devoid of EPS (Beckmann et al. 1979). Even now, only a few descriptions and/or case reports of EPS under clozapine treatment are available (Cohen et al. 1991; Grover and Sahoo 2015). Although also rare in this study, EPS—almost exclusively in form of a Pisa syndrome—could be attributed to clozapine alone in a total of 8 cases and in 24 cases, clozapine was at least one of the drugs involved. In so, EPS under clozapine occurred primarily in combination with other drugs. When using therapeutic doses of clozapine, it occupies 40–50% of D2 receptors and is therefore generally too low to induce EPS (Gerlach et al. 1996) for which the threshold lies at 80% D2 receptor occupancy (Siafis et al. 2023). When combining clozapine with other (antipsychotic) drugs with antagonist properties at D2 receptors, additive D2 receptor antagonism may induce or aggravate EPS. However, the administration of clozapine can also lead to an improvement in antipsychotic-induced EPS (Wong et al. 2022). These heterogenous effects of clozapine in causing or improving EPS render the matter inconclusive for now and call for further research. We conclude that regular monitoring of EPS—even in patients treated with clozapine—should not be neglected.
Strengths and limitations
AMSP enables the detection of even rare ADRs due to its nature as a structured multicentral drug monitoring program with an observation period of 23 years of almost half a million psychiatric inpatients. Therefore, it reflects a particularly realistic patient setting. The detection of ADRs was made clinically rather than with inclusion of determined research criteria. This is certainly an advantage for the transferability of the results to everyday care, as a “real-life” patient collective was examined, rather than a highly selected one. Unlike naturalistic studies in outpatient settings that are often only able to assess prescription rates, AMSP is able to assess actual drug utilization rates. Moreover, all ADRs recorded by AMSP are vigorously discussed allowing us to determine causality of adverse events and drugs with high certainty.
However, the study has some limitations that must be considered while interpreting the results. The data only includes inpatients from hospitals in German-speaking countries. The results may therefore not be generalizable to outpatient settings or treatment practices on an international level. Drugs such as clozapine may have a different spectrum and/or frequency of ADRs at the beginning of treatment or when adjustment such as changes in dose or concomitantly used drugs are made than in long-term outpatient-treatment or after discharge. Psychiatric inpatients are generally more severely ill and may suffer from a higher degree of comorbidity, which can influence the type and frequency of ADRs. The analysis of the risk factors for the development of a clozapine-associated ADR is limited to the patients who developed an ADR because AMSP only records the risk factors for these patients but not for all patients, so we cannot make any comparisons here. The incidence of many ADRs appears to be significantly lower compared to other studies, a reason being the sole recording of severe ADRs and AMSP’s strict inclusion criteria. Additionally, many adverse effects occur with unspecific symptoms, which may have been incorrectly assessed as not drug related. Moreover, ADRs such as sedation and weight gain are documented much less frequently than ADRs that may seem more dangerous at first glance, such as agranulocytosis or epileptic seizures. The AMSP program is an induced spontaneous reporting system, which cannot have the same reliability in recording or reporting ADRs as a clinical trial can. Bias due to inadequate reporting must be considered as individual clinicians report ADRs in addition to their regular work according to their individual resources, motivation and evaluation habits. Depending on the time, motivation and financial resources of the participating hospital, individual and institutional bias in the sense of underreporting cannot be ruled out and is difficult to combat. To reduce this risk and to ensure that data is as objective as possible, all ADRs were thoroughly analyzed prior to inclusion in the AMSP database. It can therefore be assumed that the validity of the data is high.
Conclusion
To our knowledge, the present study is the first to assess severe clozapine-associated ADRs in such a large sample of inpatients (N = 38,349 patients). The results of this study highlight the eminent importance of alertness and appropriate monitoring during treatment with clozapine. While much attention is paid to clozapine’s most prominent ADR, agranulocytosis, for which a mandatory monitoring has long been established, other perhaps even more dangerous ADR such as ileus should not be underestimated. Knowledge about the possible occurrence of ADRs under clozapine treatment, especially about the frequently occurring severe ones, is essential for everyday treatment. We recommend paying special attention to frequently overlooked symptoms such as constipation, as clozapine-associated ileus appears to have a particularly high mortality. In so, abdominal examinations, including exanimation of the intestinal peristalsis, should regularly be performed. Though they are easy to perform and low in cost, this type of examination is often neglected in clinical practice compared to laboratory tests, EEGs, and electrocardiograms. This can have fatal consequences for the patients’ health and can even lead to death. Further prospective real-world data in treatment-resistant schizophrenia is needed to assess both inpatient and outpatient drug safety quality when initiating and managing clozapine treatment. Overcoming barriers to clozapine underutilization is substantially established when maximizing drug safety in clozapine-treated patients.
Data availability
The datasets referred to and/or analyzed in the present study are available from the corresponding author on reasonable request.
References
Alvir JM, Lieberman JA, Safferman AZ et al (1993) Clozapine-induced agranulocytosis. Incidence and risk factors in the United States. N Engl J Med 329:162–167. https://doi.org/10.1056/NEJM199307153290303
Amsler HA, Teerenhovi L, Barth E et al (1977) Agranulocytosis in patients treated with clozapine. A study of the Finnish epidemic. Acta Psychiatr Scand 56:241–248. https://doi.org/10.1111/j.1600-0447.1977.tb00224.x
Balda MV, Garay OU, Papale RM et al (2015) Clozapine-associated neutropenia and agranulocytosis in Argentina (2007–2012). Int Clin Psychopharmacol 30:109–114. https://doi.org/10.1097/YIC.0000000000000060
Beckmann B, Hippius H, Rüther E (1979) Treatment of schizophrenia. Prog Neuropsychopharmacol 3:47–52. https://doi.org/10.1016/0364-7722(79)90068-7
Bishara D, Taylor D (2014) Adverse effects of clozapine in older patients: epidemiology, prevention and management. Drugs Aging 31:11–20. https://doi.org/10.1007/s40266-013-0144-2
Centorrino F, Albert MJ, Drago-Ferrante G et al (2003) Delirium during clozapine treatment: incidence and associated risk factors. Pharmacopsychiatry 36:156–160. https://doi.org/10.1055/s-2003-41201
Chandru P, Gunja N (2023) Toxicity and adverse effects in clozapine-related presentations to a medical toxicology service in Western Sydney. J Med Toxicol 19:374–380. https://doi.org/10.1007/s13181-023-00963-1
Choudhury S, Banerjee S, Chatterjee K et al (2021) Low-dose clozapine-induced agranulocytosis in patients with movement disorders-retrospective study from India. Ann Indian Acad Neurol 24:831–832. https://doi.org/10.4103/aian.AIAN_522_20
Chung S-J, Jeong S-H, Ahn Y-M et al (2002) A retrospective study of clozapine and electroencephalographic abnormalities in schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry 26:139–144. https://doi.org/10.1016/s0278-5846(01)00238-x
Cohen D (2017) Clozapine and Gastrointestinal Hypomotility. CNS Drugs 31:1083–1091. https://doi.org/10.1007/s40263-017-0481-5
Cohen BM, Keck PE, Satlin A, Cole JO (1991) Prevalence and severity of akathisia in patients on clozapine. Biol Psychiatry 29:1215–1219. https://doi.org/10.1016/0006-3223(91)90329-k
Conley RR, Kelly DL (2001) Management of treatment resistance in schizophrenia. Biol Psychiatry 50:898–911. https://doi.org/10.1016/s0006-3223(01)01271-9
Curto M, Girardi N, Lionetto L et al (2016) Systematic review of clozapine cardiotoxicity. Curr Psychiatry Rep 18:68. https://doi.org/10.1007/s11920-016-0704-3
Demler TL, Morabito NE, Meyer CE, Opler L (2016) Maximizing clozapine utilization while minimizing blood dyscrasias: evaluation of patient demographics and severity of events. Int Clin Psychopharmacol 31:76–83. https://doi.org/10.1097/YIC.0000000000000108
Doane MJ, Bessonova L, Friedler HS et al (2022) Weight gain and comorbidities associated with oral second-generation antipsychotics: analysis of real-world data for patients with schizophrenia or bipolar I disorder. BMC Psychiatry 22:114. https://doi.org/10.1186/s12888-022-03758-w
Druschky K, Bleich S, Grohmann R et al (2019) Seizure rates under treatment with antipsychotic drugs: data from the AMSP project. World J Biol Psychiatry 20:732–741. https://doi.org/10.1080/15622975.2018.1500030
Farde L, Wiesel FA, Halldin C, Sedvall G (1988) Central D2-dopamine receptor occupancy in schizophrenic patients treated with antipsychotic drugs. Arch Gen Psychiatry 45:71–76. https://doi.org/10.1001/archpsyc.1988.01800250087012
Fox SH, Katzenschlager R, Lim S-Y et al (2018) International Parkinson and movement disorder society evidence-based medicine review: Update on treatments for the motor symptoms of Parkinson’s disease. Mov Disord 33:1248–1266. https://doi.org/10.1002/mds.27372
Friedrich M-E, Winkler D, Konstantinidis A et al (2020) Cardiovascular adverse reactions during antipsychotic treatment: results of AMSP, a drug surveillance program between 1993 and 2013. Int J Neuropsychopharmacol 23:67–75. https://doi.org/10.1093/ijnp/pyz046
Frogley C, Taylor D, Dickens G, Picchioni M (2012) A systematic review of the evidence of clozapine’s anti-aggressive effects. Int J Neuropsychopharmacol 15:1351–1371. https://doi.org/10.1017/S146114571100201X
Gerlach J, Lublin H, Peacock L (1996) Extrapyramidal symptoms during long-term treatment with antipsychotics: special focus on clozapine and D1 and D2 dopamine antagonists. Neuropsychopharmacology 14:35S-39S. https://doi.org/10.1016/0893-133X(95)00203-P
Gill SS, Rochon PA, Herrmann N et al (2005) Atypical antipsychotic drugs and risk of ischaemic stroke: population based retrospective cohort study. BMJ 330:445. https://doi.org/10.1136/bmj.38330.470486.8F
Global Burden of Disease Study (2013) Collaborators (2015) Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 386:743–800. https://doi.org/10.1016/S0140-6736(15)60692-4
Glocker C, Grohmann R, Engel R et al (2021) Galactorrhea during antipsychotic treatment: results from AMSP, a drug surveillance program, between 1993 and 2015. Eur Arch Psychiatry Clin Neurosci 271:1425–1435. https://doi.org/10.1007/s00406-021-01241-3
Glocker C, Grohmann R, Burkhardt G et al (2023) Antipsychotic drug-induced neutropenia: results from the AMSP drug surveillance program between 1993 and 2016. J Neural Transm (vienna) 130:153–163. https://doi.org/10.1007/s00702-023-02589-7
Grohmann R, Engel RR, Rüther E, Hippius H (2004) The AMSP drug safety program: methods and global results. Pharmacopsychiatry 37(Suppl 1):S4-11. https://doi.org/10.1055/s-2004-815505
Grohmann R, Engel RR, Möller H-J et al (2014) Flupentixol use and adverse reactions in comparison with other common first- and second-generation antipsychotics: data from the AMSP study. Eur Arch Psychiatry Clin Neurosci 264:131–141. https://doi.org/10.1007/s00406-013-0419-y
Grover S, Sahoo S (2015) Clozapine induced akathisia: a case report and review of the evidence. Indian J Pharmacol 47:234–235. https://doi.org/10.4103/0253-7613.153441
Günther W, Baghai T, Naber D et al (1993) EEG alterations and seizures during treatment with clozapine. A retrospective study of 283 patients. Pharmacopsychiatry 26:69–74. https://doi.org/10.1055/s-2007-1014345
Hatano M, Yamada K, Matsuzaki H et al (2023) Analysis of clozapine-induced seizures using the Japanese Adverse Drug Event Report database. PLoS ONE 18:e0287122. https://doi.org/10.1371/journal.pone.0287122
Ingimarsson O, MacCabe JH, Sigurdsson E (2018) Constipation, ileus and medication use during clozapine treatment in patients with schizophrenia in Iceland. Nord J Psychiatry 72:497–500. https://doi.org/10.1080/08039488.2018.1517189
Ismail Z, Wessels AM, Uchida H et al (2012) Age and sex impact clozapine plasma concentrations in inpatients and outpatients with schizophrenia. Am J Geriatr Psychiatry 20:53–60. https://doi.org/10.1097/JGP.0b013e3182118318
Jann MW, Grimsley SR, Gray EC, Chang WH (1993) Pharmacokinetics and pharmacodynamics of clozapine. Clin Pharmacokinet 24:161–176. https://doi.org/10.2165/00003088-199324020-00005
Joy G, Whiskey E, Bolstridge M et al (2017) Hearts and minds: real-life cardiotoxicity with clozapine in psychosis. J Clin Psychopharmacol 37:708–712. https://doi.org/10.1097/JCP.0000000000000792
Kapur S, Agid O, Mizrahi R, Li M (2006) How antipsychotics work-from receptors to reality. NeuroRx 3:10–21. https://doi.org/10.1016/j.nurx.2005.12.003
Kar N, Barreto S, Chandavarkar R (2016) Clozapine monitoring in clinical practice: beyond the mandatory requirement. Clin Psychopharmacol Neurosci 14:323–329. https://doi.org/10.9758/cpn.2016.14.4.323
Kikuchi YS, Sato W, Ataka K et al (2014) Clozapine-induced seizures, electroencephalography abnormalities, and clinical responses in Japanese patients with schizophrenia. Neuropsychiatr Dis Treat 10:1973–1978. https://doi.org/10.2147/NDT.S69784
Kurisu K, Miyabe D, Furukawa Y et al (2020) Association between polypharmacy and the persistence of delirium: a retrospective cohort study. Biopsychosoc Med 14:25. https://doi.org/10.1186/s13030-020-00199-3
Lavrador M, Castel-Branco MM, Cabral AC et al (2021) Association between anticholinergic burden and anticholinergic adverse outcomes in the elderly: Pharmacological basis of their predictive value for adverse outcomes. Pharmacol Res 163:105306. https://doi.org/10.1016/j.phrs.2020.105306
Leslie DL, Rosenheck RA (2002) From conventional to atypical antipsychotics and back: dynamic processes in the diffusion of new medications. Am J Psychiatry 159:1534–1540. https://doi.org/10.1176/appi.ajp.159.9.1534
Leucht S, Cipriani A, Spineli L et al (2013) Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 382:951–962. https://doi.org/10.1016/S0140-6736(13)60733-3
Lorenzo-Villalba N, Alonso-Ortiz MB, Maouche Y et al (2020) Idiosyncratic drug-induced neutropenia and agranulocytosis in elderly patients. J Clin Med 9:1808. https://doi.org/10.3390/jcm9061808
Mahendran R, Hendricks M, Chan YH (2010) Weight gain in Asian patients on second-generation antipsychotics. Ann Acad Med Singap 39:118–121
McEvoy JP, Lieberman JA, Stroup TS et al (2006) Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry 163:600–610. https://doi.org/10.1176/ajp.2006.163.4.600
McIlwain ME, Harrison J, Wheeler AJ, Russell BR (2011) Pharmacotherapy for treatment-resistant schizophrenia. Neuropsychiatr Dis Treat 7:135–149. https://doi.org/10.2147/NDT.S12769
Meltzer HY, Bastani B, Ramirez L, Matsubara S (1989) Clozapine: new research on efficacy and mechanism of action. Eur Arch Psychiatry Neurol Sci 238:332–339. https://doi.org/10.1007/BF00449814
Meltzer HY, Alphs L, Green AI et al (2003) Clozapine treatment for suicidality in schizophrenia: international suicide prevention trial (InterSePT). Arch Gen Psychiatry 60:82–91. https://doi.org/10.1001/archpsyc.60.1.82
Möller HJ (2000) State of the art of drug treatment of schizophrenia and the future position of the novel/atypical antipsychotics. World J Biol Psychiatry 1:204–214. https://doi.org/10.3109/15622970009150593
Moore TA, Buchanan RW, Buckley PF et al (2007) The Texas Medication Algorithm Project antipsychotic algorithm for schizophrenia: 2006 update. J Clin Psychiatry 68:1751–1762. https://doi.org/10.4088/jcp.v68n1115
Moschny N, Hefner G, Grohmann R et al (2021) Therapeutic drug monitoring of second- and third-generation antipsychotic drugs-influence of smoking behavior and inflammation on pharmacokinetics. Pharmaceuticals (basel) 14:514. https://doi.org/10.3390/ph14060514
Myles N, Myles H, Xia S et al (2018) Meta-analysis examining the epidemiology of clozapine-associated neutropenia. Acta Psychiatr Scand 138:101–109. https://doi.org/10.1111/acps.12898
Nielsen J, Correll CU, Manu P, Kane JM (2013) Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided? J Clin Psychiatry 74:603–613. https://doi.org/10.4088/JCP.12r08064. (quiz 613)
Palmer SE, McLean RM, Ellis PM, Harrison-Woolrych M (2008) Life-threatening clozapine-induced gastrointestinal hypomotility: an analysis of 102 cases. J Clin Psychiatry 69:759–768. https://doi.org/10.4088/jcp.v69n0509
Pisani F, Oteri G, Costa C et al (2002) Effects of psychotropic drugs on seizure threshold. Drug Saf 25:91–110. https://doi.org/10.2165/00002018-200225020-00004
Schmauss M, Wolff R, Erfurth A, Rüther E (1989) Tolerability of long term clozapine treatment. Psychopharmacology 99(Suppl):S105-108. https://doi.org/10.1007/BF00442572
Schneider M, Pauwels P, Toto S et al (2020) Severe weight gain as an adverse drug reaction of psychotropics: Data from the AMSP project between 2001 and 2016. Eur Neuropsychopharmacol 36:60–71. https://doi.org/10.1016/j.euroneuro.2020.05.001
Shirazi A, Stubbs B, Gomez L et al (2016) Prevalence and predictors of clozapine-associated constipation: a systematic review and meta-analysis. Int J Mol Sci 17:863. https://doi.org/10.3390/ijms17060863
Siafis S, Wu H, Wang D et al (2023) Antipsychotic dose, dopamine D2 receptor occupancy and extrapyramidal side-effects: a systematic review and dose-response meta-analysis. Mol Psychiatry 28:3267–3277. https://doi.org/10.1038/s41380-023-02203-y
Siskind D, Sharma M, Pawar M et al (2021) Clozapine levels as a predictor for therapeutic response: a systematic review and meta-analysis. Acta Psychiatr Scand 144:422–432. https://doi.org/10.1111/acps.13361
Skokou M, Karavia EA, Drakou Z et al (2022) Adverse drug reactions in relation to clozapine plasma levels: a systematic review. Pharmaceuticals (basel) 15:817. https://doi.org/10.3390/ph15070817
Spina E, de Leon J (2007) Metabolic drug interactions with newer antipsychotics: a comparative review. Basic Clin Pharmacol Toxicol 100:4–22. https://doi.org/10.1111/j.1742-7843.2007.00017.x
Stroup TS, Gerhard T, Crystal S et al (2016) Comparative effectiveness of clozapine and standard antipsychotic treatment in adults with schizophrenia. Am J Psychiatry 173:166–173. https://doi.org/10.1176/appi.ajp.2015.15030332
Toto S, Grohmann R, Bleich S et al (2019) Psychopharmacological treatment of schizophrenia over time in 30 908 inpatients: data from the AMSP study. Int J Neuropsychopharmacol 22:560–573. https://doi.org/10.1093/ijnp/pyz037
Treves IA, Neufeld MY (1996) EEG abnormalities in clozapine-treated schizophrenic patients. Eur Neuropsychopharmacol 6:93–94. https://doi.org/10.1016/0924-977x(95)00057-v
Umbricht DS, Pollack S, Kane JM (1994) Clozapine and weight gain. J Clin Psychiatry 55(2):157–160
van der Horst MZ, Meijer Y, de Boer N et al (2023) Comprehensive dissection of prevalence rates, sex differences, and blood level-dependencies of clozapine-associated adverse drug reactions. Psychiatry Res 330:115539. https://doi.org/10.1016/j.psychres.2023.115539
van der Zalm Y, Foldager L, Termorshuizen F et al (2021) Clozapine and mortality: a comparison with other antipsychotics in a nationwide Danish cohort study. Acta Psychiatr Scand 143:216–226. https://doi.org/10.1111/acps.13267
Varma S, Bishara D, Besag FMC, Taylor D (2011) Clozapine-related EEG changes and seizures: dose and plasma-level relationships. Ther Adv Psychopharmacol 1:47–66. https://doi.org/10.1177/2045125311405566
Wagner E, Siafis S, Fernando P et al (2021) Efficacy and safety of clozapine in psychotic disorders-a systematic quantitative meta-review. Transl Psychiatry 11:487. https://doi.org/10.1038/s41398-021-01613-2
West S, Rowbotham D, Xiong G, Kenedi C (2017) Clozapine induced gastrointestinal hypomotility: a potentially life threatening adverse event. A review of the literature. Gen Hosp Psychiatry 46:32–37. https://doi.org/10.1016/j.genhosppsych.2017.02.004
Wheeler AJ (2008) Treatment pathway and patterns of clozapine prescribing for schizophrenia in New Zealand. Ann Pharmacother 42:852–860. https://doi.org/10.1345/aph.1K662
Wohkittel C, Gerlach M, Taurines R et al (2016) Relationship between clozapine dose, serum concentration, and clinical outcome in children and adolescents in clinical practice. J Neural Transm (vienna) 123:1021–1031. https://doi.org/10.1007/s00702-016-1573-y
Wong J, Pang T, Cheuk NKW et al (2022) A systematic review on the use of clozapine in treatment of tardive dyskinesia and tardive dystonia in patients with psychiatric disorders. Psychopharmacology 239:3393–3420. https://doi.org/10.1007/s00213-022-06241-2
Xu Y, Amdanee N, Zhang X (2021) Antipsychotic-induced constipation: a review of the pathogenesis, clinical diagnosis, and treatment. CNS Drugs 35:1265–1274. https://doi.org/10.1007/s40263-021-00859-0
Acknowledgements
The authors would like to gratefully thank all psychiatric hospitals and drug monitors participating in the AMSP project for their continuous support in data collection.
Funding
Open Access funding enabled and organized by Projekt DEAL. The presented research in this manuscript was conducted without any specific grants or funding.
The AMSP drug safety project is facilitated by non-profit associations in Germany, Austria, and Switzerland. The AMSP project has been supported with unrestricted educational and research grants since 1993 by the following companies:
German companies: Abbott GmbH & Co. KG, Aristo Pharma, AstraZeneca GmbH, Aventis Pharma Deutschland GmbH GE–O/R/N, Bayer Vital GmbH, Boehringer Mannheim GmbH, Bristol-Myers-Squibb, Ciba Geigy GmbH, Desitin Arzneimittel GmbH, Duphar Pharma GmbH & Co. KG, Eisai GmbH, Esparma GmbH Arzneimittel, GlaxoSmithKline Pharma GmbH & Co. KG, Hoffmann-La Roche AG Medical Affairs, Janssen-Cilag GmbH, Janssen Research Foundation, Knoll Deutschland GmbH, Lilly Deutschland GmbH Niederlassung Bad Homburg, Lundbeck GmbH & Co. KG, Novartis Pharma GmbH, Nordmark Arzneimittel GmbH, Organon GmbH, Otsuka-Pharma Frankfurt, Pfizer GmbH, Pharmacia & Upjohn GmbH, Promonta Lundbeck Arzneimittel, Recordati Pharma GmbH, Rhone-Poulenc Rohrer, ROVI, Sanofi-Synthelabo GmbH, Sanofi-Aventis Deutschland, Schering AG, SmithKlineBeecham Pharma GmbH, Solvay Arzneimittel GmbH, Synthelabo Arzneimittel GmbH, Dr. Wilmar Schwabe GmbH & Co., Thiemann Arzneimittel GmbH, Troponwerke GmbH & Co. KG, Upjohn GmbH, Wander Pharma GmbH and Wyeth-Pharma GmbH;
Austrian companies: Astra Zeneca Österreich GmbH, Boehringer Ingelheim Austria, Bristol-Myers Squibb GmbH, CSC Pharmaceuticals GmbH, Eli Lilly GmbH, Germania Pharma GmbH, GlaxoSmithKline Pharma GmbH, Janssen-Cilag Pharma GmbH, Lundbeck GmbH, Novartis Pharma GmbH, Pfizer Med Inform, and Wyeth Lederle Pharma GmbH.
Swiss companies: AHP (Schweiz) AG, AstraZeneca AG, Bristol-Myers Squibb AG, Desitin Pharma GmbH, Eli Lilly (Suisse) S.A., Essex Chemie AG, GlaxoSmithKline AG, Janssen-Cilag AG, Lundbeck (Suisse) AG, Organon AG, Pfizer AG, Pharmacia, Sanofi-Aventis (Suisse) S.A., Sanofi-Synthelabo SA, Servier SA, SmithKlineBeecham AG, Solvay Pharma AG, Wyeth AHP (Suisse) AG, and Wyeth Pharmaceuticals AG.
Author information
Authors and Affiliations
Contributions
This study was conducted within the scope of the doctoral thesis of Lene Bleich.
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Lene Bleich, Sermin Toto, Johanna Seifert and Renate Grohmann. The first draft of the manuscript was written by Lene Bleich and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
S. Toto is the project manager of the AMSP program and a member of the advisory board for Otsuka and Janssen-Cilag and has received speaker’s honoraria from Janssen-Cilag, Lundbeck/Otsuka, Recordati Pharma GmbH, ROVI, and Servier. D. Dabbert has received speaker’s honoraria from Aristo. A. Erfurth has received honoraria for advisory board membership and/or speaker fees from Angelini, Boehringer Ingelheim, Germania, Janssen, Lundbeck, Mylan, Neuraxpharm, Recordati, Rovi and Sandoz. All other authors state they have no competing interests to declare that are relevant to the content of this article.
Ethical approval
The AMSP project and the evaluation of the collected data were authorized by AMSP representatives from Germany, Austria, and Switzerland and the ethics committees of the Ludwig-Maximilians-University of Munich and Hannover Medical School (Nr. 8100_BO_S_2018). This study adheres to the Declaration of Helsinki and its later adaptions. The AMSP program in no way interferes with the treatment of hospitalized patients due to its nature of being an observational post-marketing drug surveillance program. Moreover, data used in this study was obtained from the anonymized data bank of the AMSP program and therefore, individual patients cannot be retraced.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sermin Toto and Johanna Seifert shared senior-authorship.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bleich, L., Grohmann, R., Greil, W. et al. Clozapine-associated adverse drug reactions in 38,349 psychiatric inpatients: drug surveillance data from the AMSP project between 1993 and 2016. J Neural Transm 131, 1117–1134 (2024). https://doi.org/10.1007/s00702-024-02818-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-024-02818-7