Abstract
While it has been hypothesized that brown adipocytes responsible for mammalian thermogenesis are absent in birds, the existence of beige fat has yet to be studied directly. The present study tests the hypothesis that beige fat emerges in birds as a mechanism of physiological adaptation to cold environments. Subcutaneous neck adipose tissue from cold-acclimated or triiodothyronine (T3)-treated chickens exhibited increases in the expression of avian uncoupling protein (avUCP, an ortholog of mammalian UCP2 and UCP3) gene and some known mammalian beige adipocyte-specific markers. Morphological characteristics of white adipose tissues of treated chickens showed increased numbers of both small and larger clusters of multilocular fat cells within the tissues. Increases in protein levels of avUCP and mitochondrial marker protein, voltage-dependent anion channel, and immunohistochemical analysis for subcutaneous neck fat revealed the presence of potentially thermogenic mitochondria-rich cells. This is the first evidence that the capacity for thermogenesis may be acquired by differentiating adipose tissue into beige-like fat for maintaining temperature homeostasis in the subcutaneous fat ‘neck warmer’ in chickens exposed to a cold environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
How different species of organisms adapt to environmental temperature is central to understanding mechanisms for animal resilience in response to climate change. In mammals, two types of thermogenic fat cells, brown adipocytes and beige adipocytes, have been identified and play key roles in the regulation of systemic energy homeostasis. Brown adipocytes contain an abundance of mitochondria and small multilocular lipid droplets. The main protein responsible for non-shivering thermogenesis (NST) is uncoupling protein 1 (UCP1), which is embedded in the mitochondrial inner membrane and dissipates the proton gradient to generate heat (Cinti et al. 1989; Nicholls et al. 1978). Similarly, brown-like adipocytes (beige adipocytes or brite [brown-in-white] adipocytes) also have a multilocular morphology and express UCP1, but can be found in white adipose depots only when animals are exposed to cold or other inducers (Paulo and Wang 2019; Himms-Hagen et al. 2000; Vitali et al. 2012; Young et al. 1984; Kajimura et al. 2015). Regarding typical similarities and differences between brown adipocytes and beige adipocytes in mammals, cell death-inducing DNA fragmentation factor like effector a (CIDEA) is expressed in both brown and beige adipocytes (Ikeda et al. 2018; Petrovic et al. 2010; Sharp et al. 2012; Wu et al. 2012), and T-box transcription factor 1 (TBX1) and transmembrane protein 26 (TMEM26) (Harms and Seale 2013; Ikeda et al. 2018; Wu et al. 2012), are specifically expressed in beige adipocytes.
For birds, morphological data suggest that there is no BAT, but BAT-resembling WAT (Barre et al. 1986a; Oliphant 1983). However, this WAT lacks the metabolic capacity characteristic of BAT and has neither sympathetic innervation nor expression of UCP1, which is the key protein for BAT-type thermogenesis (Saarela et al. 1989, 1991). Based on these observations, Hohtola (2002) hypothesized that there are no adipose tissues, such as BAT or beige fat, which can participate in thermoregulatory heat production in birds. Just before proposing his hypothesis, Raimbault et al. (2001) and Vianna et al. (2001) made the discovery of a novel uncoupling protein (avian UCP) in skeletal muscle of birds. The expression of avUCP in skeletal muscle in chickens was elevated by exposure to cold-(Collin et al. 2003a; Toyomizu et al. 2002; Ueda et al. 2005) and T3-(Collin et al. 2003b) treatment, both of which are known to be typical stimuli for the emergence of beige adipocytes in mammals. However, no studies have examined expression of avUCP in beige fat tissues to confirm its existence in birds. Studies investigating the emergence of beige adipocytes are of particular interest because it is unclear whether skeletal muscle is the only important source of cold-induced non-shivering thermogenesis in birds. Consequently, in our study, we provide evidence for the in vivo emergence of beige-like fat within neck subcutaneous fat in chickens by (1) examining the effects of cold acclimation and T3 treatment on the expression of the avUCP gene and other marker genes known to be enriched in mammalian beige fat, as well as on the morphological characteristics of neck subcutaneous and abdominal fat adipose tissues of chickens; (2) determining the mRNA and protein levels of avUCP and the protein abundance of the mitochondrial marker, voltage-dependent anion channel (VDAC); and (3) analyzing mitochondria in multilocular fat cells from the neck subcutaneous fat of T3-treated chickens using immunohistochemistry.
Materials and methods
Ethics statement
The Animal Care and Use Committee of the Graduate School of Agricultural Science, Tohoku University, approved all procedures and every effort was made to minimize pain or discomfort to all animals used in the study.
Animals and experimental design
One-day-old male white leghorn chicks (Julia) were obtained from a commercial hatchery (I-Hiyoko, Co. Ltd, Niigata, Japan). The chicks were housed in electrically heated batteries and provided with water and a commercial starter diet ad libitum. Fourteen-day-old chickens that were to be used for experiments were selected from a twofold larger population to obtain uniform body weights and kept in wire-bottomed cages under conditions of controlled temperature (24 ± 1 °C) and continuous light for 6–7 days.
In the first series of experiments, twenty-four 21-day-old chickens (235 ± 11 g), after a 3-day adaptation period for individual cages were used for confirmation of increased expression of avUCP and beige adipose tissue markers, as well as the emergence of multilocular fat cells in cold-exposed or T3-treated chickens. The chickens were randomly divided into the following three groups (n = 8 per group): a ‘control’ group, fed a commercial diet under a thermoneutral environment (24 ± 1 °C); a ‘T3’ group, fed a commercial diet containing thyroid hormone (0.6 mg/kg of diet: T3, #T2877, Sigma-Aldrich, St. Louis, MO, USA) under thermoneutral conditions; and a ‘cold exposure’ group, fed a commercial diet in a cold environment (4 ± 1 °C) and then maintained for 10 days. Individual body weight and feed intake were recorded.
In a second series of experiments, twelve 20-day-old chickens (219 ± 6 g) after a 3-day adaptation period were used to clarify the presence of beige adipocyte clusters, constituting small adipocytes in the mitochondria-rich tissue of T3-treated chickens. Six chickens were fed a commercial diet (‘control’ group), while the remainder were fed a commercial diet supplemented with T3 (0.6 mg/kg of diet) under thermoneutral conditions (‘T3’ group), and then maintained for 9 days.
The dose of T3 was based on preliminary trial (unpublished data) and previous study (Collin et al. 2003b). All chickens had free access to food and water. After they were decapitated, neck subcutaneous and abdominal fats were quickly excised. They were then weighed, frozen, ground to a powder in liquid nitrogen, and stored at − 80 °C until used for protein analyses and extraction of total RNA. For analysis of fat tissue morphology, a sample of fat excised from each chicken was fixed in 10% formalin neutral buffer solution.
Quantitative real-time RT-PCR
Total RNA was extracted and cDNA was synthesized as previously described (Kikusato et al. 2015), with minor modifications. A quantitative RT-PCR analysis was performed using a CFX Connect™ system (Bio-Rad Laboratories, Hercules, CA, USA) for measuring the expression levels of avUCP, CIDEA (Ikeda et al. 2018; Petrovic et al. 2010; Sharp et al. 2012; Wu et al. 2012), TBX1, TMEM26 (Harms and Seale 2013; Ikeda et al. 2018; Wu et al. 2012), CAR4 (carbonic anhydrase 4) (Sharp et al. 2012), SLC27A1 (solute carrier family 27 member 1, fatty acid transporter) (Sharp et al. 2012; Wu et al. 2012), CD137 (Wu et al. 2012; Harms and Seale 2013), EAR2 (NR2F6: nuclear receptor subfamily 2 group F member 6) (Wu et al. 2012), and CPT1b (carnitine palmitoyltransferase 1b) (Sharp et al. 2012). The results are presented as ratios of mRNA levels of target molecules to 18S ribosomal RNA (18S). Primer sets used to amplify each gene are listed in Table S1.
Hematoxylin and eosin (H&E) staining
Adipose tissue was collected from each chicken and fixed in 10% formalin neutral buffer solution for 24 h at room temperature (25 °C). The tissues were dehydrated with 70%, 80%, 90%, 95%, 100%, and 100% (v/v) ethanol, treated with xylene, and embedded in paraffin. Tissue sections (4 µm thick) were prepared using a microtome and dehydrated with a graded ethanol series and stained with hematoxylin and eosin (H&E) to address the cell structure under a microscope.
To measure adipocyte size distribution, H&E-stained tissue images were subjected to grayscale conversion, and further binarization was carried out by setting the pixel existing in the cell area to 1 and other pixels to 0. Regions in which binarization could not be appropriately performed, as determined by visual inspection, were either deleted or corrected manually. The maximum area where the pixels of the cell area are connected to each other is judged as one cell and the pixel number is considered as the cross sectional area of the cell. To remove noise, cells with less than 10 pixels (an extremely small area) were removed. Image processing was performed using R script (https://www.r-project.org/) and ImageJ (v1.29x, National Institute of Health, Bethesda, MD, USA).
For the detection of small cell clusters, cells with an area larger than the threshold values of (250, 500, and 1000) pixels, were removed from the binary image at the time of measurement of the distribution of the cell area, and a binarized image was created by extracting only small cells. After applying a Gaussian filter to the image, binarization was again measured. Isolated cells were left intact and clusters of small cells were classified as connected regions by the blurred boundaries. The connected region was judged to be a cluster of small cells and measurements were carried out. Calculations were performed with the NumPy library, matplotlib, and scikit-image in Python (https://www.python.org/).
Immunohistochemistry
Histological analyses were carried out as described previously (Niimi et al. 2018; Zhu et al. 2017) with some modifications. The sections were washed with water after deparaffinization in xylene and dehydration in ethanol. The sections were incubated in 3% H2O2 diluted with phosphate buffered saline (PBS) for 15 min to remove endogenous peroxidase activity and incubated in 10 mM citrate buffer for 60 min at 90 °C for antigen retrieval. After three 5-min PBS washes, the sections were blocked in 10% goat serum (#426041, Nichirei Bioscience, Tokyo, Japan) for 10 min and incubated with a polyclonal rabbit anti-VDAC primary antibody (ab15895, Abcam, Cambridge, UK) at a dilution of 1:3000 overnight at 4 °C. Normal rabbit IgG (#2729, Cell Signaling, Beverly, MA, USA) diluted to the same concentration as a primary antibody was used as the negative control. After being washed three times for 5 min in PBS, the sections were incubated in Histofine Simple Stain Max PO (#414181, Nichirei Bioscience) as a secondary antibody at room temperature for 30 min. After three 5-min washes in PBS, the signals were developed using a diaminobenzidine (DAB) substrate solution kit (#425011, Nichirei Bioscience) and counterstained with hematoxylin.
Western blotting
To measure VDAC/Porin and avUCP protein levels, western blotting was conducted as previously described, with minor modifications (Kikusato and Toyomizu 2013). The frozen tissues were solubilized/sonicated with radioimmunoprecipitation assay (RIPA) buffer. Lysates were mixed with the same amount of sodium dodecyl sulfate (SDS) buffer (containing 0.125 M Tris–HCl [pH 6.8], 4% SDS, 10% glycerol, 0.002% phenol red, and 0.1 M dithiothreitol), and then boiled for 5 min. The samples were loaded onto 12% polyacrylamide gels (Mini-PROTEAN® TGX™ Gels, Bio-Rad). The electrophoresed proteins were transferred to a polyvinylidene difluoride (PVDF) membrane (0.2 µm) using a semi-dry transfer apparatus (Trans-Blot® Turbo™, Bio-Rad) according to the manufacturer’s instructions. The membranes were blocked with Tris-buffered saline (TBS) containing 5% (w/v) skimmed milk and 0.1% (v/v) Tween 20 for 1 h at room temperature. The membranes were incubated overnight at 4 °C with rabbit anti-VDAC (ab15895, Abcam) or rabbit anti-avUCP polyclonal antibodies diluted 1:3000 and 1:100, respectively. Anti-avUCP antibody was obtained from rabbits by injection of a peptide (MVGLKPPEVPPTAAVK) coupled with the KLH protein (EFS, Etablissement français du sang, Nantes, France). After being washed, the membranes were incubated with a horseradish peroxidase (HRP)-conjugated anti-rabbit IgG secondary antibody (7074S, Cell Signaling Technology, Beverly, MA, USA) diluted 1:10,000 in blocking buffer at room temperature for 1 h. After being washed, the signal was developed with a chemiluminescent substrate solution (Chemi-Lumi One Super, Nacalai Tesque Inc., Kyoto, Japan) for 1 min. Immunoreactive proteins on the membranes were imaged using a VersaDoc Model 5000 (Bio-Rad). Protein sizes were estimated using a MagicMark™ Western protein standard (#LC5602, Thermo Fisher Scientific, Waltham, MA, USA). The VDAC/Porin protein content was quantified based on standard curves obtained from serial dilutions of recombinant human VDAC1/Porin (ab132481, Abcam) on the same membrane. Additionally, membranes were stained with Ponceau-S (#SP-4030, Aproscience, Tokushima, Japan) according to the manufacturer’s instructions to confirm similarities of protein transfer efficiency among the loading western blot samples.
Statistics
Statistical analyses were performed using BellCurve for Excel 2015 software (Social Survey Research Information Co., Ltd. Tokyo, Japan). Differences between control, T3- and cold-treated groups and between control and T3-treated groups were assessed using a Shirley-Williams test and a Student’s t-test for unpaired data, respectively. Differences in data with p < 0.05 are considered statistically significant. Pearson correlation coefficients were calculated between avUCP and PGC-1α gene levels.
Results
Body weight, food intake, and adipose tissue weight
Compared with the control group, body weight gain was marginally lower in T3-treated chickens and significantly lower in cold-acclimated chickens (control, 147 ± 13 g; T3-treated, 135 ± 15 g; cold-acclimated, 81 ± 13 g: p < 0.05 versus control). T3-treated chickens ate significantly less feed than the control, whereas cold-acclimated chickens ate significantly more (control, 831 ± 16 g; T3-treated, 770 ± 24 g: p < 0.05 versus control; cold-acclimated, 1009 ± 29 g: p < 0.05 versus control). Consequently, and as expected, feed efficiency, which is calculated as the ratio of body weight gain to feed intake, decreased in cold-acclimated animals, but not in T3-treated chickens (control, 0.177 ± 0.004; T3-treated, 0.175 ± 0.005; cold-acclimated, 0.080 ± 0.005: p < 0.05 versus control), implying that cold acclimation induced greater whole-body heat production. Neck subcutaneous fat weight was significantly lower in cold-acclimated animals than in control chickens, but there was no significant difference between T3-treated and control chickens, whereas abdominal fat weight was significantly lower in T3-treated and cold-acclimated chickens than in control chickens (Fig. 1a).
Increased mRNA expression levels of avian UCP (avUCP) and of beige adipose tissue markers in chickens exposed to 4 °C or fed a T3-supplemented diet. a Weights of neck subcutaneous fat (left) and abdominal fat (right) of control, T3-treated and cold-exposed chickens. Values are means ± S.E., n = 8 chickens in each group. b mRNA levels of avUCP in neck subcutaneous fat (left) and abdominal fat (right) of control, T3-treated and cold-exposed chickens. Values are means ± S.E., n = 8 chickens in each group. c mRNA levels of markers known to be enriched in mammalian beige adipocytes in neck subcutaneous fat (left) and abdominal fat (right) of control, T3-treated and cold-exposed chickens. Values are means ± S.E., n = 8 chickens for CIDEA, TBX1 and TMEM26 in each group; n = 5–8 chickens for CAR4, SLC27A1, CD137, EAR2 and CPT1b in each group. d Dependency of avUCP expression on avPGC-1α in neck subcutaneous fat of control, T3-treated, and cold-exposed chickens (n = 24) and effects of T3- and cold-treatments on mRNA levels of avPGC-1α. (n = 8 chickens in each group). All data for mRNA levels are shown as fold changes relative to control values. Quantitative real-time RT-PCR was used to quantify mRNA levels, and the results were normalized to 18S mRNA levels. ND means not appropriately detected for too low expression. Value in panel D represents Pearson correlation coefficient for the relationship between avUCP and PGC-1α gene levels. Differences between control, T3- and cold-treated groups were assessed using the Shirley-Williams test. *p < 0.05 compared to the control group
mRNA expression of avUCP and mammalian beige adipocyte-related genes
To determine the mRNA expression levels of typical gene markers known to be enriched in mammalian beige adipocytes, the mRNA levels of avUCP, CIDEA, TBX1, TMEM26, CAR4, SLC27A1, CD137, EAR2, and CPT1b in the neck subcutaneous and abdominal fats of T3-treated and cold-exposed chickens were analyzed by quantitative real-time RT-PCR (Fig. 1b, c). There was a statistically significant increase in avUCP mRNA levels in the neck subcutaneous fat of T3-treated and cold-acclimated chickens compared with control chickens. In contrast, in the abdominal fat avUCP mRNA was not detected in control, T3-treated or cold-acclimated chickens (Fig. 1b). In neck subcutaneous fat, the mRNA expression levels of CIDEA, TBX1, TMEM26 and CPT1b were significantly higher, though the level of CAR4 was slightly higher, in the cold-acclimated group than in the control group, while in the T3-treated group, those levels were intermediate between control and cold-acclimated groups. The levels of SLC27A1, CD137, or EAR2 mRNA did not increase in both treatments (Fig. 1c). In contrast, no differences were observed in abdominal fat CIDEA, TBX1, TMEM26, CAR4, SLC27A1, CD137, EAR2, and CPT1b mRNA expression levels except for EAR2 mRNA whose level was decreased by both treatments. To clarify whether avian peroxisome proliferator-activated receptor γ coactivator-1α (PPARGC1A, avPGC-1α) is associated with avUCP expression in neck subcutaneous fat, the dependency of avUCP expression on avPGC-1α was investigated. The results showed a positive correlation between avUCP and avPGC-1α mRNA levels of control, T3-treated and cold-exposed chickens despite the observation that avPGC-1α was not significantly increased by either treatment (Fig. 1d).
Morphological characteristics of neck subcutaneous fat and abdominal fat stained with hematoxylin and eosin
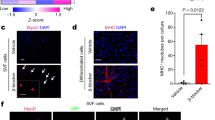
Hematoxylin and eosin (H&E) staining was carried out to investigate the emergence of the beige adipocytes by cold and T3 treatment. Islets of multilocular fat cells were observed within the neck subcutaneous fat and abdominal fat depots of T3-treated and cold-acclimated chickens though there were few multilocular fat cells in control chickens (Fig. 2a): namely, fat cells became more multilocular in T3-treated and cold-acclimated chickens, i.e., the cells were smaller. These morphological characteristics are very similar to those observed for stimuli-induced mammalian beige fat (Sidossis and Kajimura 2015). For quantitative evaluation of multilocular adipocytes, each fat depot consisting of small adipocytes was regarded as one cluster, and digital imaging was used to measure the number of clusters relative to each cluster size within the WAT depots (Fig. 2b). Scatter plots were constructed as shown in Fig. 2c. Compared with the control group, cold- or T3-treated chickens exhibited increases in the numbers of both small and larger clusters, showing an increased number of multilocular fat cell clusters within neck subcutaneous fat and abdominal fat, and providing evidence for the emergence of multilocular fat cells as a result of T3 and cold stimulation.
Emergence of multilocular fat cells in chickens exposed to cold and fed a T3-supplemented diet. a H&E stains showing multilocular fat cells within the neck subcutaneous fat (upper) and abdominal fat (lower) depots in control, T3-treated and cold-acclimated chickens. b Process for the quantitative evaluation of multilocular adipocytes. Fat depots containing small adipocytes were regarded as one cluster, such as large, medium, or small-sized cluster. c Scatter plots displaying data points of the number of clusters on the y-axis versus the cluster size on the x-axis within neck subcutaneous fat (upper) and abdominal fat (lower) tissues of control, T3-treated and cold-acclimated chickens
mRNA and protein levels of avUCP in tissue and mitochondrial VDAC protein content
In the second series of experiments, mRNA and protein levels of avUCP and the mitochondrial protein VDAC were examined to characterize the neck subcutaneous fat of T3-treated chickens. The level of avUCP mRNA expression significantly increased in the T3-treated group compared with the control group though the levels were very low in the abdominal fat of both groups (Fig. 3a). A positive correlation between avUCP and avPGC-1α mRNA levels of control and T3-treated chickens was observed (Fig. S1). These results were in agreement with findings obtained from the first series of experiments. The avUCP protein content also tended to increase with T3 treatment (Fig. 3b). The protein expression level of VDAC, which is a mitochondrial marker protein, expressed per tissue protein, significantly increased in the neck subcutaneous fat of T3-treated chickens (Fig. 3c), which means that an enhancement of mitochondrial biogenesis follows T3 stimulation in the neck subcutaneous fat. In contrast, the levels of avPGC-1α mRNA and the VDAC protein in abdominal fat were not significantly different between the groups (Fig S2 and S3).
Clusters of beige-like adipocytes densely emerge in a mitochondria-rich cell of neck subcutaneous fat in T3-treated chickens. a mRNA levels of avUCP in neck subcutaneous fat and abdominal fat tissues of control and T3-treated chickens. Quantitative real-time RT-PCR was used to quantify mRNA levels, and the results were normalized to 18S rRNA levels. Values are means ± S.E., n = 5–6 chickens in each group. b Protein levels of avUCP in neck subcutaneous fat of control and T3-treated chickens. avUCP protein levels were assessed by Western blot of tissue protein (80 μg) using an anti-avUCP antibody. Band intensities of avUCP shown as semi-quantified by densitometric tracing. Values are means ± S.E., n = 5 chickens in each group. Uncropped data and observed band size are depicted in Supplement A, Supporting Information. c VDAC protein content in neck subcutaneous fat of control and T3-treated chickens. VDAC protein levels were assessed by Western blot analysis of tissue protein (10 μg) using an anti-VDAC antibody. Band intensities of VDAC shown as semi-quantified by densitometric tracing. Values are means ± S.E., n = 6 chickens in each group. Uncropped data are depicted in Supplement B, Supporting Information. d Immunohistochemical analysis using anti-VDAC antibody for neck subcutaneous fat of control and T3-treated chickens. Data for mRNA and protein levels of avUCP or VDAC protein are shown as fold changes relative to control values for the neck subcutaneous fat. Differences between control and T3-treated chickens were assessed using the Student’s t-test for unpaired data. *p < 0.05 compared to the control group
Immunohistochemistry of neck subcutaneous fat from T3-treated chickens
Morphological and immunohistochemical characterizations of the neck subcutaneous fat of T3-treated chickens were carried out using an anti-VDAC antibody. The neck subcutaneous fat of T3-treated chickens presented many types of clusters that were positive for VDAC, corresponding to multilocular fat cells (Fig. 3d). The abdominal fat exhibited weaker VDAC signal in both groups compared with the neck subcutaneous fat, and there was no apparent difference in the signal between groups (Fig. S4). Therefore, it could be concluded that the dense clusters of beige-like adipocytes were present in the mitochondria-rich neck subcutaneous fat tissue of T3-treated chickens.
Discussion
Brown adipocytes in mammals are distinguished from the more common white fat adipocytes by having numerous small lipid droplets, elevated numbers of mitochondria, and mitochondrial expression of UCP1, the uncoupler of oxidative phosphorylation responsible for non-shivering thermogenesis (Mezentseva et al. 2008). Although the thermogenic BAT has not been described in birds, Mezentseva et al. (2008) identified in vitro inductive conditions for avian brown adipocyte-like cells and demonstrated that mesenchymal cells isolated from the embryonic chicken limb bud can differentiate into avian brown adipocyte-like cells that exhibit morphological and many biochemical properties of terminally differentiated brown adipocytes. These avian brown adipocyte-like cells could be similar to brown-like adipocytes, so-called beige adipocytes (Ishibashi and Seale 2010), or brite (brown-in-white) adipocytes (Petrovic et al. 2010) observed in mammals though the expression of any UCP homologs has never been examined. The question of whether ‘beige cells expressing avUCP’ could come from white adipose tissue of birds exposed to cold or other inducers remains an unanswered question in the literature. Here, the chicken was used as avian model to clarify if ‘beige cells’ could appear based on in vivo experiments. The potential physiological value of beige adipocytes browning obtained in the avian models can be extrapolated with care, to birds, given that some of the avian physiological characteristic relevant to energy metabolism, such as the lack of glut4 and the advent of avUCP are evidenced with chickens (Raimbault et al. 2001; Newman et al. 2013; Seki et al. 2003).
We applied cold- and T3-treatments, which are both effective stimuli to shift WAT into beige fat in mammals (Kajimura et al. 2015; Barbatelli et al. 2010; Matesanz et al. 2017; Lee et al. 2012), to laying-type chickens which have much more tolerance to heat or cold environmental stress than a meat-type chickens (Mujahid et al. 2005; Azad et al. 2010).
Enhancements in gene expression of avUCP in the neck subcutaneous adipose tissues of cold-acclimated or T3-treated chickens were observed (Fig. 1b), which are in agreement with the results obtained in mice by Guerra et al. (1998), who showed that, after adrenergic signaling stimulation by cold exposure or treatment with a β3-adrenergic agonist, increased UCP1 mRNA levels were accompanied by a corresponding increase in brown adipocytes in white retroperitoneal fat as revealed by the anti-UCP1 antibody staining. In addition to avUCP, the expression level of CIDEA [a known mammalian marker for both brown and beige adipocytes (Ikeda et al. 2018; Petrovic et al. 2010; Sharp et al. 2012; Wu et al. 2012)], and those of TBX1, TMEM26, CAR4, and CPT-1b [known mammalian beige adipocyte-specific markers (Ikeda et al. 2018; Sharp et al. 2012; Wu et al. 2012; Harms and Seale 2013)], increased in the neck subcutaneous fat of control, T3-treated and cold-acclimated group in ascending order. Expression levels of SLC27A1, CD137, and EAR2 [known mammalian beige adipocyte-specific markers (Sharp et al. 2012; Wu et al. 2012)] were not changed (Fig. 1c). Those apparent discrepancies between the mammalian results and ours in the gene expression of SLC27A1 and CD137 may have arisen from use of different species and type of animals.
The lack of increase in SLC27A1 in this study may simply be related to bird-specific lipid metabolism, considering that, different from mammals, fat deposition in birds depends on exogenous fatty acid provision much more than de novo synthesis (Griffin et al. 1992). SLC27A1 may not be a rate-limiting transporter in fatty acids uptake for mitochondrial β-oxidation, which might be orchestrated by different other transporters, CD36 and ACS1 beside SLC27A1.
Although CD137 was identified as a marker of beige adipocyte in DNA microarray screens of immortalized cell lines derived from inguinal WAT of a strain of obesity-resistant 129SVE mice (Wu et al. 2012), subsequent studies were unable to validate enrichment of CD137 in beige adipocytes, or increased CD137 expression after cold exposure or adrenergic stimulation in mice (Rosenwald et al. 2013; Srivastava et al. 2020). In agreement with those studies, we did not observe increased expression of CD137 mRNA upon cold- and T3-treatments. In these regards, additional studies will be required to clarify the chicken-specific functions of SLC27A1 and CD137 related to lipid catabolism and immunoreactivity in beige adipocytes.
On the other hand, in abdominal fat, gene expression of avUCP was scarcely detected regardless of either treatment (Fig. 1b), or even if detected, was observed to be very low in control and T3-treated chickens (Fig. 3a). Furthermore, the levels of all marker examined for both brown and beige adipocytes were not increased by either treatment in abdominal fat (Fig. 1c). Therefore, even though the islets of multilocular fat cells emerging within neck subcutaneous fat and abdominal fat following both cold- and T3 stimulation are characterized by an increased number of clusters containing small adipocytes (Fig. 2c), these results suggest that beige-like adipocytes may be predominantly present in the neck subcutaneous fat rather than in abdominal fat tissues of chicken exposed to cold- and T3-treatment.
Since the observed increase in avUCP expression as well as the number of multilocular fat cells within neck subcutaneous fat are indicative of the presence of beige adipocytes, this tissue may have thermogenic capacities to promote energy expenditure. Himms-Hagen et al. (2000) observed that multilocular fat cells were rich in mitochondria and positive for UCP1 by immunohistochemistry in the WAT of rats treated with the β3-adrenoceptor agonist CL-316243. We confirmed similar results in chicken by showing the presence of both functional mitochondria and avUCP protein in multilocular fat cells. We experimentally measured the expression of the mitochondrial outer membrane protein, VDAC (Bathori et al. 2006), and the mitochondrial inner membrane protein avUCP in the WAT of T3-treated chickens. An increased abundance of avUCP at the protein level and the VDAC protein were found as well as that of avUCP mRNA in neck subcutaneous fat tissues (Fig. 3a–c). Based on the immunohistochemical analysis, the neck subcutaneous fat of T3-treated chickens presented small clusters that were positive for VDAC, corresponding to multilocular fat cells (Fig. 3d), which shows that the dense clusters of beige-like adipocytes emerge in mitochondria-rich tissue of chickens. Our morphological and immunohistochemical results for beige-like adipose tissue in chickens are consistent with the criteria adopted by Sidossis et al. (2015), namely that the browning of subcutaneous WAT is determined by the presence of multilocular adipocytes, UCP1, and increases in mitochondrial density and respiratory capacity. Therefore, the increased avUCP transcript and protein levels within the multilocular fat cells in the neck subcutaneous fat of T3-treated chickens are evidences for the presence of beige-like adipocytes. However, this seems not to be the case for abdominal fat because it was devoid of avUCP, or exhibited low avUCP expression and VDAC protein., where triglycerides must first be also hydrolyzed and some of the resultant fatty acids delivered to the thermogenarating muscles. These depot-specific effects are supported by the differences observed in the proportions of UCP1-immunoreactive adipocytes in the subcutaneous and visceral WAT as reported in 129 Sv mice (Barbatelli et al. 2010); after 10 days’ exposure to 6 °C, brown adipocytes significantly increased in subcutaneous WAT, whereas they were not significantly increased in visceral WAT. Our results suggest that abdominal fat exhibits increased lipolysis probably without increased thermogenesis while neck subcutaneous fat has increases in both lipolysis and thermogenesis.
Is avPGC-1α involved in the upregulation of avUCP in adipose tissue after stimulation? In mammals, the transcriptional regulator gene PGC-1α in BAT is required to coordinate the expression of UCP1 (Lowell and Spiegelman 2000), while expression of PGC-1α in WAT is also induced by cold stimulation and dietary supplementation with T3, indicative of the role for PGC-1α in beige adipocytes of mammals (Barbatelli et al. 2010; Lee et al. 2012). In chickens, upregulation of avPGC-1α expression precedes an increase in avUCP expression in the mitochondria of skeletal muscle (Ueda et al. 2005). Therefore, avPGC-1α may be involved in regulating avUCP expression in neck subcutaneous adipose tissues. Results of this work showed a positive correlation between avUCP and avPGC-1α mRNA levels (Fig. 1d, Fig. S1), suggesting that avPGC-1α in neck subcutaneous adipose tissues is important for regulating the expression of mitochondrial avUCP in chickens under cold exposure or T3 treatment. This hypothesis is supported by the correlated alteration in UCP and PGC-1α mRNA levels during development of brown adipocytes in retroperitoneal white fat of mice (Xue et al. 2007).
Another important question emerging from the current study is whether avUCP functions as a thermogenic protein in chickens by mediating avUCP-induced proton leak. The functional assessment of beige adipose tissue and UCP1, and whether beige adipocytes generate a sufficient amount of heat that will substantially support thermoregulation remains unresolved in mammals (Keipert and Jastroch 2014). Even if UCP1 activity would directly produce heat, it should be noted that a robust UCP1-independent thermogenic mechanism in beige fat can also involve either enhanced ATP-dependent Ca2+ cycling by sarco/endoplasmic reticulum Ca2+-ATPase 2b (SERCA2b) and ryanodine receptor 2 (RyR2) (Ikeda et al. 2017), or a futile creatine-driven cycle coupled to mitochondria ATP synthesis (Kazak et al. 2015). Both are noncanonical thermogenic mechanisms through which beige fat controls whole-body energy homeostasis. In birds, although the physiological role of avUCP remains controversial (Emre et al. 2007; Mozo et al. 2005), it was previously reported that cold acclimation (4–6 °C for 10–12 days) induces fatty acid-mediated uncoupling of mitochondrial oxidative phosphorylation in the subsarcolemmal mitochondria isolated from chicken skeletal muscle (Toyomizu et al. 2002) and that the mitochondria from chicken skeletal muscle exposed to cold for 2 d and 2.5 d (4 °C to 6 °C) exhibited uncoupling by both endogenous and exogenous fatty acids (Ueda et al. 2005). Thus, an increased supply of non-esterified fatty acids to cold-acclimated skeletal muscles may contribute to thermogenesis in chickens via avUCP, responsible for uncoupling of mitochondrial oxidative phosphorylation processes (Barre et al. 1986b; Duchamp et al. 1992; Roussel et al. 1998; Wojtczak and Schonfeld 1993). This fatty acids-mediated uncoupling probably happens with even beige-like adipose tissue. However, it cannot be also ruled out that avUCP may play a role in alleviating the overproduction of reactive oxygen species (Abe et al. 2006; Rey et al. 2010) as shown for UCP1 of BAT (Dlasková et al. 2010), resulting from a stimulation of the ATP-consuming process of sarcoplasmic reticulum Ca2+ cycling or futile creatine substrate cycling, which could produce heat as described above.
Perspective and significance
Non-shivering thermogenesis in BAT is induced by cold via thyroid hormones and the sympathetic nervous system, and is an essential mammalian mode of protection against hypothermia, especially in young and hibernating animals (Cannon and Nedergaard 2004). Beige adipocytes detected in animals exposed to cold or other inducers also contribute to thermogenesis. However, birds do not appear to possess BAT, while in vivo emergence of beige fat has not been reported for at least the last two decades. Although previous studies have suggested that thermogenesis in birds in response to cold or thyroid hormones is mediated entirely by skeletal muscle (Newman et al. 2013), skeletal muscle thermogenesis is unlikely sufficient to maintain the body temperature of birds around some regions of the animal due to sparse feathering. Our study on chickens showed that levels of the avUCP protein are correlated with the presence of beige-like adipocytes in response of cold environments, implying that thermogenesis in chickens might be associated with avUCP in beige-like adipocytes as well. Given that some of avian physiological characteristic relevant to energy metabolism were evidenced with chickens, our current results can be used to infer that birds may acquire the capacity for non-shivering thermogenesis by converting some white adipocytes into beige-like adipocytes, thus highlighting the potential physiological value of beige-like adipocytes in birds. Our new finding warrants further investigation.
Data availability
All data in the figures, image analyses and tests are available from the authors upon reasonable request.
Abbreviations
- UCP:
-
Uncoupling protein
- VDAC:
-
Voltage-dependent anion channel
- WAT:
-
White adipose tissue
- BAT:
-
Brown adipose tissue
- CIDEA:
-
Cell death inducing DNA fragmentation factor like effector a
- TBX1:
-
T-box transcription factor 1
- TMEM26:
-
Transmembrane protein 26
- CAR4:
-
Carbonic anhydrase 4
- SLC27A1:
-
Solute carrier family 27 member 1
- CD137:
-
Cluster of differentiation 36
- NR2F6:
-
Nuclear receptor subfamily 2 group F member 6
- CPT1b:
-
Carnitine palmitoyltransferase 1b
- T3 :
-
Triiodothyronine
- avPGC-1α:
-
Avian peroxisome proliferator-activated receptor γ coactivator-1α
- 18S:
-
18S-ribosomal RNA; H&E, hematoxylin and eosin
References
Abe T, Mujahid A, Sato K, Akiba Y, Toyomizu M (2006) Possible role of avian uncoupling protein in down-regulating mitochondrial superoxide production in skeletal muscle of fasted chickens. FEBS Lett 580(20):4815–4822. https://doi.org/10.1016/j.febslet.2006.07.070
Azad KMA, Kikusato M, Hoque AM, Toyomizu M (2010) Effect of chronic heat stress on performance and oxidative damage in different strains of chickens. J Poultry Sci 47(4):333–337. https://doi.org/10.2141/jpsa.010025
Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K, Giacobino JP, De Matteis R, Cinti S (2010) The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metabol 298(6):E1244-1253. https://doi.org/10.1152/ajpendo.00600.2009
Barre H, Cohen-Adad F, Duchamp C, Rouanet JL (1986a) Multilocular adipocytes from muscovy ducklings differentiated in response to cold acclimation. J Physiol 375(1):27–38. https://doi.org/10.1113/jphysiol.1986.sp016103
Barre H, Nedergaard J, Cannon B (1986b) Increased respiration in skeletal-muscle mitochondria from cold-acclimated ducklings—Uncoupling effects of free fatty-acids. Comp Biochem Physiol B: Biochem Mol Biol 85(2):343–348. https://doi.org/10.1016/0305-0491(86)90010-6
Bathori G, Csordas G, Garcia-Perez C, Davies E, Hajnoczky G (2006) Ca2+-dependent control of the permeability properties of the mitochondrial outer membrane and voltage-dependent anion-selective channel (VDAC). J Biol Chem 281(25):17347–17358. https://doi.org/10.1074/jbc.M600906200
Cannon B, Nedergaard J (2004) Brown adipose tissue: function and physiological significance. Physiol Rev 84(1):277–359. https://doi.org/10.1152/physrev.00015.2003
Cinti S, Zancanaro C, Sbarbati A, Cicolini M, Vogel P, Ricquier D, Fakan S (1989) Immunoelectron microscopical identification of the uncoupling protein in brown adipose tissue mitochondria. Biol Cell 67(3):359–362. https://doi.org/10.1111/j.1768-322X.1989.tb00883.x
Collin A, Buyse J, van As P, Darras VM, Malheiros RD, Moraes VM, Reyns GE, Taouis M, Decuypere E (2003a) Cold-induced enhancement of avian uncoupling protein expression, heat production, and triiodothyronine concentrations in broiler chicks. Gen Comp Endocrinol 130(1):70–77. https://doi.org/10.1016/s0016-6480(02)00571-3
Collin A, Taouis M, Buyse J, Ifuta NB, Darras VM, Van As P, Malheiros RD, Moraes VM, Decuypere E (2003b) Thyroid status, but not insulin status, affects expression of avian uncoupling protein mRNA in chicken. Am J Physiol Endocrinol Metabol 284(4):E771-777. https://doi.org/10.1152/ajpendo.00478.2002
Dlasková A, Clarke KJ, Porter RK (2010) The role of UCP 1 in production of reactive oxygen species by mitochondria isolated from brown adipose tissue. Biochim Biophys Acta 1797(8):1470–1476. https://doi.org/10.1016/j.bbabio.2010.04.008
Duchamp C, Cohen-Adad F, Rouanet JL, Barre H (1992) Histochemical arguments for muscular non-shivering thermogenesis in muscovy ducklings. J Physiol 457(1):27–45. https://doi.org/10.1113/jphysiol.1992.sp019363
Emre Y, Hurtaud C, Ricquier D, Bouillaud F, Hughes J, Criscuolo F (2007) Avian UCP: the killjoy in the evolution of the mitochondrial uncoupling proteins. J Mol Evol 65(4):392–402. https://doi.org/10.1007/s00239-007-9020-1
Griffin HD, Guo K, Windsor D, Butterwith SC (1992) Adipose tissue lipogenesis and fat deposition in leaner broiler chickens. J Nutr 122(2):363–368. https://doi.org/10.1093/jn/122.2.363
Guerra C, Koza RA, Yamashita H, Walsh K, Kozak LP (1998) Emergence of brown adipocytes in white fat in mice is under genetic control—effects on body weight and adiposity. J Clin Invest 102(2):412–420. https://doi.org/10.1172/Jci3155
Harms M, Seale P (2013) Brown and beige fat: development, function and therapeutic potential. Nat Med 19(10):1252–1263. https://doi.org/10.1038/nm.3361
Himms-Hagen J, Melnyk A, Zingaretti MC, Ceresi E, Barbatelli G, Cinti S (2000) Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am J Physiol Cell Physiol 279(3):C670-681. https://doi.org/10.1152/ajpcell.2000.279.3.C670
Hohtola E (2002) Facultative and obligatory thermogenesis in young birds: a cautionary note. Comp Biochem Physiol A Mol Integr Physiol 131(4):733–739. https://doi.org/10.1016/s1095-6433(02)00011-9
Ikeda K, Kang Q, Yoneshiro T, Camporez JP, Maki H, Homma M, Shinoda K, Chen Y, Lu X, Maretich P, Tajima K, Ajuwon KM, Soga T, Kajimura S (2017) UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat Med 23(12):1454–1465. https://doi.org/10.1038/nm.4429
Ikeda K, Maretich P, Kajimura S (2018) The common and distinct features of brown and beige adipocytes. Trends Endocrinol Metab 29(3):191–200. https://doi.org/10.1016/j.tem.2018.01.001
Ishibashi J, Seale P (2010) Beige can be slimming. Science 328(5982):1113–1114. https://doi.org/10.1126/science.1190816
Kajimura S, Spiegelman BM, Seale P (2015) Brown and beige fat: physiological roles beyond heat generation. Cell Metab 22(4):546–559. https://doi.org/10.1016/j.cmet.2015.09.007
Kazak L, Chouchani ET, Jedrychowski MP, Erickson BK, Shinoda K, Cohen P, Vetrivelan R, Lu GZ, Laznik-Bogoslavski D, Hasenfuss SC, Kajimura S, Gygi SP, Spiegelman BM (2015) A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell 163(3):643–655. https://doi.org/10.1016/j.cell.2015.09.035
Keipert S, Jastroch M (2014) Brite/beige fat and UCP1—is it thermogenesis? Biochim Biophys Acta 1837(7):1075–1082. https://doi.org/10.1016/j.bbabio.2014.02.008
Kikusato M, Toyomizu M (2013) Crucial role of membrane potential in heat stress-induced overproduction of reactive oxygen species in avian skeletal muscle mitochondria. PLoS ONE 8(5):e64412–e64421. https://doi.org/10.1371/journal.pone.0064412
Kikusato M, Yoshida H, Furukawa K, Toyomizu M (2015) Effect of heat stress-induced production of mitochondrial reactive oxygen species on NADPH oxidase and heme oxygenase-1 mRNA levels in avian muscle cells. J Therm Biol 52:8–13. https://doi.org/10.1016/j.jtherbio.2015.04.005
Lee JY, Takahashi N, Yasubuchi M, Kim YI, Hashizaki H, Kim MJ, Sakamoto T, Goto T, Kawada T (2012) Triiodothyronine induces UCP-1 expression and mitochondrial biogenesis in human adipocytes. Am J Physiol Cell Physiol 302(2):C463-472. https://doi.org/10.1152/ajpcell.00010.2011
Lowell BB, Spiegelman BM (2000) Towards a molecular understanding of adaptive thermogenesis. Nature 404(6778):652–660. https://doi.org/10.1038/35007527
Matesanz N, Bernardo E, Acin-Perez R, Manieri E, Perez-Sieira S, Hernandez-Cosido L, Montalvo-Romeral V, Mora A, Rodriguez E, Leiva-Vega L, Lechuga-Vieco AV, Ruiz-Cabello J, Torres JL, Crespo-Ruiz M, Centeno F, Alvarez CV, Marcos M, Enriquez JA, Nogueiras R, Sabio G (2017) MKK6 controls T3-mediated browning of white adipose tissue. Nat Commun 8(1):856–869. https://doi.org/10.1038/s41467-017-00948-z
Mezentseva NV, Kumaratilake JS, Newman SA (2008) The brown adipocyte differentiation pathway in birds: an evolutionary road not taken. BMC Biol 6(1):17–29. https://doi.org/10.1186/1741-7007-6-17
Mozo J, Emre Y, Bouillaud F, Ricquier D, Criscuolo F (2005) Thermoregulation: what role for UCPs in mammals and birds? Biosci Rep 25(3–4):227–249. https://doi.org/10.1007/s10540-005-2887-4
Mujahid A, Ueda M, Akiba Y, Toyomizu M (2005) Expression of avian UCP and ANT in skeletal muscle of cold-exposed laying and meat-type Chickens. J Poultry Sci 42(3):230–237. https://doi.org/10.2141/jpsa.42.230
Newman SA, Mezentseva NV, Badyaev AV (2013) Gene loss, thermogenesis, and the origin of birds. Ann N Y Acad Sci 1289(1):36–47. https://doi.org/10.1111/nyas.12090
Nicholls DG, Bernson VS, Heaton GM (1978) The identification of the component in the inner membrane of brown adipose tissue mitochondria responsible for regulating energy dissipation. Effectors of Thermogenesis. Springer, Berlin, pp 89–93. https://doi.org/10.1007/978-3-0348-5559-4_9
Niimi K, Usami K, Fujita Y, Abe M, Furukawa M, Suyama Y, Sakai Y, Kamioka M, Shibata N, Park EJ, Sato S, Kiyono H, Yoneyama H, Kitazawa H, Watanabe K, Nochi T, Aso H (2018) Development of immune and microbial environments is independently regulated in the mammary gland. Mucosal Immunol 11(3):643–653. https://doi.org/10.1038/mi.2017.90
Oliphant LW (1983) First observations of brown fat in birds. Condor 85(3):350–354. https://doi.org/10.2307/1367075
Paulo E, Wang B (2019) Towards a better understanding of beige adipocyte plasticity. Cells 8(12):1552–1567. https://doi.org/10.3390/cells8121552
Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J (2010) Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 285(10):7153–7164. https://doi.org/10.1074/jbc.M109.053942
Raimbault S, Dridi S, Denjean F, Lachuer J, Couplan E, Bouillaud F, Bordas A, Duchamp C, Taouis M, Ricquier D (2001) An uncoupling protein homologue putatively involved in facultative muscle thermogenesis in birds. Biochem J 353(3):441–444. https://doi.org/10.1042/0264-6021:3530441
Rey B, Roussel D, Romestaing C, Belouze M, Rouanet JL, Desplanches D, Sibille B, Servais S, Duchamp C (2010) Up-regulation of avian uncoupling protein in cold-acclimated and hyperthyroid ducklings prevents reactive oxygen species production by skeletal muscle mitochondria. BMC Physiol 10(1):5–16. https://doi.org/10.1186/1472-6793-10-5
Rosenwald M, Perdikari A, Rülicke T, Wolfrum C (2013) Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol 15(6):659–667. https://doi.org/10.1038/ncb2740
Roussel D, Rouanet JL, Duchamp C, Barre H (1998) Effects of cold acclimation and palmitate on energy coupling in duckling skeletal muscle mitochondria. FEBS Lett 439(3):258–262. https://doi.org/10.1016/s0014-5793(98)01382-9
Saarela S, Hissa R, Pyörnilä A, Harjula R, Ojanen M, Orell M (1989) Do birds possess brown adipose tissue? Comp Biochem Physiol A Physiol 92(2):219–228. https://doi.org/10.1016/0300-9629(89)90157-6
Saarela S, Keith JS, Hohtola E, Trayhurn P (1991) Is the “mammalian” brown fat-specific mitochondrial uncoupling protein present in adipose tissues of birds? Comp Biochem Physiol B: Comp Biochem 100(1):45–49. https://doi.org/10.1016/0305-0491(91)90082-o
Seki Y, Sato K, Kono T, Abe H, Akiba Y (2003) Broiler chickens (Ross strain) lack insulin-responsive glucose transporter GLUT4 and have GLUT8 cDNA. Gen Comp Endocrinol 133(1):80–87. https://doi.org/10.1016/s0016-6480(03)00145-x
Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L, Hu H, Wang L, Pavlova Z, Gilsanz V (2012) Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS ONE 7(11):e49452-49461. https://doi.org/10.1371/journal.pone.0049452
Sidossis L, Kajimura S (2015) Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J Clin Invest 125(2):478–486. https://doi.org/10.1172/JCI78362
Sidossis LS, Porter C, Saraf MK, Borsheim E, Radhakrishnan RS, Chao T, Ali A, Chondronikola M, Mlcak R, Finnerty CC, Hawkins HK, Toliver-Kinsky T, Herndon DN (2015) Browning of subcutaneous white adipose tissue in humans after severe adrenergic stress. Cell Metab 22(2):219–227. https://doi.org/10.1016/j.cmet.2015.06.022
Srivastava RK, Moliner A, Lee E-S, Nickles E, Sim E, Liu C, Schwarz H, Ibáñez CF (2020) CD137 negatively affects “browning” of white adipose tissue during cold exposure. J Biol Chem 295(7):2034–2042. https://doi.org/10.1074/jbc.AC119.011795
Toyomizu M, Ueda M, Sato S, Seki Y, Sato K, Akiba Y (2002) Cold-induced mitochondrial uncoupling and expression of chicken UCP and ANT mRNA in chicken skeletal muscle. FEBS Lett 529(2–3):313–318. https://doi.org/10.1016/s0014-5793(02)03395-1
Ueda M, Watanabe K, Sato K, Akiba Y, Toyomizu M (2005) Possible role for avPGC-1alpha in the control of expression of fiber type, along with avUCP and avANT mRNAs in the skeletal muscles of cold-exposed chickens. FEBS Lett 579(1):11–17. https://doi.org/10.1016/j.febslet.2004.11.039
Vianna CR, Hagen T, Zhang CY, Bachman E, Boss O, Gereben B, Moriscot AS, Lowell BB, Bicudo JE, Bianco AC (2001) Cloning and functional characterization of an uncoupling protein homolog in hummingbirds. Physiol Genom 5(3):137–145. https://doi.org/10.1152/physiolgenomics.2001.5.3.137
Vitali A, Murano I, Zingaretti MC, Frontini A, Ricquier D, Cinti S (2012) The adipose organ of obesity-prone C57BL/6J mice is composed of mixed white and brown adipocytes. J Lipid Res 53(4):619–629. https://doi.org/10.1194/jlr.M018846
Wojtczak L, Schonfeld P (1993) Effect of fatty acids on energy coupling processes in mitochondria. Biochim Biophys Acta 1183(1):41–57. https://doi.org/10.1016/0005-2728(93)90004-y
Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerback S, Schrauwen P, Spiegelman BM (2012) Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150(2):366–376. https://doi.org/10.1016/j.cell.2012.05.016
Xue B, Rim JS, Hogan JC, Coulter AA, Koza RA, Kozak LP (2007) Genetic variability affects the development of brown adipocytes in white fat but not in interscapular brown fat. J Lipid Res 48(1):41–51. https://doi.org/10.1194/jlr.M600287-JLR200
Young P, Arch JR, Ashwell M (1984) Brown adipose tissue in the parametrial fat pad of the mouse. FEBS Lett 167(1):10–14. https://doi.org/10.1016/0014-5793(84)80822-4
Zhu J, Li X, Qi H, Gu Z, Song S, Yang X, Zhou G, Li C (2017) Oxidative and anti-oxidative status in muscle of young rats in response to six protein diets. Sci Rep 7(1):13184–13193. https://doi.org/10.1038/s41598-017-11834-5
Acknowledgements
We thank Dr. Yoshiki Hiruta for his technical assistance in image processing and data analysis, and our colleagues in the Laboratory of Animal Nutrition at Tohoku University Graduate School of Agricultural Science for their help in sample collection support.
Funding
This study was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI (15H04582 to M.T. and 16H06205 to M.K.) and by JSPS Core-to-Core Advanced Research Networks Program, entitled “Establishment of international agricultural immunology research-core for a quantum improvement in food safety”.
Author information
Authors and Affiliations
Contributions
Substantial contributions were made by each author over the 4-year period of the study are as follows: RS, MS degree: design of the work, animal experiments, all analyses, interpretation of data, preparation of figures, and initial drafting of manuscripts, AH, PhD: quantified multilocular adipocytes and initial drafting of the manuscript, MK, PhD: designed the work, statistics and acted as sub-supervisor, TA, PhD: performed western blot analysis of VDAC protein and data interpretation, KF, PhD (Post Doc): performed western blot analysis of avUCP protein, AK, MS degree: initial observation and first effort to discover beige-like fat, KW, PhD: performed analyses for H&E stains and immunohistochemistry, AC, PhD: prepared avUCP anti-bodies and analysis, RH, MS degree: performed RT-PCR analysis of mRNA expression, YT, MS degree: performed western blot analysis, HS, PhD: performed RT-PCR analysis of mRNA expression and western blot analysis, HT, PhD: prepared samples for analysis of mRNA expression, GW, PhD: discussion on energy metabolism of BAT & beige, TN, PhD: strategy of the work & interpretation of morphological data, TS, PhD: analysis of mRNA expression, CHW, PhD: discussion on avUCP, and assisted in drafting the manuscript, MT, PhD: supervised all works including concept, all procedures and preparation of the manuscript (Corresponding author).
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the Institutional Animal Care and Use Committee of the Graduate School of Agricultural Science, Tohoku University.
Informed consent
No informed consent is required for this study.
Additional information
Handling editor: F. Bazer.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sotome, R., Hirasawa, A., Kikusato, M. et al. In vivo emergence of beige-like fat in chickens as physiological adaptation to cold environments. Amino Acids 53, 381–393 (2021). https://doi.org/10.1007/s00726-021-02953-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-021-02953-5