Abstract
Introduction
Treadmill training (TT) is a gait training technique that has commonly been used in neurorehabilitation, and has positive effects on gait, mobility, and related outcomes in stroke survivors. Transcranial direct current stimulation (tDCS) is a non-invasive approach for modulating brain cortex excitability.
Aim
To evaluate the available scientific evidence on the effects of TT combined with tDCS on mobility, motor performance, balance function, and brain-related outcomes in stroke survivors.
Methods
Five databases namely the Cochrane library, PEDro, Web of Science, PubMed, and EMBASE, were searched for relevant studies from inception to March, 2024. Only randomized controlled trials were included, and their methodological quality and risk of bias (ROB) were evaluated using the PEDro scale and Cochrane ROB assessment tool respectively. Qualitative and quantitative syntheses (using fixed effects meta-analysis) were employed to analyze the data.
Results
The results revealed that TT combined with active tDCS had significant beneficial effects on some mobility parameters, some gait spatiotemporal parameters, some gait kinematic parameters, gait endurance, gait ability, and corticomotor excitability in stroke survivors, but no significant difference on gait speed (P > 0.05), functional mobility (P > 0.05), motor performance (P > 0.05), or some balance functions (P > 0.05), compared with the control conditions.
Conclusions
TT combined with active tDCS significantly improves some gait/mobility outcomes and corticomotor excitability in stroke survivors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Varying limitations including those in mobility, transfers, walking, navigating stairs and essential daily activities are found in stroke survivors [1]. In addition, in stroke, activities for daily locomotion such as walking on sloped surface, moving around obstacles, climbing up and down the stairs, making turn and changing directions are also impaired [2]. During the gait cycle, stroke survivors typically show altered kinetic and kinematic variables at the hip, knee and ankle joints [3]. The post-stroke gait impairments strongly contribute to overall disability in stroke survivors [4]. Because impairments in walking arise from neuromuscular control deficiency, it is important to better understand the effects of gait training on motor pattern in lower extremity [3]. High intensity treadmill training (HIT), an increasingly popular exercise training in which exercise intensity is maximized by combining short bursts of maximal effort [5], can be used to targets gait difficulties and decreased cardiorespiratory fitness [6].
Treadmill exercises, especially during the acute phase of stroke, have several positive effects, leading to improvements in numerous dimensions [7]. It has been found that turning-based treadmill training (TT) effectively improves functional reorganization of the brain underlying cortico-muscular and cortico-cortical mechanisms, leading to improvements in gait among stroke survivors [8]. Moreover, post-stroke TT has been shown to improve walking distance, but not walking speed and balance, compared with over-ground walking training [9]. TT can improve aerobic capacity more effectively than conventional rehabilitation [2]. Aerobic treadmill exercises have been reported to be used successfully in gait retraining and for enhancing cardiorespiratory fitness [4]. In addition, high intensity speed-based treadmill training has been reported to improve walking speed, walking endurance and quality of life even up to 3 months post intervention [5].
Transcranial direct current stimulation (tDCS) has been established as a non-invasive approach to improving neurorehabilitation [10] involving neuromodulation of the brain cortical areas with possible therapeutic benefits [11]. It consists of applying a low-intensity constant direct current to specific brain areas and is used for modulating neuronal activity and influencing brain function [12]. The weak electrical current induces the regulation of brain cortex activity [13,14,15]. tDCS can significantly improve walking and lower limb motor functions in stroke survivors [16]. In tDCS, constant low current is delivered between two electrodes, either anodal or cathodal in nature placed on the skull, which results in the modulation of neural excitability [17]. However, a recent systematic review, reported a lack of evidence for the use of tDCS alone for improving motor function, balance and quality of life in stroke survivors; but postulated the beneficial effects of tDCS when combined with other interventions [18].
To the extent of what we know, and given the background provided above, there has not been a systematic review on the effects of TT combined with tDCS on the outcomes of interest. Hence, the purpose of the systematic review and meta-analysis was to determine the scientific evidence on the effects of TT combined with tDCS on mobility, motor performance, balance function, and other brain related outcomes in stroke survivors.
Methods
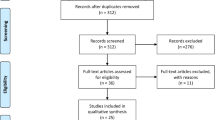
The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guide was followed in carrying out the review (Fig. 1), and the review has been registered in PROSPERO (registration number: CRD42024534889). The PICOS strategy was used to construct the following research question: What is the effect of TT combined with active tDCS (I = intervention) on mobility, motor performance, balance function and other brain-related outcomes (O = outcome) in stroke survivors (P = population), when compared with TT alone or combined with sham tDCS (C = comparison) in randomized controlled trials (RCTs) (S = study design)?
Strategy for search
Five online databases, namely the Cochrane library, PEDro, Web of Science, PubMed, and EMBASE online data bases were searched for relevant studies from inception to March; 2024. The search strategy employed in the data bases was produced using the PICOS strategy. All of the data bases were searched in accordance with their requirements. Details about the search approach employed in the online data bases are presented in Supplementary Material 1. In addition, there was a manual search of the reference lists of the relevant studies and reviews. Similarly, studies obtained from the general literature search were involved in the review. The studies found were imported into the reference manager and duplicates were removed, whereas the results that remained undergone additional screenings in the title, abstract and full text. The search was conducted independently by one author (JSU), and verified by the other two authors (TWLW and SSMN).
Criteria for eligibility
Studies that met the following inclusion criteria were included in the review: studies (i) performed in human stroke survivors and published in English in peer reviewed journals, (ii) that reported the effects of TT combined with tDCS, and (iii) that had a cross-over or parallel RCT design with full text obtainable. Abstracts presented in conferences, theses, and review studies were left out.
Data extraction and selection of studies
After expunging the duplicate studies, two authors (JSU and TWLW) independently screened the titles and abstracts of the remaining studies in line with the review selection criteria. Any disagreement between the two authors regarding the eligibility or inclusion of a study were resolved by consulting the other author (SSMN). For full text screening, the full-texts of relevant studies have been obtained, and the following data were extracted from each study: author and publication year, design, sample size, sex, age, clinical characteristics of the population (chronicity, stroke duration, stroke type, and affected side), intervention group(s), outcomes, TT protocols, evaluation periods, parameters for stimulation (type, site, intensity, period, size of electrode, number of stimulation sessions, and side effects), results and conclusions. The extracted data was inputted into Microsoft Excel.
Assessment of methodological quality (MQ) and risk of bias (ROB)
The MQ of the studies included was appraised using the PEDro scale. The scale has eleven items used to score the MQ of the studies [19], the 11 items assess the internal validity and statistical reporting of the study, excluding initial item that deals with eligibility criteria and is excluded in computing the total score [20]. Thus, the internal validity consist of 10 items that are rated as 1 or 0 signifying Yes (present) and No (absent) respectively [20]. Total scores of 0–3, 4–5, 6–8, and 9–10 are considered to indicate poor, fair, good and excellent MQ respectively [21, 22].
The Cochrane ROB tool for RCTs was applied to appraise the ROB in the studies involved in the review (Fig. 2a & b). The tool provide three decision (unclear, high or low) regarding the ROB for individual items in the following domains of bias in: selection, reporting, performance, detection, attrition and other sources [23]. Each study receives an overall judgement (unclear, high, or low) based on the total score obtained by summing the individual domain scores. Two authors (JSU and TWLW) conducted the ROB evaluation independently. Any disagreements were resolved by consulting the other author (SSMN).
Data analysis
Qualitative and quantitative syntheses were applied to analyze the data that was retrieved. In the qualitative synthesis, the MQ, ROB, and characteristics of the relevant studies were explained. The quantitative synthesis involved a fixed effects meta-analysis (FEMA) of the mean and standard deviation values of the outcomes post-intervention and the sample sizes of the relevant studies. For outcomes for which meta-analyses could not be performed, e.g., because less than two studies reported them, the mean and standard deviation values were used to compute the effect sizes (ESs) for these outcomes. The Cochrane GRADE approach was used to synthesize and assess the certainty of the evidence.
Results
Qualitative synthesis of the results
In the qualitative synthesis, the data were synthesized narratively.
Identification and selection of eligible studies
In total, 153 relevant studies were identified by the searching the online databases and added sources. Subsequent to the removal of duplicates (35), 118 studies left. Title and abstract screening; led to the exclusion of 108 studies for not conforming with the eligibility criteria. Subsequently, the full-texts of the remaining 10 studies were assessed against the eligibility criteria for the review. Eight (8) studies were found to be suitable and were involved in the review. All of the included studies were RCTs with cross-over or parallel design. Figure 1 shows the procedure of identification and selection of studies in line with the PRISMA guidelines.
MQ of the relevant included studies
Of the eight included studies, four had good and, four had excellent MQ. Regarding the domains of the PEDro scale, all of the included specified the eligibility criteria, conducted random allocation, had groups that are similar at baseline in terms of important prognostic indicators, measured the key outcomes from majority of participants initially allocated to groups, had all subjects whom outcome measures were available received intervention as allocated, and reported between group comparisons and provides point measures and measures of variability for key outcomes. In all the studies except three [24,25,26], there was allocation concealments. All studies had subjects blinding except in two studies [24, 27], half of the studies had experimenter blinding and half have not, and all the studies except three [24,25,26] adopted outcome assessor blinding. Table 1 provides the methodological details of the included studies.
ROB in the relevant included studies
All of the studies had a low ROB in the domains of attrition, reporting and other biases, while half of the studies had a low and the other half had a high ROB in detection bias; In addition, a majority of the studies had a low ROB in performance bias and selection bias (allocation concealment), and most of the studies had an unclear ROB in selection bias (random sequence generation). Figure 2a and b provides the ROB assessment results of the included studies.
Characteristics of the relevant included studies
All of the studies included chronic stroke survivors except one [25] that included subacute stroke survivors. Four studies employed a parallel RCT design, and the other four adopted a cross-over RCT design. In total, 227 subjects participated in the included studies, of whom 146 were male. The mean age range of the participants was 55.43 to 67.25 years, and the mean stroke duration ranged from 25.50 to 121.25 months. Regarding the stroke type, 122 participants had ischemic stroke, while 73 had hemorrhagic stroke. One study [26] did not report the stroke type. The affected side was right in 93 participants, and one study [25] did not report the affected side. Regarding the tDCS type, all of the studies used real/active tDCS and sham tDCS combined with TT. In terms of outcomes assessment, all of the included studies, performed pre and post intervention outcome assessments. Table 2 presents the characteristics of the included studies.
Described outcomes
Gait speed
Eight studies reported the gait speed of the participants [24,25,26,27,28,29,30,31].
Mobility
Two studies reported functional mobility [25, 27], and one study reported mobility [25]. Walking performance was reported in two studies [26, 31].
Motor function
Four studies reported motor function [25,26,27, 31].
Functional capacity
Two studies reported on functional capacity [27, 28].
Other gait spatiotemporal and related parameters
One study [28] reported stance phase, double limb support time (DBST), and step width, and one study [31] reported cadence, and step time. Step length was reported in three studies [28, 30, 31], and step length symmetry was reported in two studies [26, 30]. One study [26] reported the paretic step ratio and paretic propulsion, and one study [28] reported kinematic parameters.align=“left”
Balance-related parameters
One study reported balance performance and balance confidence [27] while another one reported trunk control [25].
Other brain-related parameters
Corticomotor excitability was reported in three studies [24, 27, 31].
Cardiovascular related parameters
The heart rate and/or its variability was reported in two studies [24, 29], while blood pressure was reported in three studies [24, 28, 29].
tDCS procedures
Types of stimulation
All of the included studies except one [31] conducted anodal tDCS, while one study [26] conducted cathodal and dual stimulations in addition to anodal stimulation. One study reported bilateral and cathodal stimulations [31].
Site of electrodes during tDCS
The anode electrode placement on the leg motor area (M1)/motor cortex was reported in all of the included studies except one [29] in which the placement was lateral to the inion. Cathode electrode placement on the orbital region was reported in four studies [24, 27, 28, 31], while the other studies reported cathode placement on the middle deltoid muscle [29], ipsilateral buccinator muscle [30], inion [25], ipsilateral shoulder [26], and contralesional M1 [26, 31].
Intensity and duration of stimulation
Five studies applied a tDCS intensity of 2.0 mA [26, 28,29,30,31], and three studies applied a tDCS intensity of 1.0 mA [24, 25, 27]. Regarding stimulation duration, five studies reported a duration of 20 min [25, 26, 28, 29, 31], two studies reported 15 min [24, 27], and one study reported 10 min [30].
Size of electrodes
With regards to the anode/active electrode size, two studies [29, 30] reported 5 × 5 cm2, one [25] reported 5 × 5 cm, while one study each reported 8cm2 [24], 5 × 2.5 cm [27], 1.75cm2 [26] and 35cm2 [31].
Number of stimulation sessions
Concerning number of stimulation sessions, when the studies were reviewed, two studies conducted twelve sessions [27, 31]. Additionally, one study each conducted one [29], two [24], three [30] four [26], seven [25], and ten [28] sessions.
Side effects of tDCS
Two studies reported side effects [27, 30]. In two studies [24, 31] there participants reported no adverse/side effects, while in another study [28], no detail information was reported about the adverse/side effects. However, three studies [25, 26, 29] reported no information on adverse/side effects linked to tDCS. Table 3 provides the details of the tDCS protocols used in the included studies.
Qualitative synthesis, and quantitative synthesis
Effects of the interventions
Effects on gait speed
Three studies evaluated the effect on comfortable gait speed [26,27,28]. The FEMA results showed no significant difference in comfortable gait speed in the initial sessions post-intervention (standardized mean difference [SMD] = -0.25, 95% confidence interval [CI] -0.59 to 0.10, P = 0.17) (Fig. 3a) (with low certainty of evidence downgraded due to ROB and imprecision), comfortable gait speed in the follow-up sessions post-intervention (SMD = -0.34, 95% CI -0.74 to 0.07, P = 0.10) (Fig. 3b) (with moderate certainty of evidence downgraded due to imprecision), or fastest walking speed (SMD=-0.36, 95%CI -0.83 to 0.11, P = 0.13) (Fig. 3c) (with low certainty of evidence downgraded due to imprecision and high ROB); among stroke survivors between the experimental and control groups. However, Speed-based high-intensity interval training (HISTT) has been found to improve gait speed in stroke survivors [27]. Additionally, TT combined with active tDCS significantly improved walking speed [25, 28], and cognitive dual task walking speed [31]. Also TT combined with active tDCS shows trend toward improving gait speed [24]. There was also positive improvement in walking speed especially in the TT with sham tDCS group post intervention [30].
Effects on mobility
The ES showed no significant difference in functional mobility (mean difference [MD] = 2.80, 95% CI -0.91 to 6.51, P = 0.14) [27], (MD = -3.10, 95% CI -6.87 to 0.67, P = 0.11) [25] between the experimental and control groups. For other outcomes, the calculated ES revealed a significant difference in performance-oriented mobility (MD = 0.56, 0.04 to 1.07, P = 0.03) between the experimental and control groups [25]. Anodal tDCS over the supplementary motor area combined with BWSTT was found to possibly improve gait ability in stroke survivors [25]. Also, TT combined with active tDCS has superior positive effect on cognitive dual task walking performance [31]. Also, it was found that clinical and biomechanical walking performance may be affected with the intervention [26].
Effects on motor performance
The calculated ES revealed no significant difference in motor function (MD = 1.20, 95% CI -1.37 to 3.77, P = 0.36) [27], (MD = -1.50, 95% CI -4.12 to 1.12, P = 0.26) [25] between experimental and control group in stroke survivors. However, TT combined with active tDCS significantly improved lower extremity motor control [31].
Effects on functional capacity
The calculated ES revealed no significant difference in the 6-minute walk test score (MD = -0.143, P = 0.18) between the experimental and control groups. HISTT was found to improve endurance in stroke survivors [27].
Effects on other gait spatiotemporal and related parameters
The calculated ES revealed no significant difference in the stance phase (after one session) (SMD = 0.21, P = 0.43), stance phase (after 10 sessions) (SMD = 0.34, P = 0.20), stance phase (at the one-month follow up) (SMD = -0.10, P = 0.70), between the experimental and control groups. Furthermore, the calculated ES revealed a significant difference in DBST (after one session) (MD = 0.56, 0.04 to 1.07, P = 0.006) between the experimental and control groups, but no significant difference in DBST (after 10 session) (MD = 0.38, P = 0.16) and DBST (at the one-month follow up) (MD = 0.02, P = 0.95) between the experimental and control groups. Similarly, no significant difference was observed in the step length (P > 0.05), 10-meter walk test score (P > 0.05), paretic step ratio (P > 0.05) and paretic propulsion (P > 0.05), but significant differences were observed in the step width (SMD = 0.93, 0.38 to 1.49, P = 0.0010) and stroke impact (SMD = -0.82, -1.46 to -0.18, P = 0.01) between the experimental and control groups. Additionally, cathodal tDCS followed by TT can result to better effects on the cognitive dual-task walking (CDTW), speed, cadence, and step time of the paretic leg than TT alone [31]. One study [28] revealed a significant improvement in gait kinematic parameters.
Effects on balance-related parameters
The calculated ES revealed no significant difference in trunk control (MD = 0.05, P = 0.84), balance performance (P > 0.05), or balance confidence (MD = -0.12, P = 0.71) between the experimental and control groups. Also, although both Berg balance test and mini-BEST test scores shows time effect, and the scores were higher at post assessment and 3 month follow up, but no group effect or group X time interaction with the interventions [27].
Effects on other brain-related parameters
The calculated ES revealed no significant difference in corticomotor excitability (CME; P > 0.05) [27]. In addition, anodal tDCS enhanced with ankle-tracking (e-tDCS) + HIIT induced an increase in the CME of the paretic tibialis anterior (TA) with a corresponding increase in non-paretic CME [24]. Cathodal tDCS followed by TT significantly increased the inhibition; and decreased the excitability of the contralesional M1 more than TT alone [31].
Effect on cardiovascular-related parameters
The calculated ES revealed significant difference in the variance of heart rate variability (HRV) post-tDCS in favor of the experimental group (MD = -370.91, P = 0.04) [29]. But, there was reported no significant between group differences in systolic blood pressure and heart rate variabilities in the evaluation periods [29].
Discussion
The key objective of this study was to evaluate the scientific evidence on the effects of TT combined with tDCS on mobility, motor performance, balance function and other brain-related outcomes in stroke survivors. The major findings of the quantitative synthesis revealed that TT combined with active tDCS had a significant impact on DBST, step width, performance-oriented mobility, and stroke impact among stroke survivors. However, no significant differences were observed in other spatiotemporal parameters such as gait speed, functional mobility, motor performance, and some balance function outcomes between the experimental and control groups. The qualitative analysis findings showed that the interventions in the experimental group led to significant improvements in gait ability, gait endurance, some gait kinematic parameters, CDTW, gait speed, cadence, step time, and corticomotor excitability in stroke survivors compared with the control group. The review included eight RCTs. Qualitative and quantitative syntheses were used for data analysis.
Quantitative findings
The results revealed that in stroke survivors, TT combined with active tDCS led to significantly better step width, performance-oriented mobility, and DBST than the control interventions. Improvements in the above parameters may go a long way in improving the balance of stroke survivors. These improvements may have occurred possibly because of the nature of the task involved in TT which has to be executed with lower extremities, and all the improved parameters involved the lower extremities. Such may be supported by the previous finding that TT is effective in improving some mobility and gait parameters posts-stroke [32]. In addition, the additional effect of active tDCS may have contributed to the improvements. Although improvements were found in the above parameters, the findings for the outcomes were from few studies involved in the meta-analysis, some findings on some outcomes were only based on qualitative synthesis of some of the RCTs and the calculated effect sizes. In addition, even though the results tended to favoring the experimental group, the results of meta-analysis showed no significant difference between the effects of TT combined with active tDCS and those of TT combined with sham on other spatiotemporal parameters such as gait speed, functional mobility, motor performance, and some balance function outcomes. Hence, further research is warranted to support the existing evidence; because the few studies included in the meta-analysis had limited sample sizes and some methodological flaws. The above-mentioned evidences limit the certainty of the conclusions drawn and calls for more research to obtain more robust conclusions.
Qualitative findings
The qualitative analysis results revealed that the interventions in the experimental group led to significant improvements in gait ability, gait endurance, some gait kinematic parameters, CDTW, gait speed, cadence, step time, lower extremity motor control and corticomotor excitability in stroke survivors compared with the control groups. This implies that TT combined with tDCS enhances gait/mobility and corticomotor excitability. These findings were supported by three studies [5, 8, 32]. It is vital to note that the findings of improvements in some of the parameters should be interpreted cautiously because the conclusions were derived considering the results of qualitative synthesis and the calculated ESs from the studies; hence, future studies are needed to confirm the findings.
Some of the studies revealed no significant differences in other spatiotemporal parameters, such as gait speed, functional mobility, motor performance, and some balance function outcomes between the experimental and control groups. This finding further supports the need for more RCTs in future on the outcomes of interest; to establish firmer conclusions.
Although; the studies included in the analysis were of good or excellent MQ, some studies did not report adequate data on the outcomes of interest to enable a meta-analysis to be conducted. Hence only ESs were calculated for such outcomes. Additionally, most of the studies included in the review had small sample sizes, and some studies also had an unclear or high ROB in selection and performance bias. These limitations highlight the limited strength of the current evidence; hence, further studies are required to address these limitations and validate the findings.
Strengths and limitations
The main strengths of this review are adherence to PRISMA guidelines for conducting and reporting the review, searching of relevant online data bases, and use of standardised tools to evaluate the MQ and ROB. The key limitation of the review is that meta-analysis was not performed for some of the outcomes of interest due to inadequate studies or data on these outcomes. Thus, further studies on these outcomes of focus are required. The main limitations of the individual relevant studies were small sample sizes in most, selection and detection bias in many, and performance bias in some of the relevant studies. Thus, caution should be exercised when interpreting the results. Future studies are needed to address these limitations.
Conclusions
TT combined with active tDCS is beneficial for improving some gait/mobility outcomes and corticomotor excitability in stroke survivors.
Data availability
Data is available from the corresponding author on reasonable request.
References
Tarihci Cakmak E, Yaliman A, Torna G, Sen EI (2024) The effectiveness of bodyweight-supported treadmill training in stroke patients: randomized controlled trial. Neurol Sci 45(7):3277–3285
da Silva RS et al (2019) Effects of inclined treadmill training on functional and cardiovascular parameters of stroke patients: study protocol for a randomized controlled trial. Trials 20:1–9
Mao Y-R et al (2015) The effect of body weight support treadmill training on gait recovery, proximal lower limb motor pattern, and balance in patients with subacute stroke. BioMed research international, 2015
Lam JM et al (2010) Predictors of response to treadmill exercise in stroke survivors. Neurorehabilit Neural Repair 24(6):567–574
Madhavan S et al (2019) Effects of high intensity speed-based treadmill training on ambulatory function in people with chronic stroke: a preliminary study with long-term follow-up. Sci Rep 9(1):1985–p
Brauer SG et al (2021) High-intensity treadmill training and self-management for stroke patients undergoing rehabilitation: a feasibility study. Pilot Feasibility Stud 7:1–10
Motaqi M, Ghanjal A, Afrand M (2022) Treadmill Exercise and its Effect on Rehabilitation of patients after ischemic strok: a narrative study. Int J Musculoskelet pain Prev 7(3):730–740
Chen I-H et al (2019) Novel gait training alters functional brain connectivity during walking in chronic stroke patients: a randomized controlled pilot trial. J Neuroeng Rehabil 16:1–14
Gelaw AY et al (2019) Effectiveness of treadmill assisted gait training in stroke survivors: a systematic review and meta-analysis. Global Epidemiol 1:100012
Kolmos M et al (2023) Patient-tailored transcranial direct current stimulation to improve stroke rehabilitation: study protocol of a randomized sham-controlled trial. Trials 24(1):216
Dumel G et al (2018) Multisession anodal transcranial direct current stimulation induces motor cortex plasticity enhancement and motor learning generalization in an aging population. Clin Neurophysiol 129(2):494–502
Rodrigues NO et al (2023) Efficacy of Transcranial Direct Current Stimulation (tDCS) on cognition, anxiety, and mobility in Community-Dwelling older individuals: a controlled clinical trial. Brain Sci 13(12):1614
Tabrizi YM et al (2019) Transcranial direct current stimulation on prefrontal and parietal areas enhances motor imagery. NeuroReport 30(9):653–657
Speth J, Speth C, Harley TA (2015) Transcranial direct current stimulation of the motor cortex in waking resting state induces motor imagery. Conscious Cogn 36:298–305
Saimpont A et al (2016) Anodal transcranial direct current stimulation enhances the effects of motor imagery training in a finger tapping task. Eur J Neurosci 43(1):113–119
Zhang XH et al (2021) The Effect of Transcranial Direct Current Stimulation and Functional Electrical Stimulation on the lower limb function of stroke patients. Front Neurosci 15:685931
Bodranghien F, Manto M, Lebon F (2016) Enhancing transcranial direct current stimulation via motor imagery and kinesthetic illusion: crossing internal and external tools. J Neuroeng Rehabil 13(1):1–3
Lima E, de Souza Neto JMR, Andrade SM (2023) Effects of transcranial direct current stimulation on lower limb function, balance and quality of life after stroke: a systematic review and meta-analysis. Neurol Res 45(9):843–853
Maher CG et al (2003) Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther 83(8):713–721
Paci M, Bianchini C, Baccini M (2022) Reliability of the PEDro scale: comparison between trials published in predatory and non-predatory journals. Archives Physiotherapy 12(1):1–9
Foley NC et al (2003) Stroke rehabilitation evidence-based review: methodology. Top Stroke Rehabil 10(1):1–7
Gonzalez GZ et al (2018) Methodologic quality and statistical reporting of physical therapy randomized controlled trials relevant to musculoskeletal conditions. Arch Phys Med Rehabil 99(1):129–136
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group (2011) The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ, 18:343
Madhavan S, Stinear J, Kanekar N (2016) Effects of a Single Session of High Intensity Interval Treadmill Training on Corticomotor Excitability following Stroke: Implications for Therapy. Neural Plasticity, 2016
Manji A et al (2018) Effects of transcranial direct current stimulation over the supplementary motor area body weight-supported treadmill gait training in hemiparetic patients after stroke. Neurosci Lett 662:302–305
Seamon BA et al (2023) Transcranial Direct Current Stimulation Electrode montages May differentially impact variables of walking performance in individuals Poststroke: a preliminary study. J Clin Neurophysiol 40(1):71–78
Madhavan S et al (2020) Cortical priming strategies for gait training after stroke: a controlled, stratified trial. J Neuroeng Rehabil 17(1):111
Dumont AJL, Cimolin V, Parreira RB, Armbrust D, Fonseca DRP, Fonseca AL, Cordeiro L, Franco RC, Duarte NAC, Galli M, Oliveira CS (2023) Effects of transcranial direct current stimulation combined with treadmill training on kinematics and spatiotemporal gait variables in stroke survivors: a randomized, triple-blind, sham-controlled study. Brain Sci, 13(1):11
Heinz G et al (2020) Effects of transcranial direct current stimulation (tDCS) and exercises treadmill on autonomic modulation of hemiparetic patients due to stroke—clinic test, controlled, randomized, double-blind. Front Neurol 10:p1402
Kumari N, Taylor D, Olsen S, Rashid U, Signal N (2020) Cerebellar Transcranial Direct Current Stimulation for Motor Learning in people with chronic stroke: a pilot randomized controlled trial. Brain Sci, 10(12):982
Wong PL et al (2023) Effects of Transcranial Direct Current Stimulation followed by Treadmill Training on Dual-Task walking and cortical activity in chronic stroke: a double-blinded Randomized Controlled Trial. J Rehabil Med 55:jrm00379
Ada L, Dean CM, Lindley R (2013) Randomized trial of treadmill training to improve walking in community-dwelling people after stroke: the AMBULATE trial. Int J Stroke 8(6):436–444
Funding
Open access funding provided by The Hong Kong Polytechnic University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare that there is no conflict of interest with any financial organization or institution with regards to the materials described in the manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Usman, J.S., Wong, T.Wl. & Ng, S.S.M. Effects of treadmill training combined with transcranial direct current stimulation on mobility, motor performance, balance function, and other brain-related outcomes in stroke survivors: a systematic review and meta-analysis. Neurol Sci (2024). https://doi.org/10.1007/s10072-024-07768-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10072-024-07768-2