Abstract
The purpose of this study is to evaluate the in-hospital mortality of community-acquired pneumonia (CAP) treated with ceftaroline in comparison with standard therapy. This was a retrospective observational study in two centers. Hospitalized patients with CAP were grouped according to the empiric regimen (ceftaroline versus standard therapy) and analyzed using a propensity score matching (PSM) method to reduce confounding factors. Out of the 6981 patients enrolled, 5640 met the inclusion criteria, and 89 of these received ceftaroline. After PSM, 78 patients were considered in the ceftaroline group (cases) and 78 in the standard group (controls). Ceftaroline was mainly prescribed in cases with severe pneumonia (67% vs. 56%, p = 0.215) with high suspicion of Staphylococcus aureus infection (9% vs. 0%, p = 0.026). Cases had a longer length of hospital stay (13 days vs. 10 days, p = 0.007), while an increased risk of in-hospital mortality was observed in the control group compared to the case group (13% vs. 21%, HR 0.41; 95% CI 0.18 to 0.62, p = 0.003). The empiric use of ceftaroline in hospitalized patients with severe CAP was associated with a decreased risk of in-hospital mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately 10 to 18% of hospitalized patients with community-acquired pneumonia (CAP) present with severe pneumonia that requires admission to the intensive care unit (ICU) [1]. Severe CAP is associated with high mortality, ranging from 25 to more than 50% [2, 3]. Prompt identification of severe pneumonia and early, adequate antibiotic therapy are crucial in managing these cases. Based on this observation, early, adequate antibiotic therapy could reduce mortality in severe CAP.
Several studies have reported the beneficial effects on the patient outcomes by using ceftriaxone plus macrolide regimen as empiric therapy [4, 5]. However, this combination is not good enough to cover Staphylococcus aureus, which is increasingly identified as a cause of severe pneumonia with high associated mortality, particularly in cases related to influenza virus co-infection or COVID-19 [1, 6, 7]. Also, the increasing prevalence of methicillin-resistant S. aureus and penicillin- and ceftriaxone-resistant Streptococcus pneumoniae in severe CAP has made this combination of antibiotics less effective [8, 9]. Ceftaroline is a broad-spectrum cephalosporin that covers gram-positive bacteria (including methicillin-susceptible and methicillin-resistant S. aureus and drug-resistant S. pneumoniae) and third-generation-susceptible gram-negative bacilli [10]. Results of the FOCUS 1 and 2 trials demonstrated the superiority of ceftaroline against ceftriaxone in bacterial CAP; however, these studies did not include severe cases and the mortality rate was low [10]. In comparison with ceftriaxone, ceftaroline has shown to have superior in vitro activity against S. aureus (≥ 16-fold more potent than ceftriaxone against MSSA and active against MRSA), S. pneumoniae, and other common CAP pathogens [11, 12].
Using the propensity score matching (PSM) method, we aim to evaluate in-hospital mortality of CAP treated with ceftaroline in comparison with standard therapy (Supplementary Table 1) in our cohort of severe CAP.
Methods
Study design
This was a retrospective analysis of prospectively collected data conducted at two Spanish hospitals (Hospital 1 from 1996 to 2020, and Hospital 2 from 2017 to 2020). The collection method was systematic, and all patients with CAP admitted to both hospitals were enrolled in the study.
Selection of patients
We enrolled all consecutive adult patients with a diagnosis of CAP admitted to hospital via the emergency department. We included patients from nursing homes, as we previously demonstrated that the microbial aetiology in this population is similar to that of CAP arising in people living in their own homes [13].
Exclusion criteria were as follows: (a) severe immunosuppression (AIDS, chemotherapy, immunosuppressive drugs [e.g., oral corticosteroid ≥ 10 mg prednisone or equivalent per day for at least 2 weeks]); (b) active tuberculosis; (c) cases with a confirmed alternative diagnosis.
The study was approved by the Ethics Committees of both institutions (Register: 2009/5451). The need for written informed consent was waived due to the non-interventional design.
Definitions
CAP was defined as a new pulmonary infiltrate on chest x-ray performed at hospital admission, combined with symptoms and signs consistent with a lower respiratory tract infection (e.g., fever, cough, sputum production, and pleuritic chest pain) in patients with no recent hospitalization or regular exposure to a healthcare system. Severe CAP was diagnosed by the presence of at least one major or three minor criteria, as set out by the Infectious Diseases Society of America/American Thoracic Society (IDSA/ATS) guideline [14]. Prior antibiotic treatment was defined as antibiotics taken within the week before disease presentation. Polymicrobial pneumonia was defined as pneumonia due to more than one pathogen.
Data collection
Demographic variables, comorbidities, and physiologic parameters were collected in the emergency department within 24 h of admission. Comorbidities of interest included chronic respiratory disease (e.g., chronic obstructive pulmonary disease, asthma, and bronchiectasis), diabetes mellitus, chronic cardiovascular disease, neurologic disease (e.g., dementia, coma, stroke, degenerative diseases, Parkinson’s disease, and Down syndrome), chronic renal disease, chronic liver disease, and previous neoplasm.
The Pneumonia Severity Index (PSI), CURB-65 score (i.e., confusion, urea nitrogen, respiratory rate, blood pressure, and age ≥ 65 years), and SOFA score were calculated at admission. During hospitalization, we recorded whether the patients had specific complications, including multilobar infiltration, pleural effusions, acute respiratory distress syndrome (ARDS), septic shock, or acute renal failure. Further details are reported elsewhere [15]. All surviving patients were visited or contacted by telephone 30 days after discharge.
Microbiologic evaluation
Microbiologic examination was performed on respiratory, urinary, and blood samples. Cultures were collected before the initiation of empiric antibiotic therapy in the emergency department. Criteria for making an aetiologic diagnosis have been reported previously[15].
Blood cultures, sputum cultures, and urine samples for S. pneumoniae and Legionella pneumophila antigen detection were obtained within 24 h of hospital admission. When available, pleural fluid, tracheobronchial aspirates, and bronchoalveolar lavage samples were collected for Gram and Ziehl–Neelsen staining and processed for bacterial, fungal, and mycobacterial pathogen detection. The results of susceptibility testing were interpreted according to EUCAST guidance (http://www.eucast.org). Blood samples for serology of atypical pathogens and respiratory virus were collected at admission and between the third and sixth week thereafter.
Respiratory viruses were diagnosed by serology, immunofluorescence assay (IFA), and isolation in cell cultures between 2005 and 2007. However, between 2008 and 2019, polymerase chain reaction (PCR) and/or cultures of nasopharyngeal swab samples were used instead. Two independent nested multiplex real-time PCR tests were performed to detect human influenza viruses (A, B, and C), respiratory syncytial virus, adenoviruses, parainfluenza viruses (1–4), coronaviruses (229E and OC43), enteroviruses, and rhinoviruses (A, B, and C).
Outcomes
Primary outcomes were in-hospital and 30-day mortality. Secondary outcomes included length of hospital stay, ICU mortality, length of stay in ICU, need of mechanical ventilation, and 30-day and 1-year mortality.
Statistical analysis
We report the number and percentage of patients for categorical variables, the median (first quartile; third quartile) for continuous variables with non-normal distributions, and the mean (standard deviation) for continuous variables with normal distributions. Categorical variables were compared using the chi-squared test or Fisher’s exact test, whereas continuous variables were compared using the t test or nonparametric Mann–Whitney U test.
Patients receiving ceftaroline in monotherapy or in combination as empirical therapy were considered the case group, whereas the remaining cohort was considered as controls. PSM was used to obtain a balance between patients in the case and control groups. To match the two cohorts, we used a 1:1 nearest neighbor matching without replacement within a match tolerance width of 0.005. Variables were chosen for inclusion in the PSM calculation according to methods set forth by Brookhart et al. [16]. Variables included were associated with the case group and outcomes (age, gender, chronic respiratory disease, chronic cardiovascular disease, diabetes mellitus, neurologic disease, chronic renal disease, chronic liver disease, previous neoplasm, fever, confusion, C-reactive protein, PaO2/FiO2, polymicrobial, bacteremia, multilobar, septic shock, ICU admission, and mechanical ventilation). An adequate model fit with discrimination and calibration of the PSM was demonstrated by the logistic model including covariates yielded a goodness-of-fit p = 0.867.
Cox proportional hazard regression models were used for in-hospital, 3-day, and 30-day mortality. The hazard ratio (HR) and its 95% confidence intervals (CI) were calculated.
We used the multiple imputation method for missing data in the covariates of the PSM model.
The level of significance was set at 0.05 (two-tailed). All statistical analyses were performed using IBM SPSS Statistics 26.0 (Armonk, NY, USA).
Results
General clinical characteristics
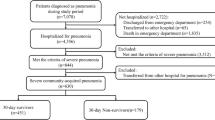
During the study period, 6981 patients with CAP were enrolled. Of these, 5640 (80%) patients met the inclusion criteria. In the full cohort, a total of 5551 (99%) received standard empiric antibiotic treatment and 89 (1%) received ceftaroline (3% monotherapy and 97% in combination therapy [46% ceftaroline + azithromycin; 16% ceftaroline + levofloxacin; and 38% other combinations]). After PSM was performed, 156 patients were finally included in the study (78 cases and 78 controls) (Fig. 1).
Table 1 summarizes the demographics and clinical characteristics of patients between case and control groups in the full cohort and after PSM. In the full cohort, and when compared to the control group, the case group was more likely to be younger and have a lower rate of influenza vaccines and a higher rate of pneumococcal vaccine. Also, the case group was less likely to have previously received inhaled corticosteroids and more frequently had chronic renal disease and a previous neoplasm. At admission, the case group less frequently presented with fever and purulent sputum than the control group. At admission, the case group had higher levels of C-reactive protein, lower lymphocyte counts, and worse oxygenation. Also, the case group at days 2 and 3 had higher levels of C-reactive protein and neutrophils, yet a lower rate of lymphocyte counts. In comparison with the control group, the case group also had a higher percentage of patients with severe CAP, with more cases of complications including bacteremia, multilobar involvement, ARDS, and acute renal failure. Pneumococcal vaccination, purulent sputum, and pleuritic pain were the only general variables not adequately balanced between both groups after PSM.
Microbial aetiology
In the full cohort, microbiologic diagnosis was more frequent in the case group (70% vs. 41%, p < 0.001) than in the control group. A higher prevalence of polymicrobial aetiology and Staphylococcus aureus, as well as a lower prevalence of Legionella pneumophila, was observed in the case group when compared to the control group. After PSM, no differences in microbiologic diagnoses were observed (73% vs. 76%, p = 0.714); however, Staphylococcus aureus was more frequent in the case group than in the control group (Table 2).
Outcomes
In the full cohort, the case group had more ICU admissions; longer length of hospital stay; longer length of ICU stay; more invasive mechanical ventilation; and higher 1-year mortality (Table 1). Cox regression (Table 3) showed that the risks of in-hospital, 3-day, and 30-day mortality did not differ between patients of either the case or control groups. After PSM, the case group was associated with a lower in-hospital mortality (adjusted HR 0.41 [95% CI 0.18 to 0.92]) and a longer length of hospital stay in comparison to the control group (Table 1 and Table 3, respectively).
Discussion
There are 3 main findings of this study. First, ceftaroline was mainly prescribed in cases of severe pneumonia with high suspicion of S. aureus infection. Second, in-hospital mortality of the PSM cohort was 13% in the ceftaroline group, and 21% in the control group. Third, after confounding variables were adjusted, the use of ceftaroline was associated with a lower in-hospital mortality rate.
Implementing new antibiotics into clinical practice often implies use of such drugs in the most severe cases, and a simple analysis of its effectiveness could be biased. Ceftaroline has been proposed as a better alternative to ceftriaxone during influenza season when S. aureus is more prevalent [17]. Severe infections characterize this population [18, 19], and it explains why ceftaroline was used mainly in critically ill patients in our cohort (67% presented with severe CAP). These patients had a higher prevalence of S. aureus and a recent history of pneumococcal vaccine. Ceftaroline is 16 times more active against MSSA (MIC90 0.25 versus 4 mg/L) than ceftriaxone and is active against MRSA as well [11]. Furthermore, available data about critically ill patients suggest that 2 g of ceftriaxone does not reach adequate plasma concentrations [20]. In the case of ceftaroline, pharmacokinetic/pharmacodynamic (PK/PD) target is achieved with a standard dosage, although PK data in critically ill patients is lacking. In addition, an animal model of pneumonia due to MRSA-producing Panton-Valentine leukocidin (PVL) showed that ceftaroline was bactericidal and also significantly reduced PVL concentration in the lung [21]. All of this data could explain why better results were obtained in 3 randomized controlled trials (RCT) [22,23,24] comparing the clinical efficacy of ceftaroline vs. ceftriaxone. In a meta-analysis of these studies, which included 1916 patients, ceftaroline (600 mg/12 h) was superior to ceftriaxone (1–2 g/24 h) in terms of clinical recovery (OR 1.66; 95% CI 1.34, 2.06) [25]; however, mortality rate in these studies was low (1.5% in each group) [25].
In our study, in-hospital mortality of the PSM cohort was 15%. This is a high mortality for CAP [26, 27], clearly demonstrating the severity of patients included in the analysis. Interestingly, in-hospital mortality was lower in the ceftaroline group (13% vs. 21%, p = 0.197). After we adjusted the analysis for confounding variables, in-hospital mortality was significantly lower in the ceftaroline group (adjusted HR 0.41 [95% CI 0.18 to 0.92]). The same trend was observed in results obtained for 30-day and 1-year mortality (adjusted HR 0.54 [95% CI 0.24 to 1.24]); however, the small number of patients may have not allowed for significance to be reached. Longer in-hospital survival in the case group may explain extended hospital stay.
There is a continuous debate about which antibiotic, a macrolide or fluoroquinolone, is the best companion to β-lactams. Both options are included as first-line choices in recent ATS guidelines [1]. Several studies have reported an association between the use of the combination of β-lactams plus macrolide and lower mortality in CAP, when compared to the use of β-lactams in monotherapy [28, 29]. However, Postma et al. [30]. found that β-lactam monotherapy was not inferior to the treatment with combination of β-lactams and macrolides or fluoroquinolone monotherapy with respect to 90-day mortality in patients with non-severe CAP. The main benefits of macrolides include a reduction in pneumolysin, immunomodulation, and activity against atypical pathogens, albeit not against S. aureus [31]. An association of macrolide with levofloxacin offers activity against S. aureus, but there is little data about its efficacy and some studies have even shown a worse prognosis [28]. In addition, the use of fluoroquinolones is associated with severe adverse effects [32, 33]. The potent activity of ceftaroline against S. aureus, the possibility to combine it with a macrolide, and the present article showing good outcomes in patients with severe CAP highlight how such a combination could offer broad-spectrum coverage, potent bactericidal activity, and the potential benefits of macrolides.
We believe that our statistical approach using a PSM is a strength in this study. There are, however, some limitations due to the final total sample size. In this study, we were able to analyze only 78 patients in each group (total N = 156). This sample size may result in a large type II error, and conclusions that can be drawn are limited. This aside, though, the rigorous approach to the study underpins our confidence in its findings and their clinical relevance. Another limitation of this study is the analysis of empiric antibiotic therapy, without consideration of dose or duration of the treatment.
Our data showed that ceftaroline was associated with a decreased risk of mortality in hospitalized patients with severe CAP. Ceftaroline is a very active β-lactam against S. pneumoniae and S. aureus, which can be associated with a macrolide without a loss of beneficial effects. This explains why ceftaroline was recommended by current ATS/IDSA guidelines [1] in the management of CAP. Our experience supports the use of ceftaroline in patients with severe CAP.
References
Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K et al (2019) Diagnosis and treatment of adults with community-acquired pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 200:e45-67. https://doi.org/10.1164/rccm.201908-1581ST
Cillóniz C, Dominedò C, Garcia-Vidal C, Torres A (2018) Community-acquired pneumonia as an emergency condition. Curr Opin Crit Care 24:531–539. https://doi.org/10.1097/MCC.0000000000000550
Ferrer M, Travierso C, Cilloniz C, Gabarrus A, Ranzani OT, Polverino E et al (2018) Severe community-acquired pneumonia: characteristics and prognostic factors in ventilated and non-ventilated patients. PLoS ONE 13:e0191721. https://doi.org/10.1371/journal.pone.0191721
Waterer G (2018) Empiric antibiotics for community-acquired pneumonia: a macrolide and a beta-lactam please! Respirology 23:450–451. https://doi.org/10.1111/resp.13248
Severiche-Bueno D, Parra-Tanoux D, Reyes LF, Waterer GW (2019) Hot topics and current controversies in community-acquired pneumonia. Breathe (Sheff) 15:216–225. https://doi.org/10.1183/20734735.0205-2019
Garcia-Vidal C, Sanjuan G, Moreno-García E, Puerta-Alcalde P, Garcia-Pouton N, Chumbita M et al (2021) Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect 27:83–88. https://doi.org/10.1016/j.cmi.2020.07.041
Cilloniz C, Dominedò C, Gabarrús A, Garcia-Vidal C, Becerril J, Tovar D et al (2021) Methicillin-susceptible staphylococcus aureus in community-acquired pneumonia: risk factors and outcomes. J Infect 82:76–83. https://doi.org/10.1016/j.jinf.2020.10.032
Cillóniz C, Dominedò C, Torres A (2019) Multidrug resistant gram-negative bacteria in community-acquired pneumonia. Crit Care 9(23):79. https://doi.org/10.1186/s13054-019-2371-3
Suaya JA, Mendes RE, Sings HL, Arguedas A, Reinert R-R, Jodar L et al (2020) Streptococcus pneumoniae serotype distribution and antimicrobial nonsusceptibility trends among adults with pneumonia in the United States, 2009–2017. J Infect 81:557–566. https://doi.org/10.1016/j.jinf.2020.07.035
File TM, Low DE, Eckburg PB, Talbot GH, Friedland HD, Lee J et al (2010) Integrated analysis of FOCUS 1 and FOCUS 2: randomized, doubled-blinded, multicenter phase 3 trials of the efficacy and safety of ceftaroline fosamil versus ceftriaxone in patients with community-acquired pneumonia. Clin Infect Dis 15(51):1395–1405. https://doi.org/10.1086/657313
Poon H, Chang MH, Fung HB (2012) Ceftaroline fosamil: a cephalosporin with activity against methicillin-resistant Staphylococcus aureus. Clin Ther 34:743–765. https://doi.org/10.1016/j.clinthera.2012.02.025
Eljaaly K, Wali H, Basilim A, Alharbi A, Asfour HZ (2019) Clinical cure with ceftriaxone versus ceftaroline or ceftobiprole in the treatment of Staphylococcal pneumonia: a systematic review and meta-analysis. Int J Antimicrob Agents 54(2):149–153. https://doi.org/10.1016/j.ijantimicag.2019.05.023
Polverino E, Dambrava P, Cillóniz C, Balasso V, Marcos MA, Esquinas C et al (2010) Nursing home-acquired pneumonia: a 10 year single-centre experience. Thorax 65:354–359. https://doi.org/10.1136/thx.2009.124776
Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC et al (2007) Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 1(44 Suppl 2):S27-72. https://doi.org/10.1086/511159
Cillóniz C, Ewig S, Polverino E, Marcos MA, Esquinas C, Gabarrús A et al (2011) Microbial aetiology of community-acquired pneumonia and its relation to severity. Thorax 66:340–346. https://doi.org/10.1136/thx.2010.143982
Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T (2006) Variable selection for propensity score models. Am J Epidemiol 15(163):1149–1156. https://doi.org/10.1093/aje/kwj149
Welte T, Kantecki M, Stone GG, Hammond J (2019) Ceftaroline fosamil as a potential treatment option for Staphylococcus aureus community-acquired pneumonia in adults. Int J Antimicrob Agents 54:410–422. https://doi.org/10.1016/j.ijantimicag.2019.08.012
Blyth CC, Webb SAR, Kok J, Dwyer DE, van Hal SJ, Foo H et al (2013) The impact of bacterial and viral co-infection in severe influenza. Influenza Other Respir Viruses 7:168–176. https://doi.org/10.1111/j.1750-2659.2012.00360.x
Bai B, Wang H, Li M, Ma X, Zheng J, Deng Q et al (2020) Two cases of influenza B virus-related fatal fulminant pneumonia complicated with Staphylococcus aureus infection in China diagnosed using next-generation sequencing (2018). Front Public Health 8:121. https://doi.org/10.3389/fpubh.2020.00121
Joynt GM, Lipman J, Gomersall CD, Young RJ, Wong EL, Gin T (2001) The pharmacokinetics of once-daily dosing of ceftriaxone in critically ill patients. J Antimicrob Chemother 47:421–429. https://doi.org/10.1093/jac/47.4.421
Croisier-Bertin D, Hayez D, Da Silva S, Labrousse D, Biek D, Badiou C et al (2014) In vivo efficacy of ceftaroline fosamil in a methicillin-resistant Panton-Valentine leukocidin-producing Staphylococcus aureus rabbit pneumonia model. Antimicrob Agents Chemother 58:1855–1861. https://doi.org/10.1128/AAC.01707-13
Zhong NS, Sun T, Zhuo C, D’Souza G, Lee SH, Lan NH et al (2015) Ceftaroline fosamil versus ceftriaxone for the treatment of Asian patients with community-acquired pneumonia: a randomised, controlled, double-blind, phase 3, non-inferiority with nested superiority trial. Lancet Infect Dis 15:161–171. https://doi.org/10.1016/S1473-3099(14)71018-7
File TM, Low DE, Eckburg PB, Talbot GH, Friedland HD, Lee J et al (2011) FOCUS 1: a randomized, double-blinded, multicentre, phase III trial of the efficacy and safety of ceftaroline fosamil versus ceftriaxone in community-acquired pneumonia. J Antimicrob Chemother 66:iii19-32. https://doi.org/10.1093/jac/dkr096
Low DE, File TM, Eckburg PB, Talbot GH, David Friedland H, Lee J et al (2011) FOCUS 2: a randomized, double-blinded, multicentre, phase III trial of the efficacy and safety of ceftaroline fosamil versus ceftriaxone in community-acquired pneumonia. J Antimicrob Chemother 66(Suppl 3):iii33-44. https://doi.org/10.1093/jac/dkr097
Taboada M, Melnick D, Iaconis JP, Sun F, Zhong NS, File TM et al (2016) Ceftaroline fosamil versus ceftriaxone for the treatment of community-acquired pneumonia: individual patient data meta-analysis of randomized controlled trials. J Antimicrob Chemother 71:1748–1749. https://doi.org/10.1093/jac/dkw136
Ramirez JA, Wiemken TL, Peyrani P, Arnold FW, Kelley R, Mattingly WA et al (2017) Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis 13(65):1806–1812. https://doi.org/10.1093/cid/cix647
Cillóniz C, Polverino E, Ewig S, Aliberti S, Gabarrús A, Menéndez R et al (2013) Impact of age and comorbidity on cause and outcome in community-acquired pneumonia. Chest 144:999–1007. https://doi.org/10.1378/chest.13-0062
Lee JH, Kim HJ, Kim YH (2017) Is β-lactam plus macrolide more effective than β-lactam plus fluoroquinolone among patients with severe community-acquired pneumonia?: a systemic review and meta-analysis. J Korean Med Sci 32:77–84. https://doi.org/10.3346/jkms.2017.32.1.77
Gilbert TT, Arfstrom RJ, Mihalovic SW, Dababneh AS, Varatharaj Palraj BR, Dierkhising RA et al (2020) Effect of β-lactam plus macrolide versus fluoroquinolone on 30-day readmissions for community-acquired pneumonia. Am J Ther 27:e177–e182. https://doi.org/10.1097/MJT.0000000000000788
Postma DF, van Werkhoven CH, van Elden LJR, Thijsen SFT, Hoepelman AIM, Kluytmans JAJW et al (2015) Antibiotic treatment strategies for community-acquired pneumonia in adults. N Engl J Med 2(372):1312–1323. https://doi.org/10.1056/NEJMoa1406330
Murphy DM, Forrest IA, Curran D, Ward C (2010) Macrolide antibiotics and the airway: antibiotic or non-antibiotic effects? Expert Opin Investig Drugs 19:401–414. https://doi.org/10.1517/13543781003636480
Stahlmann R, Lode H (2010) Safety considerations of fluoroquinolones in the elderly: an update. Drugs Aging 1(27):193–209. https://doi.org/10.2165/11531490-000000000-00000
Vouga Ribeiro N, Gouveia Melo R, Guerra NC, Nobre Â, Fernandes RM, Pedro LM et al (2020) Fluoroquinolones are associated with increased risk of aortic aneurysm or dissection: systematic review and meta-analysis. Semin Thorac Cardiovasc Surg 9(20):30404–30414. https://doi.org/10.1053/j.semtcvs.2020.11.011
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This study was supported by a grant from Pfizer (Aspire: INSPIIRE WI244153), by CIBER de Enfermedades Respiratorias (CIBERES CB06/06/0028), and by 2009 Support to Research Groups of Catalonia 911, IDIBAPS. Dr. Soriano received a grant from Pfizer (Aspire). Dr. Cilloniz received a SEPAR fellowship 2018, and a grant from the Fondo de Investigación Sanitaria (PI19/00207). SEPAR integrated respiratory infections program.
Author information
Authors and Affiliations
Contributions
AS had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: AS, AT, RM, and CC. Acquisition of data: CC, RM, HP, CG, VR, and AG. Analysis and interpretation of data: CC, HP, RM, RM, VR, CG, AT, and AS. Drafting of the manuscript: AS, CC, AT, RM, RM, CG, HP, VR, and AG. Critical revision of the manuscript for important intellectual content: AS, CC, AT, RM, CG. Statistical analysis: AG. Study supervision: AS, AT, RM, and CC.
Corresponding authors
Ethics declarations
Ethics approval
The study was approved by the Ethics Committees of both institutions (Register: 2009/5451 and 2011/0219). The need for written informed consent was waived due to the non-interventional design.
Conflict of interest
The authors declare that they have no conflicts of interest with the study. AS has received a grant from Pfizer and honoraria for lectures and advisory meetings from Pfizer, MSD, Menarini, Shionogi, Gilead, and Angelini. CGV has received honoraria for talks on behalf of Gilead Science, MSD, Novartis, Pfizer, Janssen, and Lilly, as well as a grant from Gilead Science, Pfizer, and MSD. RM has received honoraria for lectures from Pfizer.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cilloniz, C., Mendez, R., Peroni, H. et al. Impact on in-hospital mortality of ceftaroline versus standard of care in community-acquired pneumonia: a propensity-matched analysis. Eur J Clin Microbiol Infect Dis 41, 271–279 (2022). https://doi.org/10.1007/s10096-021-04378-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-021-04378-0