Abstract
Patient-reported outcomes (PROs) facilitate communication between patients and providers, enhancing patient-centered care. We report PROs for virologically suppressed people living with HIV-1 who switched to dolutegravir/lamivudine (DTG/3TC) or continued their 3- or 4-drug current antiretroviral regimen (CAR) in the phase 3 SALSA study. Secondary endpoints included change from baseline in HIV Treatment Satisfaction Questionnaire (status version; HIVTSQs) and HIV Symptom Distress Module (HIV-SDM) at Weeks 4, 24, and 48. A post hoc analysis assessed change in HIVTSQs and HIV-SDM by age (≥ 50 and < 50 years). Higher HIVTSQs scores represent greater treatment satisfaction (range, 0–60); lower HIV-SDM scores indicate less symptom bother (range, 0–80). Participants in the DTG/3TC (n = 246) and CAR (n = 247) groups reported comparable baseline HIVTSQs total scores (mean [SD], 55.2 [6.5] and 55.8 [5.5], respectively). Beginning at Week 4, mean HIVTSQs scores in the DTG/3TC group further increased vs. CAR and were sustained through Week 48. Baseline mean (SD) HIV-SDM symptom bother scores were comparable between the DTG/3TC (9.0 [9.9]) and CAR (7.9 [9.3]) groups. Small improvements in HIV-SDM scores favoring DTG/3TC were observed at Weeks 4 and 24 and sustained through Week 48 (though not significant between groups). Participants aged ≥ 50 and < 50 years who switched to DTG/3TC reported higher satisfaction and less symptom distress vs. CAR; these results were generally comparable between age groups. Participants who switched to DTG/3TC reported rapid and sustained improvements in treatment satisfaction compared with those who continued CAR, reinforcing the benefits of DTG/3TC beyond virologic suppression (NCT04021290; registration date, 7/11/2019).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Advancements in antiretroviral therapy (ART) have allowed for the availability of fixed-dose combination regimens that are highly efficacious, durable, and well tolerated with a high barrier to resistance [1]. Dolutegravir/lamivudine (DTG/3TC) is a 2-drug regimen consisting of an integrase strand transfer inhibitor (INSTI) and a nucleoside reverse transcriptase inhibitor (NRTI) that has the potential to reduce adverse events, mitigate drug-drug interactions [2], and minimize long-term toxicities compared with 3- and 4-drug regimens [3]. Dolutegravir/lamivudine is recommended for both initial therapy in people living with HIV-1 who are naive to ART and as a switch option for those who are virologically suppressed and stable on their current regimen [1, 4, 5]. In the phase 3, randomized, open-label SALSA study, switching to DTG/3TC was non-inferior to continuing a 3- or 4-drug current antiretroviral regimen (CAR) for maintaining virologic suppression at Week 48, with no confirmed virologic withdrawals and a good safety profile [6]. Importantly, the SALSA participant population was diverse with higher proportions of female participants, people aged ≥ 50 years, and people who identified as African American, of African heritage, or as Asian, supporting the generalizability of results to the broader population of people living with HIV-1 and increasing the data available among those disproportionately impacted by HIV-1.
Concomitant with improvements in ART, as well as increased accessibility to treatment, the life expectancy of people living with HIV-1 has increased and is now similar to that of the overall population [4, 7, 8], and people aged ≥ 50 years have become a rapidly expanding demographic among those living with HIV-1 [9, 10]. However, as age increases, the incidence of non–AIDS-defining comorbidities also increases, potentially impacting the effectiveness of ART by introducing complications such as polypharmacy [11, 12]. These concerns underscore the need to optimize ART on an individual basis. Though achieving and maintaining virologic suppression remain the primary focus of HIV care, efforts to meet the needs and preferences of people living with HIV, consider their social and psychological experiences, and close the gap in health-related quality of life between those living with and those living without HIV are also of great importance when selecting treatment options [13, 14]. For example, HIV care now considers a person’s perception of their ART (e.g., whether they view it as a burden or disruptive to daily life) and experiences as a patient (e.g., if they see the same provider consistently, feel they can trust their provider, and are treated fairly and respectfully by their provider), as these can impact whether someone remains in the HIV care continuum and adheres to their treatment [15,16,17,18,19]. Unfortunately, many of these concerns are not readily observable with standard biomedical clinical assessments and can easily go unaddressed. Consequently, patient-reported outcome (PRO) instruments like the HIV Treatment Satisfaction Questionnaire (HIVTSQ) and HIV Symptom Distress Module (HIV-SDM; also known as the HIV Symptom Index) [20,21,22] have proven to be valuable tools for gaining deeper insights into patient needs, experiences, preferences, and health-related quality of life and can complement clinical endpoints. Patient-reported outcomes provide unique and valuable opportunities to capture firsthand perspectives of people living with HIV on their health status and treatment outcomes. Several studies focused on people living with HIV have demonstrated the effectiveness of PROs in improving communication between patients and providers [23,24,25], identifying and monitoring symptoms and behaviors [23,24,25,26], and addressing individual challenges to successful care [24, 25]. Moreover, the US Food and Drug Administration has issued guidance for industry on how to utilize PRO measures during clinical trials to incorporate patient perspectives into the drug review process [27]. Most recently, the European AIDS Clinical Society (EACS) guidelines were updated to include a section on the importance of PROs in clinical practice to facilitate discussions, improve awareness and care, and empower patients in their conversations with their healthcare providers [5].
Here, we describe PROs among virologically suppressed people living with HIV-1 who switched to DTG/3TC or continued CAR in secondary analyses from the SALSA trial, including a post hoc analysis of treatment satisfaction and symptom bother by age (≥ 50 and < 50 years).
Methods
Study Design and Participants
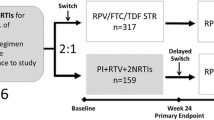
SALSA (ClinicalTrials.gov, NCT04021290) was a phase 3, randomized, multicenter, open-label, non-inferiority study to evaluate the efficacy and safety of switching to DTG/3TC compared with continuing 3- or 4-drug CAR in virologically suppressed people living with HIV-1 [6]. The study was designed in agreement with the International Conference on Harmonization Good Clinical Practice and adhered to the principles of the Declaration of Helsinki. Protocol approvals and written informed consent were obtained before participant screening.
The study design and screening criteria have been described previously [6]. Briefly, virologically suppressed adults (≥ 2 measurements of HIV-1 RNA < 50 copies/mL within 12 months of screening) on a stable first- or second-line ART regimen for ≥ 3 months were randomized 1:1 to switch to the fixed-dose combination of DTG/3TC (50 mg/300 mg) or continue CAR. Baseline regimens must have consisted of 2 NRTIs and a non-nucleoside reverse transcriptase inhibitor (NNRTI), INSTI (boosted or unboosted), or protease inhibitor (PI; boosted or unboosted). Refer to previously published data for complete study design details with inclusion and exclusion criteria [6]. Prior switches between the following agents were not considered a regimen change: cobicistat and ritonavir, 3TC and emtricitabine (FTC), and tenofovir disoproxil fumarate (TDF) and tenofovir alafenamide (TAF). Changes in regimen could not have been due to suspected or established treatment failure. The primary endpoint was the proportion of participants with HIV-1 RNA ≥ 50 copies/mL (US Food and Drug Administration Snapshot algorithm) at Week 48. Secondary endpoints included change from baseline in HIVTSQ, status version (HIVTSQs) and HIV-SDM scores at Weeks 4, 24, and 48 or withdrawal. A post hoc analysis of treatment satisfaction and symptom bother by age (≥ 50 and < 50 years) at Weeks 4, 24, and 48 using the HIVTSQs and HIV-SDM, respectively, was performed. The HIVTSQs and HIV-SDM were selected because they provide important insight into treatment satisfaction and symptom management, have demonstrated construct validity, and are commonly used PRO instruments in HIV research [21, 22, 28]. Willingness to switch was an exploratory endpoint and was assessed on Day 1 (before randomization).
Patient-Reported Outcome Measures
HIV Treatment Satisfaction Questionnaire, Status Version (HIVTSQs)
The HIVTSQs is a validated 10-item self-reported scale measuring overall satisfaction with treatment and includes 2 subscales that measure an individual’s satisfaction with the clinical aspects of their treatment (general satisfaction/clinical subscale) and with the lifestyle or ease of taking the treatment (lifestyle/ease subscale) [20, 21]. Each item on the questionnaire is graded on a scale of 0 to 6, with higher scores indicating a greater level of satisfaction. The score for both the general satisfaction/clinical and lifestyle/ease subscales ranges from 0 to 30, representing the sum of scores from responses to 5 questions, and the overall satisfaction score equals the sum of the 2 subscale values (range, 0–60). No minimal clinically important difference has been reported for the HIVTSQs.
HIV Symptom Distress Module (HIV-SDM)/Symptoms Impact Questionnaire
The HIV-SDM is a 20-item measure that addresses the presence and perceived distress linked to symptoms commonly associated with HIV or its treatment [22]. Assessments are reported as the number of symptoms present (symptom count) and the bothering level of those symptoms (symptom bother). The symptom count score ranges from 0 to 20 and the list of potential symptoms includes anxiety, appetite loss, bloating/gas, body image, cough, diarrhea, dizziness, fatigue, fever, hair loss, hand/foot pain, headache, memory loss, muscle/joint pain, nausea, sadness, sex problems, skin problems, sleep trouble, and weight loss. The symptom bother score evaluates the level of bother each symptom is causing on a 0- to 4-point Likert scale, with the overall score a summation of all individual values. Higher scores indicate a greater level of bother, with a maximum score of 80.
Willingness to Switch
Participants were verbally asked a single question, and responses were categorized as follows: tolerability issues with current regimen, concerns about long-term side effects of current regimen, difficulties with adherence to current regimen, general interest in new therapies for HIV, recommendation or request from their healthcare provider, financial considerations, and other. Respondents were not limited to 1 reason for willingness to switch.
Procedures and Statistical Analysis
The HIVTSQs and HIV-SDM were assessed at baseline and Weeks 4, 24, and 48. Any participant who withdrew from the study before Week 48 was asked to complete the HIVTSQs and HIV-SDM at withdrawal. Each questionnaire was administered on paper at the beginning of each visit, before collection of blood for analysis and other scheduled procedures. Questionnaires were provided in each participant’s preferred language. If a translation was unavailable, no assessment was performed, and the response was considered as missing in the final analysis. Willingness to switch was assessed verbally at the baseline visit.
Results of the HIVTSQs and HIV-SDM were reported as adjusted mean change from baseline or as proportions and analyzed with descriptive statistics using SAS® software version 9.4 (SAS Institute Inc, Cary, NC). The adjusted mean was the estimated mean change from baseline at each visit in each group calculated from mixed-model repeated measures adjusting for treatment, visit, baseline third agent class, age, sex, race, baseline value, treatment-by-visit interaction, and baseline value-by-visit interaction, with visit as the repeated factor. Analyses by age were also adjusted for visit-by-age, treatment-by-age, and treatment-by-visit-by-age interactions. No assumptions were made about the correlations between a participant score (i.e., the correlation matrix for within-participant errors was unstructured). Treatment differences (95% CI) in adjusted mean change from baseline and associated P values were used to detect differences between the DTG/3TC and CAR groups.
Results
Demographics and Baseline Characteristics
Participants were randomized 1:1 to switch to DTG/3TC (n = 246) or continue CAR (n = 247) [6]. The overall intention-to-treat–exposed population was diverse, with 19% (93/493) of participants identifying as African American or of African heritage, 14% (70/493) identifying as Asian, 39% (192/493) identifying as female, and 39% (193/493) aged ≥ 50 years (Table 1). Participants aged ≥ 50 years had higher concomitant medication use and more comorbidities at baseline (Online Resource 1). Participants in the DTG/3TC and CAR groups were generally well matched in median age, race, and median CD4 + cell count, although the DTG/3TC group had a higher proportion of female participants. Comparable proportions of participants in each group were taking NNRTIs, INSTIs, or PIs at baseline. The most commonly used baseline third agents were efavirenz and DTG, and the most frequently used NRTIs were FTC and TDF.
Changes in HIVTSQs from Baseline to Week 48
At baseline, mean (SD) HIVTSQs scores were high and similar between the DTG/3TC and CAR groups for total score (DTG/3TC, 55.2 [6.5]; CAR, 55.8 [5.5]), lifestyle/ease sub-score (DTG/3TC, 27.5 [3.7]; CAR, 27.8 [3.2]), and general satisfaction/clinical sub-score (DTG/3TC, 27.6 [3.5]; CAR, 28.0 [2.9]). Despite high baseline scores, small but favorable improvements in mean HIVTSQs total score and both sub-scores were observed in the DTG/3TC group as early as Week 4, which were greater than increases in the CAR group and stable through Week 48 (Fig. 1).
Adjusted mean change from baseline in HIVTSQs (a) total score, (b) lifestyle/ease sub-score, and (c) general satisfaction/clinical sub-score. Adjusted mean is the estimated mean change from baseline at each visit in each group calculated from mixed-model repeated measures adjusting for treatment, visit, baseline third agent class, age, sex, race, baseline value, treatment-by-visit interaction, and baseline value-by-visit interaction, with visit as the repeated factor. Adjusted treatment difference (95% CI) is displayed in the middle for each post-baseline study visit. All differences were P < 0.05. Dashed line represents no change from baseline. BL, baseline; CAR, current antiretroviral regimen; DTG, dolutegravir; HIVTSQs, HIV Treatment Satisfaction Questionnaire, status version; 3TC, lamivudine
From baseline to Week 48, the proportion of participants reporting the highest level of satisfaction (i.e., score of 6 out of 6) increased by ≥ 10% for all 5 lifestyle/ease and 4 of 5 general satisfaction/clinical sub-score items in the DTG/3TC group, whereas ≥ 10% increases were observed for 1 item in the CAR group (Fig. 2). In the DTG/3TC group, the highest increases occurred in the proportion of participants satisfied with continuing their current treatment (+ 28%), satisfied with the extent their treatment fits their lifestyle (+ 17%), satisfied with the side effects of their regimen (+ 16%), and who would recommend their regimen to someone else with HIV (+ 16%). In general, satisfaction with treatment (scores of ≥ 4 out of 6) was high in both groups, with > 95% of participants reporting they found their treatment to be convenient (DTG/3TC, 100%; CAR, 98%), would recommend their present treatment to others (DTG/3TC, 99%; CAR, 97%), and would be satisfied continuing their present treatment (DTG/3TC, > 99%; CAR, 96%).
Proportion of participants reporting the highest level of satisfaction (score of 6/6) on individual HIVTSQs items at baseline and Week 48. Data labels indicate difference in proportion of 6/6 scores between baseline and Week 48; lighter colored sections indicate higher proportions at Week 48. CAR, current antiretroviral regimen; DTG, dolutegravir; HIVTSQs, HIV Treatment Satisfaction Questionnaire, status version; 3TC, lamivudine. aN = 228
Changes in HIVTSQs by Age
Baseline HIVTSQs total score, lifestyle/ease sub-score, and general satisfaction/clinical sub-score were similar between the DTG/3TC and CAR groups regardless of age (Fig. 3). Among participants aged ≥ 50 years, those who switched to DTG/3TC had greater improvements in HIVTSQs total score and lifestyle/ease sub-score vs. continuing CAR at Weeks 4 and 24, with improvements remaining stable through Week 48. The general satisfaction/clinical sub-score did not meaningfully differ between treatment groups at any assessment in participants aged ≥ 50 years. Participants aged < 50 years who switched to DTG/3TC had greater improvements in mean HIVTSQs total score and both sub-scores compared with those continuing CAR at all time points assessed.
Adjusted mean change from baseline in HIVTSQs (a-b) total score, (c-d) lifestyle/ease sub-score, and (e-f) general satisfaction/clinical sub-score by age. Adjusted mean is the estimated mean change from baseline at each visit in each group calculated from mixed-model repeated measures adjusting for treatment, visit, baseline third agent class, age, sex, race, baseline value, treatment-by-visit interaction, baseline value-by-visit interaction, visit-by-age interaction, treatment-by-age interaction, and treatment-by-visit-by-age interaction, with visit as the repeated factor. Adjusted treatment difference (95% CI) is displayed in the middle for each post-baseline study visit. Differences with P < 0.05 are bolded. Dashed line represents no change from baseline. BL, baseline; CAR, current antiretroviral regimen; DTG, dolutegravir; HIVTSQs, HIV Treatment Satisfaction Questionnaire, status version; 3TC, lamivudine
From baseline to Week 48, the proportion of participants in the DTG/3TC group scoring HIVTSQs items as 6 out of 6 increased by ≥ 10% for 6 of the 10 items among those aged ≥ 50 years and 9 of the 10 items among those aged < 50 years (Online Resource 2). In the CAR group, ≥ 10% increases were observed for 1 item among participants aged ≥ 50 years and 3 items among those aged < 50 years. Overall, a higher proportion of participants aged ≥ 50 years in both treatment groups scored items as 6 out of 6 compared with participants aged < 50 years at both baseline and Week 48.
Changes in HIV-SDM from Baseline to Week 48
At baseline, mean (SD) symptom count scores were comparable between the DTG/3TC and CAR groups (DTG/3TC, 4.9 [4.6]; CAR, 4.4 [4.4]). Small reductions from baseline in mean (SD) symptom count score were observed in both treatment groups through Week 48, with values of 3.5 (4.1) and 3.8 (4.8) in the DTG/3TC and CAR groups, respectively. Symptoms reported by > 25% of participants in both groups at baseline included muscle aches/joint pain, fatigue/loss of energy, nervousness/anxiety, sadness/depression, and difficulty falling/staying asleep (Fig. 4). In the DTG/3TC group, the proportion of participants reporting each of these symptoms decreased by ≥ 10% for 4 of 5 symptoms by Week 48, with difficulty falling/staying asleep decreasing by 5%. Overall, 13 symptoms decreased by ≥ 5% among participants in the DTG/3TC group, while only 4 symptoms decreased by ≥ 5% in the CAR group.
Proportion of participants reporting each HIV-SDM symptom at baseline and Week 48 and change in proportion between time points. BL, baseline; CAR, current antiretroviral regimen; DTG, dolutegravir; HIV-SDM, HIV Symptom Distress Module; 3TC, lamivudine; W48, Week 48. aSystem organ class categorization not included in the HIV-SDM
Mean (SD) baseline symptom bother scores were 9.0 (9.9) and 7.9 (9.3) in the DTG/3TC and CAR groups, respectively. Both groups reported reductions from baseline in mean symptom bother score over time, with small and significant improvements observed in the DTG/3TC vs. CAR group at Weeks 4 and 24 (Fig. 5a). Scores were similar and not significantly different between treatment groups at Week 48.
Adjusted mean change from baseline in HIV-SDM symptom bother score in (a) the overall population, (b) participants aged ≥ 50 years, and (c) participants aged < 50 years. Adjusted mean is the estimated mean change from baseline at each visit in each group calculated from mixed-model repeated measures adjusting for treatment, visit, baseline third agent class, age, sex, race, baseline value, treatment-by-visit interaction, and baseline value-by-visit interaction, with visit as the repeated factor. Analyses by age also adjusted for visit-by-age interaction, treatment-by-age interaction, and treatment-by-visit-by-age interaction. Adjusted treatment difference (95% CI) is displayed in the middle for each post-baseline study visit. Differences with P < 0.05 are bolded. Dashed line represents no change from baseline. BL, baseline; CAR, current antiretroviral regimen; DTG, dolutegravir; HIV-SDM, HIV Symptom Distress Module; 3TC, lamivudine
Changes in HIV-SDM by Age
Among participants aged ≥ 50 years, the incidence of 18 of 20 symptoms declined by Week 48 in the DTG/3TC group, while 8 of 20 symptoms decreased in the CAR group (Online Resource 3). Five and 13 symptoms decreased by ≥ 10% and ≥ 5%, respectively, in participants aged ≥ 50 years in the DTG/3TC group. In participants aged ≥ 50 years in the CAR group, no symptoms decreased by ≥ 10% and 3 decreased by ≥ 5%. Among participants aged < 50 years, the proportion reporting each symptom was lower at Week 48 than at baseline for all 20 symptoms in both treatment groups. The extent of change between treatment groups was generally similar, favoring DTG/3TC for 10 symptoms and CAR for 6 symptoms. Three symptoms in the DTG/3TC group and 2 in the CAR group declined by ≥ 10%; 14 symptoms in the DTG/3TC group and 9 in the CAR group declined by ≥ 5%.
Mean (SD) baseline symptom bother scores were similar between treatment groups in participants aged ≥ 50 years (DTG/3TC, 10.0 [9.3]; CAR, 8.7 [9.7]) and < 50 years (DTG/3TC, 8.3 [10.2]; CAR, 7.4 [9.0]). Adjusted mean change from baseline in symptom bother score indicated improvements in both treatment groups in both age groups at Week 48, though improvements were numerically greater in the DTG/3TC treatment groups relative to the respective CAR groups (Fig. 5b-c).
Willingness to Switch
The most frequent reasons underlying a participant’s willingness to switch were interest in new HIV therapies (DTG/3TC, 71%; CAR, 80%), physician recommendation (DTG/3TC, 37%; CAR, 38%), and concerns about long-term side effects of their current regimen (DTG/3TC, 24%; CAR, 15%). Additional stated reasons, each of which were cited by ≤ 5% of the overall population, included problems tolerating their current regimen because of side effects, difficulty adhering to their current regimen, costs of their current HIV medications, and other reasons.
Discussion
In this analysis, we present PROs from virologically suppressed people living with HIV-1 in the SALSA randomized clinical trial who switched to the 2-drug regimen DTG/3TC or continued 3- or 4-drug CAR. Among participants who switched to DTG/3TC, mean increases in HIVTSQs total score and both lifestyle/ease and general satisfaction/clinical sub-scores were higher than in those continuing CAR through Week 48. Moreover, these improvements were observed as soon as 4 weeks after switch and remained stable thereafter. The DTG/3TC group also had improvements in HIV-SDM score compared with CAR at Weeks 4 and 24 and a similar HIV-SDM score at Week 48. Consistent with results in the overall study population, the subgroup of participants aged ≥ 50 years who switched to DTG/3TC had larger improvements from baseline in mean HIVTSQs total score and lifestyle/ease sub-score at Weeks 4 and 24, with numerical improvements at Week 48 compared with those who continued CAR. Participants aged ≥ 50 years in the DTG/3TC group also had numerically lower symptom bother scores vs. CAR at Weeks 4 and 24, which remained stable through Week 48. With participants in the DTG/3TC and CAR groups having an estimated median of 5.3 and 5.9 years of ART use, respectively, these results indicate that switching to DTG/3TC is associated with improved treatment satisfaction (e.g., better lifestyle fit, satisfied continuing treatment) in individuals with substantial ART experience. While both treatment groups had similar improvements in symptom distress at Week 48, switch to DTG/3TC resulted in more rapid improvements in symptom distress. Altogether, these data highlight the importance of DTG/3TC as a highly effective ART regimen that also improves treatment satisfaction and minimizes symptom burden, particularly among a population of individuals facing challenges associated with age-related comorbidities and complications such as polypharmacy.
Guidelines from EACS recommend assessing PROs in all people living with HIV to facilitate conversations between patients and healthcare providers, improve awareness of overall health, and empower patients to receive individualized care [5]. These recommendations along with the responses SALSA participants provided for the HIVTSQs and HIV-SDM underscore the importance of evaluating PROs in providing care for people living with HIV [15, 29]. In the primary SALSA safety analysis, a higher proportion of participants who switched to DTG/3TC reported adverse events (73%) and drug-related adverse events (20%) compared with those who continued CAR (70% and 6%, respectively), though the frequency of drug-related adverse events leading to discontinuation was low for both DTG/3TC (2%, n = 4) and CAR (< 1%, n = 1) [6]. A transient and higher incidence of adverse events was expected after switching from stable regimens [6, 30]. Surprisingly, some of the PRO findings in this study contrasted with the adverse event data. Specifically, the mean number of symptoms and the degree to which symptoms were bothersome after switch to DTG/3TC declined at Week 4 and remained below baseline levels; however, most drug-related adverse events were reported in the first 24 weeks of the study [6]. These findings highlight how PRO and adverse event data used together help to provide a more comprehensive understanding of treatment impact on participants.
Both participants aged ≥ 50 and < 50 years who switched to DTG/3TC reported higher levels of satisfaction and less symptom distress at the first post-baseline measurement compared with those who continued CAR, and these improvements were sustained through Week 48, though not significant between treatment groups. Differences in symptom reporting were especially pronounced in participants aged ≥ 50 years, with nearly all symptoms becoming less frequent among those who switched to DTG/3TC while fewer than half of symptoms became less frequent among those who continued CAR. Improvements were seen in participants aged < 50 years as well but to a generally similar extent in both treatment groups. Individuals living with HIV-1 aged ≥ 50 years have a higher prevalence of comorbidities and polypharmacy compared with those aged < 50 years [31,32,33], an observation also seen in this study. As these factors are associated with reduced overall quality of life and worsened health outcomes [34, 35], results here underscore the importance of an ART regimen with fewer drugs as a treatment option for an aging population of people living with HIV-1 who may be using a variety of medications to treat multiple conditions. Such a regimen could also potentially reduce drug-drug interactions and toxicities [2, 12].
Strengths of this study include the diverse study population, in which ~ 40% of participants were non-White, female, or aged ≥ 50 years, with 17 different countries represented, as this diversity increases the generalizability of the results and the data available among those most impacted by the burden of HIV. The wide variety of ART regimens used by the CAR group further increases generalizability. However, the variability in ART regimens makes measuring differences between DTG/3TC and specific regimens more difficult. The open-label study design may have contributed to bias.
Overall, participants experienced fewer and less bothersome symptoms after switching to DTG/3TC from CAR through 48 weeks in SALSA, regardless of age. Moreover, individuals who switched to DTG/3TC reported higher satisfaction with their treatment, greater ease of incorporating their treatment into their lifestyle, and more interest in continuing their treatment after the study. These results complement the virologic outcome data from SALSA, which demonstrated the non-inferior efficacy of switching to DTG/3TC vs. continuing CAR [6], as well as real-world data gathered through 3 years of the URBAN cohort study in Germany, in which treatment-experienced people living with HIV-1 who switched to DTG/3TC reported significant improvement in treatment satisfaction and stable HIV-SDM scores [36]. With the availability of multiple highly effective ART regimens, the choice of an optimal treatment for an individual should account for how therapy impacts their daily life in addition to its clinical efficacy. As age-related comorbidities develop and introduce new complications such as polypharmacy, the impact of those everyday experiences, preferences, and feelings will likely be amplified. Such a possibility underscores the crucial importance of PRO instruments in monitoring aspects of health that are not easily captured by clinical measurements. Results from this study further suggest highly effective and streamlined regimens such as DTG/3TC have the potential to provide health-related quality-of-life benefits, including among the growing population of people aged ≥ 50 years living with HIV-1.
Data Availability
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
Code Availability
Not applicable.
References
Gandhi RT, Bedimo R, Hoy JF, Landovitz RJ, Smith DM, Eaton EF, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2022 recommendations of the International Antiviral Society-USA panel. JAMA. 2023;329(1):63–84. https://doi.org/10.1001/jama.2022.22246.
Back D. 2-Drug regimens in HIV treatment: pharmacological considerations. Germs. 2017;7(3):113–4. https://doi.org/10.18683/germs.2017.1115.
Chawla A, Wang C, Patton C, Murray M, Punekar Y, de Ruiter A, et al. A review of long-term toxicity of antiretroviral treatment regimens and implications for an aging population. Infect Dis Ther. 2018;7(2):183–95. https://doi.org/10.1007/s40121-018-0201-6.
Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/adult-adolescent-arv/guidelines-adult-adolescent-arv.pdf. Accessed February 27, 2024.
European AIDS Clinical Society. Guidelines version 12.0. 2023. https://www.eacsociety.org/media/guidelines-12.0.pdf. Accessed Dec 18 2023.
Llibre JM, Brites C, Cheng C-Y, Osiyemi O, Galera C, Hocqueloux L, et al. Efficacy and safety of switching to the 2-drug regimen dolutegravir/lamivudine versus continuing a 3- or 4-drug regimen for maintaining virologic suppression in adults living with human immunodeficiency virus 1 (HIV-1): week 48 results from the phase 3, noninferiority SALSA randomized trial. Clin Infect Dis. 2023;76(4):720–9. https://doi.org/10.1093/cid/ciac130.
Antiretroviral Therapy Cohort Collaboration. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV. 2017;4(8):e349–56. https://doi.org/10.1016/s2352-3018(17)30066-8.
Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS ONE. 2013;8(12):e81355. https://doi.org/10.1371/journal.pone.0081355.
Autenrieth CS, Beck EJ, Stelzle D, Mallouris C, Mahy M, Ghys P. Global and regional trends of people living with HIV aged 50 and over: estimates and projections for 2000–2020. PLoS ONE. 2018;13(11):e0207005. https://doi.org/10.1371/journal.pone.0207005.
National Institutes of Health National Institute on Aging. HIV, AIDS, and older adults. 2024. https://www.nia.nih.gov/health/hiv-aids/hiv-aids-and-older-adults. Accessed Jan 8 2024.
McGettrick P, Barco EA, Mallon PWG. Ageing with HIV. Healthcare (Basel). 2018;6(1):17. https://doi.org/10.3390/healthcare6010017.
Back D, Marzolini C. The challenge of HIV treatment in an era of polypharmacy. J Int AIDS Soc. 2020;23(2):e25449. https://doi.org/10.1002/jia2.25449.
Remien RH, Stirratt MJ, Nguyen N, Robbins RN, Pala AN, Mellins CA. Mental health and HIV/AIDS: the need for an integrated response. AIDS. 2019;33(9):1411–20. https://doi.org/10.1097/qad.0000000000002227.
Shubber Z, Mills EJ, Nachega JB, Vreeman R, Freitas M, Bock P, et al. Patient-reported barriers to adherence to antiretroviral therapy: a systematic review and meta-analysis. PLoS Med. 2016;13(11):e1002183. https://doi.org/10.1371/journal.pmed.1002183.
Short D, Wang X, Suri S, Hsu TK, Jones B, Fredericksen RJ, et al. Risk factors for suboptimal adherence identified by patient-reported outcomes assessments in routine HIV care at 2 North American clinics. Patient Prefer Adherence. 2022;16:2461–72. https://doi.org/10.2147/ppa.s378335.
Dang BN, Westbrook RA, Hartman CM, Giordano TP. Retaining HIV patients in care: the role of initial patient care experiences. AIDS Behav. 2016;20(10):2477–87. https://doi.org/10.1007/s10461-016-1340-y.
Cooper V, Clatworthy J, Youssef E, Llewellyn C, Miners A, Lagarde M, et al. Which aspects of health care are most valued by people living with HIV in high-income countries? A systematic review. BMC Health Serv Res. 2016;16(1):677. https://doi.org/10.1186/s12913-016-1914-4.
Mukamba N, Chilyabanyama ON, Beres LK, Simbeza S, Sikombe K, Padian N, et al. Patients’ satisfaction with HIV care providers in public health facilities in Lusaka: a study of patients who were lost-to-follow-up from HIV care and treatment. AIDS Behav. 2020;24(4):1151–60. https://doi.org/10.1007/s10461-019-02712-4.
Ndirangu EW, Evans C. Experiences of African immigrant women living with HIV in the U.K.: implications for health professionals. J Immigr Minor Health. 2009;11(2):108–14. https://doi.org/10.1007/s10903-008-9116-8.
Woodcock A, Bradley C. Validation of the HIV treatment satisfaction questionnaire (HIVTSQ). Qual Life Res. 2001;10(6):517–31. https://doi.org/10.1023/a:1013050904635.
Woodcock A, Bradley C. Validation of the revised 10-item HIV treatment satisfaction questionnaire status version and new change version. Value Health. 2006;9(5):320–33. https://doi.org/10.1111/j.1524-4733.2006.00121.x.
Justice AC, Holmes W, Gifford AL, Rabeneck L, Zackin R, Sinclair G, et al. Development and validation of a self-completed HIV symptom index. J Clin Epidemiol. 2001;54(suppl 1):S77–90. https://doi.org/10.1016/s0895-4356(01)00449-8.
Crane HM, Crane PK, Tufano JT, Ralston JD, Wilson IB, Brown TD, et al. HIV provider documentation and actions following patient reports of at-risk behaviors and conditions when identified by a web-based point-of-care assessment. AIDS Behav. 2017;21(11):3111–21. https://doi.org/10.1007/s10461-017-1718-5.
Short D, Fredericksen RJ, Crane HM, Fitzsimmons E, Suri S, Bacon J, et al. Utility and impact of the implementation of same-day, self-administered electronic patient-reported outcomes assessments in routine HIV care in two North American clinics. AIDS Behav. 2022;26(7):2409–24. https://doi.org/10.1007/s10461-022-03585-w.
Fredericksen R, Short D, Fitzsimmons E, Jacobs B, Musten A, Korlipara D, et al. Patient perceptions of the utility and impact of a same-day self-administered routine electronic patient-reported outcomes (PRO) assessment in HIV care in two North American clinics. Qual Life Res. 2020;29(suppl 1):S124.
Kjaer ASHK, Rasmussen TA, Hjollund NH, Rodkjaer LO, Storgaard M. Patient-reported outcomes in daily clinical practise in HIV outpatient care. Int J Infect Dis. 2018;69:108–14. https://doi.org/10.1016/j.ijid.2018.02.015.
US Food and Drug Administration. Patient-reported outcome measures: use in medical product development to support labeling claims. Guidance for industry. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-reported-outcome-measures-use-medical-product-development-support-labeling-claims. Accessed September 20, 2023.
Akinosoglou K, Antonopoulou S, Katsarolis I, Gogos CA. Patient-reported outcomes in HIV clinical trials evaluating antiretroviral treatment: a systematic review. AIDS Care. 2021;33(9):1118–26. https://doi.org/10.1080/09540121.2020.1852160.
Kall M, Marcellin F, Harding R, Lazarus JV, Carrieri P. Patient-reported outcomes to enhance person-centred HIV care. Lancet HIV. 2020;7(1):e59–68. https://doi.org/10.1016/s2352-3018(19)30345-5.
Osiyemi O, De Wit S, Ajana F, Bisshop F, Portilla J, Routy JP, et al. Efficacy and safety of switching to dolutegravir/lamivudine versus continuing a tenofovir alafenamide-based 3- or 4-drug regimen for maintenance of virologic suppression in adults living with human immunodeficiency virus type 1: results through week 144 from the phase 3, noninferiority TANGO randomized trial. Clin Infect Dis. 2022;75(6):975–86. https://doi.org/10.1093/cid/ciac036.
Funke B, Spinner CD, Wolf E, Heiken H, Christensen S, Stellbrink HJ, et al. High prevalence of comorbidities and use of concomitant medication in treated people living with HIV in Germany - results of the BESIDE study. Int J STD AIDS. 2021;32(2):152–61. https://doi.org/10.1177/0956462420942020.
Spinelli F, Prakash M, Slater J, van der Kolk M, Bassani N, Grove R, et al. Dolutegravir-based regimens in treatment-naive and treatment-experienced aging populations: analyses of 6 phase III clinical trials. HIV Res Clin Pract. 2021;22(2):46–54. https://doi.org/10.1080/25787489.2021.1941672.
The Lancet Healthy Longevity. Ageing with HIV. Lancet Healthy Longev. 2022;3(3):e119. https://doi.org/10.1016/s2666-7568(22)00041-1.
Okoli C, de los Rios P, Eremin A, Brough G, Young B, Short D. Relationship between polypharmacy and quality of life among people in 24 countries living with HIV. Prev Chronic Dis. 2020;17:E22. https://doi.org/10.5888/pcd17.190359.
Murray MM, Lin J, Buros Stein A, Wilcox ML, Cottreau J, Postelnick M, et al. Relationship of polypharmacy to HIV RNA suppression in people aged ≥ 50 years living with HIV. HIV Med. 2021;22(8):742–9. https://doi.org/10.1111/hiv.13122.
Noe S, Scholten S, Wyen C, Sabranski M, Postel N, Degen O, et al. 3-Year outcomes for dolutegravir (DTG) + lamivudine (3TC) in ART-naive and pre-treated people living with HIV-1 (PLHIV) in Germany: real-world data from the German URBAN cohort. Presented at: 19th European AIDS Conference; October 18–21, 2023; Warsaw, Poland.
Acknowledgements
We thank the study participants; their families and caregivers; investigators and site staff who participated in the study; and the ViiV Healthcare, GSK, and Pharmaceutical Product Development study team members. Editorial assistance was provided under the direction of the authors by Marc Potempa, PhD, and Jennifer Rossi, MA, ELS, MedThink SciCom, and was funded by ViiV Healthcare.
Funding
This study was funded by ViiV Healthcare.
Author information
Authors and Affiliations
Contributions
BW, BJ, and JP contributed to the conception of the study. PK, AEC, CJ-O, MGD, SDG, CB, LH, P-LL, JO, AO, BW, BJ, and JP contributed to the design of the study. PK, AEC, CJ-O, MGD, SDG, CB, LH, and P-LL contributed to the acquisition of data. JO, AO, BW, BJ, and JP contributed to the analysis of data. All authors contributed to the interpretation of data, drafting the manuscript, critically revising the manuscript for important intellectual content, and approve the manuscript for publication.
Corresponding author
Ethics declarations
Ethical Approval
This study was designed in agreement with the International Conference on Harmonization Good Clinical Practice and adhered to the principles of the Declaration of Helsinki. Study documents were reviewed and approved by national, regional, or investigational center ethics committees or institutional review boards.
Consent to Participate
Written informed consent was obtained before participant screening.
Consent to Publish
Not applicable.
Competing Interests
PK has received grants from Gilead, Merck, Theratechnologies, and ViiV Healthcare/GSK (paid to institution); has received consulting fees from and participated in data safety monitoring/advisory boards for Gilead, Merck, and ViiV Healthcare/GSK; and holds stock/stock options in Gilead, GSK, Johnson & Johnson, Merck, Moderna, and Pfizer. AEC has received grants from Gilead, GSK, MSD, and ViiV Healthcare (paid to institution); speaker fees from MSD; travel/meeting support from Gilead and ViiV Healthcare; and has participated in advisory boards for MSD, Theratechnologies, and ViiV Healthcare. CJ-O has received consulting fees from GSK and ViiV Healthcare; honoraria from AbbVie, CCO, Gilead, GSK, Janssen-Cilag, and ViiV Healthcare; payment for expert testimony from GSK and ViiV Healthcare; travel/meeting support from CCO, Gilead, GSK, Janssen-Cilag, MSD, and ViiV Healthcare; has participated in data safety monitoring/advisory boards for Gilead, GSK, Janssen-Cilag, MSD, and ViiV Healthcare; and holds a leadership role with BAGNAE. MGD has received grants and honoraria from and participated in data safety monitoring/advisory boards for AbbVie, Gilead, Janssen, MSD, and ViiV Healthcare; has received meeting/travel support from Gilead and Janssen; and has received equipment/materials from Gilead. SDG has no competing interests. CB has received research grants from GSK and Pfizer and received speaker fees from and participated in advisory boards for Gilead, GSK, and Merck. LH has received honoraria and meeting/travel support from Gilead, MSD, and ViiV Healthcare. P-LL has no competing interests. JO, AO, BW, BJ, LAE, DF, MK, and JP are employees of GSK or ViiV Healthcare and may own stock in GSK.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kumar, P., Clarke, A.E., Jonsson-Oldenbüttel, C. et al. Patient-Reported Outcomes After Switching to a 2-Drug Regimen of Fixed-Dose Combination Dolutegravir/Lamivudine: 48-Week Results from the SALSA Study. AIDS Behav (2024). https://doi.org/10.1007/s10461-024-04479-9
Accepted:
Published:
DOI: https://doi.org/10.1007/s10461-024-04479-9