Abstract
Noise-induced hidden hearing loss (HHL) is a newly uncovered form of hearing impairment that causes hidden damage to the cochlea. Patients with HHL do not have significant abnormalities in their hearing thresholds, but they experience impaired speech recognition in noisy environments. However, the mechanisms underlying HHL remain unclear. In this study, we developed single-cell transcriptome profiles of the cochlea of mice with HHL, detailing changes in individual cell types. Our study revealed a transient threshold shift, reduced auditory brainstem response wave I amplitude, and decreased number of ribbon synapses in HHL mice. Our findings suggest elevated oxidative stress and GDF15 expression in cochlear hair cells of HHL mice. Notably, the upregulation of GDF15 attenuated oxidative stress and auditory impairment in the cochlea of HHL mice. This suggests that a therapeutic strategy targeting GDF15 may be efficacious against HHL.
Graphical Abstract
-
1.
HHL mice had a transient threshold shift, reduced ABR wave I amplitude, and decreased number of ribbon synapses.
-
2.
HHL mice's cochlear hair cells exhibited increased oxidative stress and elevated GDF15 expression.
-
3.
Upregulation of GDF15 attenuated oxidative stress and auditory damage in the cochlea of HHL mice, implying that GDF15-targeted treatment techniques may be useful for HHL.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hearing loss is now recognized as a global health hazard and a public health issue. Over 1.5 billion people are thought to be affected by hearing loss globally (Wilson and Tucci 2021; Xu et al. 2023). As a newly uncovered form of hearing loss, noise-induced hidden hearing loss (HHL) has drawn attention in recent years (Valderrama et al. 2022). In contrast to noise-induced hearing loss (NIHL), which has been the subject of more in-depth research in the past, individuals with HHL generally have normal pure tone hearing thresholds, yet they often experience a decline in speech recognition ability in noisy environments (Trevino and Lobarinas 2022; Colla et al. 2023). Previous studies have indicated that HHL may involve recoverable temporary hair cell injury, primarily characterized by damaged ribbon synapses and abnormal hair cell function. However, the detailed pathogenesis of HHL remain poorly understood, necessitating further in-depth research (Li et al. 2023a, b).
Reactive oxygen species (ROS) serve as signaling molecules, controlling a range of biological activities within cells such as gene expression, cell division, and survival (Xiong et al. 2021). Endogenous antioxidant enzymes such as superoxide dismutase (SOD) moderate intracellular ROS levels to maintain intracellular homeostasis. Oxidative stress happens when the intracellular production of ROS exceeds the antioxidant capacity (Fetoni et al. 2019). The cochlea, as one of the tissues with high metabolic needs, is especially susceptible to oxidative stress (Hajam et al. 2022). And oxidative stress can lead to varying degrees of ROS-mediated damage to intracellular components, which in turn impairs their physiological function (Wang et al. 2021a, b).
As a stress-responsive cytokine, growth differentiation factor 15 (GDF15) circulates at low levels under normal physiological conditions (Johann et al. 2021; Huang et al. 2021). Studies have shown that increased GDF15 is associated with metabolic stress conditions and plays an irreplaceable role therein, especially in obesity and its associated metabolic diseases. GDF15, for example, inhibits hepatic steatosis in non-alcoholic fatty liver disease by suppressing oxidative stress and AIM2 inflammasome activation, and it also contributes to the control of glucose homeostasis and energy balance in the mitochondrial integrated stress response. Since GDF15 levels are significantly elevated in a range of diseases, such as renal failure, atherosclerosis and diabetes mellitus, it has become a typical marker and therapeutic target for these conditions (May et al. 2021; Galuppo et al. 2022; Kobayashi et al. 2023; Zhu et al. 2023). However, the link between GDF15 levels and noise-induced HHL has not been clearly established (Tsui et al. 2015; Tzikas et al. 2019; Zhu et al. 2019; Milon et al. 2021; You et al. 2022). Our objectives were to investigate the connection among GDF15, oxidative stress, and HHL and to explore potentially effective therapeutic targets of HHL.
Materials and methods
Animal groups
Four-week-old male C57BL/6J mice, sourced from the Laboratory Animal Center of Air Force Medical University, were used herein. The mice were housed in an animal room with a 12-hour light/dark cycle and regular changes of clean food and water. Every mouse utilized in the study had normal baseline hearing and had never previously been exposed to noise. All procedures for this study were authorized by the University's Animal Care and Use Committee (No. 20230375).
Each group in the animal experiments of this study contained six randomly assigned mice. The control group did not undergo any reagent treatment or noise exposure. The HHL group was exposed to noise exposure of 110 dB for 2 hours. The NIHL group received noise exposure of 115 dB for 2 hours for 3 consecutive days. The GDF15 group was given 10 μL of recombinant GDF15 at 10 ng/mL via the cochlear round window of mice. The HHL + GDF15 group was given 10 μL of recombinant GDF15 at 10 ng/mL via the cochlear round window of mice and then received a 2-hour noise exposure of 110 dB after 2 h.
Noise stimulation
The noise used in the experiment is broadband noise, which is recorded from the engine noise of a certain kind of military helicopter operated by the Chinese aviation army. It was transmitted to speakers (IT-12, RADIN, China) for playback via an amplifier (AV-502BT, BGL, China). A soundproof room with a sound absorption coefficient > 0.99 was used for the noise stimulation. For noise exposure, mice were housed in 10.5 cm × 5.5 cm × 5.5 cm cages with the loudspeaker mounted on top of the cage. It is worth noting that the animals were kept awake during the noise exposure.
ABR measurement
After induction of anesthesia by intraperitoneal injection of sodium pentobarbital (0.3 ml/100 g) in mice, the ABR measurements were performed using the ABR-evoked potential instrument (Otometrics, Denmark) in a soundproof room. Short clicks were used to measure hearing thresholds in mice, and the frequencies for the short pure tones were set at 1, 2, 4, and 8 kHz. The lowest sound level of recognizable wave III is considered to be the hearing threshold of the mouse. The amplitude and latency of ABR wave I were also noted.
Tissue preparation
Mice were anesthetized and euthanized via cervical dislocation, and the cochlea was then extracted. The cochlea was submerged in a 40 g/L paraformaldehyde solution, then carefully trimmed using ophthalmic scissors and microtome forceps under a stereomicroscope (SZN71, Sunny Optical Technology, China) to remove excess bone and tissue, and the oval window was opened. After being perfused with paraformaldehyde, the cochlea was kept at 4°C for the whole night. The cochlea was subsequently decalcified in 100 g/L EDTA (AR1071, Boster, China) for three days. Excess tissue was excised, and the cochlear basilar membrane was carefully removed and divided into apical, middle, and basal turns based on its location. Mouse serum was prepared by orbital blood sampling. After blood collection, the blood was injected into test tubes containing anticoagulant, centrifuged, and the supernatant was taken as serum.
Immunofluorescence
Isolated cochlear basilar membrane samples were treated with 10 mL/L TritonX-100 and then blocked using goat serum (AR0009, BOSTER, China) for 1 h. Then, rabbit anti-CtBP2 antibody (ab128871, Abcam, UK), mouse anti-4-HNE antibody (ab48506, Abcam, UK), or mouse anti-3-NT antibody (ab110282, Abcam, UK) was put in and underwent incubation at 4℃ overnight. Afterwards, Cy3-labeled goat anti-rabbit IgG (for CtBP2, SA00009-2, Proteintech, United States), 488 (for 4-HNE, CL488-16, Proteintech, United States), or 594 (for 3-NT, 8890S, CST, United States)-labeled goat anti-mouse IgG were put in and underwent incubation for 2 h. The basilar membrane was transferred to a slide, and then a DAPI-containing sealing solution (P0131, Beyotime, China) was added. One cochlea per animal was randomly selected for immunofluorescence. Immunofluorescence was observed by confocal microscopy (LSM 800, Zeiss, Germany). The software Image J was used for fluorescence quantification.
Quantification of ribbon synapses
Each group contained six cochlear basilar membrane samples, and one field of view was selected in each turn for each sample to be photographed and counted. Cochlear basilar membrane sections were observed using a confocal microscope with 63 × magnification. The CtBP2 antibody-labeled synapses within these fields were counted. Quantification of ribbon synapses was performed using the software Image J. The number of ribbon synapses per inner hair cell (IHC) was calculated by dividing the total number of ribbon synapses by the number of hair cells in the field of view.
SOD and CAT detection
Four cochlea, extracted from two mice in the same group, were combined to form a single sample. These were then prepared as a 50% homogenate in 0.9% saline. The assays for SOD and CAT were conducted using the SOD assay kit (A001-3, Jiancheng Biotechnology, China) and the CAT assay kit (A003-1, Jiancheng Biotechnology, China), respectively, following the guidelines provided by the manufacturer. Each group's SOD and CAT activity were assessed using a microplate reader (BioTek, United States).
GDF15 Injection through round window
After anesthetizing the mice and ensuring that they were unresponsive to painful stimuli, the hair behind the ears was removed with depilatory cream, and the surgical area behind the ears was disinfected with 75% ethanol. The drugs were administered in a single ear of mice. An incision was made behind the outer ear, and the soft tissue was gently pushed aside to expose the auditory vesicle. A syringe was used to perforate the auditory vesicle near the round window. A microsyringe pump (RSP01-BG2, Biotaor, China) and capillary pipette (10 μL, San'ai Instrument, China) were used to inject 10 μL of GDF15 solution (10 ng/mL) slowly into the auditory vesicle through the small hole. The GDF15 solution was made by dissolving recombinant GDF15 in 0.9% saline. After the injection, the perforation was covered with adipose tissue, the posterior auricular muscle and adipose tissue were restored to their normal positions, and the wound was softly sutured and disinfected. The mice were placed in a warm and clean environment for observation and care while waiting for their recovery.
Culture of house ear institute-organ of corti 1 (HEI-OC1) cells and H2O2 Exposure
HEI-OC1 cells were grown in Dulbecco's Modified Eagle's Medium (DMEM; PM150210, Procell, China) at 10% CO2 and 33°C. All experiments involving this cell line were performed during the logarithmic growth phase. To determine the optimal dose and duration of hydrogen peroxide (H2O2) exposure, HEI-OC1 cells were grown in culture medium containing varying concentrations of H2O2 (25, 50, 75, 100, 125, 150, 175, and 200 µM) for 24 h. The concentration of H2O2 that resulted in 70% survival of the hair cells after treatment was chosen as the optimal H2O2 treatment concentration.
RNA Extraction and real-time PCR
The RNA was extracted from the cochlea using the TRIzol reagent (15,596,018CN, Invitrogen, USA), and its concentration was measured by an RNA quantifier. Reverse transcription of RNA was achieved through a cDNA synthesis kit (11201ES03, Yeasen, China). The primers used for amplification were made by Beijing Tsingke Biotech, with their sequences detailed in Table 1. A CFX Connect Real-Time PCR Detection System (BioRad, USA) and a reaction mixture containing SYBR Green fluorescent dye were used to perform the real-time PCR. We used β-actin as an internal reference gene. The relative RNA expression was estimated using the 2−ΔΔCt method.
Cell Viability assay
Cells were cultured with 5 subwells per group before the experiment. Then the cells in each well were treated with varying concentrations of H2O2 according to the group. After adding 100 μL/mL of Cell Counting Kit-8 reagent (CK04, Dojindo, Japan) to each well, the mixture was incubated for two hours at 37°C. Next, the absorbance of the samples was quantified with a microplate reader.
ROS Detection
We used the Reactive Oxygen Species Assay Kit (S0033S, Beyotime, China) for ROS detection of HEI-OC1 cells in keeping with the manufacturer’s instructions. Following incubation of the cells with DCFH-DA at 37°C, the fluorescence intensity was assessed by flow cytometry.
Enzyme-Linked Immunosorbent Assay (ELISA)
Mouse cochlea and cells were harvested and stored at −80°C. The GDF15 levels in HHL mouse cochlea, serum, and HEI-OC1 cells were quantified using the ELISA kit (SEC034Mu, Cloud-Clone Corporation, Houston, USA) according to the protocol provided by the manufacturer, with absorbance readings taken on a microplate reader. Notably, there was no significant cross-reactivity or interference observed between GDF15 and its analogs.
Apoptosis Assay
The HEI-OC1 cells were washed with PBS and resuspended using binding buffer. Subsequently, the suspension was supplemented with Annexin V-FITC and propidium iodide (PI), well mixed, and incubated for 30 min at 4°C under light protection. Following incubation, the cell suspension was supplemented with a new binding buffer, and flow cytometry was used for analyzing the samples.
Isolation of Cochlear Cells
After being dissected, the cochlea underwent incubation in DMEM supplemented with DNase I and thermolysin for 10 min at 37°C. The cochlea was carefully trimmed and incubated in 0.25% trypsin-EDTA (C100C1, NCM Biotech, China) at 37°C for 15 min. Gentle grinding was performed every 5 min during this period. Then, FBS was added to inactivate the trypsin. The isolated cells were then filtered using a 40-μm mesh to form pellets, which were subsequently resuspended in DMEM containing 10% FBS.
Single Cell Preparation and Library Construction
Quality control and counting of single-cell suspensions were first carried out when cell viability was higher than 80%. Cells were placed onto the 10× Chromium Single Cell Platform (10× Genomics) using the Gel Bead Kit v.3 and Single Cell 3′ Library. This was followed sequentially by the generation of emulsion gel beads (GEMs), barcoding, GEM-RT purification, cDNA amplification, and library construction. The Illuminutesa Nova6000 device was used to sequence the final library pool utilizing paired-end reads consisting of 150 base pairs.
Processing of Single-cell RNA sequencing Data
Cellranger software (version 2.1.0) was used to count genes and perform quality control. R software (Seurat package version 2.2) was utilized for clustering. The analysis focused on cells expressing > 200 genes and < 10% mitochondrial genes. The first 2000 highly variable genes were used as the basis for principle component analysis. The Louvain Modularity Optimization approach was applied to cell clustering, and the t-distributed stochastic neighbor embedding (t-SNE) was used for visualization.
The Seurat FindMarkers function’s implementation of the "bimod" test was used to calculate each cluster’s differential expression. Marker genes were identified based on a log2 mean expression difference of 0.585 and P < 0.05. Canonical markers of known cell types were used to annotate cell clusters. A series of visual analyses, such as Gene Ontology (GO) enrichment analysis and Gene Set Enrichment Analysis (GSEA), were then carried out through the R package. A cutoff of FDR (adjusted P-value) of ≤ 0.05 was established for identifying significantly enriched terms and pathways.
Data sources
We used the following public database resources in this study in addition to our own experimental data. The oxidative stress gene set was derived from Wang et al. DOI: (https://doi.org/10.7717/peerj.14784). The transcriptome of acoustic trauma in the inner ear was obtained from the gene-expression analysis resource (gEAR; https://umgear.org/NIHL).
Statistical Analysis
SPSS 26.0 (IBM Corporation, USA) and GraphPad Prism 7.0 (GraphPad Software, USA) were used to statistically analyze the data. The experimental data results were displayed as the mean ± SEM. The experimental groups were compared through the LSD test, and the experimental and control groups were compared using the Dunnett test or one-way ANOVA. A difference was deemed statistically significant if P < 0.05.
Results
Hidden Hearing Loss in Mice After Noise Exposure
Firstly, we observed the auditory functional and morphological changes in C57BL/6J mice before and after noise exposure to confirm that 110 dB 2 h noise exposure induces HHL in mice (Fig. 1a). The results of ABR measurement showed that the hearing thresholds of mice at click were elevated one day following 110 dB 2 h noise exposure compared with the control group (P < 0.01). They gradually returned to pre-exposure levels 14 days post-exposure (P > 0.05) (Fig. 1b). We then evaluated the ABR thresholds at 1, 2, 4, and 8 kHz one day following 110 dB 2 h noise exposure and found that the ABR thresholds at each frequency were significantly elevated (P < 0.01). These thresholds gradually recovered, but at different rates of elevation and decline (Fig. 1c). Additionally, we measured the ABR wave I amplitude and latency in mice before and after noise exposure. The results revealed that ABR wave I amplitude decreased one day post-exposure (P < 0.01), which could not be restored to the pre-exposure level after 14 days (P < 0.05) (Fig. 1d). ABR wave I latency was prolonged one day post-exposure (P < 0.05) but recovered to pre-exposure levels by 14 days (Fig. 1e). Thus, mice exhibited auditory function changes characteristic of HHL after 110 dB for 2 h of noise exposure.
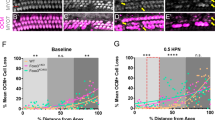
Phenotypes of noise-induced hidden hearing loss in mouse cochlea. a Schematic diagram of the experimental procedure. b Variations in hearing thresholds at click in mice at different time points before and after noise exposure. c Changes in ABR thresholds at 1, 2, 4, and 8 kHz in mice at different time points. d-e ABR wave I amplitude and latency in various groups of mice. f Fluorogram representing changes in the number of ribbon synapse. Scale bar = 20 μm. g The number of ribbon synapses per IHC at different time points. h The number of ribbon synapses per IHC at different locations. n = 6. *P < 0.05, **P < 0.01, as compared with the control group
To investigate how noise exposure affects ribbon synapses, we employed DAPI labeling of the arrangement of hair cell nuclei and fluorescence-specific labeling of CtBP2, a presynaptic membrane protein between the spiral ganglion and the inner hair cell, to quantify ribbon synapses (Fig. 1f). One day after noise exposure, the noise group exhibited considerably fewer ribbon synapses per IHC (8.97 ± 0.70, P < 0.01) than the control group (16.65 ± 0.88). Although its number of ribbon synapses partially recovered after 7 days (11.24 ± 0.41) and 14 days (11.56 ± 0.77), it remained far lower than that of the control group (P < 0.05) (Fig. 1g). According to statistical analysis, the decrease in the number of ribbon synapses was more significant in the basal turn of the basilar membrane, the region of the cochlea perceiving high-frequency sounds (Fig. 1h). These changes in ribbon synapses are consistent with the morphological characteristics of HHL.
Composition and Classification of Cochlear Cells
To explore the changes of gene expression in noise-induced hidden hearing loss, we constructed high-throughput single-cell transcriptome profiles of the mouse cochlea. Specifically, cochlea from three groups of mice (control, HHL, and NIHL) were extracted, and single-cell sequencing was conducted following exposure to varying noise intensities for each group (Fig. 2a). After rigorous quality control, we acquired a total of 39,092 single cells for downstream analysis. We employed t-SNE analysis to clarify the distribution of cell types in each sample.
Construction of single-cell transcriptome landscape. a Study design protocol for single-cell sequencing. b The distribution of various cell types in the cochlea. c A t-SNE plot showing the clustering results of each cell type within the cochlea. d The normalized log-transformed expression values of the marker genes for each cell type compared with all other cells showen by violin plots. e Z-score expression profiles of the first 50 cell type-specific genes and corresponding GO terms for each cell type. f Different cell types and their proportions in the cochlea
Based on gene expression profiles and distinct cell-specific markers, we identified 18 major cell types integral to hearing. These were distributed across five groups: cells within the organ of Corti, the modiolus, the stria vascularis, the spiral ligament, and various immune cells (Fig. 2b-c). We compared marker gene expression in each cell type with other cell types to verify the accuracy of cell type identification based on particular markers (Fig. 2d). Cells around the organ of Corti include hair cell (HC, Cnmd + , S100a16 +), Deiters’ and pillar cell (DC + PC, Col1a1 + , Gas1 +), inner phalangeal and inner border cell (IPhC + IBC, Cdh2 + , Lbsp +), Hensen’s cell (HeC, Bgn + , Tpm1 +), outer sulcus cell and inter sulcus cell (OSC + ISC, Apod + , Cavin2 +) (Ranum et al. 2019; Pavlinkova 2020; Chen et al. 2022; Piekna-Przybylska et al. 2022; Xu et al. 2022; You et al. 2022; Tu and Zuo 2023). Cells in the modiolus comprise spiral ganglion neuron (SGN, Car1 + , Ces2g +), satellite glial cell (SGC, Plk1 + , Rad18 +), Schwann cell (SC, Mpz + , Plp1 +), and chondrocyte (CC, Coch + , Cald1 +) (Li et al. 2019; Kalucka et al. 2020; Brosius et al. 2022; Petitpre et al. 2022; Li et al. 2023a, b). The stria vascularis contains capillary endothelial cell (CEC, S100a4 + , Vcan +), while the spiral ligament have fibroblast (FB, Igfbp5 + , Rgs2 +) and smooth muscle cell (SMC, Acta2 + , Myl9 +) (Zhou 2019; Pan et al. 2020). Additionally, the classification encompasses various immune cells within the cochlea (Wood and Zuo 2017; Rai et al. 2020). By analyzing the functional enrichment of the top 50 genes specifically expressed in each cell type, we demonstrated the unique functions corresponding to each cell type (Fig. 2e), thereby affirming the accuracy of our cell categorization. For instance, HC is linked to the cellular response to mechanical stimulus, SGN to the regulation of neuron death, and CEC to the regulation of angiogenesis. We also show the number and proportion of cells in each part of the cochlea and each cell type to better visualize the cochlear cell composition (Fig. 2f, Supplementary Table 1). In summary, our creation of an extensive high-throughput scRNA-seq atlas provides a detailed cellular classification of the adult mouse cochlea.
General Characteristics of Mouse Cochlear Cells
Initially, we compared the interactions between various cell types in the cochlea of HHL mice and normal mice. Our analysis revealed that certain cells, including macrophages (Mφ), OSCs + ISCs, and HeCs, exhibit significantly more connections with HCs in HHL (Fig. 3a). Subsequently, we compiled and analyzed the differentially expressed genes (DEGs) in the organ of Corti, a critical component of the cochlea responsible for auditory perception, under different hearing loss conditions. By identifying the common up- and down-regulated genes in both NIHL and HHL and examining these genes for KEGG pathway enrichment, we discovered that these DEGs are associated with ECM-receptor interaction, the PI3K-Akt signaling pathway, and the NOD-like receptor signaling pathway. This suggests that these genes and pathways, already known to be significant in NIHL, also play a crucial role in HHL (Fig. 3b-c). To delve deeper into cell type-specific changes in the cochlea during HHL, we assessed the DEGs for each cell type. We discovered the majority of cell types showed significant reactions to HHL. The three cell types with the highest number of DEGs in HHL were found to be FB, SC, and HC. Notably, when the stimulus intensity increased, as in cases of NIHL, HCs showed the highest number of DEGs (Fig. 3d). Given the unique alterations observed in HCs and their significant role in auditory cognition, further investigation is warranted.
General characteristics of mouse cochlear cells. a Cell-to-cell communication in the control and HHL groups, respectively. b Up- and down-regulated genes in different groups in the organ of Corti. c Pathway enrichment of differentially expressed genes in organ of Corti revealed by KEGG pathway analysis. d The number of differentially expressed genes (DEGs) in each cell type
Transcriptional Characterization of Cochlear Hair Cells
To further investigate transcriptome changes in cochlear hair cells, which are crucial for hearing, we conducted a volcano plot analysis, highlighting the top 50 genes with differential expression between groups. Notably, GDF15 was among these differentially expressed genes (Fig. 4a). We focused specifically on the top ten differentially expressed genes within hair cells affected by HHL, exhibiting the most significant changes. These included the up-regulation of Dclk1, Slc26a4, Gsn, Spp1, Anxa1, and Camp, and the down-regulation of Fabp3, Scd1, Dnajb1, and Isg15. Dclk1 encodes a microtubule-associated serine/threonine kinase involved in intracellular microtubule polymerization and cell migration facilitation. The Slc26a4 gene is responsible for encoding Pendrin, a transmembrane transporter protein essential for maintaining the body's ionic composition homeostasis, which is highly expressed in the cochlea. The proteins translated from the Gsn, Spp1, and Anxa1 genes are crucial in numerous biological functions, such as governing cellular inflammatory reactions and apoptosis. Camp is crucial for the cAMP signaling pathway, indicating significant alterations in energy metabolism during hidden hearing loss. Fabp3 is involved in regulating fatty acid metabolism and signaling. Dnajb1 encodes a molecular chaperone protein that enhances the ATPase activity of HSP70. Isg15 encodes the protein engaged in several signaling pathways, including NF-κB, JNK, and IRF-3. Overall, the findings on differential gene expression and protein interactions in hair cells with HHL indicated the abnormalities in cellular structure, signaling, energy metabolism, and various biological processes, including inflammation, impacting their normal physiological functions.
Transcriptional characterization of cochlear hair cells during HHL. a Volcano plot of top 50 up- and down-regulated genes in HCs. b GSEA analysis showing oxidative stress of HCs in HHL and NIHL. c Intersection of up- and down-regulated genes in HCs with oxidative stress-related genes. d GSEA analysis showing enrichment of DEGs in inflammation and lipid metabolism in HCs of HHL mice. e Pathway enrichment in HCs of mice with HHL revealed by KEGG pathway analysis. f Expression of GDF15 in OHCs of mice with NIHL. g Locations of GDF15 expression in the cochlea of mice with NIHL (gEAR; https://umgear.org/NIHL). h Expression of GDF15 in HCs of mice with HHL
Oxidative stress, recognized as a risk factor for various diseases, was a focal point of the present study. GSEA showed that similar to mice with NIHL, the redox imbalance was also present in mice with HHL (Fig. 4b), aligning with previous literature (Molina et al. 2016; Liu et al. 2022; Tan and Song 2023). Concurrently, we observed that both up-regulated and down-regulated genes in HHL mice exhibited significant enrichment in oxidative stress gene set (Fig. 4c), suggesting the occurence of oxidative stress in hair cells. The oxidative stress gene set was derived from the study by Wang et al. (Supplementary Table 2) (Wang et al. 2022). Furthermore, we noted enrichment of both up-regulated and down-regulated genes in pathways related to inflammation and lipid metabolism in HHL-affected hair cells (Fig. 4d-e). Considering the anti-inflammatory and anti-obesity properties of GDF15, we hypothesized a connection between HHL and GDF15. In an analysis of data from Beatrice Milon’s study (Milon et al. 2021) we found elevated levels of GDF15 in hair cells of mouse cochlea with NIHL (Fig. 4f-g). Pursuing this line of inquiry, we then analyzed GDF15 expression in various cochlear cells and also discovered increased GDF15 expression in hair cells of HHL mice compared with controls (Fig. 4h), supporting our speculation.
Recognition and Analysis of HC and SGN Subtypes
In our study, we conducted a detailed analysis of two crucial cell types in the cochlea related to hearing: the HC and the SGN. Using gene expression profiles and classical marker genes, we identified two subtypes of HCs: the inner hair cell (IHC) and the outer hair cell (OHC) (Fig. 5a-c). Significantly altered genes (e.g., Bcam and Map1b) in the OHCs of HHL mice revealed possible abnormalities in multiple biological processes, including defects in microtubule assembly and protein synthesis (Fig. 5d). Further analyses indicated that DEGs in HHL-affected OHCs were notably enriched in the oxidative stress gene set and oxidative stress-related pathways (Fig. 5e-f). An increased expression of GDF15 was also observed in both IHCs and OHCs in the case of HHL (Fig. 5g). KEGG analysis showed that DEGs in OHCs were significantly enriched in processes involving leukocyte transendothelial migration and oxidative phosphorylation pathways (Fig. 5h).
Recognition and analysis of HC subtypes. a The distribution of subtypes of HCs shown by the t-SNE plot. b Gene expression characterization of IHCs and OHCs shown by heatmap. c The expression levels of marker genes for IHCs and OHCs shown by violin plots. d Volcano plot of top 50 up- and down-regulated genes in OHCs. e Intersection of up- and down-regulated genes in IHCs with oxidative stress-related genes. f GSEA analysis showing oxidative stress in HCs of mice with HHL. g Relative expression of GDF15 in IHCs and OHCs of mice with HHL. h KEGG pathway enrichment in OHCs of mice with HHL
Furthermore, we distinguished four SGN subtypes according to their unique gene expression traits. These subtypes were classified as type IA (Ligp1 +), type IB (Mgst3 +), type IC (Hist1h1a +), and type II (Arhgdib +) (Fig. S1a and S1b). Notably, type IA serves as an afferent neuron in the inner ear, primarily forming ribbon synaptic structures with inner hair cells (Goutman et al. 2015; Reijntjes and Pyott 2016). So we focused on analyzing the transcriptomic changes in the type IA subtype. According to KEGG analysis, these alterations in mice with HHL were substantially enriched in pathways linked to ribosome, cellular senescence, ferroptosis, and necroptosis (Fig. S1c). DEGs such as Clk1 in the HHL group suggest that type IA SGNs may have impaired bioprotein synthesis (Fig. S1d). Enrichment of their DEGs with oxidative stress gene set suggests that their functional abnormalities may be associated with oxidative stress (Fig. S1e). SCENIC analysis further revealed that inflammation-related transcription factors (e.g., IRF7, Mafg, and Zmiz1) were up-regulated in type IA SGNs of cochlea with HHL (Figure S1f).
Elevated Oxidative Stress and GDF15 in Cochlea and HEI-OC1 Cells in Hidden Hearing Loss
Bioinformatics analysis revealed that the cochlea and hair cells of mice with HHL showed elevated levels of GDF15 and oxidative stress. We therefore conducted studies on animals and cells to validate these results. Initially, we measured the degree of oxidative stress in mouse cochlea before and after noise exposure. The findings showed a marked decrease in the activities of the antioxidant enzymes SOD and CAT in the cochlea one day after noise exposure (P < 0.05), and this reduction persisted for up to 14 days (P < 0.01) (Fig. 6a-b). We also assessed the cochlea expression levels of several markers of oxidative stress. Notably, the anti-oxidative stress molecule FOXO1 significantly decreased one day post-exposure (P < 0.01) and gradually recovered over time. The expression levels of other molecules related to oxidative stress, such as p53, p21 and IL-1β, also showed significant changes post-exposure (P < 0.05) (Fig. 6c-d, Fig. S2a-b). These results suggest that oxidative stress levels increased in the cochlea with HHL. Additionally, the expression of GDF15 and its downstream molecule Smad2 exhibited significant changes during this process, with GDF15 (P < 0.01) and Smad2 (P < 0.05) expressions notably increasing one day after exposure and then recovering over time (Fig. 6e-f).
Oxidative stress and GDF15 expression in cochlear and hair cells. a, b The activities of SOD and CAT in the cochlea of mice before and after noise exposure. c-f The mRNA expression levels of FOXO1, p53, GDF15, and Smad2 in the cochlea of mice with HHL. g-i Protein expression levels of GDF15 in HHL mouse cochlea, serum, and HEI-OC1 cells. n = 6.*P < 0.05, **P < 0.01, as compared with the control group
We further conducted cellular experiments using the H2O2-treated HEI-OC1 cell line, a widely recognized model for studying noise-induced hearing loss (Tian et al. 2024; Fan et al. 2023; Liu et al. 2021). To determine the appropriate H2O2 concentration, we created a gradient, treating the HEI-OC1 cell line with varying concentrations of H2O2 (0, 25, 50, 75, 100, 125, 150, 175, 200 μM). We selected 50 μM H2O2 as the optimal concentration, as it resulted in 70% cell survival of hair cells post-treatment (Fig. S2c). After H2O2 treatment, the antioxidant enzymes SOD and CAT showed a decrease in intracellular activities. (P < 0.05) (Fig. S2d-e), indicating the increase of oxidative stress in the treated hair cells. Moreover, we evaluated GDF15 expression in the cochlea and serum pre- and post-noise exposure and in hair cells pre- and post-H2O2 treatment. The results showed significantly increased GDF15 protein levels in the cochlea, serum, and hair cells of mice with HHL compared with the control group (Fig. 6g-i). These results corroborate our initial findings from the bioinformatics analysis.
GDF15 Attenuates Oxidative Stress Damage in HEI-OC1 Cells
To gain insights into the role of GDF15 and oxidative stress in HHL, we established control, GDF15, H2O2, and H2O2 + GDF15 groups using the HEI-OC1 cells. No treatment was administered to the control group. The remaining groups received 50 μM H2O2 or 400 μM recombinant GDF15, respectively. The results showed that ROS in HEI-OC1 cells increased following H2O2 treatment (P < 0.05) and decreased by recombinant GDF15 treatment (P < 0.05) (Fig. 7a). Furthermore, the activities of antioxidant enzymes SOD (P < 0.01) and CAT (P < 0.01) were considerably decreased in HEI-OC1 cells post-H2O2 treatment but elevated (P < 0.05) following recombinant GDF15 treatment (Fig. 7b-c). Flow cytometry measurements of each group’s apoptosis levels showed that hair cells exhibited a higher rate of apoptosis following H2O2 treatment (P < 0.001), and the apoptosis levels could be lowered by recombinant GDF15 treatment (P < 0.05) (Fig. 7d). These results implied that GDF15 treatment significantly reduced the damage caused by oxidative stress in HEI-OC1 cells.
Effect of GDF15 on oxidative stress damage in HEI-OC1 cells. a Fluorescence intensities of intracellular ROS with or without recombinant GDF15 treatment. b-c The activities of SOD and CAT in HEI-OC1 cells with or without recombinant GDF15 treatment. d Apoptosis rates of HEI-OC1 cells in different groups. n = 6. *P < 0.05, **P < 0.01, ***P < 0.001
GDF15 Alleviates Oxidative Stress in Cochlear Hair Cells and Protects Auditory Function
To look into the specific impacts of GDF15 on cochlear hair cells and auditory function in mice, we first performed immunofluorescence detection of 4-HNE and 3-NT in cochlear hair cells in each group. The results showed that there was no statistically significant change in their concentration in cochlear hair cells in the GDF15 group compared with the control group (P > 0.05), while they were significantly higher (P < 0.01) in the HHL group. However, the HHL + GDF15 group displayed notably reduced 4-HNE (P < 0.05) and 3-NT (P < 0.01) in the cochlear hair cells compared with the HHL group (Fig. 8a-b, S2f-g).
Effects of GDF15 on oxidative stress and auditory function in mouse cochlear hair cells. a Immunofluorescence of 4-HNE in cochlear hair cells of mice. Scale bar = 30 μm. b Relative expression of 4-HNE in hair cells. c Changes in hearing thresholds of mice in each group. d Changes in ABR wave I latency in various groups of mice. e Fluorogram representing changes in the number of ribbon synapses. Scale bar = 20 μm. f Changes in the number of ribbon synapses per IHC of the cochlea. n = 6. *P < 0.05, **P < 0.01, ***P < 0.001
We then compared the hearing thresholds of mice one day after noise exposure in each group and found that the hearing thresholds of GDF15 group (7.50 ± 2.51 dB) were not significantly different from those of the control group (8.33 ± 2.20 dB) (P > 0.05), while the HHL group's (44.17 ± 4.80 dB) mice's hearing thresholds were significantly elevated (P < 0.001). The hearing thresholds of mice in the HHL + GDF15 group (22.5 ± 4.24 dB) were remarkably lower (P < 0.05) than those of mice in the HHL group (Fig. 8c). Furthermore, we discovered that the ABR wave I latency was slightly prolonged in HHL mice (P < 0.05), whereas GDF15 treatment significantly reduced it (P < 0.05) (Fig. 8d).
In addition, we observed the effects of noise and GDF15 on the number of ribbon synapses and found that each turn of the cochlear basilar membrane responded slightly differently to these treatments. Overall, the number of ribbon synapses per IHC in the GDF15 group did not significantly change one day after noise stimulation. However, it did considerably decrease in the HHL group. Compared with the HHL group, the HHL + GDF15 group had a greater number of ribbon synapses per IHC. To be more precise, the HHL group had 6.51 ± 0.38 ribbon synapses per IHC in the basal turn, which was less than the control group's 12.22 ± 0.33 (P < 0.05). However, he HHL + GDF15 group's number of ribbon synapses per IHC did not differ significantly from the HHL group's (P > 0.05). The ribbon synapses per IHC in the middle and apical turns of the HHL group were 12.25 ± 1.13 and 8.17 ± 0.77, which were significantly lower than those of the control group (22.74 ± 2.65 and 14.67 ± 0.96) (P < 0.05), while those of the HHL + GDF15 group were 16.84 ± 0.75 and 13.06 ± 1.79, respectively, being markedly higher than those of the HHL group (P < 0.05) (Fig. 8e-f).
Discussion
Noise-induced HHL is a newly uncovered form of hearing impairment that has attracted considerable attention (Fernandez et al. 2021). Unlike NIHL, patients with HHL typically exhibit normal hearing thresholds but show difficulties in speech recognition in noisy environments (Bakay et al. 2018; Bajin et al. 2022). Recent findings suggest that HHL, while not manifesting as severe hearing loss, may heighten the cochlea's vulnerability to noxious stimuli (Song et al. 2016; Huang 2020).
Previous studies have suggested that HHL may involve a recoverable, temporary impairment of auditory function which does not significantly affect hearing thresholds (Aedo and Aguilar 2020; Wei et al. 2020). In our animal experiments involving C57BL/6J mice, noise exposure was used to create a model of hidden hearing loss. These mice exhibited a temporary hearing threshold shift after noise exposure, but some irreversible changes were also observed, including a decrease in ABR wave I amplitude and prolonged latency. Besides, our study discovered a permanent decrease in cochlear ribbon synapses in HHL mice, which is in keeping with the findings of prior research (Liberman and Kujawa 2017; Budak et al. 2021; Seo et al. 2022). The extent of ribbon synapse damage varied across different sites, with those located in the basal turn of the basilar membrane, characterized by low spiking rates and high threshold nerve fibers, being more severely affected.
The cochlea is one of the vital structures for conducting and sensing sound waves. Structural changes and functional decline in cochlear cells are intimately linked to hearing impairment (Gratias et al. 2021; Hough et al. 2022). However, although several studies have already explored transcriptomic changes in acoustic injury, no studies have identified particular transcriptomic characteristics in HHL at the single-cell level to date (Wang et al. 2021a, b; Sanders and Kelley 2022). In this research, we have established a single-cell transcriptional profile of the cochlea in C57BL/6J mice, identifying and presenting five major cell types within the mouse cochlea according to their location and function. This formed a solid foundation for exploring the unique alterations of each cell type under varying physiological and pathological conditions.
Hair cells and nerve fiber cells in the cochlea are key to auditory perception and conduction (Driver and Kelley 2020). The OHCs act as effectors of mechanical acoustic stimulation; the IHCs convert acoustic signals into nerve impulses; and SGNs are responsible for sending auditory signals to the auditory centre (Cunningham and Muller 2019; Mu et al. 2020; Goutman et al. 2015; Reijntjes and Pyott 2016; Fettiplace 2017). In this study, we concentrate on analyzing the transcriptome characteristics of OHCs and type IA SGNs due to their predominance in both percentage and function, to elucidate the impairment of auditory function during HHL. This includes, but is not limited to, changes in the expression of auditory-related molecules and the abnormal enrichment of pathways like oxidative stress and inflammation.
Oxidative stress represents a pathological condition that arises from an imbalance between oxidative and antioxidative processes, which can cause certain degrees of damage to cells and tissues (Ji et al. 2019; Forman and Zhang 2021). In the prior study, our team found that the auditory cortex and cochlea had increased levels of oxidative stress following five days of constant noise exposure at 115 dB for four hours (Chen et al. 2020). However, the specific relationship and mechanisms between HHL and oxidative stress remain to be fully illuminated. In our study, we discovered that oxidative stress is engaged in cochlear hair cells of HHL mice, as evidenced by single-cell gene expression profiling in the cochlea. Significant changes in the expression of oxidative stress-related molecules, such as FOXO1, were also observed. We validated these findings at the in vivo and in vitro levels, respectively.
GDF15 is expressed across various human tissues, including the heart and brain (Wischhusen et al. 2020; Rochette et al. 2021; Wang et al. 2023). Notably, basal circulatory levels of GDF15 in human are higher at 0.2–1.2 ng/mL than those of other cytokines (Tsai et al. 2018). GDF15 expression increases with age or in stress-related diseases such as diabetic nephropathy (Assadi et al. 2020; Oshita et al. 2023). To explore the relationship of GDF15 with auditory function and HHL, we first innovatively demonstrated the expression of GDF15 in cochlear hair cells and their subtypes in HHL mice by single-cell sequencing results. By RNA and protein level experiments, we found that GDF15 levels were elevated in cochlear hair cells of HHL mice compared with controls, and the application of recombinant GDF15 significantly reduced oxidative stress and apoptosis in hair cells. These results suggest that there is an elevated expression of GDF15, specifically in the cochlear hair cells of HHL mice, and that GDF15 has a mitigating effect on oxidative stress damage in hair cells.
Recently, scientists have been investigating methods to hinder or cure noise-induced hearing loss, such as counteracting cochlear inflammation and promoting cochlear synapse regeneration after noise exposure (Kalinec et al. 2017; Nevoux et al. 2021). However, this area is still understudied. In our research, we investigated the impact of GDF15 on oxidative stress and auditory function in mice. By comparing the 4-HNE and 3-NT, the number of cochlear ribbon synapses, and the auditory function in each group, we found that neither oxidative stress nor the autitory function were significantly impacted by the administration of GDF15 alone. However, GDF15 treatment prior to noise remarkably attenuated the noise-induced oxidative stress in mouse cochlear hair cells and mitigated the noise-induced damage to the number of ribbon synapses and the hearing threshold. These findings suggest that GDF15 can protect auditory function and shield mouse cochlear hair cells from oxidative damage. However, the limitation of this study is that the relevant mechanisms downstream of GDF15 were not investigated. Combined with the existing stress-related studies of GDF15, we speculate that the MAPK signaling pathway and antioxidant molecules like Nrf2 may be involved in GDF15's ameliorative effect on HHL (Li et al. 2018; Lin et al. 2024). This will also be the direction and goal of our next study.
Overall, this study constructed a single-cell transcriptome profile of the cochlea in HHL mice, which lays the foundation for a deeper understanding of the pathogenesis of HHL. Our findings indicate that increased oxidative stress and GDF15 levels are important characteristics of cochlear lesions in HHL mice. Targeting GDF15 and oxidative stress specifically may provide novel strategies for the prevention and management of HHL.
Data availability
All data will be shared upon reasonable request to the corresponding authors.
Abbreviations
- ABR:
-
Auditory brainstem response
- CAT:
-
Catalase
- CC:
-
Chondrocyte
- CEC:
-
Capillary endothelial cell
- DC + PC:
-
Deiters’ and pillar cell
- DEGs:
-
Differentially expressed gene
- DMEM:
-
Dulbecco’s Modified Eagle Medium
- ELISA:
-
Enzyme-linked immunosorbent assay
- FB:
-
Fibroblast
- FBS:
-
Fetal bovine serum
- GDF15:
-
Growth differentiation factor 15
- GEMs:
-
Gel bead emulsions
- GO:
-
Gene Ontology
- GSEA:
-
Gene Set Enrichment Analysis
- H2O2 :
-
Hydrogen peroxide
- HC:
-
Hair cell
- HeC:
-
Hensen’s cell
- HHL:
-
Hidden hearing loss
- HEI-OC1:
-
House Ear Institute-Organ of Corti 1
- IHC:
-
Inner hair cell
- IPhC + IBC:
-
Inner phalangeal and inner border cell
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- Mφ:
-
Macrophage
- NAFLD:
-
Non-alcoholic fatty liver disease
- NIHL:
-
Noise-induced hearing loss
- OS:
-
Oxygen species
- OSC + ISC:
-
Outer sulcus cell and inter sulcus cell
- PI:
-
Propidium iodide
- ROS:
-
Reactive oxygen species
- SC:
-
Schwann cell
- scRNA-seq:
-
Single-cell RNA sequencing
- SGC:
-
Satellite glial cell
- SGN:
-
Spiral ganglion neuron
- SMC:
-
Smooth muscle cell
- SOD:
-
Superoxide dismutase
- TGF-β:
-
Transforming growth factor-β
- t-SNE:
-
t-distributed stochastic neighbor embedding
References
Aedo C, Aguilar E. Cochlear synaptopathy: new findings in animal and human research. Rev Neurosci. 2020;31(6):605–15. https://doi.org/10.1515/revneuro-2020-0002.
Assadi A, Zahabi A, Hart RA. Gdf15, an update of the physiological and pathological roles it plays: a review. Pflugers Archiv-European Journal of Physiology. 2020;472(11):1535–46. https://doi.org/10.1007/s00424-020-02459-1.
Bajin MD, Dahm V, Lin V. Hidden hearing loss: current concepts. Curr Opin Otolaryngol Head Neck Surg. 2022;30(5):321–5. https://doi.org/10.1097/MOO.0000000000000824.
Bakay W, Anderson LA, Garcia-Lazaro JA, Mcalpine D, Schaette R. Hidden hearing loss selectively impairs neural adaptation to loud sound environments. Nat Commun. 2018;9(1):4298. https://doi.org/10.1038/s41467-018-06777-y.
Brosius LA, Lucas TA, Carson GA, Caneda C, Zhou L, Barres BA, Buckwalter MS, Sloan SA. An rna-sequencing transcriptome of the rodent schwann cell response to peripheral nerve injury. J Neuroinflammation. 2022;19(1):105. https://doi.org/10.1186/s12974-022-02462-6.
Budak M, Grosh K, Sasmal A, Corfas G, Zochowski M, Booth V. Contrasting mechanisms for hidden hearing loss: synaptopathy vs myelin defects. PLoS Comput Biol. 2021;17(1):e1008499. https://doi.org/10.1371/journal.pcbi.1008499.
Chen XM, Ji SF, Liu YH, Xue XM, Xu J, Gu ZH, Deng SL, Liu CD, Wang H, Chang YM, Wang XC. Ginsenoside rd ameliorates auditory cortex injury associated with military aviation noise-induced hearing loss by activating sirt1/pgc-1alpha signaling pathway. Front Physiol. 2020;11:788. https://doi.org/10.3389/fphys.2020.00788.
Chen J, Gao D, Chen J, Hou S, He B, Li Y, Li S, Zhang F, Sun X, Jin Y, Sun L, Yang J. Pseudo-temporal analysis of single-cell rna sequencing reveals trans-differentiation potential of greater epithelial ridge cells into hair cells during postnatal development of cochlea in rats. Front Mol Neurosci. 2022;15:832813. https://doi.org/10.3389/fnmol.2022.832813.
Colla MF, Lunardelo PP, Dias F. Cochlear synaptopathy and hidden hearing loss: a scoping review. Codas. 2023;36(2):e20230032. https://doi.org/10.1590/2317-1782/20232023032pt.
Cunningham CL, Muller U. 2019. Molecular structure of the hair cell mechanoelectrical transduction complex. Cold Spring Harbor Perspectives in Medicine 9(5). https://doi.org/10.1101/cshperspect.a033167.
Driver EC, Kelley MW. 2020. Development of the cochlea. Development 147(12). https://doi.org/10.1242/dev.162263.
Fan B, Lu F, Du WJ, Chen J, An XG, Wang RF, Li W, Song YL, Zha DJ, Chen FQ. Pten inhibitor bisperoxovanadium protects against noise-induced hearing loss. Neural Regen Res. 2023;18(7):1601–6. https://doi.org/10.4103/1673-5374.358606.
Fernandez KA, Watabe T, Tong M, Meng X, Tani K, Kujawa SG, Edge AS. 2021. Trk agonist drugs rescue noise-induced hidden hearing loss. Jci Insight 6(3). https://doi.org/10.1172/jci.insight.142572.
Fetoni AR, Paciello F, Rolesi R, Paludetti G, Troiani D. Targeting dysregulation of redox homeostasis in noise-induced hearing loss: oxidative stress and ros signaling. Free Radical Biol Med. 2019;135:46–59. https://doi.org/10.1016/j.freeradbiomed.2019.02.022.
Fettiplace R. Hair cell transduction, tuning, and synaptic transmission in the mammalian cochlea. Compr Physiol. 2017;7(4):1197–227. https://doi.org/10.1002/cphy.c160049.
Forman HJ, Zhang H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discovery. 2021;20(9):689–709. https://doi.org/10.1038/s41573-021-00233-1.
Galuppo B, Agazzi C, Pierpont B, Chick J, Li Z, Caprio S, Santoro N. Growth differentiation factor 15 (gdf15) is associated with non-alcoholic fatty liver disease (nafld) in youth with overweight or obesity. Nutr Diabetes. 2022;12(1):9. https://doi.org/10.1038/s41387-022-00187-2.
Goutman JD, Elgoyhen AB, Gomez-Casati ME. Cochlear hair cells: the sound-sensing machines. FEBS Lett. 2015;589(22):3354–61. https://doi.org/10.1016/j.febslet.2015.08.030.
Gratias P, Nasr J, Affortit C, Ceccato JC, Francois F, Casas F, Pujol R, Pucheu S, Puel JL, Wang J. 2021. Impulse noise induced hidden hearing loss, hair cell ciliary changes and oxidative stress in mice. Antioxidants 10(12). https://doi.org/10.3390/antiox10121880.
Hajam YA, Rani R, Ganie SY, Sheikh TA, Javaid D, Qadri SS, Pramodh S, Alsulimani A, Alkhanani MF, Harakeh S, Hussain A, Haque S, Reshi MS. 2022. Oxidative stress in human pathology and aging: molecular mechanisms and perspectives. Cells 11(3). https://doi.org/10.3390/cells11030552.
Hough K, Verschuur CA, Cunningham C, Newman TA. Macrophages in the cochlea; An immunological link between risk factors and progressive hearing loss. Glia. 2022;70(2):219–38. https://doi.org/10.1002/glia.24095.
Huang H, Chen Z, Li Y, Gong K, Xiao L, Fu H, Yang J, Wang X, Meng Q. Gdf-15 suppresses atherosclerosis by inhibiting oxldl-induced lipid accumulation and inflammation in macrophages. Evid Based Complement Alternat Med. 2021;2021:6497568. https://doi.org/10.1155/2021/6497568.
Huang L. 2020. [Hidden hearing loss and early identification]. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 34(7): 668–671. https://doi.org/10.13201/j.issn.2096-7993.2020.07.023.
Ji L, Lee HJ, Wan G, Wang GP, Zhang L, Sajjakulnukit P, Schacht J, Lyssiotis CA, Corfas G. Auditory metabolomics, an approach to identify acute molecular effects of noise trauma. Sci Rep. 2019;9(1):9273. https://doi.org/10.1038/s41598-019-45385-8.
Johann K, Kleinert M, Klaus S. 2021. The role of gdf15 as a myomitokine. Cells 10(11). https://doi.org/10.3390/cells10112990.
Kalinec GM, Lomberk G, Urrutia RA, Kalinec F. Resolution of cochlear inflammation: novel target for preventing or ameliorating drug-, noise- and age-related hearing loss. Front Cell Neurosci. 2017;11:192. https://doi.org/10.3389/fncel.2017.00192.
Kalucka J, de Rooij L, Goveia J, Rohlenova K, Dumas SJ, Meta E, Conchinha NV, Taverna F, Teuwen LA, Veys K, Garcia-Caballero M, Khan S, Geldhof V, Sokol L, Chen R, Treps L, Borri M, de Zeeuw P, Dubois C, Karakach TK, Falkenberg KD, Parys M, Yin X, Vinckier S, Du Y, Fenton RA, Schoonjans L, Dewerchin M, Eelen G, Thienpont B, Lin L, Bolund L, Li X, Luo Y, Carmeliet P. Single-cell transcriptome atlas of murine endothelial cells. Cell. 2020;180(4):764–79. https://doi.org/10.1016/j.cell.2020.01.015.
Kobayashi S, Yamazaki H, Imamura T, Fujioka H, Kakeshita K, Koike T, Kinugawa K. Implication of serum growth differentiation factor-15 level in patients with renal diseases. Int Urol Nephrol. 2023;55(11):2935–41. https://doi.org/10.1007/s11255-023-03580-7.
Li S, Ma YM, Zheng PS, Zhang P. Gdf15 promotes the proliferation of cervical cancer cells by phosphorylating akt1 and erk1/2 through the receptor erbb2. J Exp Clin Cancer Res. 2018;37(1):80. https://doi.org/10.1186/s13046-018-0744-0.
Li Q, Cheng Z, Zhou L, Darmanis S, Neff NF, Okamoto J, Gulati G, Bennett ML, Sun LO, Clarke LE, Marschallinger J, Yu G, Quake SR, Wyss-Coray T, Barres BA. Developmental heterogeneity of microglia and brain myeloid cells revealed by deep single-cell rna sequencing. Neuron. 2019;101(2):207–23. https://doi.org/10.1016/j.neuron.2018.12.006.
Li H, Jiang X, Xiao Y, Zhang Y, Zhang W, Doherty M, Nestor J, Li C, Ye J, Sha T, Lyu H, Wei J, Zeng C, Lei G. Combining single-cell rna sequencing and population-based studies reveals hand osteoarthritis-associated chondrocyte subpopulations and pathways. Bone Res. 2023a;11(1):58. https://doi.org/10.1038/s41413-023-00292-7.
Li P, Li S, Wang L, Li H, Wang Y, Liu H, Wang X, Zhu X, Liu Z, Ye F, Zhang Y. Mitochondrial dysfunction in hearing loss: oxidative stress, autophagy and nlrp3 inflammasome. Front Cell Dev Biol. 2023b;11:1119773. https://doi.org/10.3389/fcell.2023.1119773.
Liberman MC, Kujawa SG. Cochlear synaptopathy in acquired sensorineural hearing loss: manifestations and mechanisms. Hear Res. 2017;349:138–47. https://doi.org/10.1016/j.heares.2017.01.003.
Lin H, Luo Y, Gong T, Fang H, Li H, Ye G, Zhang Y, Zhong M. Gdf15 induces chemoresistance to oxaliplatin by forming a reciprocal feedback loop with nrf2 to maintain redox homeostasis in colorectal cancer. Cell Oncol. 2024. https://doi.org/10.1007/s13402-024-00918-w.
Liu W, Xu L, Wang X, Zhang D, Sun G, Wang M, Wang M, Han Y, Chai R, Wang H. Prdx1 activates autophagy via the pten-akt signaling pathway to protect against cisplatin-induced spiral ganglion neuron damage. Autophagy. 2021;17(12):4159–81. https://doi.org/10.1080/15548627.2021.1905466.
Liu YH, Jiang YH, Li CC, Chen XM, Huang LG, Zhang M, Ruan B, Wang XC. Involvement of the sirt1/pgc-1alpha signaling pathway in noise-induced hidden hearing loss. Front Physiol. 2022;13:798395. https://doi.org/10.3389/fphys.2022.798395.
May BM, Pimentel M, Zimerman LI, Rohde LE. 2021. Gdf-15 as a biomarker in cardiovascular disease. Arquivos Brasileiros De Cardiologia 116(3): 494–500. https://doi.org/10.36660/abc.20200426.
Milon B, Shulman ED, So KS, Cederroth CR, Lipford EL, Sperber M, Sellon JB, Sarlus H, Pregernig G, Shuster B, Song Y, Mitra S, Orvis J, Margulies Z, Ogawa Y, Shults C, Depireux DA, Palermo AT, Canlon B, Burns J, Elkon R, Hertzano R. A cell-type-specific atlas of the inner ear transcriptional response to acoustic trauma. Cell Rep. 2021;36(13):109758. https://doi.org/10.1016/j.celrep.2021.109758.
Molina SJ, Miceli M, Guelman LR. Noise exposure and oxidative balance in auditory and extra-auditory structures in adult and developing animals. Pharmacological approaches aimed to minimize its effects. Pharmacol Res. 2016;109:86–91. https://doi.org/10.1016/j.phrs.2015.11.022.
Mu Y, Su H, Wu F, Yang J, Li D. Research progress of hair cell protection mechanism. Neural Plast. 2020;2020:8850447. https://doi.org/10.1155/2020/8850447.
Nevoux J, Alexandru M, Bellocq T, Tanaka L, Hayashi Y, Watabe T, Lahlou H, Tani K, Edge A. An antibody to rgma promotes regeneration of cochlear synapses after noise exposure. Sci Rep. 2021;11(1):2937. https://doi.org/10.1038/s41598-021-81294-5.
Oshita T, Watanabe S, Toyohara T, Kujirai R, Kikuchi K, Suzuki T, Suzuki C, Matsumoto Y, Wada J, Tomioka Y, Tanaka T, Abe T. Urinary growth differentiation factor 15 predicts renal function decline in diabetic kidney disease. Sci Rep. 2023;13(1):12508. https://doi.org/10.1038/s41598-023-39657-7.
Pan H, Xue C, Auerbach BJ, Fan J, Bashore AC, Cui J, Yang DY, Trignano SB, Liu W, Shi J, Ihuegbu CO, Bush EC, Worley J, Vlahos L, Laise P, Solomon RA, Connolly ES, Califano A, Sims PA, Zhang H, Li M, Reilly MP. Single-cell genomics reveals a novel cell state during smooth muscle cell phenotypic switching and potential therapeutic targets for atherosclerosis in mouse and human. Circulation. 2020;142(21):2060–75. https://doi.org/10.1161/CIRCULATIONAHA.120.048378.
Pavlinkova G. 2020. Molecular aspects of the development and function of auditory neurons. International Journal of Molecular Sciences 22(1). https://doi.org/10.3390/ijms22010131.
Petitpre C, Faure L, Uhl P, Fontanet P, Filova I, Pavlinkova G, Adameyko I, Hadjab S, Lallemend F. Single-cell rna-sequencing analysis of the developing mouse inner ear identifies molecular logic of auditory neuron diversification. Nat Commun. 2022;13(1):3878. https://doi.org/10.1038/s41467-022-31580-1.
Piekna-Przybylska D, Na D, Zhang J, Baker C, Ashton JM, White PM. Single cell rna sequencing analysis of mouse cochlear supporting cell transcriptomes with activated erbb2 receptor indicates a cell-specific response that promotes cd44 activation. Front Cell Neurosci. 2022;16:1096872. https://doi.org/10.3389/fncel.2022.1096872.
Rai V, Wood MB, Feng H, Schabla NM, Tu S, Zuo J. The immune response after noise damage in the cochlea is characterized by a heterogeneous mix of adaptive and innate immune cells. Sci Rep. 2020;10(1):15167. https://doi.org/10.1038/s41598-020-72181-6.
Ranum PT, Goodwin AT, Yoshimura H, Kolbe DL, Walls WD, Koh JY, He D, Smith R. Insights into the biology of hearing and deafness revealed by single-cell rna sequencing. Cell Rep. 2019;26(11):3160–71. https://doi.org/10.1016/j.celrep.2019.02.053.
Reijntjes D, Pyott SJ. The afferent signaling complex: regulation of type i spiral ganglion neuron responses in the auditory periphery. Hear Res. 2016;336:1–16. https://doi.org/10.1016/j.heares.2016.03.011.
Rochette L, Dogon G, Zeller M, Cottin Y, Vergely C. 2021. Gdf15 and cardiac cells: current concepts and new insights. International Journal of Molecular Sciences 22(16). https://doi.org/10.3390/ijms22168889.
Sanders TR, Kelley MW. Specification of neuronal subtypes in the spiral ganglion begins prior to birth in the mouse. Proc Natl Acad Sci USA. 2022;119(48):e2091032177. https://doi.org/10.1073/pnas.2203935119.
Seo HW, Lee SY, Byun H, Lee SH, Chung JH. 2022. Possible existence of cochlear synaptopathy in patients completely recovered from idiopathic sudden sensorineural hearing loss. Journal of Clinical Medicine 11(3). https://doi.org/10.3390/jcm11030875.
Song Q, Shen P, Li X, Shi L, Liu L, Wang J, Yu Z, Stephen K, Aiken S, Yin S, Wang J. Coding deficits in hidden hearing loss induced by noise: the nature and impacts. Sci Rep. 2016;6:25200. https://doi.org/10.1038/srep25200.
Tan W, Song L. Role of mitochondrial dysfunction and oxidative stress in sensorineural hearing loss. Hear Res. 2023;434:108783. https://doi.org/10.1016/j.heares.2023.108783.
Tian C, Yang Y, Wang R, Li Y, Sun F, Chen J, Zha D. Norepinephrine protects against cochlear outer hair cell damage and noise-induced hearing loss via alpha(2a)-adrenergic receptor. BMC Neurosci. 2024;25(1):5. https://doi.org/10.1186/s12868-024-00845-4.
Trevino M, Lobarinas E. Current topics in hearing research: deafferentation and threshold independent hearing loss. Hear Res. 2022;419:108408. https://doi.org/10.1016/j.heares.2021.108408.
Tsai V, Husaini Y, Sainsbury A, Brown DA, Breit SN. The mic-1/gdf15-gfral pathway in energy homeostasis: implications for obesity, cachexia, and other associated diseases. Cell Metab. 2018;28(3):353–68. https://doi.org/10.1016/j.cmet.2018.07.018.
Tsui KH, Hsu SY, Chung LC, Lin YH, Feng TH, Lee TY, Chang PL, Juang HH. Growth differentiation factor-15: a p53- and demethylation-upregulating gene represses cell proliferation, invasion, and tumorigenesis in bladder carcinoma cells. Sci Rep. 2015;5:12870. https://doi.org/10.1038/srep12870.
Tu S, Zuo J. Systematic single cell rna sequencing analysis reveals unique transcriptional regulatory networks of atoh1-mediated hair cell conversion in adult mouse cochleae. PLoS ONE. 2023;18(12):e284685. https://doi.org/10.1371/journal.pone.0284685.
Tzikas S, Vassilikos V, Keller T. Gdf-15 as a risk stratification biomarker for cardiovascular disease. Int J Cardiol. 2019;292:246–7. https://doi.org/10.1016/j.ijcard.2019.06.009.
Valderrama JT, de la Torre A, Mcalpine D. The hunt for hidden hearing loss in humans: from preclinical studies to effective interventions. Front Neurosci. 2022;16:1000304. https://doi.org/10.3389/fnins.2022.1000304.
Wang D, Day EA, Townsend LK, Djordjevic D, Jorgensen SB, Steinberg GR. Gdf15: emerging biology and therapeutic applications for obesity and cardiometabolic disease. Nat Rev Endocrinol. 2021a;17(10):592–607. https://doi.org/10.1038/s41574-021-00529-7.
Wang S, Lee MP, Jones S, Liu J, Waldhaus J. Mapping the regulatory landscape of auditory hair cells from single-cell multi-omics data. Genome Res. 2021b;31(10):1885–99. https://doi.org/10.1101/gr.271080.120.
Wang H, Tian RF, Liang X, Fan J, Duan ZC, Fan XY, Zhang JJ, Yao DS, Chen ZN, Li L. A four oxidative stress gene prognostic model and integrated immunity-analysis in pancreatic adenocarcinoma. Front Oncol. 2022;12:1015042. https://doi.org/10.3389/fonc.2022.1015042.
Wang D, Townsend LK, Desormeaux GJ, Frangos SM, Batchuluun B, Dumont L, Kuhre RE, Ahmadi E, Hu S, Rebalka IA, Gautam J, Jabile M, Pileggi CA, Rehal S, Desjardins EM, Tsakiridis EE, Lally J, Juracic ES, Tupling AR, Gerstein HC, Pare G, Tsakiridis T, Harper ME, Hawke TJ, Speakman JR, Blondin DP, Holloway GP, Jorgensen SB, Steinberg GR. Gdf15 promotes weight loss by enhancing energy expenditure in muscle. Nature. 2023;619(7968):143–50. https://doi.org/10.1038/s41586-023-06249-4.
Wei M, Wang W, Liu Y, Mao X, Chen TS, Lin P. Protection of cochlear ribbon synapses and prevention of hidden hearing loss. Neural Plast. 2020;2020:8815990. https://doi.org/10.1155/2020/8815990.
Wilson BS, Tucci DL. Addressing the global burden of hearing loss. Lancet. 2021;397(10278):945–7. https://doi.org/10.1016/S0140-6736(21)00522-5.
Wischhusen J, Melero I, Fridman WH. Growth/differentiation factor-15 (gdf-15): from biomarker to novel targetable immune checkpoint. Front Immunol. 2020;11:951. https://doi.org/10.3389/fimmu.2020.00951.
Wood MB, Zuo J. The contribution of immune infiltrates to ototoxicity and cochlear hair cell loss. Front Cell Neurosci. 2017;11:106. https://doi.org/10.3389/fncel.2017.00106.
Xiong H, Lai L, Ye Y, Zheng Y. Glucose protects cochlear hair cells against oxidative stress and attenuates noise-induced hearing loss in mice. Neurosci Bull. 2021;37(5):657–68. https://doi.org/10.1007/s12264-020-00624-1.
Xu Z, Tu S, Pass C, Zhang Y, Liu H, Diers J, Fu Y, He D, Zuo J. Profiling mouse cochlear cell maturation using 10x genomics single-cell transcriptomics. Front Cell Neurosci. 2022;16:962106. https://doi.org/10.3389/fncel.2022.962106.
Xu K, Xu B, Gu J, Wang X, Yu D, Chen Y. Intrinsic mechanism and pharmacologic treatments of noise-induced hearing loss. Theranostics. 2023;13(11):3524–49. https://doi.org/10.7150/thno.83383.
You D, Guo J, Zhang Y, Guo L, Lu X, Huang X, Sun S, Li H. The heterogeneity of mammalian utricular cells over the course of development. Clin Transl Med. 2022;12(10):e1052. https://doi.org/10.1002/ctm2.1052.
Zhou Y. Beyond fibroblast heterogeneity: what single-cell rna sequencing tells us. Am J Respir Cell Mol Biol. 2019;61(1):7–8. https://doi.org/10.1165/rcmb.2019-0120ED.
Zhu X, Zhang Y, Liang F, Yin J, Jiang L, Cai W, Lu J, Zhang C, Xiao Y, Teng H, Ge W, Hu Y, Lu Y, Su J, Zhang J, Wu M. Relationship between plasma growth differentiation factor 15 levels and complications of type 2 diabetes mellitus: a cross-sectional study. Can J Diabetes. 2023;47(2):117–23. https://doi.org/10.1016/j.jcjd.2022.09.116.
Zhu Y, Scheibinger M, Ellwanger DC, Krey JF, Choi D, Kelly RT, Heller S, Barr-Gillespie PG. 2019. Single-cell proteomics reveals changes in expression during hair-cell development. Elife 8. https://doi.org/10.7554/eLife.50777.
Acknowledgements
We thank the other partners of our laboratory for their helpful suggestions.
Funding
The study was supported by the National Natural Science Foundation of China (No. 82373610).
Author information
Authors and Affiliations
Contributions
Xiaocheng Wang, Bai Ruan, and Min Zhang conceived and designed the experiment. Yihong Jiang, Zeyu Zheng, and Jing Zhu completed the main part of the experiment, including data collection and analysis. Peng Zhang, Shaoheng Li, Yang Fu, Fei Wang, Zhuoru Zhang, and Tong Chang completed part of the experiment and provided suggestions. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
All animal studies were approved by the Institutional Animal Care and Use Committee of Air Force Medical University.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
10565_2024_9912_MOESM2_ESM.doc
Supplementary Material 2: Supplementary Figure Legends (Fig. S1 Recognition and analysis of SGN subtypes; Fig. S2 Oxidative stress levels in cochlear hair cells and HEI-OC1 cells).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jiang, Y., Zheng, Z., Zhu, J. et al. The role of GDF15 in attenuating noise-induced hidden hearing loss by alleviating oxidative stress. Cell Biol Toxicol 40, 79 (2024). https://doi.org/10.1007/s10565-024-09912-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10565-024-09912-2