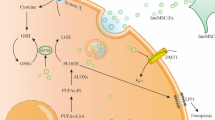
Abstract
Human umbilical cord mesenchymal stem cell-derived exosomes (hucMSC-Ex) have emerged as a new treatment strategy for inflammatory bowel disease (IBD) due to their immunoregulatory function. N6-methyladenosine (m6A) plays a crucial role in regulating intestinal immunity, especially in IBD where macrophages play an important role, although its mechanism is not yet fully understood. From this perspective, this research aimed to evaluate the effect of hucMSC-Ex on m6A modification of macrophages in IBD. In the process of alleviating inflammation, hucMSC-Ex promotes macrophage polarization toward the M2 type and regulates intracellular m6A levels by upregulating the expression of m6A “Writer” METTL3 and “Reader” YTHDF1. Solute Carrier Family 37 Member 2 (Slc37a2) was identified by Methylation RNA immunoprecipitation sequencing as the target molecule of the hucMSC-Ex. Mechanically, hucMSC-Ex promoted the binding of METTL3 to the Slc37a2 mRNA complex, and enhanced the binding of Slc37a2 to YTHDF1 to upregulate the intracellular expression of Slc37a2, thereby attenuating the pro-inflammatory function of macrophage. This study confirms the modulatory role of hucMSC-Ex on the m6A modification of macrophages in IBD, providing a new scientific basis for the treatment of IBD with hucMSC-Ex.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
IBD is a non-specific intestinal inflammatory disease that includes ulcerative colitis (UC) and Crohn’s disease (CD). The clinical manifestation is an abnormal response of the intestinal mucosal immune system, and its incidence is increasing year by year (Kuenzig et al. 2022; Ng et al. 2019). At present, the specific pathogenesis of IBD is not completely clarified. Individual genetic susceptibility, environmental risk factors, intestinal microbial community imbalance, intestinal mucosal abnormal immunity, and other factors all play an important role in the occurrence and development of IBD (Ananthakrishnan et al. 2018; Ni et al. 2017; Greuter and Vavricka 2019). The abnormal and excessive immune response of the colorectal mucosa may be affected by many of these factors, contributing to the progression of IBD.
As one of the important immune cells of the intestinal mucosal barrier, macrophages are crucial in the progression of IBD (Bain and Mowat 2014). M1-type (classically activated) macrophages can be differentiated by IFN-γ and LPS stimulation, secrete IL-1β, TNF-α, IL-6, IL-18, and other pro-inflammatory factors, and highly express markers such as CD80/86 and iNOS. However, IL-4/IL-23 stimulation could produce M2 macrophages, which mainly secret anti-inflammatory factors such as TGF-β and IL-10, and highly express CD206 and Arg1 (Funes et al. 2018). Generally, they promote immune balance by expressing anti-inflammatory phenotypes. More research has shown that macrophages are instrumental in maintaining intestinal homeostasis and are also intimately involved in the pathogenesis of IBD. The balance between the phenotype and function of inflammatory M1 cells and anti-inflammatory M2 cells, regulated by extracellular and intracellular stimuli, determines the mode of progression of IBD (Seyedizade et al. 2020). In IBD and other conditions, pro-inflammatory macrophages in the colorectal mucosa increase and highly express inflammatory factors such as IL-1β, IL-6, and TNF-α (Cai et al. 2021), and the degree of increase is highly related to the progression and deterioration of the disease (Na et al. 2019). Yang and his colleagues found that M2 macrophage-secreted exosomes can alleviate DSS-induced colitis in mice by raising Treg cells and IL-4 and reducing the production of pro-inflammatory factors (IL-1β, IL-6, and IL-17A) (Yang et al. 2019). Zhou et al. showed that YAP deletion in macrophages alleviates chemically induced IBD by enhancing M2 polarization, inhibiting IL-6 production in M1 macrophages, and altering the homeostasis of the gut microbiota (Zhou et al. 2019). IL-10, which is mainly produced by M2-type macrophages, is an important mechanism for the treatment of IBD with anti-TNF-α agents, which increase IL-10 expression by acting on Fcγ receptor signaling, which in turn promotes the repolarization of M1-type intestinal macrophages into CD206 + M2-type macrophages (Koelink et al. 2020). Tian et al. demonstrated that Astragaloside IV (AS-IV) can ameliorate experimental colitis by blocking macrophage M1-type polarization through inhibition of the STAT1 signaling pathway (Tian et al. 2021). Therefore, the polarization of M1/M2 macrophages plays an important role in the inflammatory response and resolution of IBD. The development of related targeted drugs to effectively regulate the polarization of macrophages to M2 type is of great significance for the clinical resolution of IBD.
In recent years, the role and mechanism of mesenchymal stem cells (MSCs) in regenerative medicine have attracted much attention. Studies have shown that MSCs are mainly involved in the repair of tissue damage through the paracrine pathway (van Poll et al. 2008; Aghajani et al. 2017), and exosomes are the main effect components of MSCs that exert its paracrine effect, which has great application prospects in the treatment of IBD (Baghaei et al. 2019). Moreover, human umbilical cord mesenchymal stem cell-derived exosome (hucMSC-Ex) can effectively alleviate IBD and play a regulatory role through a variety of mechanisms (Wang et al. 2020a; Zhang et al. 2022a; Xu et al. 2022). In the microenvironment of IBD, exosomes regulate immune cells, intestinal microbiota, and intestinal barrier cells, which are important participants in the mechanism of repairing damage and restoring intestinal mucosal function (Ocansey et al. 2020).
N6-methyladenosine (m6A) has attracted much attention due to its dynamic regulation and reversible post-transcriptional regulation. Its interaction with a variety of RNAs and signaling pathways makes it important in the development of diseases (Zhang et al. 2020a). The dynamic modification of m6A is mainly performed by three components, namely “Writers”, “Erasers” and “Readers” (Xu et al. 2021). Among them, “Writers” are represented by adenosine methyltransferase, and the methylation location of specific regions depends on the polyprotein adenosine methyltransferase complex. METTL3 (methyltransferase like 3), METTL14 (methyltransferase like 14) and WTAP (Wilms tumor 1-associated protein) are common complexes (Cao et al. 2016). “Erasers” represent demethylases, including ALKBH5 (AlkB homolog 5) (Zheng et al. 2013) and FTO (Fat mass and obesity-associated protein) (Jia et al. 2011). “Readers” refer to m6A-binding proteins, including YTH domain-containing proteins (Xiao et al. 2016; Zhang et al. 2020b; Chang et al. 2020; Shi et al. 2019), heterogeneous nuclear ribonucleoprotein (hnRNP) (Kwon et al. 2019), and eukaryotic initiation factor (eIF). Among them, YTHDC1, YTHDC2, YTHDF1, YTHDF2, YTHDF3, and other proteins have YTH domains at the C terminus, which can overlap with the m6A consensus motif to mediate RNA-specific binding. YTHDF1-3 specifically recognizes m6A-modified mRNA in the cytoplasm (Shi et al. 2017) and regulates the translation, decay, and processing of transcripts into the nucleus.
M6A modification involved in all parts of the intestinal mucosal barrier plays an irreplaceable role in the process of mucosal immunity. Wu et al. showed that dynamic epigenetic modifications in the gut strongly mediate crosstalk between gut microbes and the intestinal mucosal barrier. Lactobacillus and Bifidobacterium in the intestinal commensal flora can synthesize folate to increase DNA methylation and mRNA m6A modification to ensure normal intestinal development (Wu et al. 2022). Similarly, Jabs S and colleagues found that gut microbial community changes are associated with m6A modification in the cecum and, to some extent, affect m6A modification in the liver, affecting pathways related to metabolism, inflammation, and antimicrobial response (Jabs et al. 1344). Han and colleagues demonstrated that YTHDF1 is highly expressed in intestinal stem cells (ISCs) to promote Wnt/β-catenin signaling, which is required for the maintenance of ISCs regeneration and tumorigenesis and is essential for normal intestinal development in mice (Han et al. 2020). Wang et al. identified that insulin-like growth factor 2 messenger RNA-binding protein 2 (IGF2BP2), one of the m6A-binding proteins, could regulate the polarization of macrophages to M2-type by stabilizing PPARγ mRNA, thus controlling the development of DSS-induced colitis (Wang et al. 2021). In summary, m6A modification plays an important role in intestinal development and related diseases.
In this research, we constructed a DSS-induced IBD in a mouse model and treated it with hucMSC-Ex to identify novel targets of hucMSC-Ex that regulate colorectal m6A modifications in IBD. The specific mechanism by which hucMSC-Ex regulates macrophage m6A modification and mediates its phenotypic polarization to alleviate IBD was explored.
Results
HucMSC-Ex regulates macrophage phenotype polarization to alleviate LPS-induced inflammation
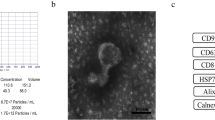
In this study, hucMSCs were isolated, cultured, and identified according to the protocol previously described. As shown in Figure S1A, long spindle cells grew around the umbilical cord tissue. Flow cytometry was used to assess negative markers (CD34, CD45, CD11b) and positive markers (CD73, CD105, CD29) on the surface of hucMSCs, confirming that the isolated cells were hucMSCs (Fig. S1B). HucMSCs culture supernatant was collected and exosomes were extracted according to the method established before (Yuan et al. 2021). The results of the nanoparticle tracking analyzer (NanoSight) and transmission electron microscope showed that hucMSC-Ex had a diameter of about 100 nm, complete cell membrane, low electron density in the cavity, and concave circular membrane vesicle structure (Fig. S1C, D). Western-blot results showed that extracted hucMSC-Ex expressed exosomes surface labeled proteins CD81, CD9, HSP70, and Alix (Fig. S1E). These results indicated that hucMSC-Ex was extracted successfully, which provided a material guarantee for the subsequent experiments.
In the next phase of the study, LPS was used to stimulate the inflammation of RAW264.7 cells. Following stimulation with LPS, the cell morphology underwent a transformation from round to long fusiform, thereby establishing a successful in vitro model for the inflammation process. CCK8 analysis showed a significant decrease in cell proliferation after hucMSC-Ex treatment(Fig. 1A). The expression of pro-inflammatory cytokines IL-1β, IL-6, and TNF-α decreased, while the expression of anti-inflammatory cytokines IL-10 increased after hucMSC-Ex treatment (Fig. 1B). These results indicated that hucMSC-Ex could alleviate LPS-induced macrophage inflammation. At the same time, the transcription levels of M1-type macrophage functional gene iNOS and MCP-1, and M2 functional gene Arg1 were measured by qRT-PCR. The results showed that the expressions of iNOS and MCP-1 were significantly up-regulated after LPS stimulation, and down-regulated after hucMSC-Ex treatment. The expression of Arg1 was further increased after hucMSC-Ex treatment (Fig. 1C), and cell culture supernatant NO assay showed that the level of NO released from macrophages decreased after hucMSC-Ex treatment (Fig. 1D). Moreover, the M1-type surface marker CD86 and M2-type surface marker CD206 were detected by immunofluorescence (IF). The expression of CD206 in the LPS group was significantly decreased, while that in the hucMSC-Ex group was enhanced (Fig. 1E-F). By flow cytometry of RAW264.7, the expression of CD206 in the hucMSC-Ex group was significantly increased compared with the LPS group (Fig. 1G). These results indicate that hucMSC-Ex promotes macrophage polarization toward the M2 type to alleviate inflammation.
hucMSC-Ex regulates the henotypic transition of macrophages to relieve LPS-induced macrophage inflammation. A Cell proliferation detection by CCK8 (n = 3 independent experiments). B The expression of pro-inflammatory cytokines IL-1β, IL-6, TNF-α, and anti-inflammatory cytokines IL-10 as detected by qRT-PCR (n = 3 independent experiments). C M1-type macrophages functional gene iNOS, MCP-1, and M2-type functional gene Arg1 as detected by qRT-PCR (n = 3 independent experiments). D The concentration of NO in the supernatant of macrophage culture (n = 3 independent experiments); E M1-type surface marker CD86 and M2-type surface marker CD206 (600 ×) as detected by immunofluorescence; F The mean gray value analysis of the immunofluorescence of CD86 and CD206. G M1-type surface marker CD86 and M2-type surface marker CD206 as detected by flow cytometry. Data are presented as mean ± SD.*P < 0.05, **P < 0.01, ***P < 0.001
HucMSC-Ex regulates macrophage m6A modification
We used qRT-PCR to initially screen the significantly different m6A-related "writers" (METTL3, METTL14), "erasers" (FTO, ALKBH5), and "readers" (YTHDF1, YTHDF2) in mouse macrophage cell lines RAW264.7. The results showed that the expression of “writer” METTL3 and “reader” YTHDF1 was significantly down-regulated after LPS stimulation, and increased after hucMSC-Ex treatment (Fig. 2A), preliminarily revealing the changes of m6A modification in RAW264.7. The changes of METTL3 and YTHDF1 in RAW264.7 were verified by Western-blot and IF, which were consistent with the transcription level (Fig. 2B, D, E). Consistent with the change of METTL3, the modification level of m6A was significantly up-regulated after hucMSC-Ex treatment (Fig. 2C), suggesting that hucMSC-Ex regulated m6A modification in mouse macrophage cell strain RAW264.7.
hucMSC-Ex regulates m6A modification in RAW264.7. A Transcription levels of "writers", "erasers" and "readers" associated with m6A modification in macrophages as detected by qRT-PCR. B Western-blot analysis of the expression of METTL3 and YTHDF1 (n = 3 independent experiments). C The m6A RNA methylation quantitative assay in RAW264.7 cells (n = 3 independent experiments). D The expression of METTL3 in RAW264.7 was detected by cellular immunofluorescence (600 ×) and the mean gray value analysis. E The expression of YTHDF1 in RAW264.7 as detected by cellular immunofluorescence (600 ×) and the mean gray value analysis. Data are presentd as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001
To further verify this effect, we examined the expression of m6A-related molecules in human monocytic leukemia cell line THP-1 and clinical colon tissue specimens. After the addition of Phorbol 12-myristate 13-acetate (PMA) to induce the differentiation of THP-1 cells into macrophages, LPS was also added to stimulate cellular inflammation. QRT-PCR and Western-blot further verified the expressions of differential molecules METTL3 and YTHDF1, which were consistent with the results of RW264.7 cells. Their expressions were down-regulated after LPS stimulation, but significantly increased after hucMSC-Ex treatment(Fig. 3A, B). Consistent with the changes in METTL3, m6A% of total RNA in cells was significantly upregulated after hucMSC-Ex treatment (Fig. 3C). These results also suggest that hucMSC-Ex regulates m6A modification in THP-1 cells. In order to further observe the expression of m6A-related regulatory molecules, we collected colorectal tissues from 18 pairs of clinical IBD patients and healthy subjects to detect the expression of METTL3 and YTHDF1. The results of qRT-PCR showed that METTL3 and YTHDF1 were decreased in IBD patients compared with healthy subjects (Fig. 3D, E). Figure 3F further showed H&E staining of colon tissue sections of clinical patients. H&E results showed clear glandular structure of colonic tissue in healthy subjects, whereas IBD patients had disturbed colonic tissue results with loss of glandular structure and massive infiltration of inflammatory cells. IHC results also confirmed the differential expression of METTL3 and YTHDF1 in IBD tissues (Fig. 3G).
hucMSC-Ex regulates the expression of m6A-related molecules in THP-1 cells. A The transcription level of -METTL3 and YTHDF1 in THP-1 as detected by qRT-PCR (n = 3 independent experiments). B The protein level of METTL3 and YTHDF1 in THP-1 as detected by Western-blot. C The m6A RNA methylation quantitative assay in THP-1 cells (n = 3 independent experiments). D, E The expression of METTL3 and YTHDF1 in colon tissues of IBD and healthy subjects as detected by qRT-PCR (n = 18 patients). F H&E staining of colon tissues of patients and healthy subjects (200 ×). G METTL3 and YTHDF1 expression in colon tissues of patients as detected by IHC (200 ×) and the average optical density analysis. Data are presented as mean ± SD. *P < 0.05, ***P < 0.001
HucMSC-Ex alleviates DSS-induced IBD in mice by regulating colorectal m6A modification
To verify the repair effect of hucMSC-Ex on DSS-induced IBD in mice, we constructed a DSS-induced IBD mice model and treated it with hucMSC-Ex. Both the body weight and DAI score showed that hucMSC-Ex could relieve the weight loss and bloody stool of colitis mice to a certain extent (Fig. 4A, B). It was observed that the colorectal tissues of mice in the DSS group were significantly shortened, and that of mice treated with hucMSC-Ex were significantly restored (Fig. 4D, E). The spleen, as a major immune organ, has been found to have impaired splenic function in a significant percentage of IBD patients (Lenti et al. 2020). As shown in Fig. 4C, the spleen tissue was significantly enlarged in the DSS group but hucMSC-Ex treatment restored the spleen morphology close to that of the NEG group. The colorectal weight/length ratio of mice increased in the DSS group but decreased in the hucMSC-Ex group (Fig. 4F). Colorectal tissue protein was extracted for the expressions of tight junction proteins Occludin and Claudin1, and PCNA by Western-blot. The results showed that the expression of Occludin, Claudin1, and PCNA decreased in the DSS group, indicating that the colorectal barrier integrity and cell proliferation ability were impeeded. However, the colorectal barrier in the hucMSC-Ex group was relatively intact, and the cell proliferation ability was significantly restored (Fig. 4G). H&E staining results of colorectal tissues showed that the intestinal mucosal tissue structure of DSS-induced IBD mice was severely damaged, while that of the hucMSC-Ex group had better integrity (Fig. 4H). Moreover, H&E staining of the spleen tissue showed that the destruction of the spleen colony was reduced in the hucMSC-Ex group. (Fig. 4I). Compared with the NEG group, the expression of pro-inflammatory factors (IL-1β, IL-6, TNF-α) was increased in the colorectal tissues of the DSS group, but significantly decreased in the hucMSC-Ex group. The expression of the anti-inflammatory factor IL-10 was highly increased in the hucMSC-Ex group (Fig. 4J). In the colon tissue, M1-related molecules iNOS and MCP-1 significantly increased in IBD condition but significantly decreased after hucMSC-Ex treatment, and M2-related molecule Arg1 increased after hucMSC-Ex treatment (Fig. 4K). The co-localization of M1-type surface marker CD86 and M2-type surface marker CD206 of colon tissue macrophages by immunofluorescence technology showed that the number of macrophages expressing CD86 in the colon tissue of IBD mice was significantly increased, and the number of CD206-expressing positive cells decreased. In the hucMSC-Ex group, CD86-positive cells decreased significantly but CD206-positive macrophages increased, indicating that intestinal macrophage phenotype changed under the hucMSC-Ex effect (Fig. 4L). The above results indicate that hucMSC-Ex effectively repaired DSS-induced IBD in mice and mediated the phenotypic polarization of macrophages in colorectal tissues.
hucMSC-Ex alleviates DSS-induced IBD in mice by regulating macrophage phenotypic transformation of colorectal tissue. A Body weight changes of mice (n = 3 independent experiments). B DAI score (n = 3 independent experiments). C Gross view of mice spleen. D Gross view of mice colorectum. E Colorectal length(cm) (n = 3 independent experiments). F Colorectal weight/length ratio (n = 3 independent experiments). G The expression of Occludin, PCNA, and Claudin-1 in colorectal tissue by Western-blot. H H&E staining of mice colorectal tissues (100 ×). I H&E staining of mice spleen tissue (100 ×). J The expression levels of pro-inflammatory and anti-inflammatory cytokines (IL-1β, IL-6, TNF-α, IL-10) in mice colorectal tissues by qRT-PCR (n = 3 independent experiments). K The quantitative results of iNOS, MCP-1, and Arg1 in colon tissue (n = 3 independent experiments). L M1-type surface marker CD86 and M2-type surface marker CD206 (200 ×) by IF. Data are presented as mean ± SD.*P < 0.05, **P < 0.01, ***P < 0.001
We further explored whether hucMSC-Ex regulates the expression level of m6A regulatory factor in mice colorectal tissue. Western-blot and qRT-PCR were used to detect the transcription and protein levels of METTL3 and YTHDF1. The results showed that METTL3 and YTHDF1 were reduced in the DSS group but significantly increased in the hucMSC-Ex group, which was consistent with the results of cell models (Fig. 5A, B). This indicates that hucMSC-Ex up-regulates the m6A modification level in colorectal tissues of IBD mice (Fig. 5C). IHC staining results further verified the regulatory effect of hucMSC-Ex on METTL3 and YTHDF1 (Fig. 5D).
hucMSC-Ex regulates the expression of m6A-related molecules in the colorectal tissue of mice. A Transcription levels of METTL3 and YTHDF1 in mice colon tissue (n = 3 independent experiments). B The protein expression of METTL3 and YTHDF1 was detected by Western-blot. C The level of m6A modification in colon tissue by m6A RNA methylation quantitative detection kit (n = 3 independent experiments). D The expression of METTL3 and YTHDF1 in colon tissue of mice by IHC (200 ×) and the average optical density analysis. Data are presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001
Slc37a2 serves as the target of hucMSC-Ex in regulating m6A modification of macrophages by MeRIP-Sequencing
MeRIP-Sequencing was used to analyze the differential m6A modification in colonic tissues of IBD mice treated with hucMSC-Ex. The differential m6A-modified genes (Fig. S2A) and peaks (Fig. S2B) between the hucMSC-Ex group and the DSS group are presented in a Venn diagram. Figure S2C statistically demonstrates the regional distribution characteristics of methylation sites on genes in the colon tissue of the hucMSC-Ex group, which were mainly distributed in the CDS, startC, stopC, 5’UTR, and 3’UTR regions. MetaGene peak density map showed that the peak density of m6A modification in the 3’UTR region of the hucMSC-Ex group was significantly higher than that of the DSS group, while there was little difference between the two in the CDS region (Fig. S2D). The m6A peak that was significantly up-regulated in the hucMSC-Ex group was screened for motif map, and the GGACU motif was obtained, while m6A mainly occurred in RRACH (R = G/A; H = A/C/U), and the identified motif was included, indicating that the identified peak has high confidence (Fig. S2E). The mRNA GO enrichment analysis showed significant up- or down-regulation of the top 10 functional differences (Fig. S2F, G). For example, genes related to vascular endothelial growth factor receptor binding function and CXCR chemokine receptor binding-related genes, which participate in inflammation were down-regulated in the hucMSC-Ex group. However, genes related to O-methyltransferase activity were upregulated. Similarly, KEGG signaling pathway analysis of mRNAs with different m6A modification levels between the hucMSC-Ex group and the DSS group showed that the inflammation-related pathways PI3k-Akt, IL-17, and TNF-related signaling pathways were significantly down-regulated in the hucMSC-Ex group, while the pathways related to amino acid synthesis were significantly up-regulated (Fig. S2H, I), suggesting that hucMSC-Ex may play an important role in the regulation of amino acid metabolism-related molecules in the process of up-regulating m6A modification to alleviate IBD.
Based on the MeRIP-Seq results, we identified Slc37a2 and Serpinb1c as the key molecules related to inflammatory response since it was significantly up-regulated in the hucMSC-Ex group compared with the DSS group from the differentially modified m6A sites. As is shown in Fig. 6A, the 3’UTR region of Slc37a2 showed high m6A modification in the hucMSC-Ex group compared to the DSS group, while the peak of high m6A modification of Serpinb1c appeared in the CDS region. To confirm the reliability of the sequencing results, we used qRT-PCR to assess the differential expression of Slc37a2 and Serpinb1c in RAW264.7 cells and mouse colorectal tissues (Fig. 6B, D). As shown in the figure, Slc37a2 and Serpinb1c expression were both significantly decreased in DSS-induced IBD mice colorectal tissues. However, after treatment with hucMSC-Ex, only Slc37a2 expression level was significantly enhanced in RAW264.7, so only slc37a2 was further studied in subsequent experiments. Western-blot and IHC analysis revealed a consistent pattern of Slc37a2 expression both in vitro and in vivo (Fig. 6C, E, F).
hucMSC-Ex regulates Slc37a2 m6A modification to influence its stability in mice colon. A The differential m6A peak of Slc37a2 and Serpinb1c between DSS and hucMSC-Ex groups was visualized by IGV software. B The transcription level of Slc37a2 and Serpinb1c in RAW264.7 cells by qRT-PCR (n = 3 independent experiments). C The protein expression of Slc37a2 in RAW264.7 cells by Western-blot. D The transcription level of Slc37a2 and Serpinb1c in colon tissue of mice by qRT-PCR (n = 3 independent experiments). E The protein expression of Slc37a2 in colon tissue of mice by Western-blot. F Slc37a2 expressed in colon tissue by IHC (200 ×) and the average optical density analysis. G Detection of the stability of Slc37a2 transcription in RAW264.7 cells by qRT-PCR (n = 3 independent experiments). H The stability of pro-inflammatory cytokines IL-1β, IL-6, TNF-α, and anti-inflammatory cytokine IL-10 was detected by qRT-PCR (n = 3 independent experiments). Data are presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001
After verifying the up-regulation effect of hucMSC-Ex on the expression of Slc37a2, we further explored its mechanism. The decay rate of Slc37a2 in macrophages was measured at different time points after blocking the transcription of cells with 5 μg/ml actinomycin D. The results showed that the addition of hucMSC-Ex slowed down the decay rate of Slc37a2 at 2 h and 4 h compared with LPS stimulation (Fig. 6G). In addition, hucMSC-Ex accelerated the decay rate of pro-inflammatory cytokine IL-1β (mainly produced by M1 Mϕ) at 2 and 4 h, but had no effect on the decay rate of pro-inflammatory cytokines IL-6 and TNF-α, while the decay rate of anti-inflammatory cytokine IL-10 (mainly produced by M2 Mϕ) was slowed down (Fig. 6H). These results suggest that hucMSC-Ex can slow down mRNA attenuation of IL-10, and accelerate IL-1β, which may related to the alterations in the level of m6A modification, but this still needs further validation. It also reveals a new mechanism by which hucMSC-Ex regulates m6A modification of Slc37a2 to slow their decay rate and related downstream inflammatory factors, thereby alleviating the pro-inflammatory function of macrophages.
hucMSC-Ex up-regulates m6A modification of macrophage through the METTL3-Slc37a2-YTHDF1 axis
After confirming the regulatory effect of hucMSC-Ex on Slc37a2, we further verified that the specific m6A site of Slc37a2 which hucMSC-Ex acted on according to the MeRIP-Seq result. MeRIP-qPCR was used to verify the up-regulation effect of hucMSC-Ex on this site in vitro and in vivo (Fig. 7A, B). To clarify that hucMSC-Ex plays a role in regulating the amount of METTL3 binding to the m6A site of Slc37a2, the dual luciferase reporter assay was employed. Results showed that this site was the binding site of METTL3 (Fig. 7C). YTHDF1, as an RNA binding protein downstream of m6A, promotes the translation and expression of m6A-modified mRNA. We further validated the binding of YTHDF1 to this sequence, and the data also showed that hucMSC-Ex treatment enhanced Slc37a2-YTHDF1 binding (Fig. 7D, E), revealing a new mechanism by which hucMSC-Ex regulates METTL3-Slc37a2-YTHDF1 axis to up-regulate m6A modification in macrophages.
hucMSC-Ex enhances m6A modification via the METTL3-Slc37a2-YTHDF1 axis. A The m6A modification sites of Slc37a2 in RAW264.7 cells as detected by MeRIP-qPCR (n = 3 independent experiments). B The m6A modification sites of Slc37a2 in mouse colon tissue as detected by MeRIP-qPCR (n = 3 independent experiments). C Construction of the m6A WT and MUT fragment vector of Slc37a2, and double luciferase reporter assay verifying hucMSC-Ex's regulation of METTL3 binding to the target m6A site (n = 3 independent experiments). D RIP-qPCR detection of the expression of Slc37a2 binding to YTHDF1 in RAW264.7 cells (n = 3 independent experiments). E RIP-qPCR detection of the expression of Slc37a2 binding to YTHDF1 in colon tissue of mice (n = 3 independent experiments). Data are presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001
The regulatory effect of hucMSC-Ex on Slc37a2 is dependent on METTL3
To further clarify the mechanism by which hucMSC-Ex regulates the METTL3-Slc37a2-YTHDF1 axis of macrophages to mediate phenotype polarization and alleviate inflammation, we treated samples with hucMSC-Ex after knocking down METTL3 in macrophages. First, the optimal knockdown condition of si-METTL3 in RAW264.7 cells was determined (Fig. S3A, B). Next, we examined whether hucMSC-Ex could still regulate the expression of METTL3 and YTHDF1 after the knockdown of METTL3. The results of both mRNA and protein levels showed that compared with the non-knockdown group, the addition of the same dose of hucMSC-Ex did not upregulate the expression of METTL3 and YTHDF1 (Fig. S3C, D). The result was further validated by cellular immunofluorescence (Fig. S3E), indicating that METTL3 is an important target for hucMSC-Ex in exerting its regulatory effect.
We further examined whether hucMSC-Ex could mediate phenotypic polarization to alleviate cellular inflammation after the knockdown of METTL3 in RAW264.7 cells. The expression of M1- and M2-related inflammatory factors was measured by qRT-PCR. The results showed that IL-1β expression increased significantly after the knockdown of METTL3, followed by LPS stimulation. IL-10 and IL-6 showed a certain degree of reduction, while TNF-α appeared to be unaffected; but none was regulated by hucMSC-Ex (Fig. S4A). The expression of M1-type functional genes iNOS and MCP-1 increased to a certain extent after METTL3 knockdown, and the expression of M2-type functional gene Arg1 decreased more significantly. Similarly, these changes could not be reversed after hucMSC-Ex treatment (Fig. S4B). The expression of NO in the supernatant of macrophages cultured medium also showed similar results (Fig. S4C). Flow cytometry assays for CD86 and CD206 showed that the proportion of CD206 was significantly decreased after METTL3 knockdown, indicating that the polarization process of macrophages to M2 was inhibited (Fig. S4D). The results of macrophage markers CD86 and CD206 by IF showed that while the expression of CD86 was not significantly up-regulated after METTL3 knockdown, CD206 was significantly decreased (Fig. S4E). Therefore, we hypothesized that hucMSC-Ex exerted anti-inflammatory effects mainly by targeting METTL3 to mediate macrophage polarization to the M2 type.
Finally, we verified whether hucMSC-Ex could still regulate the m6A modification of Slc37a2 to influence its stability after the knockdown of METTL3. The results of qRT-PCR and western-blot showed that the expression of Slc37a2 was not enhanced by hucMSC-Ex treatment after METTL3 knockdown (Fig. 8A, B). The stability of Slc37a2 was measured at 2 h and 4 h, and the results showed that METTL3 knockdown accelerated the decay rate of Slc37a2 compared with the LPS stimulation group without METTL3 knockdown (Fig. 8C), while the decay rate of hucMSC-Ex did not change at this time (Fig. 8D). The MeRIP-qPCR results (Fig. 8E) also showed that the expression of m6A modification of Slc37a2 in the hucMSC-Ex group was not significantly different from that of the LPS group after knocking down METTL3. The above results further confirm that METTL3 is an important target for hucMSC-Ex to play its regulatory role, and hucMSC-Ex could not regulate macrophage Slc37a2 m6A modification after the knockdown of METTL3.
The regulatory effect of hucMSC-Ex on Slc37a2 depends on METTL3. A qRT-PCR detection of Slc37a2 mRNA stability in the LPS group and si-MEETL3 + LPS group (n = 3 independent experiments). B The protein expression of Slc37a2 was detected by Western blot. C-D qRT-PCR detection of Slc37a2 mRNA stability in the si-MEETL3 + LPS group and si-MEETL3 + hucMSC-Ex group (n = 3 independent experiments). E The m6A modified sequence of Slc37a2 in RAW264.7 cells was detected by MeRIP-qPCR (n = 3 independent experiments). F The mechanism of hucMSC-Ex regulating macrophage m6A modification to alleviate IBD. Data are presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001
Discussion
Macrophages are widely distributed in many organs and tissues of the body, playing a complex dual role in the inflammatory environment, and their function mainly depends on epigenetic reprogramming (Kuznetsova et al. 2020). As the most common modification of mRNA transcriptome found in recent years, the important role of m6A modification in the inflammatory environment mediated by macrophages has been gradually recognized. Studies have shown that m6A modification participates in the inflammatory process of macrophages and thus plays an important role in several diseases, including atopic dermatitis (Yang et al. 2022), atherosclerosis (Sun et al. 2022), liver fibrosis (Shu et al. 2021), septic acute kidney injury (Mao et al. 2023), and other inflammatory diseases. RAW264.7 cells are widely used in the establishment of inflammation-related models. In our research, we used the mouse-derived macrophage RAW264.7 as the research object. Meanwhile, the non-immunogenic feature of hucMSC-Ex has made it widely used in the study of clinically relevant mouse models, which is adopted in many studies (Cai et al. 2021; Tan et al. 2022; Wei et al. 2023; Wang et al. 2024). Our results suggest that hucMSC-Ex may alleviate intestinal inflammation by regulating the transformation of macrophages to M2-type. In a study by Song and colleagues, the treatment of IBD mice with hucMSC-Ex by intraperitoneal injection promoted tissue homeostasis and repair of the mucosal barrier by causing the repolarization of macrophages (Song et al. 2017), which confirms our findings. In recent years, MSC-derived exosomes (MSC-Ex) have opened a new paradigm of "cell-free therapy" and shown promising results due to their multiple efficacies in cell survival, immunomodulation, angiogenesis, and tissue regeneration. However, the content of MSC-Ex is not static but is derived from the nature and activity of MSC and its neighboring cells. Therefore, in terms of cytokines, signaling lipids, mRNA and regulatory miRNA carried by MSC-Ex, its therapeutic effect depends to a large extent on the quality of its source MSC production, reproducibility, and potency (Song et al. 2017). Therefore, to ensure the efficacy of this new treatment strategy and reduce risks, proper standardization of the extraction and identification of MSC-Ex will be an important problem in future research.
As the catalytic core of methyltransferase, the specific regulatory function of METTL3 in the pro-inflammatory function of macrophages is still unclear. Shu and colleagues showed that METTL3 upregulates MALAT1 level through m6A modification, promotes its interaction with PTBP1, and enhances the degradation of USP8 mRNA, while reduced USP8 is involved in regulating TAK1 ubiquitination and protein stability, thereby promoting macrophage pyroptosis and inflammatory response (Shu et al. 2021). Another study showed that METTL3 synergizes with YTHDF2 to promote PGC-1α mRNA degradation, increase the accumulation of mitochondrial reactive oxygen species, and promote an inflammatory response in oxidized low-density lipoprotein-induced monocyte inflammation (Zhang et al. 2021a). In contrast, Wang et al. showed that METTL3 attenuates LPS-induced inflammatory responses in macrophages through the NF-κB signaling pathway (Wang et al. 2019). Confined to the limited studies available on this subject, the role of METTL3 and its mediated m6A modification in the pro-inflammatory function of macrophages remains uncertain. In this study, we confirm that METTL3 and YTHDF1 are down-regulated in both LPS-induced macrophage inflammation and the colon of IBD mice, and correspond with a decreased level of m6A modification. The addition of hucMSC-Ex could reverse these changes and alleviate the inflammatory response. Thus, there is a certain relationship between the m6A modification regulated by hucMSC-Ex and the phenotypic polarization mediated by hucMSC-Ex in the process of alleviating IBD.
The pathophysiological research of IBD shows large numbers of complex inflammatory pathways involved in the occurrence and development of IBD. Among them, reactive oxygen species released from tissue cell injury can promote IκB kinase activation of NF-κB through the PI3k/Akt/PTEN pathway, which plays an important role in the progression of CD (Tokuhira et al. 2015). The IL-23/IL17 axis also plays an important role in the pathogenesis of UC by promoting the immune response mediated by Th17 cells and related pro-inflammatory cytokines and chemokines (Noviello et al. 2021). In addition, the roles of JAK/STAT (Salas et al. 2020), Wnt/β-catenin (Zhang et al. 2021b), AMPK/mTOR, HMGB1/RAGE and Nrf2/HO-1 (Arab et al. 2021) pathways in IBD have been gradually recognized. TNF-α, as the main proinflammatory factor secreted by macrophages, has been widely known in IBD, and anti-TNF-α antagonists have been formulated as first-line drugs in clinical practice. In this study, our MeRIP-Seq results show that hucMSC-Ex significantly down-regulates m6A modification of PI3k-Akt, IL-17, TNF, and other inflammation-related signaling pathway molecules in the colorectal tissues of mice compared with the untreated DSS group, revealing a new mechanism of hucMSC-Ex in alleviating IBD and providing evidence for our previous hypothesis.
In recent years, with the continuous exploration of epigenetic modifications such as methylation, several studies have reported that m6A plays an important role in the regulation of intestinal mucosal homeostasis and IBD. A study found that gut microbial community changes correlated with m6A modification in the cecum and, to some extent, affect m6A modification in the liver, influencing pathways related to metabolism, inflammation, and antimicrobial response (Jabs et al. 1344). A recent study also showed that dietary gluten can induce a higher m6A modification level in the 5’UTR of XPO1 RNA in intestinal epithelial cells, which in turn produces more protein mediated by YTHDF1, thereby enhancing downstream NF-κB activity and stimulating epithelial cells to produce a large number of IL-8, promoting inflammatory response of colonic epithelial cells (Olazagoitia-Garmendia et al. 2022). A study by Nie et al. found that m6A regulators in IBD show broad differential expression in four independent discovery cohorts (IGF2BP2, HNRNPA2B1, ZCCHC4, and EIF3I), affecting the immunophenotype and clinical inflammatory state of IBD (Nie et al. 2021). Wang and colleagues found that DSS-induced m6A modification in colonic tissues of IBD mice is different from that of normal controls (Wang et al. 2022), while a recent study showed that METTL14 deficiency leads to the dysfunction of Treg cells and causes spontaneous colitis in mice (Lu et al. 2020). METTL14 deficiency decreases RORγt expression in Treg cells and impairs the induction of naive T cells to inducible Treg cells. Adoptive transfer of wild-type Treg cells could alleviate the induced colitis. In addition, METTL14 could limit cell death to maintain cell homeostasis by regulating Nfkbia mRNA stability and the NF-κB pathway in colonic epithelial cells. Inhibition of NF-κB signaling promotes cell death, which triggers the loss of colon stem cells and the occurrence of colitis (Zhang et al. 2022b). In an experiment to explore the regulatory effect of m6A on T cells, METTL3 prevented the development of colitis by targeting the IL-7/STAT5/SOCS pathway in the adoptive lymphatic transfer mouse model, which prevented the steady-state expansion of naive T cells and maintained them in the naive state for up to 12 weeks (Li et al. 2017). The above findings indicate that m6A modification is involved in various components of the intestinal mucosal barrier and plays an important role in IBD. Again, hucMSC-Ex repairs IBD by regulating macrophage-related functions. Therefore, we hypothesized that hucMSC-Ex can promote the repair of IBD by regulating m6A modification in macrophages.
Slc37a2 is a member of solute carrier family 37 and composed of four sugar phosphate exchangers A1, A2, A3, and A4, which is mainly responsible for sugar phosphate exchange. Its main function is to maintain the activity of glucose 6-phosphate: inorganic phosphate antitransporter, involved in glucose-6-phosphate transport and phosphate ion transmembrane transport, and is a component of the endoplasmic reticulum membrane. Interestingly, Slc37a2 is shown to exhibit the highest level of transcript abundance in neutrophils and macrophages (Chou et al. 2013), suggesting that it may play an important role in regulating innate immune function. However, Wang and colleagues showed that Slc37a2 acts as an early suppressor to inhibit macrophage inflammatory activation and promote the resolution of inflammation during acute inflammatory activation by down-regulating glycolytic reprogramming (Wang et al. 2020b), confirming its molecular mechanism in regulating macrophage inflammatory response. Unfortunately, there are very limited studies on this molecule in macrophages as well as in IBD, and there is no direct evidence on what role Slc37a2 plays in IBD.
In our study, we found for the first time that the expression of Slc37a2 is decreased in IBD, which may be related to the decreased m6A modification level. hucMSC-Ex upregulates the m6A modification levels of Slc37a2 through the METTL3-Slc37a2-YTHDF1 axis and enhances their mRNA stability to increase expression in macrophages, thereby alleviating IBD (Fig. 8F). The loss of METTL3 leads to the down-regulation of Slc37a2 expression and affects its downstream inflammatory response. The findings of the present study suggest that the new m6A regulatory mechanisms in inflammation mitigation. However, our study has not yet found what substances in hucMSC-Ex act on METTL3 and SLC37A2, which is the limitation of this study and needs further research in the future. It is still unclear how Slc37a2 interacts with the related inflammatory pathway, and the downstream molecules of inflammation involved in this process still need to be further explored.
Conclusion
In our study, hucMSC-Ex exerted anti-inflammatory effects by targeting METTL3 and up-regulating m6A modification to polarize macrophages to the M2 type. On this basis, MeRIP-Seq technology was used to screen and verify the target molecule Slc37a2 regulated by hucMSC-Ex. HucMSC-Ex enhanced the expression of Slc37a2 in macrophages to alleviate inflammatory response by affecting its mRNA stability. In conclusion, this study elucidated the molecular mechanism by which hucMSC-Ex regulates the METTL3-Slc37a2-YTHDF1 axis in macrophages to alleviate IBD, suggesting that hucMSC-Ex may be a promising therapeutic agent for IBD.
Materials and methods
Ethics approval and consent to participate
The study was approved by the Ethical Committee of Jiangsu University (2012258). Animal experiments were carried out following the U.K. Animals (Scientific Procedures) Act, 1986, and associated guidelines.
Cell culture
HucMSCs were obtained from newborn umbilical cords after securing ethical approval and maternal consent and identified following the previous method (Qiao et al. 2008; Kestendjieva et al. 2008). HucMSCs were cultured in α-MEM medium (Invitrogen, Grand Island, NY, USA). Mouse mononuclear macrophage leukemia cells RAW264.7 were purchased from Beiner Biotechnology Company (Beijing, China) and cultured in DMEM high glucose medium (Invitrogen) containing 10% fetal calf serum (FBS; Excell, Uruguay). Human myeloid leukemia mononuclear cells THP-1 were cultured in RPMI 1640 medium (Invitrogen) containing 10% FBS, which was also purchased from Beiner Biotechnology Company. All the above cells were cultured at 37 °C in humid air with 5% CO2. Cell morphology was observed using a general light microscope (Nikon, Japan).
Extraction and identification of exosomes
Exosomes were extracted by ultra-centrifugation method as previously described (Yuan et al. 2021; Yang et al. 2021). hucMSCs surface markers CD34, CD45, CD11b, CD73, CD105, and CD29 were identified by using the FACS Calibur (Beckman Coulter, USA). A transmission electron microscope (Philips, Amsterdam, The Netherlands) was used to observe the morphology of exosomes. Nanoparticle Tracking Analysis (NTA) was used to analyze the size distribution and concentration of exosomes by ZetaView (Malvern Panalytical, Malvern, UK). In addition, Western-blot was used to detect the expression of marker proteins Calnexin, CD9, CD81, HSP70, and Alix.
In vitro experiment
An in vitro experiment was conducted with LPS (Sigma Aldrich, USA), stimulating the inflammation of RAW264.7 and THP-1 cells. THP-1 cells were induced to macrophage-like phenotype by adding 50 ng/ml PMA (Sigma Aldrich) for 16 h before the experiment. The LPS group received only LPS stimulation, whereas, in the hucMSC-Ex group, hucMSC-Ex was added to the culture system following LPS stimulation. The concentrations of LPS and hucMSC-Ex used in this experiment were 1 μg/ml and 200 μg/ml, respectively.
In vivo experiment
Male BALB/c mice (20 ± 3 g) were purchased from the Animal Research Center of Jiangsu University (Zhenjiang, China). Mice were randomly allocated into three groups (n = 5/group): the negative control (NEG) group, the DSS-induced colitis (DSS) group, and the hucMSC-Ex-treated colitis (hucMSC-Ex) group. Mice in the NEG group were fed autoclaved water during the experiment, while mice in the DSS and hucMSC-Ex groups were fed autoclaved water containing 3% DSS (MP Biomedicals, USA). A total of 1 mg of hucMSC-Ex was administered by caudal vein to mice in the hucMSC-Ex group on the 3rd, 6th, and 9th day of modeling, while mice in other groups were injected with PBS. All mice were sacrificed when severe hematochezia and weight loss were observed in the DSS group. The disease progression of mice in the different groups was evaluated by weight change, DAI score, colorectal length, and weight ratio. After the mice were sacrificed, the general view of the spleen and colorectum of the mice was observed. The colorectal mucosa and spleen tissues of the mice in each group were separated, and RNA and protein of the tissues were extracted for subsequent experiments.
CCK8
RAW264.7 cells (5000 cells per well) were seeded in a 96-well plate for CCK8 assay. 1 μg/ml LPS and 200 μg/ml hucMSC-Ex were cultured with cells for 12 h. CCK8 reagent (Vazyme, Nanjing, China) was then added to each well and incubated away from light for 30 min. Absorbance was measured at 450 nm with a microplate reader (Thermo Fisher Scientific).
RNA extraction and qRT-PCR
RNA from the tissues and cells was obtained via TRIzol reagent (Invitrogen, USA) and chloroform extractions, followed by reverse transcription using HiScript® III 1st Strand cDNA Synthesis Kit (+ gDNA wiper) (Vazyme). qRT-PCR was performed using AceQ® Universal SYBR qPCR Master Mix (Vazyme). mRNA expression was normalized to the internal control β-actin. The qRT-PCR primers are shown in Table 1.
Western-blot
Cell lysates were obtained using RIPA lysate (Thermo Fisher Scientific, MA, USA). Protein concentration was determined using the BCA protein detection kit (Vazyme). The sample (20 μg protein) was separated by 10% SDS-PAGE, then transferred to a PVDF membrane (Millipore, Billerica, USA), and incubated in a closed buffer solution (5% skim milk powder dissolved in Tris-buffered brine (TBS) with 0.1% Tween20) for 1 h. The membrane was then treated at 4 °C overnight with anti-Calnexin (1:500; Affinity, China), anti-CD9 (1:500; Affinity, China), anti-CD81 (1:1000; abcam, UK), anti-HSP70 (1:500; Affinity, China), anti-Alix (1:500; Affinity, China), anti-METTL3 (1:1000; abcam, UK), anti-YTHDF1 (1:1000; proteintech, China), Anti-PCNA (1:1000; abcam, UK), anti-Occludin(1:500; proteintech, China), anti-Claudin-1 (1:1000; proteintech, China), anti-SLC37A2 (1:500; proteintech, China) and β-actin (1:8000; Abclonal, Boston, MA, USA) antibodies. Secondary incubation of the membrane was performed using goats against rabbits/mice (1:10000; proteintech, China) at room temperature for 1 h. A chemical gel imaging system (GE Healthcare Life Sciences China, Beijing, China) was used to visualize protein bands and generate images.
Nitric Oxide (NO) measurement
The supernatant of cultured RAW264.7 cells was collected and centrifuged at 600 g for 5 min to remove dead cells for subsequent detection. According to the instructions provided by the manufacturer (Beyotime, China), NO production in cells was measured using the Griess method.
Immunofluorescence (IF)
RAW264.7 cells were fixed with 4% paraformaldehyde for 20 min, followed by 0.1% Triton X-100 for 20 min to break the membrane, then 5% bovine serum albumin (BSA) for blocking non-specific binding. The target antibodies were diluted (CD86, 1:300, Novus; CD206, 1: 200, CST; METTL3, 1:1000, Abcam; YTHDF1, 1:1000, Proteintech), and incubated with cells at 4℃ overnight, followed by the corresponding fluorescent secondary antibodies (FITC and Carolight594,1:200, Proteintech) at room temperature for 2 h. The nuclei were stained with DAPI (1:500, Sigma) at room temperature for 10 min away from light. The fluorescence was observed and photographed with a laser confocal microscope (Nikon, Japan).
Flow cytometry
RAW264.7 cells were collected into sterile EP tubes, centrifuged at room temperature at 500 g for 5 min, then the cell precipitates were re-suspended in 100 μl PBS. First, 0.5 μl CD86 antibody (Novus) was added to each tube and incubated at 4℃ for 30 min away from light. Then, 100 μl PBS and 100 μl IC Fixation Buffer (Thermo Fisher) were added, respectively. After incubating at 4℃ for 20 min away from light, the cells were suspended in 40 μl washing buffer, then 1 μl CD206 antibody (Biolegend) was added to each tube and incubated at 4℃ for 45 min-1 h. Finally, the cells were centrifuged and suspended in 200 μl PBS in each tube for immediate detection.
Immunohistochemical staining (IHC)
Paraffin-embedded mouse colorectal tissues were exposed to 3% hydrogen peroxide at room temperature for 30 min after dewaxing and then steamed in a citric acid buffer for 30 min to repair antigens. Non-specific antigens were blocked by covering the tissues with a 5% bovine serum albumin (BSA) solution. The primary antibody (METTL3, 1:1000, Abcam; YTHDF1, 1:1000, Proteintech; Slc37a2, 1:300, Proteintech) was incubated overnight at 4℃, followed by the goat and mouse secondary antibody (Bost Biological, Wuhan)) at 37 °C for 30 min. Then Strept Avidin Biotin Complex (SABC) was added and incubated at 37 °C for 30 min. Finally, 3,3′-Diaminobenzidine (DAB) substrate was applied to the sections and then re-stained with hematoxylin for observation.
m6A content analysis
As mentioned above, total RNA was extracted using TRIzol lysate (Invitrogen). RNA quality was assessed by NanoDrop (Thermo Fisher Scientific, USA). The m6A modification level of total RNA was examined via EpiQuik m6A RNA methylation quantitative kit (Epigentek Group Inc., USA). In simple terms, the assay well was coated with 200 ng RNA and an m6A standard, followed by the application of antibody solution and antibody detection solution. The m6A level was quantified through colorimetry by reading the absorbance of each well at 450 nm.
Methylated RNA immunoprecipitation sequencing (MeRIP-Seq)
The m6A-IP-Seq service was provided by CloudSeq Inc. (Shanghai, China). Total RNA was subjected to immunoprecipitation with the GenSeq® m6A-IP Kit (GenSeq Inc.) by following the manufacturer’s instructions. Briefly, RNA was randomly fragmented to ~ 200 nt by RNA Fragmentation Reagents. Protein A/G beads were coupled to the m6A antibody by rotating the sample at room temperature for 1 h. The RNA fragments were incubated with the bead-linked antibodies and rotated at 4 °C for 4 h. After incubation, the RNA/antibody complexes were washed several times, and then, the captured RNA was eluted from the complexes and purified. RNA libraries for IP and input samples were then constructed with GenSeq® Low Input Whole RNA Library Prep Kit (GenSeq, Inc.) by following the manufacturer’s instructions. Libraries were qualified using an Agilent 2100 bioanalyzer and then sequenced in a NovaSeq platform (Illumina).
mRNA stability measurement
The transcription of RAW264.7 cells was blocked by adding Actinomycin D with a concentration of 5 μg/ml into the cell well and incubating for 0 h, 2 h, and 4 h, respectively. Cells were collected, RNA extracted, and mRNA levels measured by qRT-PCR.
MeRIP
The m6A modification of Slc37a2 was determined using the Magna EpiQuik CUT&RUN m6A RNA Enrichment (MeRIP) kit (Epigentek, USA) according to the manufacturer’s instructions. In brief, 10 μg total RNA was taken for m6A immunoprecipitation, and 1/10 was retained as the input control group. First, an immune capture solution containing m6A antibody, non-immune IgG, affinity beads, immune capture buffer, and RNA samples was prepared and swirled at room temperature for 90 min to immunologically capture m6A RNA. The RNA was then cleaved using a lyase mixture, and the m6A-containing RNA was purified by adding protease K and RNA purification solution to the immunoprecipitation complex to remove excess proteins. Finally, the immunoprecipitated m6A RNA was recovered with an elution buffer and its level was detected by qRT-PCR.
RNA immunoprecipitation (RIP)
RNA immunoprecipitation kit (Geneseed, Guangzhou, China) was used to detect the combination of Scl37a2 and YTHDF1 according to the manufacturer’s instructions. Briefly, 3 × 107 RAW264.7 cells were collected, cleaved with RIP lysis buffer, and incubated with magnetic beads conjugated with antibodies against IgG (proteintech, China) or YTHDF1(proteintech, China). The coprecipitated RNA was detected by qRT-PCR.
Dual-luciferase reporter assay
The luciferase reporter vectors of Slc37a2 3’UTR m6A modification sites were constructed, and gene fragments carrying mutant or wild type of Slc37a2 m6A modification sites were cloned into pGL3 alkaline vector (Fenghbio, China). It was co-transfected into RAW264.7 cells with METTL3 overexpressed vector or simulated vector. Cells were harvested 48 h later and firefly and Renera luciferase activity were measured using the Dual-Luciferase Reporting and Detection System Kit (Vazyme).
Cell transfection
si-RNA specifically targeting METTL3 (si-METTL3) and the corresponding non-targeting control siRNA (si-NC) were purchased from Genepharma, China. Transfections of siRNAs were performed using Lipofectamine 2000 (Invitrogen) following the manufacturer’s instructions. The si-METTL3 sequence is shown in the table below (Table 2).
Statistical analysis
All data were shown as mean ± standard deviation (SD). Each experiment was repeated three times. Statistical analysis was performed using GraphPad Prism software (GraphPad Software, San Diego, CA, USA). Comparisons between multiple groups were assessed by student t-test or one-way ANOVA with the Bonferroni post hoc test. P < 0.05 was considered significant.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- hucMSC-Ex:
-
Human umbilical cord mesenchymal stem cell-derived exosome
- IBD:
-
Inflammatory bowel disease
- m6A:
-
N6-methyladenosine
- DSS:
-
Dextran sulfate sodium salt
- LPS:
-
Lipopolysaccharide
- MeRIP-Seq:
-
Methylation RNA immunoprecipitation sequencing
- UC:
-
Ulcerative colitis
- CD:
-
Crohn’s disease
- METTL3:
-
Methyltransferase like 3
- METTL14:
-
Methyltransferase like 14
- WTAP:
-
Wilms tumor 1-associated protein
- ALKBH5:
-
AlkB homolog 5
- hnRNP:
-
Heterogeneous nuclear ribonucleoprotein
- eIF:
-
Eukaryotic initiation factor
- ISCs:
-
Intestinal stem cells
- IGF2BP2:
-
Insulin-like growth factor 2 messenger RNA-binding protein 2
- PMA:
-
Phorbol 12-myristate 13-acetate
- MSC-Ex:
-
MSC-derived exosomes
- Slc37a2:
-
Solute Carrier Family 37 Member 2
References
Aghajani Nargesi A, Lerman LO, Eirin A. Mesenchymal stem cell-derived extracellular vesicles for kidney repair: current status and looming challenges. Stem Cell Res Ther. 2017;8(1):273. https://doi.org/10.1186/s13287-017-0727-7.
Ananthakrishnan AN, Bernstein CN, Iliopoulos D, Macpherson A, Neurath MF, Ali RAR, et al. Environmental triggers in IBD: a review of progress and evidence. Nat Rev Gastroenterol Hepatol. 2018;15(1):39–49. https://doi.org/10.1038/nrgastro.2017.136.
Arab HH, Al-Shorbagy MY, Saad MA. Activation of autophagy and suppression of apoptosis by dapagliflozin attenuates experimental inflammatory bowel disease in rats: targeting AMPK/mTOR, HMGB1/RAGE and Nrf2/HO-1 pathways. Chem Biol Interact. 2021;335:109368. https://doi.org/10.1016/j.cbi.2021.109368.
Baghaei K, Tokhanbigli S, Asadzadeh H, Nmaki S, Reza Zali M, Hashemi SM. Exosomes as a novel cell-free therapeutic approach in gastrointestinal diseases. J Cell Physiol. 2019;234(7):9910–26. https://doi.org/10.1002/jcp.27934.
Bain CC, Mowat AM. Macrophages in intestinal homeostasis and inflammation. Immunol Rev. 2014;260(1):102–17. https://doi.org/10.1111/imr.12192.
Cai X, Zhang ZY, Yuan JT, Ocansey DKW, Tu Q, Zhang X, et al. hucMSC-derived exosomes attenuate colitis by regulating macrophage pyroptosis via the miR-378a-5p/NLRP3 axis. Stem Cell Res Ther. 2021;12(1):416. https://doi.org/10.1186/s13287-021-02492-6.
Cao G, Li HB, Yin Z, Flavell RA. Recent advances in dynamic m6A RNA modification. Open Biol. 2016;6(4):160003. https://doi.org/10.1098/rsob.160003.
Chang G, Shi L, Ye Y, Shi H, Zeng L, Tiwary S, et al. YTHDF3 induces the translation of m(6)A-enriched gene transcripts to promote breast cancer brain metastasis. Cancer Cell. 2020;38(6):857-871.e7. https://doi.org/10.1016/j.ccell.2020.10.004.
Chou JY, Sik Jun H, Mansfield BC. The SLC37 family of phosphate-linked sugar phosphate antiporters. Mol Aspects Med. 2013;34(2–3):601–11. https://doi.org/10.1016/j.mam.2012.05.010.
Funes SC, Rios M, Escobar-Vera J, Kalergis AM. Implications of macrophage polarization in autoimmunity. Immunology. 2018;154(2):186–95. https://doi.org/10.1111/imm.12910.
Greuter T, Vavricka SR. Extraintestinal manifestations in inflammatory bowel disease - epidemiology, genetics, and pathogenesis. Expert Rev Gastroenterol Hepatol. 2019;13(4):307–17. https://doi.org/10.1080/17474124.2019.1574569.
Han B, Yan S, Wei S, Xiang J, Liu K, Chen Z, et al. YTHDF1-mediated translation amplifies Wnt-driven intestinal stemness. EMBO Rep. 2020;21(4):e49229. https://doi.org/10.15252/embr.201949229.
Jabs S, Biton A, Bécavin C, Nahori MA, Ghozlane A, Pagliuso A, et al. Impact of the gut microbiota on the m(6)A epitranscriptome of mouse cecum and liver. Nat Commun. 2020;11(1):1344. https://doi.org/10.1038/s41467-020-15126-x.
Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y, et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7(12):885–7. https://doi.org/10.1038/nchembio.687.
Kestendjieva S, Kyurkchiev D, Tsvetkova G, Mehandjiev T, Dimitrov A, Nikolov A, et al. Characterization of mesenchymal stem cells isolated from the human umbilical cord. Cell Biol Int. 2008;32(7):724–32. https://doi.org/10.1016/j.cellbi.2008.02.002.
Koelink PJ, Bloemendaal FM, Li B, Westera L, Vogels EWM, van Roest M, et al. Anti-TNF therapy in IBD exerts its therapeutic effect through macrophage IL-10 signalling. Gut. 2020;69(6):1053–63. https://doi.org/10.1136/gutjnl-2019-318264.
Kuenzig ME, Fung SG, Marderfeld L, Mak JWY, Kaplan GG, Ng SC, et al. Twenty-first century trends in the global epidemiology of pediatric-onset inflammatory bowel disease: systematic review. Gastroenterology. 2022;162(4):1147-1159.e4. https://doi.org/10.1053/j.gastro.2021.12.282.
Kuznetsova T, Prange KHM, Glass CK, de Winther MPJ. Transcriptional and epigenetic regulation of macrophages in atherosclerosis. Nat Rev Cardiol. 2020;17(4):216–28. https://doi.org/10.1038/s41569-019-0265-3.
Kwon J, Jo YJ, Namgoong S, Kim NH. Functional roles of hnRNPA2/B1 regulated by METTL3 in mammalian embryonic development. Sci Rep. 2019;9(1):8640. https://doi.org/10.1038/s41598-019-44714-1.
Lenti MV, Mengoli C, Vernero M, Aronico N, Conti L, Borrelli de Andreis F, et al. Preventing infections by encapsulated bacteria through vaccine prophylaxis in inflammatory Bowel disease. Front Immunol. 2020;11:485. https://doi.org/10.3389/fimmu.2020.00485.
Li HB, Tong J, Zhu S, Batista PJ, Duffy EE, Zhao J, et al. m(6)A mRNA methylation controls T cell homeostasis by targeting the IL-7/STAT5/SOCS pathways. Nature. 2017;548(7667):338–42. https://doi.org/10.1038/nature23450.
Lu TX, Zheng Z, Zhang L, Sun HL, Bissonnette M, Huang H, et al. A new model of spontaneous colitis in mice induced by deletion of an RNA m(6)A methyltransferase component METTL14 in T cells. Cell Mol Gastroenterol Hepatol. 2020;10(4):747–61. https://doi.org/10.1016/j.jcmgh.2020.07.001.
Mao Y, Jiang F, Xu XJ, Zhou LB, Jin R, Zhuang LL, et al. Inhibition of IGF2BP1 attenuates renal injury and inflammation by alleviating m6A modifications and E2F1/MIF pathway. Int J Biol Sci. 2023;19(2):593–609. https://doi.org/10.7150/ijbs.78348.
Na YR, Stakenborg M, Seok SH, Matteoli G. Macrophages in intestinal inflammation and resolution: a potential therapeutic target in IBD. Nat Rev Gastroenterol Hepatol. 2019;16(9):531–43. https://doi.org/10.1038/s41575-019-0172-4.
Ng SC, Kaplan GG, Tang W, Banerjee R, Adigopula B, Underwood FE, et al. Population density and risk of inflammatory bowel disease: a prospective population-based study in 13 countries or regions in Asia-Pacific. Am J Gastroenterol. 2019;114(1):107–15. https://doi.org/10.1038/s41395-018-0233-2.
Ni J, Wu GD, Albenberg L, Tomov VT. Gut microbiota and IBD: causation or correlation? Nat Rev Gastroenterol Hepatol. 2017;14(10):573–84. https://doi.org/10.1038/nrgastro.2017.88.
Nie K, Yi J, Yang Y, Deng M, Yang Y, Wang T, et al. A broad m6A modification landscape in inflammatory Bowel disease. Front Cell Dev Biol. 2021;9:782636. https://doi.org/10.3389/fcell.2021.782636.
Noviello D, Mager R, Roda G, Borroni RG, Fiorino G, Vetrano S. The IL23-IL17 immune axis in the treatment of ulcerative colitis: successes, defeats, and ongoing challenges. Front Immunol. 2021;12:611256. https://doi.org/10.3389/fimmu.2021.611256.
Ocansey DKW, Zhang L, Wang Y, Yan Y, Qian H, Zhang X, et al. Exosome-mediated effects and applications in inflammatory bowel disease. Biol Rev Camb Philos Soc. 2020;95(5):1287–307. https://doi.org/10.1111/brv.12608.
Olazagoitia-Garmendia A, Zhang L, Mera P, Godbout JK, Sebastian-DelaCruz M, Garcia-Santisteban I, et al. Gluten-induced RNA methylation changes regulate intestinal inflammation via allele-specific XPO1 translation in epithelial cells. Gut. 2022;71(1):68–76. https://doi.org/10.1136/gutjnl-2020-322566.
Qiao C, Xu W, Zhu W, Hu J, Qian H, Yin Q, et al. Human mesenchymal stem cells isolated from the umbilical cord. Cell Biol Int. 2008;32(1):8–15. https://doi.org/10.1016/j.cellbi.2007.08.002.
Salas A, Hernandez-Rocha C, Duijvestein M, Faubion W, McGovern D, Vermeire S, et al. JAK-STAT pathway targeting for the treatment of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020;17(6):323–37. https://doi.org/10.1038/s41575-020-0273-0.
Seyedizade SS, Afshari K, Bayat S, Rahmani F, Momtaz S, Rezaei N, et al. Current status of M1 and M2 macrophages pathway as drug targets for inflammatory Bowel disease. Arch Immunol Ther Exp (Warsz). 2020;68(2):10. https://doi.org/10.1007/s00005-020-00576-4.
Shi H, Wang X, Lu Z, Zhao BS, Ma H, Hsu PJ, et al. YTHDF3 facilitates translation and decay of N(6)-methyladenosine-modified RNA. Cell Res. 2017;27(3):315–28. https://doi.org/10.1038/cr.2017.15.
Shi Y, Fan S, Wu M, Zuo Z, Li X, Jiang L, et al. YTHDF1 links hypoxia adaptation and non-small cell lung cancer progression. Nat Commun. 2019;10(1):4892. https://doi.org/10.1038/s41467-019-12801-6.
Shu B, Zhou YX, Li H, Zhang RZ, He C, Yang X. The METTL3/MALAT1/PTBP1/USP8/TAK1 axis promotes pyroptosis and M1 polarization of macrophages and contributes to liver fibrosis. Cell Death Discov. 2021;7(1):368. https://doi.org/10.1038/s41420-021-00756-x.
Song JY, Kang HJ, Hong JS, Kim CJ, Shim JY, Lee CW, et al. Umbilical cord-derived mesenchymal stem cell extracts reduce colitis in mice by re-polarizing intestinal macrophages. Sci Rep. 2017;7(1):9412. https://doi.org/10.1038/s41598-017-09827-5.
Sun Z, Chen W, Wang Z, Wang S, Zan J, Zheng L, et al. Matr3 reshapes m6A modification complex to alleviate macrophage inflammation during atherosclerosis. Clin Immunol. 2022;245:109176. https://doi.org/10.1016/j.clim.2022.109176.
Tan Y, Huang Y, Mei R, Mao F, Yang D, Liu J, et al. HucMSC-derived exosomes delivered BECN1 induces ferroptosis of hepatic stellate cells via regulating the xCT/GPX4 axis. Cell Death Dis. 2022;13(4):319. https://doi.org/10.1038/s41419-022-04764-2.
Tian L, Zhao JL, Kang JQ, Guo SB, Zhang N, Shang L, et al. Astragaloside IV alleviates the experimental DSS-induced colitis by remodeling macrophage polarization through STAT signaling. Front Immunol. 2021;12:740565. https://doi.org/10.3389/fimmu.2021.740565.
Tokuhira N, Kitagishi Y, Suzuki M, Minami A, Nakanishi A, Ono Y, et al. PI3K/AKT/PTEN pathway as a target for Crohn’s disease therapy (Review). Int J Mol Med. 2015;35(1):10–6. https://doi.org/10.3892/ijmm.2014.1981.
van Poll D, Parekkadan B, Cho CH, Berthiaume F, Nahmias Y, Tilles AW, et al. Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology. 2008;47(5):1634–43. https://doi.org/10.1002/hep.22236.
Wang J, Yan S, Lu H, Wang S, Xu D. METTL3 attenuates LPS-induced inflammatory response in macrophages via NF-κB signaling pathway. Mediators Inflamm. 2019;2019:3120391. https://doi.org/10.1155/2019/3120391.
Wang G, Yuan J, Cai X, Xu Z, Wang J, Ocansey DKW, et al. HucMSC-exosomes carrying miR-326 inhibit neddylation to relieve inflammatory bowel disease in mice. Clin Transl Med. 2020a;10(2):e113. https://doi.org/10.1002/ctm2.113.
Wang Z, Zhao Q, Nie Y, Yu Y, Misra BB, Zabalawi M, et al. Solute carrier family 37 member 2 (SLC37A2) negatively regulates murine macrophage inflammation by controlling glycolysis. iScience. 2020b;23(5):101125. https://doi.org/10.1016/j.isci.2020.101125.
Wang X, Ji Y, Feng P, Liu R, Li G, Zheng J, et al. The m6A reader IGF2BP2 regulates macrophage phenotypic activation and inflammatory diseases by stabilizing TSC1 and PPARγ. Adv Sci (Weinh). 2021;8(13):2100209. https://doi.org/10.1002/advs.202100209.
Wang J, Huang J, Fang L. Inhibition of TLR4 suppresses the inflammatory response in Inflammatory Bowel Disease (IBD) by modulating the PDK1-induced metabolism reprogramming via a m6a-denpendent manner. Comput Math Methods Med. 2022;2022:1335562. https://doi.org/10.1155/2022/1335562.
Wang M, Yang D, Li L, Wu P, Sun Y, Zhang X, et al. A dual role of mesenchymal stem cell derived small extracellular vesicles on TRPC6 protein and mitochondria to promote diabetic wound healing. ACS Nano. 2024;18(6):4871–85. https://doi.org/10.1021/acsnano.3c09814.
Wei Z, Hang S, Wiredu Ocansey DK, Zhang Z, Wang B, Zhang X, et al. Human umbilical cord mesenchymal stem cells derived exosome shuttling mir-129–5p attenuates inflammatory bowel disease by inhibiting ferroptosis. J Nanobiotechnol. 2023;21(1):188. https://doi.org/10.1186/s12951-023-01951-x.
Wu J, Zhao Y, Wang X, Kong L, Johnston LJ, Lu L, et al. Dietary nutrients shape gut microbes and intestinal mucosa via epigenetic modifications. Crit Rev Food Sci Nutr. 2022;62(3):783–97. https://doi.org/10.1080/10408398.2020.1828813.
Xiao W, Adhikari S, Dahal U, Chen YS, Hao YJ, Sun BF, et al. Nuclear m(6)A Reader YTHDC1 Regulates mRNA Splicing. Mol Cell. 2016;61(4):507–19. https://doi.org/10.1016/j.molcel.2016.01.012.
Xu X, Huang J, Ocansey DKW, Xia Y, Zhao Z, Xu Z, et al. The emerging clinical application of m6A RNA modification in inflammatory Bowel disease and its associated colorectal cancer. J Inflamm Res. 2021;14:3289–306. https://doi.org/10.2147/jir.S320449.
Xu Y, Tang X, Fang A, Yan J, Kofiwireduocansey D, Zhang X, et al. HucMSC-Ex carrying miR-203a-3p.2 ameliorates colitis through the suppression of caspase11/4-induced macrophage pyroptosis. Int Immunopharmacol. 2022;110:108925. https://doi.org/10.1016/j.intimp.2022.108925.
Yang R, Liao Y, Wang L, He P, Hu Y, Yuan D, et al. Exosomes derived from M2b macrophages attenuate DSS-induced colitis. Front Immunol. 2019;10:2346. https://doi.org/10.3389/fimmu.2019.02346.
Yang C, Gao C, Liu N, Zhu Y, Zhu X, Su X, et al. The effect of traumatic brain injury on bone healing from a novel exosome centered perspective in a mice model. J Orthop Translat. 2021;30:70–81. https://doi.org/10.1016/j.jot.2021.09.003.
Yang L, Fu J, Han X, Zhang C, Xia L, Zhu R, et al. Hsa_circ_0004287 inhibits macrophage-mediated inflammation in an N(6)-methyladenosine-dependent manner in atopic dermatitis and psoriasis. J Allergy Clin Immunol. 2022;149(6):2021–33. https://doi.org/10.1016/j.jaci.2021.11.024.
Yuan X, Li T, Shi L, Miao J, Guo Y, Chen Y. Human umbilical cord mesenchymal stem cells deliver exogenous miR-26a-5p via exosomes to inhibit nucleus pulposus cell pyroptosis through METTL14/NLRP3. Mol Med. 2021;27(1):91. https://doi.org/10.1186/s10020-021-00355-7.
Zhang H, Shi X, Huang T, Zhao X, Chen W, Gu N, et al. Dynamic landscape and evolution of m6A methylation in human. Nucleic Acids Res. 2020a;48(11):6251–64. https://doi.org/10.1093/nar/gkaa347.
Zhang C, Huang S, Zhuang H, Ruan S, Zhou Z, Huang K, et al. YTHDF2 promotes the liver cancer stem cell phenotype and cancer metastasis by regulating OCT4 expression via m6A RNA methylation. Oncogene. 2020b;39(23):4507–18. https://doi.org/10.1038/s41388-020-1303-7.
Zhang X, Li X, Jia H, An G, Ni J. The m(6)A methyltransferase METTL3 modifies PGC-1α mRNA promoting mitochondrial dysfunction and oxLDL-induced inflammation in monocytes. J Biol Chem. 2021a;297(3):101058. https://doi.org/10.1016/j.jbc.2021.101058.
Zhang M, Yang D, Yu H, Li Q. MicroRNA-49.7 inhibits inflammation in DSS-induced IBD model mice and lipopolysaccharide-induced RAW2647 cells via Wnt/β-catenin pathway. Int Immunopharmacol. 2021b;101(Pt B):108318. https://doi.org/10.1016/j.intimp.2021.108318.
Zhang L, Yuan J, Kofi Wiredu Ocansey D, Lu B, Wan A, Chen X, et al. Exosomes derived from human umbilical cord mesenchymal stem cells regulate lymphangiogenesis via the miR-302d-3p/VEGFR3/AKT axis to ameliorate inflammatory bowel disease. Int Immunopharmacol. 2022a;110:109066. https://doi.org/10.1016/j.intimp.2022.109066.
Zhang T, Ding C, Chen H, Zhao J, Chen Z, Chen B, et al. m(6)A mRNA modification maintains colonic epithelial cell homeostasis via NF-κB-mediated antiapoptotic pathway. Sci Adv. 2022b;8(12):eabl5723. https://doi.org/10.1126/sciadv.abl5723.
Zheng G, Dahl JA, Niu Y, Fedorcsak P, Huang CM, Li CJ, et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. 2013;49(1):18–29. https://doi.org/10.1016/j.molcel.2012.10.015.
Zhou X, Li W, Wang S, Zhang P, Wang Q, Xiao J, et al. YAP aggravates inflammatory bowel disease by regulating M1/M2 macrophage polarization and gut microbial homeostasis. Cell Rep. 2019;27(4):1176-1189.e5. https://doi.org/10.1016/j.celrep.2019.03.028.
Funding
This study was sponsored by the National Natural Science Fund of China (Grant no. 82250410378), 2022 Jiangsu Excellent postdoctoral program (Grant no. 2022ZB634), the Guiding Science and Technology Plan Project for Social Development of Zhenjiang Health Commission (Grant no. FZ2020031 to Y.H.), Zhenjiang key research and development plan (social development) (Grant no. SH2022062, SH2022091 and SH2023050) and Project of Suzhou Science and Technology (Grant no. SKY2022027).
Author information
Authors and Affiliations
Contributions
Conceptualization, M.F.; funding acquisition, D.-K.W.O.; project administration, X.-X.W.; software and visualization, P-J.H. and W.-N.J.; writing—original draft, D.-K.W.O. and Z.X.. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, X., Peng, J., Wang, N. et al. hucMSC-Ex alleviates inflammatory bowel disease in mice by enhancing M2-type macrophage polarization via the METTL3-Slc37a2-YTHDF1 axis. Cell Biol Toxicol 40, 74 (2024). https://doi.org/10.1007/s10565-024-09921-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10565-024-09921-1