Abstract
Bacterial wilt (BW) is caused by Ralstonia solanacearum species complex (RSSC) and can lead to severe losses in a wide range of crops, including many traditional African vegetables (TAV). Given the critical role of TAV in African food security, investigations of BW incidence, distribution, and effective breeding strategies are needed to support public and private TAV breeding programs. In this review, we address key questions related to the diversity of BW pathogens, susceptible TAV hosts, distribution, incidence, breeding strategies, sources of resistance, and gaps in the development of resistant TAV varieties in Africa. We also discuss the potential of multiomics integration to enhance our understanding of the host plant defense system against BW in Solanaceae crops. We curated BW strain databases obtained from several online platforms, representing a total of 948 BW strains. Using a refined database, we highlighted the diversity of RSSC and TAV crops affected by RSSC in different regions of Africa. Out of 29 species documented to be affected by BW in Africa, ten are TAV, including widely consumed TAV such as Amaranths and nightshades. In addition, phylotypes I and III are reported to affect TAV, and the incidence can reach up to 72.4% in farmers’ fields. An overview of the first reports revealed that the disease has become a serious threat to TAV in the past decade. Finally, this review proposes a schematic map of possible avenues for successful breeding of BW-resistant TAV using Gboma eggplant as a case study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacterial wilt (BW) is a soil-borne disease caused by the Ralstonia solanacearum species complex (RSSC) that threatens agricultural productivity and food security worldwide (Janse 2004; Mamphogoro et al. 2020). RSSC pathogens are aerobic nonspore-forming and gram-negative bacteria (Munyaneza and Bizimungu 2022). They can spread through water, farm tools, infected seeds, and previously infested crop residue, leading to whole plant wilting and drying (Uwamahoro et al. 2018). In Africa, the disease was first reported in South Africa and Zimbabwe in the 1920s and has since been observed in various African countries (McClean 1930; Robertson 1998). Approximately 450 plants species worldwide, spanning 54 botanical families, are affected by BW, with up to 100% yield loss for solanaceous crops (Sharma et al. 2021). The broad host range of RSSC underscores its threat to agricultural productivity. Major vegetable crops, such as tomatoes and eggplants, and field crops, such as potatoes, which are crucial for food security and farmer income, are widely affected (N’Guessan et al. 2012; Kurabachew and Ayana 2017; Uwamahoro et al. 2018; Nikuze et al. 2020). Although there are numerous first reports of BW, little is known about its impact on traditional African vegetable (TAV) crops. TAV encompass both indigenous vegetables and introduced crops assimilated into local culinary traditions and have become part of cropping systems or agricultural landscapes (Towns and Shackleton 2018). TAV’s consumption have the potential to significantly impact the promotion of healthy, well-balanced diets while also serving as a source of income for people in Africa (Ochieng et al. 2018; Sogbohossou et al. 2019). Several TAV exhibit varying levels of susceptibility to RSSC in tropical and subtropical regions including Solanum macrocarpon, Amaranthus spp. and Ocimum spp. in Benin, Solanum aethiopicum in Mali, and Solanum nigrum in Kenya. (Sikirou et al. 2015, 2021; Bihon et al. 2020; Mafuta et al. 2022). Disease susceptibility levels depend on plant species and genotype, pathogen phylotype and ecotype, environmental factors, and crop management practices (Wei et al. 2015; Mamphogoro et al. 2020). The development of cultivars resistant to bacterial wilt, either for direct production or for use as rootstocks in grafting, is the most efficient strategy for mitigating the impact of this disease (Huet 2014; Sharma et al. 2021). Fast-tracking the development of resistant TAV varieties requires prior knowledge of the diversity of RSSC strains, their effectors, and access to BW-resistant sources for breeding, either collected from the wild, from genebank collections, or mutagenesis or genetic engineering programs.
There have been tremendous advances in understanding the interactions between RSSC and Solanaceae crops (Jourda et al. 2019) and in tapping into this knowledge to breed resistant varieties (Hanson et al. 2016; Pandiyaraj et al. 2019). However, research on breeding for resistance in traditional African vegetables (TAV) is scarce. It has become imperative to leverage the success of developing bacterial-resistant global vegetable crop varieties to accelerate the development of resistant TAV varieties. The importance of such an endeavour lies in bridging the existing knowledge gaps and promoting resistance breeding for TAV, benefiting farmers and other stakeholders. In this review, we addressed key questions related to the distribution of the BW pathogen, breeding strategies for susceptible TAV hosts, and gaps in developing resistant TAV varieties in Africa based on studies conducted on common Solanaceae crops. This paper provides plant pathologists and breeders with information to speed up the development of BW-resistant TAV cultivars.
Methodology
Three data repository websites (EPPO Global Database accessed on 16/05/2023, GitHub—lowepowerlab/Ralstonia_Global_Diversity: Curated by UC Davis—Global Disease Biology Students accessed on 03/07/2023, GBIF accessed on 13/06/2023) were screened, and occurrence data (countries, regions), susceptible hosts, phylotypes and other related information such as subphylotypes, sequevars, clades, and biovar related to strains from Africa were extracted using Microsoft Excel. We cross-checked the extracted information across the databases and removed all duplicates based on strain names and references. We identified a total of 948 RSSC strains, 19 of which were sequenced by different research groups (Table S1). Among the 19 genome files, we used five genomes from Africa discovered by Sabbagh et al. (2019); these genomes are part of a worldwide database of complete genome sequences of RSSC. The five strains were used to identify common TE3 effectors between three common Solanaceae (tomato, eggplant, and potato) and black nightshade, a TAV crop. All the details on the functionally characterized RSSC T3Es of the five strains that induced susceptibility in tomato, eggplant, potato, and black nightshade are summarized in Table S3. All the analyses and graphs generated in this review were generated in R statistical software version 4.2.2 (R Core Team 2022).
History and progress of the control of BW in Africa
Diversity of the pathogen in Africa
A recent taxonomic revision of the RSSC allowed the classification of the bacterium into different phylotypes, with each phylotype corresponding to specific geographic origins such as phylotype I (Asia), phylotype II (America), phylotype III (Africa), and phylotype IV (Indonesia). In Africa, all these phylotypes (I, II, III, and IV) are present and classified into three species within the RSSC. The RSSC comprises R. solanacearum, which includes all phylotype II strains; Ralstonia pseudosolanacearum, encompassing phylotypes I and III; and Ralstonia syzygi, associated with phylotype IV strains (Safni et al. 2014). Nortje's 2015 study highlighted that historically, the predominant phylotypes in Africa were I and II, encompassing highly virulent IIB1 strains (Waals and Krüger 2020). Overall, the wide distribution and host range of RSSC in Africa pose significant threats to the region's food security and agricultural productivity. West and East Africa stand out as critical regions due to the extensive diversity of isolates in terms of phylotypes and sequevars. Specifically, phylotypes I, II, and III have been documented in West Africa, while all phylotypes have been observed in East Africa.
Historically, taxonomic lineages for interspecific and intraspecific classification of Ralstonia spp. were based only on single-gene 16S rRNA and 16-23S intergenic sequences (Kumar et al. 2013). Although very popular, these methods have failed to accurately differentiate related strains and species in the RSSC complex. As a result, a sequevar system was developed as a phylogeny-based taxonomy for the within-species classification of RSSC. A sequevar, also called a sequence variant, is a highly conserved sequence within a sequenced genomic area (Sharma et al. 2021). The assignment of a sequevar occurs solely when two or more strains exhibit comparable sequences of the endoglucanase (egl) gene (Fegan and Prior 2005). The strains grouped within the same sequevar exhibit less than 1% variation in the nucleotide sequence of a 750 bp region of egl (Carstensen et al. 2017). Currently, 60 sequevars have been described worldwide, including approximately 30 in Africa. However, the threshold for subdividing sequevars lacks phylogenetic justification, and the correspondence between sequevars and ecotypes becomes increasingly tenuous with the growing number of available sequences. It is worth noting that sequevar classification is likely to be replaced by a "Sequence Type" (ST) classification, which aligns better with the practices of epidemiological surveillance in medical or animal microbiology (Guinard 2012).
Based on our compiled database from the literature, we observed a prevalence of sequevar 31 followed by sequevars 18 and 46 in phylotype I (Fig. 1). However, many strains for which the corresponding sequevars have not yet been studied are classified as unknown. Phylotype III consists predominantly of strains with undetermined sequevars not yet identified by sequencing. Phylotype II is categorized into two variants, A and B, with variant A exhibiting a broader range of hosts and variant B being more specific to banana species. Several phylotype II strains are also classified as unknown since any study has not determined their sequevar or has not identified them in the database. This is sometimes due to the study type often limited to the biochemical determination of strains or the strain nomenclature not being sufficiently detailed in the paper. Such information is vital since identifying sequevars enables the subclassification of strains into distinct phylotypes, thereby enhancing their classification accuracy. Moreover, some sequevars are species specific, rendering this knowledge crucial for breeding for resistance to the disease. For instance, Akarapisan et al. (2022) reported that sequevar 30 was pathogenic only to Zingiberaceous plants.
First reports and evolution of the disease
Bacterial wilt disease caused by RSSC was reported for the first time in Africa in 1924 in South Africa and affects the production of potatoes (McClean 1930; Robertson 1998). The disease was subsequently reported in several African countries in the 1930s and 1940s (Busolo-Bulafu 1998). However, there are contradictory reports on which geographical region where the disease was first reported. There are reports that Egypt may have been the first African country where the disease was identified in potato fields (Farag et al. 1999). Conversely, reports from southern Africa indicate that Zimbabwe and South Africa were potential locations for its initial discovery. Later, many reports were published on the disease in Africa, which began by drawing attention to its propagation and the irreversibility of the damage caused even after applying disease control measures. Bacterial wilt caused by RSSC has been documented in several countries in Eastern and Southern Africa (Muthoni et al. 2012). More recently, several investigations were conducted in West Africa, with the first reports from 2009 to 2018 of common Solanaceae crops and later reports of TAV (Table 1). Furthermore, Madagascar was not spared, and the disease was identified in 2009 on potatoes (Ravelomanantsoa et al. 2018).
Currently, few data are available on the propagation of RSSC in Africa. Despite the important role that plant health plays in production and, consequently, in food security, it is a great concern that until today, there are no means to control the spread of the disease in sub-Saharan Africa. However, the incidence of this disease has been well studied in America and Asia (Machmud 1986; Fortnum and Martin 1998), and statistics on losses of important crops at the farm, country, and regional levels have started to be documented (Elphinstone 2005; Liao 2005; Sarkar and Chaudhuri 2016; Jiang et al. 2017). Bacterial wilt caused by RSSC could be classified as an emerging plant disease in Africa. According to Anderson et al. (2004) and Ristaino et al. (2021), emergent plant diseases have (1) increased in incidence, dissemination, or host range (Genin 2010; de Morais et al. 2015); (2) changed pathogenesis (Haywared 1991; Genin 2010); (3) newly evolved (Haywared 1991; Elphinstone 2005); or (4) were discovered or newly recognized (Mansfield et al. 2012).
Distribution, incidence and susceptible crops in Africa
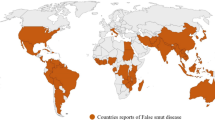
RSSC has been reported to cause bacterial wilt disease in 29 countries across all regions of Africa, with tomato being the most affected, with 35–100% losses depending on the country (Fig. 2, Table S2). In West Africa, pepper is most commonly reported after tomato, with losses ranging from 10–50% in Togo and up to 80% in Mali, but potato and eggplant are more prevalent in East Africa (Table S2). Reports on the incidence of BW on TAV are scarce, with only one reported on Gboma eggplant in southern Benin and one on a nightshade in Kenya (Schippers 2000; Sikirou et al. 2015). Different members of RSSC infect different host plants, with R. solanacearum infecting tomato and potato plants, Ralstonia pseudosolanacearum targeting many Solanaceae crops, and R. syzygi infecting only potato plants (Table 2). To date, 29 host species from 9 families and 9 orders were reported among 948 strains collected and recorded in Africa. Therefore, effective disease management strategies should prioritize horizontal resistance development rather than gene-by-gene-based resistance in crops most affected by various pathotypes of the pathogen. As the pathogen is highly diverse, effective management strategies combining resistant cultivars with sanitation measures and cultural practices that prevent BW infection and spread are crucial for controlling the disease.
Traditional vegetables susceptible to bacterial wilt disease caused by Ralstonia spp. in Africa
An RSSC can cause severe losses in a wide range of TAV. The following traditional vegetables, namely, Amaranthus spp., Bidens pilosa, Ocimum basilicum, Solanum macrocarpon, Solanum nigrum, Solanum scrabrum, and Vernonia amygdalina, have been reported to be infested by Ralstonia spp., causing bacterial wilt disease at various levels of incidence in farmers’ fields (Fig. 3). In addition, 50% of the species affected by phylotype I strains are TAV. The severity of the disease can vary depending on the host plant, with some crops such as amaranth being highly susceptible, with up to 72.4% incidence and an equivalent level of yield loss due to damage to the entire plant (Sikirou et al. 2021), while other wild or cultivated traditional vegetables such as purslane (Portulaca olearacea), whose leaves is consumed as vegetables in Africa (Daunay et al. 1991), is considered hosts. For instance, cowpea serves to contain the spread of wilt from diseased to healthy plants when intercropped with potatoes, whereas purslane is a symptomless host (French 1987). Previous studies revealed BW resistance in several vegetable species, including TAV such as nightshades and African eggplant (Solanum americanum syn Solanum nigrum, Solanum aethiopicum grp. aculeatum, and Solanum scrabrum). However, recent findings provide evidence that these species are increasingly susceptible to BW (Fig. 3). Although many TAV are known for their excellent adaptation to their environment and resilience to biotic and abiotic stresses, the processes of domestication, intensified production and genetic exchange between countries could weaken their resistance to diseases and spread new races of pathogens (MacLeod et al. 2010; Graziosi et al. 2016; Ferrero et al. 2020). The impact of RSSC on TAV can be devastating for smallholder farmers who rely on these crops for their livelihoods. In addition, this disease can lead to the loss of valuable genetic resources and traditional food cultures. Therefore, it is essential to prioritize research on BW on TAV and initiate efforts to breed BW-resistant TAV genotypes.
Breeding strategies for resistance to bacterial wilt disease
Screening methods
Various methods have been adopted to screen genetic resources for bacterial wilt resistance, including field screening/sick plot methods and artificial inoculation methods, such as the soil drenching method, leaf-clipping method, axil-puncturing method, root dipping method, and hydroponically grown seedling inoculation (Hussain et al. 2005; Hacisalihoglu et al. 2009; Artal et al. 2012; Kwon et al. 2021; Maxwell et al. 2022). These methods have strengths and limitations, and their effectiveness is influenced by the specific plant species under investigation. A quick desk review or a pre-test of a selected method and optimization if needed is critical to select the most efficient method for a given crop or circumstance. Isolation, culturing, and inoculation require specialized skills and facilities that may not be available in many research institutions.
Field screening is another effective way to identify BW-resistant lines. A field with a uniform and moderate level of infestation varying between 30 and 50% wilt incidence in the previous cropping season is needed. To account for insufficient inoculum pressure and unevenly infested plots, fields are recommended to be planted with a susceptible crop variety during previous cropping seasons. The infested plants were uniformly spread on the field and buried at harvest to homogenize and enhance soil inoculum levels. For most studies on screening for bacterial resistance in natural fields, a randomized complete block design (RCBD) with at least 3–4 replications is often used for fewer than 30 accessions or entries (Namisy et al. 2019; Zohoungbogbo et al. 2021). Other experimental designs, such as lattice or alpha designs, are recommended when experimental units consist of plots arranged in more than one row or when there are more than 30 entries, including controls (Muthoni et al. 2014; Mathai 2022). In both cases, a minimum of 3–5 plants/plot are acceptable, specifically for potatoes (Mihovilovich et al. 2017), and 10–20 plants/plot are acceptable for other vegetable crops (Sood et al. 2023; Wang et al. 1998a, b). Appropriate experimental design is necessary to avoid escape. Symptomatic plants can be quickly tested in the field using the water test to confirm whether or not the causal agent is RSSC.
Progress in traditional breeding approaches: evaluation and selection, crossbreeding, mutation breeding
A wide range of breeding strategies have been used to develop inbred lines resistant to bacterial diseases, particularly bacterial wilt. These strategies were mainly focused on crops such as tomatoes, eggplants, and potatoes and encompassed many conventional breeding techniques. These techniques involve screening large germplasm collections for bacterial resistance following crossing susceptible and resistant parents to introgress resistance into desired genotypes and subsequent backcrossing to restore the recurrent phenotype.
Traditional breeding approaches have made significant progress in selecting and developing new tomato, pepper, and eggplant varieties with improved resistance to bacterial wilt. The most promising lines or parents tested for BW resistance in West Africa are tomato lines such as the well-known Hawaii7996, which showed consistent results across different environments. Some local landraces from Uganda, Kenya, and the West Indies were tested and suggested as additional sources of resistance to BW (Osiru et al. 2001; Kathimba et al. 2021). Other sources of resistance are conserved at the World Vegetable Center, the University of California Davis, and INRA (Institut National de Recherche Agronomique) of Guadeloupe (Table 3). Segregating populations were obtained from crosses between susceptible and resistant parents to map BW resistance loci such as Bwr-12, Bwr-6a, Bwr-c, and Bwr6-d (Bihon et al. 2022), and markers have been developed to accelerate breeding through marker-assisted selection (Hanson et al. 2016). Several eggplant species (Solanum macrocarpon and Solanum melongena) have been tested in Benin and Côte d'Ivoire and have shown some level of resistance to BW. Other TAV, namely, Solanum scrabrum, Solanum nigrum, and Solanum villosum, have been screened for their resistance to BW in Kenya, and five accessions of Solanum scrabrum were found to be resistant to the disease (Mafuta et al. 2022). Several sources of BW resistance and potentially resistant breeding materials for major vegetable crops, such as peppers, tomatoes, potatoes, and eggplants, evaluated in Africa are summarized in Table 3.
Crossbreeding has contributed to the development of Solanaceae crop inbred lines resistant to BW disease worldwide. The BW-resistant source Hawaii7996 is an S. lycopersicum inbred line with BW resistance from S. pimpinellifolium PI 127805A (Hanson et al. 1998). It is one of the few breeding lines with good resistance to diverse R. solanacearum and R. pseudosolanacearum strains, with the best combining ability in many crosses performed worldwide, and is a parent of most commercial wilt-resistant tomatoes (Scott et al. 2004; Lopes et al. 2022). The BW-12 and BW-6 QTLs used in tomato breeding are derived from H7996 (Wang et al. 2013). Hybridization has also been performed in tomato breeding programs to develop varieties resistant to BW in Africa. For instance, the crosses between MT55, MT74, MT15, and MT164 in Uganda allowed the development of segregating populations, and after a joint regression analysis, they revealed an additive plus dominance model for bacterial wilt resistance with no evidence of epistasis and found two genes controlling resistance. Researchers have conducted successful crossbreeding programs in African eggplant and obtained a commercial F1 hybrid cultivar called Kalenda from a crossing between S. aethopicum and S. melongena, which is resistant to bacterial wilt (BW) and has been introduced in French West Indies (Ano et al. 1991).
Mutation breeding involves inducing variations in coding and/or regulatory gene sequences in plant genomes to create new genetic variations. The limited access to BW-resistant genotypes suggested that mutation breeding is an alternative approach for screening biodiverse accessions to develop resistant genotypes (Jyothi and Santhosha. 2012). O’Herlihy et al. (2010) reported that bacterial wilt resistance in S. lycopersicum mutants improved by 10–80% compared to that in the control. In the same study, however, screening of potato mutant populations for resistance to bacterial wilt using a pathogenicity test was inconclusive. However, variations in response were observed, suggesting the potential effects of mutagen dose rates and variety reactions (Chepkoech et al. 2020). Peiris et al. (2008) successfully developed the bacterial wilt-resistant variety 'M 127' using mutation breeding. However, it is crucial to address issues such as the stability of the selected resistant genotypes in hot and humid climates and their suitability for target agroecological regions.
Progress in molecular and biotechnology approaches
QTLs and molecular markers in global Solanaceae crops
Many studies have focused on the development of molecular markers for marker-assisted breeding to develop varieties resistant to bacterial wilt in Solanaceae crops (Table 4). Wang et al. (2013) identified two key quantitative trait loci (QTLs), namely, Bwr-6 on chromosome 6 and Bwr-12 on chromosome 12, associated with bacterial wilt resistance in the tomato inbred line 'Hawaii7996'. Their findings also showed that Bwr-12 was effective against phylotype I, and Bwr-6 was associated with resistance to race 1-phylotype I and race 3-phylotype II strains. However, the effectiveness of the combination of Bwr-12 and Bwr-6 on a given RSSC strain or on coinfected strains has yet to be elucidated. A trade-off was observed between fruit size and those loci in the 1990s and was later broken in the early 2000s. To further elucidate the underlying mechanisms of Bwr-6 and Brw-12 QTLs, a set of six cleaved amplified polymorphic site (CAPS) markers, along with derived CAPS (dCAPS) markers, were developed within the genomic region associated with resistance. Then, two markers, RsR6-5 on chromosome 6 and RsR12-1 on chromosome 12, were selected based on genotypic and phenotypic analysis to effectively distinguish resistant and susceptible cultivars (Abebe et al. 2020). In addition, Shin et al. (2020) found a total of seven QTLs associated with BW resistance to race 1-phylotype I and/or race 3-phylotype II strains located on chromosomes 6 (Bwr-6.1, 6.2, 6.3, and 6.4) and 12 (Bwr-12.1, Bwr-12.2, and Bwr-12.3). Previous studies have shown cases in which the marker‒trait associations were weak due to the distance between the markers and the resistance genes, reducing their effectiveness for MAS (Perez-de-Castro et al. 2012; Nguyen et al. 2021). A genome-wide association study (GWAS) conducted on a core collection of 191 tomato accessions detected eight markers‒trait associations (MTAs) for bacterial wilt resistance on chromosomes 4 and 12 corresponding to QTLs Bwr-4 and Bwr-12 (Nguyen et al. 2021). Furthermore, multilocation testing confirmed QTLs on chromosomes 1, 4, 6 and 12, with four environment-specific QTLs on chromosomes 1 and 8–10. Previously, the same QTLs were detected in Hawaii7996, with putative QTLs on chromosomes 8 and 10 (Thoquet et al. 1996). Multiple QTLs associated with BW resistance indicate that resistance is polygenic (Thoquet et al. 1996). Barchenger et al. (2022) identified candidate genes contributing to resistance beyond Bwr-6 and Bwr-12. The results revealed a sequence variant previously identified as Bwr3 on chromosome 3 that was captured by the marker Bwr3.2dCAPS, which is located in the Asc (Solyc03g114600.4.1) gene and has a significant association with resistance. However, this finding did not completely explain the resistance phenotype. In pepper, six QTLs were mapped in a double haploid population derived from the cross Capsicum annuum var. Yolo Wonder × Capsicum annuum var. PM687 and a recombinant inbred line (RIL) population from the cross Yolo Wonder × CM334 (Lafortune et al. 2005; Mahbou-Somo-Toukam 2010). This suggests that BW resistance in pepper is also polygenic.
QTLs for bacterial wilt resistance have also been identified in several resistant potato lines. Bacterial wilt resistance genes in potato are quantitative, with additive, nonadditive, or epistatic effects and environment- and pathogen strain-specific responses (Chakrabarti et al. 1994; López and Biosca 2005). The first attempt at marker identification used a combination of seven RAPD primers in which three primers amplified the markers associated with BW resistance (Sallam et al. 2013). Successive QTL analyses were performed and identified five QTLs (qBWR-1 to -5) on chromosomes 1, 3, 7, 10, and 11 using a dense linkage map comprising 4193 SNPs obtained from a bi-parental cross between the resistant diploid potato clone 10-03-30 and the susceptible diploid clone F-1-1 (Habe and Miyatake 2022; Habe et al. 2023, 2019). Subsequently, composite interval mapping was performed using resistant and susceptible parents of the potato species Solanum phureja, Solanum chacoense, and Solanum tuberosum, and five major and five minor resistance QTLs on chromosomes 1, 3, 5, 6, 7, 10, and 11 were identified. The major QTLs (PBWR-3 and PBWR-7) conferred stable resistance against phylotype I and IV strains, whereas PBWR-6b was strain-specific against phylotype I/biovar 3 and was more effective in cold environments. Another study compared the nucleotide sequences of one of the candidate genes located within the QTL region of PBWR-6b between the susceptible and resistant parents used for the previous QTL analysis, resulting in an allele-specific molecular marker (Rbw6-1) for PBWR-6b.
Significant effort has been made to map BW-resistance genes or QTLs in eggplant (Fukuoka et al. 2010; Nunome et al. 1998). Nunome et al. (1998) identified two QTLs associated with BW resistance in eggplant in an intraspecific F2 population of Solanum melongena derived from a cross between the Indian BW-resistant accession WCGR112-8 and the breeding line EPL1. Bulk segregant analysis (BSA) was also used to develop a SCAR marker named Rs-762 (S401, 762 bp), which is linked to a single dominant BW resistance gene from the Chinese accession E31 (Bi-hao et al. 2009). Bulk segregant analysis is widely used to rapidly discover marker‒trait associations for traits conferred by a single gene or a few genes. The primary benefit of using BSA compared to traditional QTL analysis is the reduced workload for genotyping within a segregating population. Instead, the approach involves grouping plants based on extreme phenotypes of a specific trait (e.g., resistance and susceptibility to a disease) and extracting DNA from bulks of resistant and susceptible genotypes. This significantly reduces the workload for genotyping, as only two DNA pools are genotyped.
Genetic engineering, transcriptomics, metabolomics, and effectoromics analysis
Genetic engineering for crop improvement involves the use of gene transfer methods to insert genes of interest into a plant. These methods use vectors and agrobacterium-mediated or other methods to bring the vector into the host plant. A powerful genetic engineering tool is RNA interference (RNAi), which uses genetic transformation to stimulate the host system against specific double-stranded cellular or viral RNAs. This mechanism involves the generation of small noncoding RNAs such as microRNAs (miRNAs) and small interfering RNAs (siRNAs), which interfere with target mRNA translation and repress gene expression (Kiran and Abdin 2022).
AtEFR is a resistance gene of Arabidopsis thaliana that increases resistance to bacterial pathogens (Boschi et al. 2017). Specifically, AtEFR expression in tomato (S. lycopersicum variety Moneymaker) confers resistance to R. solanacearum (Lacombe et al. 2010). Specifically, for BW resistance, Kunwar et al. (2018) conducted field trials to evaluate the resistance of transgenic tomato lines expressing the EFR and Bs2 genes to bacterial wilt. Compared with those in the other lines, the expression of EFR or Bs2/EFR in the lines significantly reduced the incidence of bacterial wilt and increased the total yield under BW pressure. Furthermore, transgenic potato lines expressing AtEFR-Tu exhibited improved resistance to bacterial wilt. The transgenic expression of AtEFR-Tu in the lines had a significant additive effect on the resistance QTLs. As a result, the authors suggested that combining the heterologous expression of AtEFR with quantitative resistance introgressed from wild relatives is a promising strategy for developing bacterial wilt resistance in potato (Bostick et al. 2004).
In contrast, overexpression of the NAC family transcription factor SmNAC reduced BW resistance in transgenic eggplant lines through reduced accumulation of salicylic acid (SA) and reduced expression of ICS1 (encoding isochorismate synthase 1, involved in SA biosynthesis), demonstrating that low SA biosynthesis plays a role in BW resistance (Na et al. 2016). The same results were obtained for transgenic potato lines expressing the AP1, Cecropin B, and Shiva genes (Jia et al. 1993; Xuping et al. 1996).
RNAi technology has also been applied to obtain transgenic lines that exhibit a high degree of resistance to bacterial wilt by targeting the RNA transcript of the PAP2 gene, which is responsible for plant wilting via the accumulation of reactive oxygen species/hypersensitive response (Lavale et al. 2022a). RNAi transgenic lines targeting the PAP2 gene showed reduced PAP2 expression, leading to increased expression of the jasmonic acid marker gene PR-4 and decreased expression of the salicylic acid marker gene PR1, resulting in resistance (Chakrabarti et al. 2019). It is worth noting that these findings are proof that genetic engineering could be used to develop BW-resistant crop varieties, but there have not been any commercialized BW-resistant varieties resulting from this technology. For instance, virus-induced gene silencing (VIGS) has been used to understand the role of the NLRs SlADR1 and SlNRG1, the key nodes of effector-triggered immunity (ETI) pathways, in the resistance of Hawaii7996 to bacterial wilt (Xu et al. 2023). However, to date, no reports on CRISPR approaches for enhancing bacterial resistance in Solanaceae plants have been reported.
Understanding the molecular mechanisms of pathogen-plant interactions through omics is important to design better disease management strategies. To date, several studies, known as in planta transcriptomics, have analyzed the transcriptome of Ralstonia strains in plant tissues during pathogenesis. In planta transcriptomic studies revealed many pathways involved in plant infection, including genes in the scrABY cluster, which are components of the bacterial sucrose uptake and catabolism pathway. The presence of sucrose in tomato xylem fluid and the dependence of R. solanacearum strains on sucrose in infected plants are important factors for virulence. In contrast, lines with low sucrose levels could generate wilt-resistant crops (Jacobs et al. 2013). A detailed investigation revealed that the overexpression of other genes, such as TSRF1, Ferredoxin-I, and Xa21, successfully improved resistance to BW in tomato plants. The transcriptome and metabolome data of eggplants after R. solanacearum infection revealed 2,896 DEGs and 63 metabolite differences. Further analysis revealed that the biosynthesis pathways for phytohormones, phenylpropanoids, and flavonoids were altered after inoculation. The results also revealed seven resistance genes and 24 genes involved in the jasmonic acid signaling pathway in the defense response of eggplant to bacterial wilt (Xiao et al. 2023). Transcriptome analysis of plant roots in response to R. solanacearum in wild potato (S. commersonii) using RNA-seq identified 221 and 644 DEGs in S. commersonii accessions resistant (F118) and susceptible (F97) to the pathogen, respectively. In pepper, gene expression analysis revealed 115 resistance-specific (R-response) and 109 susceptibility-specific (S-response) DEGs in the root system. R-responsive genes were associated with xyloglucan biosynthesis and cell wall organization, while S-responsive genes were related to stress response and cell death. Genes that play vital roles in cell wall restructuring and reinforcement were found to restrict bacterial movement in xylem vessels (Hwang et al. 2011). Dual RNA-seq technology revealed changes in the transcriptomes of the susceptible chili pepper line CM334 and the pathogen R. solanacearum strain Rs-SY1. The hypocotyls of pepper plants showed differentially expressed genes (PDEGs), reflecting the suppression of photosynthesis, the induction of ethylene production, the downregulation of polysaccharide metabolism, and the weakening of cell wall defenses by R. solanacearum for successful infection. Pathogen-specific differentially expressed genes (RDEGs) in Ralstonia were also detected, showing enhanced starch and sucrose metabolism and upregulation of virulence factors (Du et al. 2022).
Recent metabolomics experiments identified 41 metabolites involved in resistance against BW in tomatoes. The metabolites of the phenylpropanoid pathway accumulate rapidly in infected roots of tobacco (Shi et al. 2022), and other metabolites, including flavonoids, hydroxycinnamic acids, putrescine, dopamine, the auxin pathway, and tyramine derivatives, are also found in the root, stem, and leaf tissues of tomato and pepper plants (Lavale et al. 2022b; Zeiss et al. 2019). Further analyses revealed flavonoids in root tissues and hydroxycinnamic acids in leaf and stem tissues as primary metabolites from the phenylpropanoid pathway in tomato defense against bacterial wilt (Zeiss et al. 2019). The metabolic response was also reoriented to the phenylpropanoid pathway and its subbranches in tomato plants infected by bacteria after pretreatment with csp22. It elicited increased resistance to R. solanacearum infection (Zeiss et al. 2022). RSSC infection enriches xylem sap with 22 different metabolites, including putrescine and trehalose, which promote bacterial colonization of plant vessels (French et al. 2018; Lowe-Power 2017; Zeiss et al. 2018, 2019). However, Murti et al. (2021) recently reported that leucine and valine in leaf tissues are significantly distinguishable metabolites between the resistant cultivars Permata and Hawaii7996 and the susceptible cultivar GM2 in tomato. Identifying higher metabolite levels in Solanaceae crops after Ralstonia spp. infection and linking them with resistance resulted in noteworthy findings. These metabolites could create an avenue for developing new varieties with enhanced resistance to bacterial wilt through direct selection of lines with naturally higher levels of these metabolites. These lines could be crossbred with elite cultivars to transfer resistance through marker-assisted selection. However, it is paramount to characterize the identified metabolites and validate their roles and candidate genes because of their accumulation in gene expression studies, metabolic pathway analysis, and virulence validation in controlled and field experiments.
Effectoromics is a specialized omics field in which high-throughput functional screening of germplasm effectors is used to accelerate and improve the exploitation of resistance genes such as nucleotide-binding leucine-rich repeat (NLR) resistance genes (Du and Vleeshouwers 2017). It has contributed to classical resistance breeding and genetically modified approaches and has shown effectiveness; hence, it is referred to as “effector-assisted breeding” (Vleeshouwers and Oliver 2014). Ralstonia spp. pathogen-associated molecular patterns (PAMPs) and effector proteins are secreted into plant cells, where they activate and suppress plant immunity, thereby affecting susceptible plants. Approximately twelve family of effectors plus one family of candidate effectors have been reported to trigger hypersensitive reactions (HRs) in common Solanaceae cultivated crops from Africa’s strains (Table S3). The effector proteins of RSSC are under strong and diverse positive selection and contribute to bacterial pathogenicity and dampening plant defense responses (Peeters et al. 2013b; Deslandes and Genin 2014). The accessibility and advances of high-throughput sequencing technologies have allowed the sequencing of eleven RSSC strains spanning four existing phylotypes and enabled the identification of the bacterial effector repertoire (Peeters et al. 2013a, b). These core effectors are presumed to be ancestral and pivotal for interactions between R. solanacearum and its hosts. Thus, effector-assisted breeding has also been instrumental in evaluating the potential of new resistance genes for broad and long-lasting protection. This involves testing the ability of resistance genes to recognize different effector variants within a pathogen population (Vleeshouwers and Oliver 2014). Recent effectoromics studies investigated the T3E repertoires of twelve plants pathogenic Ralstonia strains, representing 12 strains spread throughout all the phylotypes of the RSSC complex. This generated a pangenome repertoire to construct a comprehensive database of type III effectors (T3Es) and identified new allelic versions of specific T3Es (102 T3Es and 16 hypothetical T3Es) (Sabbagh et al. 2019). Considering the considerable genetic diversity within Ralstonia spp., optimizing effectoromics could involve focusing on bacterial strains and plant germplasms coevolving in the same geographic region. Although the aforementioned studies tried to connect T3E diversity to the host specificity of RSSC strains and could identify some host specificity determinants, the power of such studies has usually been largely limited by the lack of exhaustive strain host range empirical data (Landry et al. 2020). In this review, we also compared T3Es among four isolated hosts (tomato, eggplant, potato, and black nightshade), as shown in Fig. 4, using curated African-sequenced strains from the worldwide database of complete genome sequences of Ralstonia spp. (Sabbagh et al. 2019). Ninety-four genes (86 T3Es and eight putative T3Es) were associated with virulence (Table S3), mainly in eggplant (83 genes), tomato (68 genes), black nightshade (66 genes), and potato (39 genes).
Venn diagram depicting conserved T3Es among different phylotypes of five sequenced African strains (Burkina-Faso, Cote d’Ivoire, Cameroon and Egypt) and different Solanaceae hosts from which they were isolated; Comparison of conserved T3Es between phylotypes I, III and IIB (right); Comparison of conserved T3Es between black nightshade and other widely cultivated Solanaceae crops (left). (Color figure online)
Lessons learned from BW resistance research on major solanaceous crops and perspectives for improving resistance of traditional vegetables to bacterial wilt: a case study of Solanum macrocarpon
The BW-borne crop system is intricate, but several observations and implications arise from studies conducted on Solanaceae crops and are conclusive for breeding BW-TAV-resistant varieties. Successful breeding for BW resistance in TAV might rely on the following points:
Germplasm and biodiversity
Germplasm conservation and increased access to biodiversity, including mostly crop wild relatives, have proven their fundamental importance in enhancing bacterial wilt resistance in solanaceous crops through screening and crossbreeding. For instance, access to wild relatives was important for finding resistant sources in tomato (S. pimpinellifolium PI 127805A), and several wild eggplant species have exhibited consistent and reliable resistance to BW. Moreover, several accessions of Solanum macrocarpon and Solanum aethiopicum, two largely consumed traditional vegetables in West and East Africa, have been tested under different combinations of scion and rootstock eggplant, and two of these accessions were reported to be resistant to bacterial wilt caused by RSSC (Musa et al. 2021).
The information gathered from TAV collections is provided through platforms such as Genesys (https://www.genesys-pgr.org/welcome), an international resource providing information on genebank collections worldwide. The World Vegetable Center is a key institution for the conservation of vegetable germplasm and holds in trust the largest public sector vegetable collection of approximately 61,000 active accessions (https://genebank.worldveg.org/). For instance, more than 300 accessions of Solanum macrocarpon from Africa are available at the World Vegetable Center and could be exploited to conduct a systematic screening with known RSSC strains under controlled conditions to reveal BW-resistant material for research and breeding.
Understanding pathogen diversity
Insight into pathogen diversity is essential for accessing the right RSSC strains for resistance screening. A better understanding of the diversity and distribution of BW would help identify crops at risk, identify disease hotspots, and determine the most virulent strains. Evaluations of pathogens should include an assessment of their biochemical and molecular characteristics, host range, and pathogenicity under various environmental conditions.
Germplasm screening
Efficient screening of core collections could be achieved through inoculation under controlled conditions with known RSSC pathotypes using fast-tracking screening methods to identify narrow and broad resistance and enable pyramiding of R genes. A variety of screening methods have been developed for vegetables worldwide, and these methods can be used or refined, if necessary, for screening large germplasm collections. The collection of germplasms from regions where the disease is prevalent and their characterization can help to identify new sources of resistance. There is currently scarce information on BW resistance screening of TAV, although some sources of tolerance have been identified for Solanum macrocarpon and Solanum nigrum complex (Oussou et al. 2020; Mafuta 2022). Some TAV could also benefit as rootstocks for bacterial wilt resistance in common crops such as eggplant and tomato.
R gene identification and integration of omics and gene engineering
The collection of BW-resistant lines after germplasm screening can further reveal which R gene(s) are needed for our varieties based on the strains highly infecting the region. At this stage, prebreeding and backcrossing or gene pyramiding are essential activities for the creation of high-yielding BW-resistant varieties. Hence, the use of omics and gene engineering can play an intermediary role in BW-breeding of TAV. Although limited literature is available, since 2015, approximately eight TAV have been fully sequenced, and their draft genomes are available (Kamenya et al. 2021), facilitating the development of a dense genome-wide molecular marker set. Moreover, other TAV, including A. blitum, A. cruentus, A. tricolor, Basella alba, Brassica carinata, Celosia argentea, Corchorus olitorius, Crassocephalum rubens, Moringa oleifera, Solanum scrabrum, and Talinum fruticosum, are on the agenda of the African Orphan Crops Consortium (AOOC) (Sogbohossou 2019).
Global market-oriented breeding
TAV usually have a localized market, but through value addition, their market can be considerably expanded. Breeding competitive TAV varieties requires an in-depth understanding of current and future clients’ (seed producers, farmers, processors, consumers) needs. This information is essential for defining market segments and target product profiles to prioritize investments and guide decision-making in breeding programs. This approach is routinely used to develop global vegetable varieties. Market segmentation, along with target product profiles, can be used to attract funding to TAV breeding programs, which are currently underfunded.
The prospects of bacterial wilt resistance breeding in TAV are promising, as evidenced by the success achieved in developing resistant cultivars using traditional breeding methods and molecular tools on significant vegetable crops. However, some challenges are still associated with the use of molecular breeding techniques, such as limited genomic resources, the complex genetic architecture of bacterial wilt resistance, and a lack of understanding of host‒interactions. To elaborate a comprehensive action plan that integrates conventional breeding strategies and quantitative genomics breeding for resistance to bacterial wilt in TAV, we used Gboma eggplant (Solanum macrocarpon) as a case study. Details of the action plan are illustrated in Fig. 5.
Data availability
No datasets were generated or analysed during the current study.
References
Abebe AM, Choi J, Kim Y, Oh C-S, Yeam I, Nou I-S, Lee JM (2020) Development of diagnostic molecular markers for marker-assisted breeding against bacterial wilt in tomato. Breed Sci 70:462–473
Akarapisan A, Kumvinit A, Nontaswatsri C, Puangkrit T, Kositratana W (2022) Phylotype sequevar and pathogenicity of Ralstonia solanaceaum species complex from Northern Thailand. J Phytopathol 170:176–184
Anderson PK, Cunningham AA, Patel NG, Morales FJ, Epstein PR, Daszak P (2004) Emerging infectious diseases of plants: pathogen pollution climate change and agrotechnology drivers. Trends Ecol Evol 19:535–544
Ano G, Hebert Y, Prior P, Messiaen CM (1991) A new source of resistance to bacterial wilt of eggplants obtained from a cross: Solanum aethiopicum L.× Solanum melongena L. Agronomie 11:555–560
Artal RB, Gopalakrishnan C, Thippeswamy B (2012) An efficient inoculation method to screen tomato brinjal and chilli entries for bacterial wilt resistance. Pest Manag Hortic Ecosyst 18:70–73
Barchenger DW, Hsu Y, Ou J, Lin Y, Lin Y, Balendres MAO, Hsu Y, Schafleitner R, Hanson P (2022) Whole genome resequencing and complementation tests reveal candidate loci contributing to bacterial wilt (Ralstonia sp) resistance in tomato. Sci Rep 12:8374. https://doi.org/10.1038/s41598-022-12326-x
Bi-hao C, Jian-jun L, Yong W, Guo-ju C (2009) Inheritance and identification of SCAR marker linked to bacterial wilt-resistance in eggplant. Afr J Biotechnol 8:5201–5207
Bihon W, Chen J-R, Kenyon L (2020) Identification and characterization of Ralstonia spp causing bacterial wilt disease of vegetables in Mali. J Plant Pathol 102:1029–1039. https://doi.org/10.1007/s42161-020-00631-1
Bihon W, Ognakossan KE, Tignegre J-B, Hanson P, Ndiaye K, Srinivasan R (2022) Evaluation of different tomato (Solanum lycopersicum L.) entries and varieties for performance and adaptation in Mali West Africa. Horticulturae 8:579. https://doi.org/10.3390/horticulturae8070579
Boakye-Mensah IN (2020) Evaluation of tomato (Solanum lycopersicum L.) genotypes for bacterial wilt (Ralstonia solanacearum) resistance in Ghana. In: Dissertation, University of Cape Coast
Boschi F, Schvartzman C, Murchio S, Ferreira V, Siri MI, Galván GA, Matthew S, Stranselfd L, Zipfel C, Vilaró FL, Dalla-Rizza M (2017) Enhanced bacterial wilt resistance in potato through expression of Arabidopsis EFR and introgression of quantitative resistance from Solanum commersonii. Front Plant Sci 8:1642
Bostick M, Lochhead SR, Honda A, Palmer S, Callis J (2004) Related to ubiquitin 1 and 2 are redundant and essential and regulate vegetative growth auxin signaling and ethylene production in Arabidopsis. Plant Cell 16:2418–2432
Busolo-Bulafu CM (1998) Resistance to bacterial wilt in Uganda Bact Wilt Dis. Mol Ecol Asp 306–308
Bouriquet G (1946) The diseases of cultivated plants in Madagascar. Encycl Mycol 12:545
Carstensen GD, Venter SN, Wingfield MJ, Coutinho TA (2017) Two Ralstonia species associated with bacterial wilt of Eucalyptus. Plant Pathol 66:393–403
Chae S-Y, Lee K, Do J-W, Hong S-C, Lee K-H, Cho M-C, Yang E-Y, Yoon J-B (2022) QTL mapping of resistance to bacterial wilt in pepper plants (Capsicum annuum) using genotyping-by-sequencing (GBS). Horticulturae 8:115. https://doi.org/10.3390/horticulturae8020115
Chakrabarti SK, Gadewar AV, Gopal J, Shekhawat GS (1994) Performance of triploid x diploid (TD) crosses of potato for bacterial wilt resistance in India. Australian Centre for International Agricultural Research Bact Wilt Newsl 10
Chepkoech E, Kinyua M, Ochuodho J, Kiplagat O, Bado S, Kinyua Z (2020) Application of gamma induced mutation in breeding potato for bacterial wilt disease resistance. Int J Pathog Res 5:28–38
Daunay M-C, Lester RN, Laterrot H (1991) The use of wild species for the genetic improvement of brinjal egg-plant (Solanum melongena) and tomato (Lycopersicon esculentum). Kew: The Royal Botanic Gardens. pp 389–412
de Morais TP, Lopes CA, Tebaldi ND, Luz JMQ (2015) Occurrence and diversity of Ralstonia solanacearum populations in Brazil. Biosci J Uberlândia 31:1722–1737
Deslandes L, Genin S (2014) Opening the Ralstonia solanacearum type III effector tool box: insights into host cell subversion mechanisms. Curr Opin Plant Biol 20:110–117
Du H, Yang J, Chen B, Zhang X, Xu X, Wen C, Geng S (2022) Dual RNA-seq reveals the global transcriptome dynamics of Ralstonia solanacearum and pepper (Capsicum annuum) hypocotyls during bacterial wilt pathogenesis. Phytopathology® 112:630–642
Du J, Vleeshouwers VG (2017) New strategies towards durable late blight resistance in potato. Potato Genome. https://doi.org/10.1007/978-3-319-66135-3_10
Elphinstone JG (2005) The current bacterial wilt situation: A global overview bacteria wilt Dis Ralstonia Solanacearum species complex. APS Press, pp 9–28
Farag N, Stead DE, Janse JD (1999) Ralstonia (Pseudomonas) solanacearum race 3 biovar 2 detected in surface (irrigation) water in Egypt. J Phytopathol 147:485–487
Fegan M, Prior P (2005) How complex is the Ralstonia solanacearum species complex. Bact Wilt Dis Ralstonia Solanacearum Species Complex 1:449–461
Ferrero V, Baeten L, Blanco-Sánchez L, Planelló R, Díaz-Pendón JA, Rodríguez-Echeverría S, Haegeman A, de la Peña E (2020) Complex patterns in tolerance and resistance to pests and diseases underpin the domestication of tomato. New Phytol 226:254–266
Fortnum B A, Martin S B (1998) Disease management strategies for control of bacterial wilt of tobacco in the southeastern USA In Bacterial wilt disease: molecular and ecological aspects, Springer, Berlin, pp 394–402
French ER (1987) Strategies for bacterial wilt control. In: Report of the planning conference on bacterial diseases of the potato, pp 133–142
French E, Kim BS, Rivera-Zuluaga K, Iyer-Pascuzzi AS (2018) Whole root transcriptomic analysis suggests a role for auxin pathways in resistance to Ralstonia solanacearum in tomato. Mol Plant Microbe Interact 31:432–444
Fukuoka H, Miyatake K, Nunome T, Negoro S, Yamaguchi H, Ohyama A (2010) Development of an integrated linkage map using genomic SSR and gene-based SNPs markers in eggplant. Adv Genet Breed Capsicum Eggplant 359–375
Gbonamou M, Nguessan AC, Chen J-R, Kone D, Bihon W, Kenyon L (2020) First phylotype analysis of Ralstonia solanacearum causing Eggplant bacterial wilt in the Republic of Guinea. J Anim Plant Sci 46:8187–8196. https://doi.org/10.35759/JAnmPlSci.v46-2.3
Genin S (2010) Molecular traits controlling host range and adaptation to plants in Ralstonia solanacearum. New Phytol 187:920–928
Graziosi I, Minato N, Alvarez E, Ngo DT, Hoat TX, Aye TM, Pardo JM, Wongtiem P, Wyckhuys KA (2016) Emerging pests and diseases of South-east Asian cassava: a comprehensive evaluation of geographic priorities management options and research needs. Pest Manag Sci 72:1071–1089
Guinard J (2012) Structuration génétique d’une population parcellaire de Ralstonia solanacearum et impact d’une culture d’aubergine résistante Montpellier SupAgro
Habe I, Miyatake K (2022) Identification and characterization of resistance quantitative trait loci against bacterial wilt caused by the Ralstonia solanacearum species complex in potato. Mol Breed 42:50
Habe I, Miyatake K, Nunome T, Yamasaki M, Hayashi T (2019) QTL analysis of resistance to bacterial wilt caused by Ralstonia solanacearum in potato. Breed Sci 69:592–600
Habe I, Sakamoto Y, Matsumoto K (2023) The development and efficient utilization of molecular markers for the major quantitative trait locus of bacterial wilt resistance in potato. Euphytica 219:68
Hacisalihoglu G, Momol MT, Wen A, Olson S (2009) Effect of pH on bacterial wilt incidence and plant growth in hydroponic tomato. Acta Hortic. https://doi.org/10.17660/ActaHortic.2009.808.47
Hanson PM, Licardo O, Hanudin Wang J-F, Chen J (1998) Diallel analysis of bacterial wilt resistance in tomato derived from different sources. Plant Dis 82:74–78
Hanson P, Lu SF, Wang JF, Chen W, Kenyon L, Tan CW, Tee KL, Wang Y, Hsu Y, Schafleitner R, Ledesma D, Yang RY (2016) Conventional and molecular marker-assisted selection and pyramiding of genes for multiple disease resistance in tomato. Sci Hortic 201:346–354
Haywared AC (1991) Biology and Epidemiology of bacterial wilt caused by Pseudomonas solanacearum. Ann Rev Phytopathol 29:65–87
Huet G (2014) Breeding for resistances to Ralstonia solanacearum. Front Plant Sci 5:715
Hussain MZ, Rahman MA, Bashar MA (2005) Screening of brinjal accessions for bacterial wilt caused by Ralstonia solanacearum Bangladesh. J Bot 34:53–58
Hwang J, Youngmi CHOI, Jumsoon KANG, Suntae KIM, Myeongcheoul CHO, Mihalte L, Younghoon PARK (2011) Microarray analysis of the transcriptome for bacterial wilt resistance in pepper (Capsicum annuum L.). Not Botanicae Horti Agrobot Cluj-Napoca 39:49–57
Jacobs JM, Milling A, Mitra RM, Hogan CS, Ailloud F, Prior P, Allen C (2013) Ralstonia solanacearum requires PopS an ancient AvrE-family effector for virulence and to overcome salicylic acid-mediated defenses during tomato pathogenesis. Am Soc Microbiol MBio. https://doi.org/10.1128/mbio00875-13
Janse JD (2004) Diagnostic protocols for regulated pests: Ralstonia solanacearum. OEPP EPPO Bull 34:173–178
Jia S-R, Xic Y, Tang T, Feng L-X, Cao D-S, Zhao Y-L, Yuan J, Bai Y-Y, Jiang C-X, Jayncs JM (1993) Genetic engineering of Chinese potato cultivars by introducing antibacterial polypeptide gene. In: Biotechnology in agriculture: proceedings of the first Asia-Pacific conference on agricultural biotechnology Beijing China, August 1992. Springer, pp 208–212
Jiang G, Wei Z, Xu J, Chen H, Zhang Y, She X, Macho AP, Ding W, Liao B (2017) Bacterial wilt in China: history current status and future perspectives. Front Plant Sci 8:1549
Jourda C, Salgon S, Lebeau A, Daunay M C, Prior P, Wicker E, Guinard J, Morel A, Peeters N, Poussier S, Yahiaoui N, Dintinger J (2019) A decade of studies in France to decipher the genetic, molecular basis of eggplant resistance to bacterial wilt INRA
Jyothi HK, Santhosha HM (2012) Recent advances in breeding for bacterial wilt (Ralstonia solanacearum) resistance in tomato-review. Curr Biot 6:370–398
Kamenya SN, Mikwa EO, Song B, Odeny DA (2021) Genetics and breeding for climate change in orphan crops. Theor Appl Genet 134:1787–1815. https://doi.org/10.1007/s00122-020-03755-1
Kathimba FK, Kimani PM, Narla RD, Kiirika LM (2021) Characterization of tomato germplasm accessions for breeding research. J Agric Biotechnol Sustain Dev 13:20–27
Kiran U, Abdin MZ (2022) Technologies in plant biotechnology and breeding of field crops, Springer
Kumar A, Prameela TP, Suseelabhai R (2013) A unique DNA repair and recombination gene (rec N) sequence for identification and intraspecific molecular typing of bacterial wilt pathogen Ralstonia solanacearum and its comparative analysis with ribosomal DNA sequences. J Biosci 38:267–278. https://doi.org/10.1007/s12038-013-9312-0
Kunwar S, Iriarte F, Fan Q, Evaristo da Silva E, Ritchie L, Nguyen NS, Freeman JH, Stall RE, Jones JB, Minsavage GV, Colee J, Scott JW, Vallad GE, Zipfel C, Horvath D, Westwood J, Hutton S, Paret ML (2018) Transgenic expression of EFR and Bs2 genes for field management of bacterial wilt and bacterial spot of tomato. Phytopathology 108:1402–1411
Kunwar S, Bamazi B, Banito A, Carter M, Weinstein S, Steidl O, Hayes MM, Allen C, Paret M (2021) First report of bacterial wilt disease of tomato pepper and gboma caused by Ralstonia solanacearum species complex in Togo. Plant Dis 105:484. https://doi.org/10.1094/PDIS-08-20-1665-PDN
Kurabachew H, Ayana G (2017) Bacterial wilt caused by Ralstonia solanacearum in Ethiopia: status and management approaches—a review. Int J Phytopathol 5:107–119
Kwon JS, Nam JY, Yeom SI, Kang WH (2021) Leaf-to-whole plant spread bioassay for pepper and Ralstonia solanacearum interaction determines inheritance of resistance to bacterial wilt for further breeding. Int J Mol Sci 22:2279. https://doi.org/10.3390/ijms22052279
Lacombe S, Rougon-Cardoso A, Sherwood E, Peeters N, Dahlbeck D, Van Esse HP, Smoker M, Rallapalli G, Thomma BHJ, Staskawicz B, Jones JDG, Zipfel C (2010) Interfamily transfer of a plant pattern-recognition receptor confers broad-spectrum bacterial resistance. Nat Biotechnol 28:365–369
Lafortune D, Béramis M, Daubèze A-M, Boissot N, Palloix A (2005) Partial resistance of pepper to bacterial wilt is oligogenic and stable under tropical conditions. Plant Dis 89:501–506
Landry D, González-Fuente M, Deslandes L, Peeters N (2020) The large diverse and robust arsenal of Ralstonia solanacearum type III effectors and their in planta functions. Mol Plant Pathol 21:211377–211388
Lavale SA, Debnath P, Mathew D, Abdelmotelb KF (2022a) Two decades of omics in bacterial wilt resistance in Solanaceae what we learned? Plant Stress 5:100099
Lavale SA, Debnath P, Mathew D, Abdelmotelb KF (2022b) Two decades of omics in bacterial wilt resistance in Solanaceae, what we learned? Plant Stress 5:100099
Lebeau A, Daunay M-C, Frary A, Palloix A, Wang J-F, Dintinger J, Chiroleu F, Wicker E, Prior P (2011) Bacterial wilt resistance in tomato pepper and eggplant: genetic resources respond to diverse strains in the Ralstonia solanacearum species complex. Phytopathology 101:154–165
Lebeau A, Gouy M, Daunay M-C, Wicker E, Chiroleu F, Prior P, Frary A, Dintinger J (2013) Genetic mapping of a major dominant gene for resistance to Ralstonia solanacearum in eggplant. Theor Appl Genet 126:143–158
Liao B (2005) A broad review and perspective on breeding for resistance to bacterial wilt Bact Wilt Dis Ralstonia Solanacearum species complex, vol 46. American Phytopathological Society, APS Press, pp 225–238
Lopes GL, Lopes CA, Nomura JV, Nandi G, Piotto FA (2022) Combining ability of tomato inbred lines to bacterial wilt resistance. Bragantia 81:e3222
López MM, Biosca EG (2005) Potato bacterial wilt management: new prospects for an old problem. Bact Wilt Dis Ralstonia Solanacearum species complex, vol 80. American Phytopathological Society, APS Press, pp 205–224
Lowe-Power TM (2017) Metabolic analyses of Ralstonia solanacearum during plant pathogenesis. The University of Wisconsin-Madison
Machmud M (1986) Bacterial wilt in Indonesia. Bact Wilt Dis Asia S Pac 13:30–34
MacLeod A, Pautasso M, Jeger MJ, Haines-Young R (2010) Evolution of the international regulation of plant pests and challenges for future. Plant Health Food Secur 2:49–70
Mafuta JN, Onamu R, Shibairo SI, Wamocho LS (2022) Evaluation of resistance of African nightshade (Solanum nigrum complex) accessions to bacterial wilt (Ralstonia solanacearum) in Western Kenya. Afr J Educ Sci Technol 7:47–57
Mafuta NJ (2022) Genetic diversity and resistance of African nightshade Solanum nigrum L. Complex to bacterial wilt Ralstonia solanacearum in Western Kenya
Mahbou-Somo-Toukam G (2010) Diversité de Ralstonia Solanacearum au Cameroun et bases génétiques de la résistance chez le piment (Capsicum annuum) et les Solanacées AgroParisTech
Mamphogoro TP, Babalola OO, Aiyegoro OA (2020) Sustainable management strategies for bacterial wilt of sweet peppers (Capsicum annuum) and other Solanaceous crops. J Appl Microbiol 129:496–508
Mansfield J, Genin S, Magori S, Citovsky V, Sriariyanum M, Ronald P, Dow MAX, Verdier V, Beer SV, Machado MA (2012) Top 10 plant pathogenic bacteria in molecular plant pathology. Mol Plant Pathol 13:614–629
Mathai FW (2022) Inheritance of bacterial wilt resistance among wild tomato and cultivated tomato germplasm. Doctoral dissertation Egerton University, Kenya Egerton University
Maxwell L A, Chang H C, Chen J R, Kenyon L, Ramasamy S (2022) Evaluation of biocontrol agents against bacterial wilt in tomato using seedling screening. In: International symposium Southeast Asia vegetable 2021 (SEAVEG 2021), Atlantis Press, pp 415–422
McClean APD (1930) The bacterial wilt disease of peanut (Arachis hypogaea L.). Sci Bull 87:14
Mihovilovich E, López C, Gutarra, L, Lindqvist-Kreuze H, Aley P, Priou S, Bonierbale MW (2017) Protocol for assessing bacterial wilt resistance in greenhouse and field conditions. International cooperators’ guide
Mimura Y, Kageyama T, Minamiyama Y, Hirai M (2009) QTL analysis for resistance to Ralstonia solanacearum in Capsicum accession ‘LS2341.’ J Jpn Soc Hortic Sci 78:307–313
Munyaneza JE, Bizimungu B (2022) Management of potato pests and diseases in Africa. Insect Pests Potato. https://doi.org/10.1016/B978-0-12-821237-0.00016-0
Murti RH, Afifah EN, Nuringtyas TR (2021) Metabolomic response of tomatoes (Solanum lycopersicum L.) against bacterial wilt (Ralstonia solanacearum) using 1H-NMR spectroscopy. Plants 10:1143
Musa I, Rafii MY, Ahmad K, Ramlee SI, Md Hatta MA, Magaji U, Muhammad I, Chukwu SC, Mat Sulaiman NN (2021) Influence of wild relative rootstocks on eggplant growth, yield and fruit physicochemical properties under open field conditions. Agriculture 11:943
Muthoni J, Shimelis H, Melis R (2012) Management of bacterial wilt [Ralstonia solanacearum Yabuuchi et al, 1995] of potatoes: opportunity for host resistance in Kenya. J Agric Sci 4:64
Muthoni J, Shimelis H, Melis R, Kinyua ZM (2014) Response of potato genotypes to bacterial wilt caused by Ralstonia solanacearum (Smith)(Yabuuchi et al.) in the tropical highlands. Am J Potato Res 91:215–232
N’Guessan CA, Abo K, Fondio L, Chiroleu F, Lebeau A, Poussier S, Wicker E, Koné D (2012) So near and yet so far: the specific case of Ralstonia solanacearum populations from Cote d’Ivoire in Africa. Phytopathology 102:733–740
Na C, Shuanghua W, Jinglong F, Bihao C, Jianjun L, Changming C, Jin J (2016) Overexpression of the eggplant (Solanum melongena) NAC family transcription factor S mNAC suppresses resistance to bacterial wilt. Sci Rep 6:31568
Namisy A, Chen J-R, Prohens J, Metwally E, Elmahrouk M, Rakha M (2019) Screening cultivated eggplant and wild relatives for resistance to bacterial wilt (Ralstonia solanacearum). Agriculture 9:157
Naresh P, Meenu K, Acharya GC, Reddy AC, DC LR (2018) Genetics and molecular markers for resistance to major soil borne pathogens in chilli (Capsicum annuum L.)
Nguyen TT, Le NT, Sim S-C (2021) Genome-wide association study and marker development for bacterial wilt resistance in tomato (Solanum lycopersicum L.). Sci Hortic 289:110418
Nikuze T, Ateka EM, Ambuko J, Owino WO (2020) Identification of microsatellite markers linked to bacterial wilt resistance in African eggplant. Afr J Hortic Sci 17:127–142
Nortje P (2015) Bacterial wilt on potato: the South African experience [document on the Internet]. [cited 2024 Aug 01]. Available from https://drive.google.com/file/d/16kzi6ntkiOY9cyh-HAKXy92s_uppqAHA/view
Nunome T, Yoshida T, Hirai M (1998) Genetic linkage map of eggplant. In: Proceedings of the 10th Eucarpia meeting on genetics and breeding of capsicum and eggplant, Avignon, pp 239–242
O’Herlihy EA, Doyle Prestwich BM, Wall G (2010) An in vitro study to examine the potential of mutation breeding to create resistance to bacterial wilt (Ralstonia solanacearum) in tomato in: XXVIII International horticultural congress on science and horticulture for people (IHC2010). In: International symposium on new, vol 935, pp 101–106
Ochieng J, Afari-Sefa V, Karanja D, Kessy R, Rajendran S, Samali S (2018) How promoting consumption of traditional African vegetables affects household nutrition security in Tanzania. Renew Agric Food Syst 33:105–115
Osiru MO, Rubaihayo PR, Opio AF (2001) Inheritance of resistance to tomato bacterial wilt and its implication for potato improvement in Uganda. Afr Crop Sci J 9:9–16
Otipa MJ, Wakahiu MW, Kinyae P, Thuo DN (2003) A report on survey of the bacterial wilt of potatoes caused by Ralstonia solanacearum and its spread in the major potato growing areas. International Potato Centre Kenya, pp 33–35
Oussou GF, Sikirou R, Afoha SA, Dossoumou ME, Boukari SA, Komlan FA, Zocli B (2020) Resistance assessment of tomato (Solanum lycopersicum L.) and Gboma (Solanum macrocarpon L.) cultivars against bacterial wilt caused by Ralstonia solanacearum in Benin. Pak J Phytopathol 32:241–249
Pandiyaraj P, Singh TH, Reddy KM, Sadashiva AT, Gopalakrishnan C, Reddy AC, Pattanaik A, Reddy DL (2019) Molecular markers linked to bacterial wilt (Ralstonia solanacearum) resistance gene loci in eggplant (Solanum melongena L.). Crop Prot 124:104822
Peeters N, Carrère S, Anisimova M, Plener L, Cazalé A-C, Genin S (2013a) Repertoire unified nomenclature and evolution of the type III effector gene set in the Ralstonia solanacearum species complex. BMC Genom 14:1–19
Peeters N, Guidot A, Vailleau F, Valls M (2013b) Ralstonia solanacearum a widespread bacterial plant pathogen in the post-genomic era. Mol Plant Pathol 14:651–662
Peiris R, Wickramasinghe TK, Indrasena SP (2008) M 127–a promising tomato variety developed through induced mutation technique. In Induced plant mutations in the genomics era. In: Proceedings of an international joint FAO/IAEA symposium. Rome: Food and Agriculture Organization of the United Nations, pp 379–380
Perez-de-Castro AM, Vilanova S, Cañizares J, Pascual L, Blanca JM, Diez M, Prohens J, Picó B (2012) Application of genomic tools in plant breeding. Curr Genomics 13:179–195
Ravelomanantsoa S, Vernière C, Rieux A, Costet L, Chiroleu F, Arribat S, Cellier G, Pruvost O, Poussier S, Robène I, Guérin F, Prior P (2018) Molecular epidemiology of bacterial wilt in the Madagascar highlands caused by Andean (Phylotype IIB-1) and African (Phylotype III) brown rot strains of the Ralstonia solanacearum species complex. Front Plant Sci 8:2258
Ristaino JB, Anderson PK, Bebber DP, Brauman KA, Cunniffe NJ, Fedoroff NV, Finegold C, Garrett KA, Gilligan CA, Jones CM (2021) The persistent threat of emerging plant disease pandemics to global food security. Proc Natl Acad Sci 118:e2022239118
Robertson AE (1998) Factors affecting the population of Ralstonia Solanacearum in a naturally infested field planted to tobacco. In: Bacterial wilt disease: molecular and ecological aspects, pp 369–375
Sabbagh CRR, Carrere S, Lonjon F, Vailleau F, Macho AP, Genin S, Peeters N (2019) Pangenomic type III effector database of the plant pathogenic Ralstonia spp. PeerJ 7:e7346
Safni I, Cleenwerck I, De Vos P, Fegan M, Sly L, Kappler U (2014) Polyphasic taxonomic revision of the Ralstonia solanacearum species complex: proposal to emend the descriptions of Ralstonia solanacearum and Ralstonia syzygii and reclassify current R. syzygii strains as Ralstonia syzygii subsp. syzygii subsp. Nov., R. solanacearum phylotype IV strains as Ralstonia syzygii subsp. Indonesiensis subsp. Nov., banana blood disease bacterium strains as Ralstonia syzygii subsp. celebesensis subsp. nov. and R. solanacearum phylotype I and III strains as Ralstonia pseudosolanacearum sp. nov. Int J Syst Evol Microbiol 64:3087–3103
Salgon S, Jourda C, Sauvage C, Daunay M-C, Reynaud B, Wicker E, Dintinger J (2017) Eggplant resistance to the Ralstonia solanacearum species complex involves both broad-spectrum and strain-specific quantitative trait loci. Front Plant Sci 8:828
Sallam MA, Abd El-Ghafar NY, Abd El-Said WM, Hajhamed AA (2013) Molecular markers associated with some resistance potato cultivars to bacterial wilt disease. Glob J Mol Sci 8:08–16
Schippers RR (2000) African indigenous vegetables: an overview of the cultivated species. Natural Resources Institute/ACP-EU Technical Centre for Agricultural and Rural Cooperation
Sarkar S, Chaudhuri S (2016) Bacterial wilt and its management. Curr Sci 110:1439–1445
Scott JW, Wang JF, Hanson PM (2004) Breeding tomatoes for resistance to bacterial wilt a global view. Int Symp Tomato Dis 695:161–172
Sharma S, Katoch V, Banyal DK (2021) Review on harnessing biotechnological tools for the development of stable bacterial wilt resistant solanaceous vegetable crops. Sci Hortic 285:110158
Shi H, Xu P, Yu W, Cheng Y, Ding A, Wang W, Shengxin W, Sun Y (2022) Metabolomic and transcriptomic analysis of roots of tobacco varieties resistant and susceptible to bacterial wilt. Genomics 114:110471
Shin IS, Hsu J-C, Huang S-M, Chen J-R, Wang J-F, Hanson P, Schafleitner R (2020) Construction of a single nucleotide polymorphism marker-based QTL map and validation of resistance loci to bacterial wilt caused by Ralstonia solanacearum species complex in tomato. Euphytica 216:1–20
Sikirou R, Zocli B, Paret ML, Deberdt P, Coranson-Beaudu R, Huat J, Assogba-Komlan F, Dossoumou M, Simon S, Wicker E (2015) First report of bacterial wilt of Gboma (Solanum macrocarpon) caused by Ralstonia solanacearum in Benin. Plant Dis 99:1640–1640
Sikirou R, Dossoumou ME, Honfoga J, Afari-Sefa V, Srinivasan R, Paret M, Bihon W (2021) Screening of Amaranthus sp. varieties for resistance to bacterial wilt caused by Ralstonia solanacearum. Horticulturae 7:465
Simbwa-Bunnya M (1972) Resistance of groundnut varieties to bacterial wilt (Pseudomonas solanacearum) in Uganda East. Afr Agric for J 37:341–343
Sogbohossou ED, Kortekaas D, Achigan-Dako EG, Maundu P, Stoilova T, Van Deynze A, de Vos RC, Schranz ME (2019) Association between vitamin content plant morphology and geographical origin in a worldwide collection of the orphan crop Gynandropsis gynandra (Cleomaceae). Planta 250:933–947
Sogbohossou ED (2019) Orphan no more: ethnobotany and genetic analysis of leaf yield and secondary metabolites content in Gynandropsis gynandra (Cleomaceae). In: Dissertation, Wageningen University and Research
Sood T, Sood S, Sood VK, Badiyal A, Anuradha Kapoor S (2023) Assessment and validation of resistance to bacterial wilt (Ralstonia solanacearum) through field and molecular studies in bell pepper. J Plant Pathol 105:1–9
Subedi N, Gilbertson RL, Osei MK, Cornelius E, Miller SA (2014) First report of bacterial wilt caused by Ralstonia solanacearum in Ghana West Africa. Plant Dis 98:840–840
Team R C (2022) R: A language and environment for statistical computing R Foundation for Statistical Computing, version 4.2. 2. Vienna
Thakur PP, Mathew D, Nazeem PA, Abida PS, Indira P, Girija D, Shylaja MR, Valsala PA (2014) Identification of allele specific AFLP markers linked with bacterial wilt [Ralstonia solanacearum (Smith) Yabuuchi et al] resistance in hot peppers (Capsicum annuum L.). Physiol Mol Plant Pathol 87:19–24
Thoquet P, Olivier J, Sperisen C, Rogowsky P, Prior P, Anais G, Mangin BB, Bazin B, Nazer R, Grimsley N (1996) Polygenic resistance of tomato plants to bacterial wilt in the French West Indies. Mol Plant Microbe Interact 9:837–842
Towns AM, Shackleton C (2018) Traditional, indigenous, or leafy? A definition, typology, and way forward for African vegetables. Econ Bot 72:461–477. https://doi.org/10.1007/s12231-019-09448-1
Uwamahoro F, Berlin A, Bucagu C, Bylund H, Yuen J (2018) Potato bacterial wilt in Rwanda: occurrence risk factors farmers’ knowledge and attitudes. Food Secur 10:1221–1235. https://doi.org/10.1007/s12571-018-0834-z
Vleeshouwers VG, Oliver RP (2014) Effectors as tools in disease resistance breeding against biotrophic hemibiotrophic and necrotrophic plant pathogens. Mol Plant-Microbe Interact 27:196–206
Waals JE, Krüger K (2020) Emerging potato pathogens affecting food security in southern Africa: recent research. South Afr J Sci 116:1–7
Wang J-F, Ho F-I, Truong HTH, Huang S-M, Balatero CH, Dittapongpitch V, Hidayati N (2013) Identification of major QTLs associated with stable resistance of tomato cultivar ‘Hawaii7996’to Ralstonia solanacearum. Euphytica 190:241–252
Wang J-F, Chen N-C, Li H-M (1998) Resistance sources to bacterial wilt in eggplant (Solanum melongena). In: Bacterial wilt disease. Molecular and ecological aspects, Springer, pp 284–289
Wang J F, Hanson P, Barnes J A (1998) Worldwide evaluation of an international set of resistance sources to bacterial wilt in tomato. In: Bacterial wilt disease. Molecular and ecological aspects, Springer, Berlin, pp 269–275
Wei Z, Huang J-F, Hu J, Gu Y-A, Yang C-L, Mei X-L, Shen Q-R, Xu Y-C, Friman V-P (2015) Altering transplantation time to avoid periods of high temperature can efficiently reduce bacterial wilt disease incidence with tomato. PLoS ONE 10:e0139313
Wicker E, Grassart L, Coranson-Beaudu R, Mian D, Guilbaud C, Fegan M, Prior P (2007) Ralstonia solanacearum strains from Martinique (French West Indies) exhibiting a new pathogenic potential. Appl Environ Microbiol 73:6790–6801
Xiao XO, Lin W, Feng E, Ou X (2023) Transcriptome and metabolome response of eggplant against Ralstonia solanacearum infection. PeerJ 11:e14658
Xi’ou X, Bihao C, Guannan L, Jianjun L, Qinghua C, Jin J, Yujing C (2015) Functional characterization of a putative bacterial wilt resistance gene (RE-bw) in eggplant. Plant Mol Biol Report 33:1058–1073
Xu A, Wei L, Ke J, Peng C, Li P, Fan C, Xiao Y, Li B (2023) ETI signaling nodes are involved in resistance of Hawaii7996 to Ralstonia solanacearum-induced bacterial wilt disease in tomato. Plant Signal Behav 18:2194747
Xuping L, Xuejian L, Guangjin W, Xianxing L, Junxin C, Shaochang X (1996) The epidemic law of bacterial wilt in Eucalyptus. J Cent-South for Coll 16:49–55
Zeiss DR, Mhlongo MI, Tugizimana F, Steenkamp PA, Dubery IA (2018) Comparative metabolic phenotyping of tomato (Solanum lycopersicum) for the identification of metabolic signatures in cultivars differing in resistance to Ralstonia solanacearum. Int J Mol Sci 19:2558
Zeiss DR, Mhlongo MI, Tugizimana F, Steenkamp PA, Dubery IA (2019) Metabolomic profiling of the host response of tomato (Solanum lycopersicum) following infection by Ralstonia solanacearum. Int J Mol Sci 20:3945
Zohoungbogbo H, Quenum A, Honfoga J, Chen J-R, Achigan-Dako E, Kenyon L, Hanson P (2021) Evaluation of resistance sources of tomato (Solanum lycopersicum L.) to phylotype i strains of Ralstonia solanacearum species complex in Benin. Agronomy 11:1513
Funding
Belchrist Eliel Sossou is a PhD student at the University of Abomey-Calavi, Republic of Benin. This research was carried out with funding from (i) the European Union and the Ministry of Foreign Affairs of the Netherlands through the project "Safe locally produced vegetables for West Africa’s consumers (SafeVeg)"—ID-4000003936, part of the DeSIRA program and implemented by the World Vegetable Center, CIRAD and Wageningen University & Research (WUR); and (ii) the CGIAR Research Initiative on Fruit and Vegetables for Sustainable Healthy Diets (FRESH); we would like to thank all funders who supported this research through their contributions to the CGIAR Trust Fund. The views expressed in this document can in no way be taken to reflect the official opinion of the funders. We also acknowledge long-term strategic donors to the World Vegetable Center: Taiwan, UK aid from the UK government, United States Agency for International Development (USAID), Australian Centre for International Agricultural Research (ACIAR), Germany, Thailand, Philippines, Korea, and Japan.
Author information
Authors and Affiliations
Contributions
Enoch G. Achigan-Dako, Roland Schafleitner, Mathieu Anatole Tele Ayenan, and Rachidatou Sikirou supervised, conceived and designed the study. Belchrist Eliel Sossou, Mathieu Anatole Tele Ayenan, and Roland Schafleitner designed and outlined the manuscript. Belchrist Eliel Sossou wrote the first draft. Enoch Gbenato Achigan-Dako, Roland Schafleitner, Mathieu Anatole Tele Ayenan, and Rachidatou Sikirou critically revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sossou, B.E., Ayenan, M.A.T., Schafleitner, R. et al. Breeding for resistance to bacterial wilt in Solanaceae crops: lessons learned and ways forward for Gboma eggplant (Solanum macrocarpon L.), a traditional African vegetable. Euphytica 220, 153 (2024). https://doi.org/10.1007/s10681-024-03393-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10681-024-03393-4