Abstract
The nitrogen fertilizer replacement value (NFRV) quantifies the value of organic amendments as a nitrogen (N) fertilizer, and is commonly defined as the extent to which organic fertilizer N can replace mineral fertilizer N. NFRVs can be calculated by comparing the crop N uptake from equal N application rates of mineral and organic fertilizer, or by comparing the N rates of both fertilizers needed to obtain equal crop N uptake. Currently, NFRVs are mainly known for animal manure, whereas other organic waste products may become available as fertilizer products in the future. In this study, a pot experiment with spring wheat was performed to (1) assess NFRVs of a range of organic amendments; (2) compare NFRVs based on equal N application with NFRVs based on equal N uptake; and (3) assess which product characteristics explain observed variation. Observed NFRVs varied between 6.2 and 78.8%, with the lowest value for raw food waste and the highest for fishmeal. NFRVs were overestimated when calculated based on equal N application rate (with on average 6.9% point), and more so at high N application rate (9.0% point). NFRVs should therefore be calculated based on equal N uptake from organic and mineral fertilizers. Nitrogen concentration of the organic fertilizer provided the best explanation of variation observed in NFRVs (R2 = 0.86). These findings give valuable insights into the large variation in value of organic waste streams as organic fertilizer and can support decisions on sustainable N application rates, to increase crop N uptake and reduce N losses to the environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recycling of nutrients from organic waste streams is a key component of circular agriculture. By using organic waste streams as a nutrient source for crop production, nutrients such as nitrogen (N) can be recycled, thereby reducing the need for mineral fertilizers as external inputs (Tsachidou et al. 2019; Valve et al. 2020; van Zwieten 2018). Currently, N fertilizer values of many organic waste streams are unknown, which hampers their application in farming. Improved understanding of variability in N supply from organic waste products would allow for improved predictions of their fertilizer value and more accurate fertilizer recommendations. This should prevent decreased yields and unnecessary N losses to the environment.
The N Fertilizer Replacement Value (NFRV) is a well-known means to quantify the value of organic products as a N fertilizer and is a helpful tool to estimate correct N application rates. The NFRV is commonly defined as the extent to which organic fertilizer N can replace mineral fertilizer N. NFRVs increase over time when organic fertilizers are applied year after year and can therefore represent either first-year or long-term NFRV (Gutser et al. 2005; Tsachidou et al. 2019).
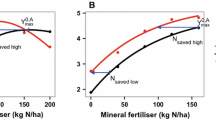
Two calculation methods to quantify NFRVs can be found in literature (Fig. 1). Method 1 (equal N application rates, Eq. 1) is the most common approach (Gutser et al. 2005; Schröder 2005; Sigurnjak et al. 2017; Tsachidou et al. 2019). It divides the N uptake from an organic fertilizer treatment (UOF,N=x) by the N uptake from a mineral fertilizer treatment (UMF,N=x) at equal Ntotal application rate (N = x), while correcting for the N uptake in the control treatment without addition of organic fertilizer (UN=0). The NFRV is thus expressed as the percentage of organic fertilizer N taken up by a crop, compared to N uptake from equal amounts of mineral fertilizer N. Although the approach with N uptake is considered more appropriate, this method can also be conducted based on equal (grain) yield or based on a comparison of agronomic use efficiencies (defined as the additional grain yield per kg N applied) (Jensen 2013; Schils et al. 2020).
Illustration of the derivation of parameters for both Nitrogen Fertilizer Replacement Value (NFRV) calculation methods. A: NFRV at equal N application rate (NFRV1, Eq. 1). N uptake from the unfertilized soil (UN=0) and from the mineral fertilizer (UMF,N=150) are derived from the fitted mineral fertilizer dose-response curve. N uptake from the organic fertilizer (UOF,N=150) is determined in the experiment. B: NFRV at equal N uptake (NFRV2, Eq. 2) where the corresponding N rate MFU=80 is derived from the N uptake (U = 80) of an organic fertilizer as determined in the experiment. MF = mineral fertilizer; OF = organic fertilizer
Method 2 (equal N uptake, Eq. 2) is based on equal plant N uptake from mineral and organic fertilizers: it compares the N rates from mineral and organic fertilizer treatments needed to obtain the same plant N uptake (U = x). Here, an experimental set-up is required with multiple N application rates to estimate a dose-response curve, i.e. N uptake (y-axis) versus mineral fertilizer application rate (x-axis). This curve can then be used to identify the amount of mineral fertilizer (Nrate MF U=x) needed to achieve a N uptake equal to that from the organic fertilizer (Nrate OFU=x). This method is used by Delin et al. (2012) and Hijbeek et al. (2018), and can also be conducted based on equal (grain) yield or based on a comparison of agronomic N use efficiencies.
To date, the two methods have not been compared or evaluated in an experimental setup. The understanding of the implications and potential (dis)advantages of these two methods is therefore limited. A comparison of methods would require a setup with corresponding N fertilizer application rates for mineral- and organic fertilizer treatments as well as a range of mineral fertilizer N treatments for the plotting of a dose-response curve. In all cases, to determine NFRV, N should be a growth limiting factor with all other nutrients sufficiently available.
Field experiments can be conducted to assess long-term NFRVs for specific organic amendments, but these are time, space, labour and capital intensive. It would therefore be useful to derive relations between easily obtainable chemical properties of organic fertilizer and their NFRVs. A high total N concentration together with a high mineralization rate from the organic amendment would lead to a higher N availability for the crop (Pinto et al. 2020; Reussi Calvo et al. 2018). It has furthermore been suggested that NFRVs of organic fertilizers are negatively correlated with their carbon (C):N ratio (Delin et al. 2012; Gutser et al. 2005), since a lower C:N ratio would result in higher N mineralization rates from the organic fertilizer. However, Teglia et al. (2010) and Probert et al. (2005) argue that the C:N ratio alone cannot explain all differences in N mineralization since it does not consider bioavailable fractions of C and N. Indicators for stability and biodegradability such as cellulose:lignin and the mineralizable N fraction could therefore explain part of the variation in NFRVs (Nkoa 2014; Teglia et al. 2010).
The aim of this study was threefold: to (1) assess NFRVs of a range of organic amendments; (2) evaluate calculation methods used to determine the NFRV of organic fertilizers; and (3) explain the variability in NFRVs. We hypothesize that the mineral N concentration (i.e. directly available N) together with a combination of their total N concentration, C:N ratio and a proxy for the rate at which this mineralizes may best explain variation in NFRVs. Data are obtained from a pot experiment where the short-term NFRVs of organic fertilizers of different origins and compositions are determined based on N uptake by spring wheat.
Methods
Organic fertilizers
Organic waste products from different origins were used as organic fertilizers in a pot experiment, and were selected based on their relevance for the Dutch food industry and potentially upcoming recycling systems. The organic fertilizers were from any of the following categories: by-products from the food industry; residual products from anaerobic digestion; manure from larvae; and commonly used organic amendments. Raw organic by-products from the food industry were rapeseed meal, fish meal, sugar beet pulp and wheat bran. Three residual products from anaerobic digestion of food waste (thermophilic monodigestion) were included: anaerobic digestate, aerobic sludge and a dried mix of these products. The raw food waste (liquified and hydrolysed) of the anaerobic digestion facility was also tested, as well as manure from larvae of the black soldier fly (Hermetia illucens) fed with the raw food waste. Farmyard manure and compost from household food and garden waste served as reference organic fertilizers.
Analyses of chemical properties
The organic fertilizers were stored in a fridge at 3 °C in closed containers prior to the experiment, no longer than 2 months in advance. The organic fertilizers were analysed for their dry matter (DM) concentration (24 h 70 °C), total N and phosphorus (P) concentration (N/Ptotal; segmented flow analyser after digestion in a H2SO4/H2O2/Se mixture), and mineral N (1:10 soil/solution ratio, 1 M KCl). In addition the C:N ratio (element analyser), the pH (1:5 extraction in water) and cellulose:lignin ratio (Soest and McQueen 1973; Van Soest 1973) were determined.
The Potential Mineralizable N (PMN) was measured in triplicate in a 7 days incubation experiment after addition of the fertilizers to the soil (Table 1). Fresh organic fertilizers were mixed with the soil at a rate equivalent to 200 kg N ha−1, after which a soil slurry (10 g fertilized soil mixed with 25 ml demineralized water) was incubated for 7 days at 40 °C (Delin et al. 2012; Waring and Bremner 1964). The mineral N concentration was determined with segmented flow analysis (SFA; San++ System, Skalar, Breda, The Netherlands) after 0 and 7 days of incubation. The amount of PMN was calculated by subtracting the total amount of mineral N at t = 0 from the total amount of mineral N at t = 7 and correcting for the PMN from the control treatment without any addition of fertilizer.
Together, the products covered a wide range of C:N ratios, total N concentrations and PMN (3.64–28.7; 14.3–102 g N kg−1 and 3.04–68.6 mg N kg−1 respectively, Table 1). The residual products from anaerobic digestion, rapeseed meal and fishmeal had relatively high total N concentrations, were high in PMN, and had a low C:N ratio compared to the other organic fertilizers. Cellulose:lignin ratios were not available for fishmeal since it is an animal-derived product, nor for larvae manure because the sample bag was lost during the analysis. Among the characteristics, total N correlated with C:N and PMN; PMN is correlated with all other characteristics (total N concentration, C:N, cellulose:lignin and mineral N concentration); and C:N is correlated with cellulose:lignin (p < 0.05, Supplementary Table 4).
The pot experiment
A pot experiment was carried out with spring wheat (Triticum aestivum, var. Quintus, Wiersum Plantbreeding) as a test crop. Seeds were pre-sown in wet tissue paper to ensure germination. After 3 days, fifteen germinated seeds were transplanted to each pot (depth 15 cm; diameter of 19 cm). After good establishment the number of plants were reduced to ten plants per pot, equivalent to 3.3 * 106 germinated seeds ha−1.
The soil used for the pot experiment was a sandy soil collected from ‘Droevendaal Agricultural Farm’ near Wageningen University and Research (NL). The soil had been organically managed and had a low N supplying capacity. The soil was dried and sieved (5 mm) and tested for the water holding capacity (WHC, 220 ml kg dry soil−1). WHC was calculated from the difference in weight after gradually adding water to the soil until shimmering but not leaking. The soil characteristics are summarized in Table 2 .
N application rates were derived from rates expressed in kg ha−1 based on the surface area of the pot (2.84 * 10−6 ha−1) and the total N concentration of the product. All organic fertilizers were applied at two rates: 100 and 200 kg Ntotal ha−1. The mineral fertilizer treatments were 0, 25, 50, 75, 100, 125, 150 and 200 kg Ntotal ha−1 from a solution of 1 M of ammonium nitrate (NH4NO3). The control treatment without any N fertilizer application served as a measure for the soil N supply. All other essential nutrients were applied in ample amounts: 15 kg ha−1 P (as NaH2PO4·H2O); 30 kg ha−1 S (as H2SO4); 100 kg ha−1 K (as KCl) and 70 kg ha−1 Ca (as CaCl2·2H2O). Per pot 3 ml of a trace element solution was added (in mg l−1: B 0.5; Mn 0.5; Zn 0.05; Cu 0.02; Mo 0.01). For each pot, 3 kg of dry soil was thoroughly mixed with the designated amount and type of fertilizer and water to reach 60% WHC. The pots were placed in a complete randomized block design with all treatments in triplicate.
The wheat plants were grown from 6 November to 2020 to 8 January 2021 in a greenhouse compartment at Unifarm (Wageningen University and Research), with artificial lighting, a constant humidity of 65%, and minimum day and night temperatures of 20 °C and 16 °C respectively. As the experiment was carried out in winter, temperatures did most likely not exceed the minimum temperature substantially. The water concentration was kept at 60% of WHC during the whole experiment. After the growth period, all above-ground biomass of the crop was harvested and the DM weight was determined (70 °C for 48 h). Subsamples of ground material were digested in a H2SO4/H2O2/Se mixture and subsequently analysed with SFA for Ntotal and Ptotal concentration.
Data processing
All data analyses in this study were performed using R software (version 4.0.3). Dplyr- and rstatix R packages were used for data manipulations.
Total N uptake in the above-ground biomass was calculated by multiplying the total N concentration (g N kg DW−1) with the shoot DW (g) per pot. This number was converted to kg N ha−1 taken up based on the surface area of the pot.
The PMN and N uptake data were tested for normality using a Shapiro-Wilk normality test (p ≤ 0.05), and visually with QQ-plots and residual plots. Since normality could be assumed and no outliers were detected, no data transformations were performed and no replicates were omitted from the data. Mean N uptake from different treatments was compared performing an ANOVA test, followed by a Tukey post-hoc test.
To estimate a dose-response curve of crop N uptake vs. mineral fertilizer N application, a regression curve was plotted through the N uptake values of the mineral fertilizer treatments (0–200 kg N ha−1) using lm and nls functions of the stats R package. Four models commonly used to predict DM yield from fertilizer application were tested for their goodness of fit (Supplementary Fig. 3): the Mitscherlich model (Sala et al. 2015), the George model (George 1984), a Square root model (Cerrato and Blackmer 1990) and a quadratic curve (Van Grinsven et al. 2022). All models were compared based on their Residual Standard Error (RSE), since the R2 for non-linear models was not provided by R. The simple quadratic model was judged to be most suitable for further calculations because it had the lowest RSE and a sufficiently high R2adj (0.965). The quadratic equation can be described as y = a + bx + cx2 where x is the N application rate of the fertilizer, and y is the total N uptake in the above-ground biomass at this application rate. The intercept (a) represents the N uptake from the soil (without fertilizer).
Calculating NFRV of the organic fertilizers using two methods
After fitting the curve and deriving the values for the parameters of the quadratic function, NFRVs were calculated with the two different methods (Eqs. 1 and 2). For Eq. 1, N uptake from mineral fertilizer (UMF,N=x) at low and high (100 and 200 kg N ha−1 respectively) N application rates were derived by filling in the application rates for x in the quadratic equation. N uptake from the soil (UN=0) was derived as the intercept of the quadratic function (Fig. 1A). For Eq. 2, the response curve was used to assess how much mineral fertilizer N (Nrate MFU=x) would be needed to achieve the same total N uptake as for each organic fertilizer treatment using the uniroot.all function of rootSolve package (Fig. 1B).
Assessing the difference between NFRV1 and NFRV2
For each organic fertilizer type and N application rate, NFRV was calculated using both methods for each replicate (11 × 2 × 2 × 3, resulting in 132 NFRV estimates). For each organic fertilizer, the influence of calculation method on NFRV was assessed with a t test (across replicates, Supplementary Tables 1 and 2). For each organic fertilizer, the influence of N application rate was also assessed using a t test (across replicates, Supplementary Table 3). Hence, for each organic fertilizer type the mean difference in NFRV was calculated between calculation methods as well as between N application rates.
In addition, the influence of calculation method and N application rate on NFRV was also assessed for the whole dataset. In this case, the inverse of the summed variance of the underlying replicates was taken as a weight, to account for variation in the replicates.
Assessing the effect of chemical properties on NFRV
To explore covariance of C:N ratio, cellulose:lignin ratio, mineral N concentration, total N concentration and PMN (mg mineral N kg soil−1) with the observed NFRV, single and multiple linear regression was performed with the lm function of the stats R package. Forward step-wise multiple linear regression of the covariates was performed with the ‘steps’ function of the stats R package. This method automatically chooses the best subset of the predictor variables (covariates) for the final model, i.e. the subset with the lowest residual sum of squares. In each step, the explanatory variables were considered based on the Akaike Information Criterion (AIC), providing means for model selection whilst dealing with the risk of overfitting as well as underfitting. This procedure was performed for all possible combinations of C:N ratio, cellulose:lignin ratio, total N concentration and PMN. Parameters were considered uninformative when the decrease in AIC was smaller than 2 (Arnold 2010). Performance of linear regression models with different sets of covariates were also compared among each other by calculating the mean of the absolute differences between predicted and observed NFRVs.
Results
N uptake from organic and mineral fertilizers in above-ground biomass
Shoot N uptake after 65 days of growth varied between 22 and 90 kg N ha−1 in the treatments with organic fertilizers, when 100 kg N ha−1 was applied (Fig. 2). It ranged between 30 and 161 kg N ha−1 at an application rate of 200 kg N ha−1. Shoot N uptake was lowest for raw food waste and sugar beet pulp, and highest for rapeseed meal and fish meal, regardless of the application rate.
Nitrogen uptake in the treatments with mineral fertilizers increased almost linearly with N application rates up to 150 kg N ha−1, above which N uptake did not increase further (Fig. 3). The dose-response curve could be described with a quadratic model (p < 0.0001), with N application as the explanatory variable x and N uptake as the response variable y.
NFRV1 (calculated at equal N application rate) was significantly larger at higher N application rate (200 kg N ha−1) than at lower N application rate (100 kg N ha−1) for seven of the eleven organic fertilizers (p ≤ 0.0001; Fig. 4A). In contrast, NFRV2 (calculated at equal N uptake) was similar across N application rates (p = 0.596; Fig. 4B; Supplementary Tables 1 and 2). When testing at individual product level, NFRV1 was higher than NFRV2 in all cases at high N application and in five out of eleven cases at low N application (p < 0.05; Supplementary Table 3). Across the entire dataset, NFRV1 was on average 6.94% point larger than NFRV2 (p < 0.0001). At high N application, NFRV1 was on average 8.98% point larger than NFRV2 (p < 0.0001).
Nitrogen Fertilizer Replacement Values (NFRVs) of eleven organic fertilizers at two N application rates as calculated with A Eq. (1) (equal N application rate) or B Eq. (2) (equal N uptake). Error bars represent standard error of the mean (n = 3). The black asterisks above the bars indicate significant differences between the means of the two application rates. The grey asterisks between the panels indicate significant differences between the two calculation methods (ns: p > 0.05; *: p ≤ 0.05; **: p ≤ 0.01; ***: p ≤ 0.001 ***). MF = mineral fertilizer; OF = organic fertilizer
Correlations between chemical properties of the organic fertilizers and NFRVs
As NFRV2 was found to be more reliable, correlations with chemical properties of the organic fertilizers and NFRV are only shown for NFRV2. NFRV correlated with total N concentration, PMN and C:N ratio (Fig. 5). Total N explained the variation in NFRV best (R2 = 0.86; p < 0.0001), followed by PMN (R2 = 0.75; p < 0.0001). Together, total N and PMN could explain 89% of the variation when using a multiple linear regression model (p < 0.05). NFRV was less strongly correlated to C:N ratios (R2 = 0.42; p < 0.001), although the R2 of the model with PMN and total N increased further to 0.91 when C:N was included (p < 0.05). Cellulose:lignin ratio and mineral N did not correlate with NFRV (p > 0.05), and were insignificant parameters when added to the multiple linear regression model (p > 0.5).
Covariance of the observed NFRVs (at equal N uptake, NFRV2) and (A) total N concentration, B Potential Mineralizable Nitrogen content, C C:N ratio, D cellulose:lignin ratio and E mineral N concentration. The letters correspond to the following organic fertilizers: a raw food waste; b digestate (AD); c wastewater sludge (AD); d sludge + digestate (dried) (AD); e rapeseed meal; f fishmeal; g sugarbeet pulp; h wheatbran; i larvae manure; j compost; k, Farmyard manure
Including all characteristics as explanatory variables in a step-wise multiple linear regression, total N resulted in the strongest decrease in AIC (− 25.9), followed by PMN (− 4.1) and cellulose:lignin (− 2.3) (Supplementary Table 5). Carbon:nitrogen and mineral N were excluded as being uninformative from the procedure. Total N was always the main informative parameter (i.e. causing the largest reduction in AIC) when included in the step-wise multiple linear regression procedure with any subset of chemical properties. PMN is the second most important informative parameter (after total N concentration), and always significantly reduces the AIC (> 2) when included in a subset of chemical properties. C:N ratio resulted only in a significant decrease in AIC (> 2) if total N concentration is not included, and/or if the procedure started with 20 degrees of freedom (multiple linear regression procedures where the cellulose:lignin ratio was taken along started with only 17 degrees of freedom, because of the missing values for larvae manure and fishmeal). Although cellulose:lignin ratio is not a significant parameter by itself (Fig. 5D), it can be an informative parameter and reduce the AIC (> 2) in some combinations of chemical properties (Supplementary Table 5).
Comparing the observed NFRVs to the predicted NFRVs based on linear models with (1) only total N, (2) total N and PMN and (3) total N, PMN and C:N shows that these models perform similarly well (Fig. 6). On average, the absolute difference between the predicted values and the observed values is 5.2; 4.8 and 4.1% point or 30, 32 and 28% respectively (Supplementary Table 6).
Discussion
NFRVs should be calculated at equal N uptake or equal yield
This study shows that calculating NFRVs at similar N application levels for organic and mineral fertilizer N consistently leads to an overestimation of the NFRV, especially at higher N applications. We therefore conclude that NFRV2 (calculated at equal N uptake) is the preferred method because its value is independent of the N application rate.
Calculating NFRV at equal N application (NFRV1) leads to an overestimation because the N uptake response is subject to the law of diminishing returns, while N supply between organic and mineral fertilizer differs. As a result, N uptake from the organic fertilizer is still in the linear part of the curve, whereas at equal total N application, N uptake from the mineral fertilizer is in the part of the curve which is levelling off. In our study, above a N application of 150 kg ha−1, the mineral fertilizer dose-response curve levelled off and consequently NFRV1 was increasingly overestimated at a higher dose (200 kg N ha−1). Calculation method NFRV1 is therefore only valid when applied in the linear part of a N dose-response curve for both organic and mineral fertilizer.
As it is unknown beforehand at which point N response curves start levelling off, calculating NFRVs at equal N uptake or equal yields (NFRV2) is a better method for estimating NFRVs. NFRV2 is not sensitive to diminishing returns of N application on N uptake (or yield) nor to varying N application rates between experiments and farmers’ practice. This method however requires a range of mineral fertilizer N treatments (at least four) as a reference and is therefore more time- and capital intensive than method 1, where one mineral fertilizer treatment at equal N application rate could suffice.
In a previous meta-analysis of long-term experiments, Hijbeek et al. (2018) assessed long term NFRVs for eight field experiments with an average duration of 25 years, based on equal yield. With continuous use of organic fertilizer N over multiple years, the soil N pool increases (proportionally more than for continuous use of mineral fertilizer N), which is why long term NFRVs tend to increase compared to short term NFRVs (Schröder 2005). Besides generally larger NFRVs, Hijbeek et al. (2018) also found NFRV to be increasing at higher N application rates. As explained in the paragraphs above, the explanation for this difference cannot be of a methodological nature as method 2 (at equal yield) was used in this study. Rather, the time duration of the field experiments might be an explanatory factor. When adding organic amendments over many years, the soil fertility will change (beyond the effect of N application). Apparently, this change in soil fertility is more pronounced at higher N application rates.
Chemical properties can explain NFRVs
We have shown that short-term NFRVs can be explained by chemical properties of the organic fertilizers, namely total N, PMN and C:N ratio. Both single- and multiple linear regressions resulted in total N concentration as the best explanatory variable for the NFRV (86%), followed by PMN (75%) and C:N (42%). A combination of all three characteristics explained the variability in NFRVs of the organic amendments best (91%). This confirms our hypothesis for total N and PMN. In contrast to what was expected, directly available N (i.e. the mineral N concentration) did not explain any variation in NFRVs.
The trends between chemical characteristics and NFRVs are applicable for a wider range of organic materials than the ones included in this study, and also hold under field conditions. Our results correspond well with findings from De Notaris et al. (2018), Stadler et al. (2006) and Delin et al. (2012), who also found strong relationships between total N concentration, PMN and/or C:N, and NFRVs or crop N uptake. In a pot experiment by Delin et al. (2012), PMN and C:N ratio are found to best explain variation in NFRV. Based on pot and incubation experiments with a range of soils and organic fertilizers, Stadler et al. (2006) conclude that total N concentration is positively correlated with crop N utilization as well as with net N mineralization. De Notaris et al. (2018) found a strong positive relationship between the total N concentration and NFRV (R2 > 0.73) in a field experiment.
In contrast to our study, De Notaris et al. (2018) found a significant correlation with ammonium-N concentration and the NFRV in a field experiment. However, this was only the case when the organic fertilizer was applied during crop growth, but not when the organic fertilizer was applied prior to crop establishment, as we did in our study. If the organic fertilizer is applied prior to the establishment of the crop, the eventual mineralization of total N might overrule the effect of directly available mineral N. Moreover, the organic fertilizers in our experiment had only a small proportion of mineral N, namely between 0.2 and 4.9% of total N (equivalent to 0.4–9.8 kg mineral N ha−1 at a dose of 200 kg total N ha−1). This may explain why we did not find a significant correlation between NFRV and the mineral N concentration.
We have shown that total N concentration and PMN correlate well with NFRVs. The method of determining the mineralization rate (as PMN) may however be relevant. Delin et al. (2012) carried out a quick and an extensive incubation trial (7 vs. 28 days) to measure the PMN (mg Nmin kg soil−1), in which the extensive aerobic trial of 28 days led to a higher R2 compared to the 7 days anaerobic method (0.78 vs. 0.34). However, in our study the 7 day anaerobic PMN method did appear to be an adequate predictor for NFRV (R2 = 0.75). Differences with the results of Delin et al. (2012) might be explained by the fact that they used a lower application rate (0.14 vs. 0.19 mg N g soil−1) and a different set of fertilizers with a smaller range of C:N ratios (2.3–13.5 vs. 3.6–28.7) and a larger range in total N (3.5–144 vs. 14.3–102 g N kg−1). Since the relationship between NFRV and C:N ratio appears less strong when C:N > 10, this would also explain why Delin et al. (2012) found a much stronger negative correlation between C:N and NFRV than we did in our study. Whereas most studies find the total N concentration to be a good predictor, it appears that the predictive power of C:N and PMN is affected by amendment type and analytical method.
In synthesis, we argue that it is possible to make relatively good estimations of the short-term NFRV of an organic fertilizer based on only its total N concentration. The increased R2 suggests that PMN and C:N ratio could lead to even better estimations, although the contributions of these characteristics were small compared to total N. By comparing the observed and the predicted NFRVs we show that the model with only total N performs equally well as the models including PMN and C:N (30 vs. 28% deviation respectively). The relatively large deviation from the observed value (30%) is mainly caused by deviations in the low NFRVs, where a small deviation leads to a large percentual difference. In percent points, the deviation is only 5.2% when using only total N as an explanatory variable. There are no clear outliers, suggesting that the model performs well for all organic fertilizers. We hereby show that solely the total N concentration of an organic amendment is sufficient to make a relatively good prediction of its NFRV, and there is no need for elaborate incubation trials nor chemical analyses to make accurate fertilizer recommendations. The dataset of this study may however be too limited to extrapolate the correlation between total N and NFRV (Fig. 5) accurately to other conditions and crop types. Predicted NFRVs calculated from the total N concentration of the organic fertilizers in De Notaris et al. (2018) and Delin et al. (2012) deviate on average 5, 17 and 24% point from the observed values for winter wheat, rye grass and spring barley respectively (Supplementary Table 7). For the purpose of developing fertilizer recommendations based on chemical characteristics, the strength of the correlation between chemical parameters and NFRVs should be further investigated using experimental field studies on different soils and in different climate zones.
Limitations of the methodological approach
The absolute values of the short-term NFRVs calculated in this study cannot immediately be used for long-term NFRVs in field conditions for several reasons. As shown by Pinto et al. (2020) patterns of N mineralization from organic fertilizers can be different between laboratory- and field incubations. This is most likely also the case for pot and field conditions. Compared to field conditions, the N mineralization rates in a pot experiment are expected to be higher due to the relatively high average temperature in the greenhouse, leading to higher short-term NFRVs. The temporal and spatial availability of both mineral N and mineralized N from organic fertilizers are expected to be more limited in field conditions due to losses and a lower root density. In subsequent years N mineralization and thereby long-term NFRVs are expected to increase, provided that the losses of N through volatilization and leaching remain limited. Nonetheless, our conclusion that NFRVs should be calculated at equal N uptake instead of N application rates holds under field conditions as yield response curves are known to have similar shapes under field conditions. In addition, we expect that total N, PMN and C:N can still predict NFRVs in field conditions. The relationships may however become less strong because mineralization is affected by other confounding factors such as soil type, climatic conditions and timing and placement of the fertilizers. Calculation of long-term NFRVs based on chemical properties and/or short-term NFRVs would require consideration of all these factors.
The chemical properties of the organic amendments have not been measured in triplicate in our study (except for PMN), although some degree of heterogeneity can be expected in organic substances. This may have led to some inaccuracy in the N application rates and the regression analysis, and may explain why at 200 kg N ha−1 the N uptake in the fishmeal treatment was higher than in the mineral N treatment. Another explanation could be that fishmeal has some other properties which are beneficial for crop growth, beyond the range of macro and micronutrients applied in all treatments. If N uptake from an organic fertilizer is larger than the top of the response curve to mineral fertilizer N, NFRV cannot be calculated using Eq. (2) (at equal N uptake) as there is no corresponding yield available from the response curve, something to bear in mind when making an experimental set-up.
Conclusions
Standardization of NFRV calculation methods is necessary to enable a better and faster evaluation of upcoming organic fertilizers, to make more specific N fertilizer recommendations, and to support farm profitability and enhance environmental sustainability. This pot experiment has provided interesting insights in the effect of N application rates and calculation methods on the NFRVs and gives an indication of the N supplying capacity of organic fertilizers of different origins and compositions. Based on the results of this study we recommend calculating NFRV based on equal N uptake (or yield) from organic- and mineral fertilizers, as opposed to a calculation based on equal N application rates.
To determine the long-term NFRVs of upcoming organic fertilizers, new and long-term field experiment are needed. We have shown that short-term NFRVs can be explained well based on a number of chemical characteristics, especially total N concentration. Field experiments are required to test if the relations remain valid for long-term NFRVs and if or how the coefficients might change. If so, this would enable the development of specific recommendations for upcoming organic fertilizers based on a limited set of product characteristics.
References
Arnold TW (2010) Uninformative parameters and model selection using Akaike’s information criterion. J Wildl Manag 74(6):1175–1178. https://doi.org/10.1111/j.1937-2817.2010.tb01236.x
Cerrato ME, Blackmer AM (1990) Comparison of models for describing; corn yield response to nitrogen fertilizer. Agron J 82(1):138–143. https://doi.org/10.2134/agronj1990.00021962008200010030x
De Notaris C, Sørensen P, Møller HB, Wahid R, Eriksen J (2018) Nitrogen fertilizer replacement value of digestates from three green manures. Nutr Cycl Agrosyst 112(3):355–368. https://doi.org/10.1007/s10705-018-9951-5
Delin S, Stenberg B, Nyberg A, Brohede L (2012) Potential methods for estimating nitrogen fertilizer value of organic residues. Soil Use Manag 28(3):283–291. https://doi.org/10.1111/j.1475-2743.2012.00417.x
George BJ (1984) Design and interpretation of nitrogen response experiments. In Nitrogen Requirement of Cereals: Proceedings of a Conference Organised by the Agricultural Development and Advisory Service, September 1982. https://agris.fao.org/agris-search/search.do?recordID=US201301429842
Gutser R, Ebertseder T, Weber A, Schraml M, Schmidhalter U (2005) Short-term and residual availability of nitrogen after long-term application of organic fertilizers on arable land. J Plant Nutr Soil Sci 168(4):439–446. https://doi.org/10.1002/jpln.200520510
Hijbeek R, ten Berge HFM, Whitmore AP, Barkusky D, Schröder JJ, van Ittersum MK (2018) Nitrogen fertiliser replacement values for organic amendments appear to increase with N application rates. Nutr Cycl Agrosyst 110(1):105–115. https://doi.org/10.1007/s10705-017-9875-5
Jensen LS (2013) Animal manure fertiliser value, crop utilisation and soil quality impacts. Anim Manure Recycl Treat Manag. https://doi.org/10.1002/9781118676677.ch15
Nkoa R (2014) Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: a review. Agron Sustain Dev 34(2):473–492
Pinto R, Brito LM, Coutinho J (2020) Nitrogen mineralization from organic amendments predicted by laboratory and field incubations. Commun Soil Sci Plant Anal 51(4):515–526. https://doi.org/10.1080/00103624.2020.1717510
Probert ME, Delve RJ, Kimani SK, Dimes JP (2005) Modelling nitrogen mineralization from manures: representing quality aspects by varying C:N ratio of sub-pools. Soil Biol Biochem 37(2):279–287. https://doi.org/10.1016/j.soilbio.2004.07.040
Reussi Calvo NI, Wyngaard N, Orcellet J, Rozas S, Echeverría HE (2018) Predicting field-apparent nitrogen mineralization from anaerobically incubated nitrogen. Soil Sci Soc Am J 82(2):502–508. https://doi.org/10.2136/sssaj2017.11.0395
Sala F, Boldea M, Rawashdeh H, Nemet I (2015) Mathematical model for determining the optimal doses of mineral fertilizers for wheat crops. Pak J Agric Sci 52:609–617
Schils R, Schröder J, Velthof G (2020) Fertilizer replacement value. In: Meers E, Velthof G, Michels E, Rietra R (eds) Biorefinery of inorganics. Wiley, New Jersey, pp 189–214. https://doi.org/10.1002/9781118921487.ch5-1
Schröder J (2005) Revisiting the agronomic benefits of manure: a correct assessment and exploitation of its fertilizer value spares the environment. Bioresour Technol 96(2):253–261. https://doi.org/10.1016/j.biortech.2004.05.015
Sigurnjak I, Michels E, Crappé S, Buysens S, Biswas JK, Tack FMG, Neve SD, Meers E (2017) Does acidification increase the nitrogen fertilizer replacement value of bio-based fertilizers? J Plant Nutr Soil Sci 180(6):800–810. https://doi.org/10.1002/jpln.201700220
Soest PJV, McQueen RW (1973) The chemistry and estimation of fibre. Proc Nutr Society 32(3):123–130. https://doi.org/10.1079/PNS19730029
Stadler C, von Tucher S, Schmidhalter U, Gutser R, Heuwinkel H (2006) Nitrogen release from plant-derived and industrially processed organic fertilizers used in organic horticulture. J Plant Nutr Soil Sci 169(4):549–556. https://doi.org/10.1002/jpln.200520579
Teglia C, Trémier A, Martel JL (2010) Characterization of solid digestates: part 1, review of existing indicators to assess solid digestates agricultural use. Waste Biomass Valoriz 2(1):43–58. https://doi.org/10.1007/s12649-010-9051-5
Tsachidou B, Daigneux B, Hissler C, George I, Delfosse P (2019) Biogas residues in substitution for chemical fertilizers: converting organic waste into resource [Application/pdf]. In Proceedings of the 27th European biomass conference and exhibition, 27–30 May 2019, 7 Pages. https://doi.org/10.5071/27THEUBCE2019-4AV.3.32
Valve H, Ekholm P, Luostarinen S (2020) The circular nutrient economy: needs and potentials of nutrient recycling. Handb Circ Econ. https://doi.org/10.4337/9781788972727.00037
van Grinsven HJM, Ebanyat P, Glendining M, Gu B, Hijbeek R, Lam SK, Lassaletta L, Mueller ND, Pacheco FS, Quemada M, Bruulsema TW, Jacobsen BH (2022) Establishing long-term nitrogen response of global cereals to assess sustainable fertilizer rates. Nat Food. https://doi.org/10.1038/s43016-021-00447-x. (Nat Food 1–11 and ten Berge, H. F. M)
Van Soest PJ (1973) Collaborative study of acid-detergent fiber and lignin. J Assoc Off Anal Chem 56(4):781–784. https://doi.org/10.1093/jaoac/56.4.781
van Zwieten L (2018) The long-term role of organic amendments in addressing soil constraints to production. Nutr Cycl Agrosyst 111(2):99–102. https://doi.org/10.1007/s10705-018-9934-6
Waring SA, Bremner JM (1964) Ammonium production in soil under waterlogged conditions as an index of nitrogen availability. Nature 201(4922):951–952. https://doi.org/10.1038/201951a0
Acknowledgements
We thank Willeke van Tintelen, Tamas Salanki, Hennie Halm and Jelmar Jongman for their practical support during the experiments. We would like to thank Ben van Selm and Alejandro Parodi for their support in sourcing the larvae manure and animal feed products. We would furthermore like to thank Twan Geraedts (EcoFuels) for supplying the anaerobic digestion products, and Andries Siepel (Droevendaal farm) and Adrie Veeken (Attero) for supplying the farmyard manure and compost respectively.
Author information
Authors and Affiliations
Contributions
DW, RH and EH have designed the study. DW performed the experiments. DW and RH conducted the statistical analysis. DW, RH and EH wrote the main manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Westerik, D., Hoffland, E. & Hijbeek, R. Nitrogen fertilizer replacement values of organic amendments: determination and prediction. Nutr Cycl Agroecosyst (2023). https://doi.org/10.1007/s10705-023-10316-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10705-023-10316-7