Abstract
Heart failure with preserved ejection fraction (HFpEF) is rapidly growing as the most common form of heart failure. Among HFpEF phenotypes, the cardiometabolic/obese HFpEF — HFpEF driven by cardiometabolic alterations — emerges as one of the most prevalent forms of this syndrome and the one on which recent therapeutic success have been made. Indeed, pharmacological approaches with sodium-glucose cotransporter type 2 inhibitors (SGLT2i) and glucagon-like peptide-1 receptor agonists (GLP-1RA) have proved to be effective due to metabolic protective effects. Similarly, lifestyle changes, including diet and exercise are crucial in HFpEF management. Increasing evidence supports the important role of diet and physical activity in the pathogenesis, prognosis, and potential reversal of HFpEF. Metabolic derangements and systemic inflammation are key features of HFpEF and represent the main targets of lifestyle interventions. However, the underlying mechanisms of the beneficial effects of these interventions in HFpEF are incompletely understood. Hence, there is an unmet need of tailored lifestyle intervention modalities for patients with HFpEF. Here we present the current available evidence on lifestyle interventions in HFpEF management and therapeutics, discussing their modalities and potential mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heart Failure with preserved ejection fraction (HFpEF) currently represents the most common form of heart failure (HF) [1], and its prevalence is increasing by 10% per decade relative to HF with reduced ejection fraction (HFrEF) [2]. This gap is expected to increase further in the coming years as a result of the cardiovascular aging of the population and the increasing prevalence of HFpEF-predisposing conditions, such as hypertension, obesity, metabolic syndrome (MetS), and diabetes in particular [3, 4]. Although HFpEF presents with similar symptoms as in HFrEF, it shows different pathophysiological mechanisms, with the transition from HFpEF to HFrEF being rare [3, 5]. HFrEF cornerstone neurohormonal therapies have failed to improve outcomes in HFpEF, shifting the therapeutic target in HFpEF towards metabolic-based pharmacological strategies [6]. Indeed, only novel pharmacological approaches such as sodium-glucose cotransporter type 2 inhibitors (SGLT2i) and glucagon-like peptide-1 receptor agonists (GLP-1RA) have revealed favorable impacts on clinical outcomes in HFpEF, improving quality of life of patients due to their metabolic protective effects [7, 8].
HFpEF presents a large phenotypical heterogeneity coupled with a high comorbidity burden and a complex multiorgan systemic pathophysiology [9]. Among various HFpEF phenotypes, the cardiometabolic/obese HFpEF — elicited by metabolic alterations — represents the most prevalent form of this syndrome [10]. According to the World Obesity Atlas 2023 report, 38% of the population worldwide is currently either overweight or obese, and by 2035, the global overweight and obesity prevalence is expected to reach 51% [11]. A body mass index (BMI) of > 25 kg/m2 is associated with a greater risk of HFpEF than HFrEF [12] and more than 80% of patients with HFpEF are overweight or obese [13]. Obesity contributes to risk factors for MetS, a condition characterized by the coexistence of visceral adiposity, dyslipidemia, type 2 diabetes, and hypertension strongly predicting HFpEF [14]. The increasing prevalence of diabetes is also reported worldwide by epidemiological data, raising from 30 to 400 million people since 1985 [15]. Western diet (WD), composed of high saturated fat and sugar [16] and associated with a Western lifestyle of sedentary behavior in the form of prolonged sitting during work and transportation [17], is an important modifiable risk factor for cardiometabolic HFpEF. Saturated fats and refined carbohydrates produce a high caloric influx into adipose tissue and often exceed the storage capacity of adipocytes. This causes increased serum lipids, enhanced lipid uptake by non-adipose tissues, and ectopic lipid accumulation [18].
The American Heart Association created “Life’s simple 7” measures to achieve ideal cardiovascular health including (1) quitting smoking, (2) eating healthy, (3) being active, (4) losing weight, (5) managing blood pressure (BP), and (6) controlling of cholesterol and (7) plasma glucose levels [19]. Diet and lifestyle changes play a pivotal role in the prevention and treatment of cardiovascular disease and beneficial effects based on AHA measures are well documented in HF [20]. Complying with these measures seems to be particularly important for HFpEF [21]. Indeed, dietary habits have been involved in the pathogenesis [22,23,24], prognosis [25,26,27], and potential reversal of HFpEF [28]. Similarly, in HFpEF, exercise training shows beneficial effects on diastolic disfunction, enhances skeletal muscle structure and function, and reduces adiposity and inflammation [29,30,31,32]. However, the specific impact of different types of lifestyle intervention on mechanisms of HFpEF remains largely unknown.
Metabolic derangements and systemic inflammation in cardiometabolic HFpEF
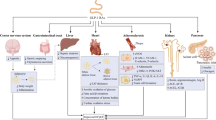
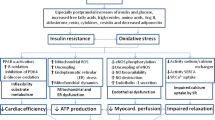
Metabolic derangements and systemic inflammation are reported as the main pathophysiological features of HFpEF (Fig. 1) [18]. The presence of insulin resistance (IR) and oxidative stress is well documented in HFpEF, resulting as the common hallmarks of cardiometabolic comorbidities [15, 33, 34]. IR worsens glucose uptake and utilization in cardiomyocyte and triggers cardiac metabolic remodeling, shifting from glucose oxidation to fatty acids oxidation (FAO) via the Randle cycle [35]. Altered cardiac substrate utilization in HFpEF is another key aspect of HFpEF pathophysiology triggering metabolic remodeling. Clinical [36, 37] and pre-clinical [38] studies suggested suppressed fatty acids (FAs) metabolism in HFpEF hearts, using indirect measurements of cardiac energy metabolism. However, direct flux measurements revealed an altered metabolic profile towards a switch in substrate utilization from glucose oxidation to FAO [39]. These findings are in line with previous results obtained in obesity and diabetes, showing up-regulated FAO [39,40,41] accompanied by decreased glucose oxidation [42,43,44]. Increased lipolysis in adipose tissue due to IR [45] and excessive reliance on free FAs [18] are linked to up-regulated uptake of FAs in cardiomyocytes [39]. The resulting lipids overload leads to the accumulation of lipotoxic intermediates — as diacylglycerols (DAGs), ceramides, and triglycerides (TGs) [35] — oxidative stress [18], and altered ATP production [46].
A healthy adult heart requires around 6 kg of ATP per day, representing a highly energy-demand organ [47]. Diastole — in which ATP is used to break actomyosin cross-links allowing cardiac relaxation — represents the most energetically demanding phase of cardiac cycle [48]. Most of ATP heart sources rely on free fatty acids (FFAs) oxidation (~ 70%), with glucose, ketone bodies (KBs), and amino acids playing a complementary role as alternative substrates [35]. Healthy cardiac tissue is metabolically flexible, adapting its substrate usage based on nutrient availability, local and systemic conditions, allowing ATP generation to continue in fed, fasting, and high-demand states [49]. Conversely, a failing heart is typically characterized by a loss of metabolic flexibility [49] and fails to respond to dynamic changes in energy demand. Indeed, patients with HFpEF show a 20–27% reduction in phosphocreatine (PCr)/adenosine triphosphate (ATP) ratio, which represents an index of the energetic state of the heart and reflects the balance of energy consumption and energy supply in the heart [50,51,52]. ATP provides a direct energy source for cellular reactions, while PCr acts as an energy storage and transport compound via the “creatine kinase-PCr energy shuttle” [53]. PCr buffers ATP in cardiomyocytes during high demand conditions. A low ratio between these high-energy phosphate compounds in human hearts, as non-invasively assessed with 31Phosphorus magnetic resonance spectroscopy (31P-MRS), suggests compromised mitochondrial function [54]. Previous clinical studies demonstrated that this ratio is reduced in failing human myocardium [53]. In obesity and diabetes loss of metabolic flexibility is associated with impaired glucose oxidation and concomitant cardiac hypertrophy and dysfunction [55]. Thus, strategies aiming to restore the resilience between energy substrates are warranted to maintain the ATP production in HFpEF [56].
A systemic low-grade inflammation stemming from comorbidities-driven metabolic derangements (i.e., meta-inflammation) represents the other key feature of HFpEF [57], implying an increased burden of oxidative and nitrosative stress [58, 59]. Metabolic derangements, such as hyperglycemia and increased adiposity, promote the release of cytokines and pro-inflammatory adipokines, triggering systemic inflammation and immune alterations [18, 60, 61]. Moreover, evidence in hypertensive patients reports an association between hyperglycaemia and increased risk of diastolic dysfunction even in the absence of diabetes [62]. Adipocyte-derived saturated FAs (SFAs) activate toll-like receptor 4 (TLR4) in macrophages, causing the release of tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) [18]. The latter affects directly cardiomyocytes, stimulating mitogen-activated protein kinases (MAPKs) and nuclear factor kappa-light-chain-enhancer of activated B (NFkB) signaling, inhibiting Akt, and promoting diastolic dysfunction [63, 64]. Systemic meta-inflammation, together with the paracrine effects of epicardial tissue, elicits HFpEF cardiac remodeling through increasing cardiomyocyte hypertrophy and myocardial fibrosis [18].
Moderate weight loss of 5–10 kg through dietary and exercise interventions results in a clinically meaningful reduction of cardiometabolic risk [65]. Interestingly, weight loss leads to lower myocardial oxygen consumption and decreased myocardial FAO [66], increasing myocardial glycolysis, myocardial glucose oxidation [67], and PCr/ATP in obese patients [68]. In addition, weight loss decreases circulating lipids, improves IR and inflammation [69, 70] and reduces systolic BP by at least 1 mmHg per kg of weight loss [71].
Dietary management of HFpEF
Dietary management of HFpEF provides benefits to the cardiovascular and muscle-skeletal system as a whole [20]. Importantly, most of the evidence to date were collected in HF mixed populations, with a limited number of studies focusing on HFpEF subjects (Table 1).
Manipulation of micro/macronutrients or modulation of specific clinical traits
Manipulation of single micro/macronutrients or modulation of a specific clinical trait has been adopted as a potential dietary strategy for patients with HFpEF.
Management of salt intake has been associated with significant amelioration in quality of life (QoL) and outcomes in HF subjects, reducing congestion and edema [72,73,74,75]. However, the effects of sodium restriction in HFpEF remain controversial. Aggressive sodium and fluid restriction in 53 decompensated HFpEF patients showed no neurohormonal benefits [76]. In addition, an observational study [77] analyzing data from the TOPCAT trial [78] found an association between overstrict dietary salt restriction and worse prognosis in HFpEF patients.
Unsaturated fatty acids (UFAs) comprise monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs) and are associated with favorable cardiovascular outcomes in obese and hypertensive patients [27, 79]. Foods rich in MUFAs are olive oil, avocados, nuts, and seeds, while sources of PUFAs are fatty fish, flaxseeds, chia seeds, walnuts, sunflower, and corn oil [79, 80]. Most MUFAs and ω-3 PUFAs, such as alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) show protective effects for metabolic and physiological processes, as well as inflammatory response [80]. The beneficial effect of UFAs on improving insulin sensitivity has been reported in vitro [81, 82] and in vivo studies [83, 84]. Studies on evaluating the effects of UFAs for HFpEF patients are extremely limited. Only one completed trial (NCT03310099) reported that an UFA-rich foods diet consumption for 84 days in 9 obese symptomatic HFpEF patients improved cardiorespiratory fitness (CRF) and clinical outcomes [85] and one on-going trial [the UFA-Preserved 2 (NCT03966755)] is set to follow up on this. Further studies are needed to fully understand the role of UFAs in the management of HFpEF.
Carbohydrate manipulation may represent another dietary strategy in HFpEF. Significant weight reduction following a low carbohydrate diet for 16 weeks (ACTRN12620001278921) is reported in patients with diabetic cardiomyopathy (DMCM) [86]. A low carbohydrate diet, which falls below 130 g of carbohydrates per day, may improve systemic IR, whole-body metabolism, and tissue functions [87]. Moreover, it shows favorable effects on low-grade inflammation in patients with type 2 diabetes (T2D) [88]. A low carbohydrate diet for 2 months was found to improve oxygen saturation in HF [89], but the clinical relevance could not be established [90].
A strategy for obesity management is calorie restriction (CR). Indeed, CR — i.e., reduction of caloric intake by 30–40% — shows positive effects on cell metabolism, resulting in weight loss and reducing systemic inflammation and oxidative stress [91]. Moreover, CR improves metabolic parameters, such as insulin sensitivity and lipid metabolism [92, 93]. CR or interventions aiming to rescheduling the time of feeding during the day [intermittent fasting and time restricting eating (TRE)] demonstrated to reduce cardiovascular events in obesity, diabetes, and metabolic syndrome [91]. A significant improvement in VO2 peak was shown in 100 obese patients with HFpEF treated with CR and aerobic exercise training for 20 weeks (NCT00959660), suggesting an additive effect of both interventions in obese HFpEF [94]. In addition, a 6-month CR diet program in 38 obese hypertensive HFpEF patients followed by > 5 kg weight reduction, led to reduction in NT-proBNP circulating levels. This was followed by an improvement in diastolic function and 6 minute walk distance (6MWD) [95]. CR inhibits the IGF-1/insulin pathway and improves protein quality control in skeletal muscle [96]. CR may reverse mitochondrial dysfunction in aging muscle stem cells (MuSCs) restoring myofiber growth and intrinsic muscle function, showing beneficial effects on muscle oxygen supply, exercise capacity, and QoL of HFpEF patients [97]. In support of this, intermittent fasting, reached by limiting caloric intake to 8 hours during the day-time, reduced cardiovascular risk in resistance-trained men [98]. In addition, a program of 10 hours-TRE for 12 weeks reduced BP and LDL cholesterol levels in patients with MetS [99]. Intermittent fasting and TRE induced a shift from fat to ketone metabolism and modulation of cellular adaptive responses, such as autophagy [91, 100]. A calorie-restricted high-protein diet for 3 months (30% protein, 40% carbohydrates, and 30% fat) in a HF population, including a 43.3% of HFpEF subjects [90], reduced cardiometabolic risk with significant improvements in BP in comparison to a standard-content protein diet (15% protein, 55% carbohydrates, and 30% fat) [101]. Thus, CR shows significant cardiometabolic effects, such as improving cardiorespiratory fitness (CRF), reducing body weight, ameliorating insulin sensitivity and glucose metabolism, improving lipid profile, and reducing systemic inflammation. These effects make CR potentially clinically relevant in the treatment of patients with HFpEF.
Supplementation of several synthetic and natural compounds — known as calorie restriction mimetics (CRMs) — may represent a valid alternative to CR, mimicking its physiological and molecular effects [91]. Examples of CMRs are represented by spermidine, resveratrol, curcumin, and epigallocatechin-3-gallate, which have shown promising results in mouse models [91]. In particular, spermidine — a naturally occurring polyamine found in soybeans, mature cheese, mushrooms, and broccoli — promotes cardioprotective autophagy [102] and attenuates cardiac senescence due to prevention of oxidative stress and improvement in mitochondrial function in preclinical HFpEF [103, 104]. In addition, the anti-inflammatory properties of spermidine are reported through inducing anti-inflammatory (M2) macrophage expression and decreasing TNF-α levels [105, 106]. Clinical implications of spermidine supplementation in HFpEF and cardiometabolic diseases are currently still unclear.
Dietary supplementations
Dietary supplementation studies tested the effects of dietary micro/macronutrients in HFpEF in the form of tablet, capsule, liquid, or powder.
Inorganic nitrate/nitrite (IN) supplementation in HFpEF showed BP lowering effect, especially during exercise [107]. Moreover, the anti-inflammatory effects of IN supplementation in atherosclerosis and systemic inflammation have been reported [108]. However, a recent clinical trial in 92 patients with HFpEF (NCT02713126) demonstrated that IN supplementation (40 mg, three times daily) for 12 weeks did not provide additional benefits from exercise training [109]. A meta-analysis of 8 randomized controlled trials (RCTs) confirmed the absence of benefits of IN supplementation in improving exercise capacity in HFpEF [107].
The REDUCE-IT trial revealed the potential therapeutic benefit of icosapent ethyl supplementation in reducing cardiovascular risk by targeting inflammation in patients with HF (NCT01492361). Icosapent ethyl showed anti-inflammatory properties by reducing the level of high-sensitivity C-reactive protein (hs-CRP) as an inflammatory biomarker, alongside its lowering effects on triglyceride levels from baseline to 2 years compared to placebo [110]. Further studies are needed to confirm the veracity of these effects in the context of systemic inflammation in patients with HFpEF.
Coenzyme Q10 (CoQ10) supplementation in T2D db/db mouse models revealed attenuation of diastolic dysfunction and cardiac remodeling [111]. Supplementation of CoQ10 is also associated with a rise in adiponectin levels, which in turn leads to a decrease in inflammatory response mediated by TNF-α [112]. The Q-SYMBIO trial [113] reported that a long-term CoQ10 supplementation reduced major adverse cardiovascular events and improved symptoms in patients with chronic HF, including 7% HFpEF [90]. In addition, a short-term CoQ10 supplementation (30 days) in 30 HFpEF patients led to statistically significant within-group changes in diastolic function, despite these were not significantly different from the control [114]. Ubiquinol — the active form of CoQ10 — and D-ribose showed a positive impact on HFpEF symptoms in a RCT study (NCT03133793) [115]. However, a RCT in elderly HFpEF patients (NCT02779634) reported no effects of CoQ10 supplementation (100 mg, three times daily) on diastolic function [116].
L-carnitine is an amino acid derivative that plays a critical role in lipid metabolism through transporting long-chain FAs to mitochondria for oxidation [117]. Decreased L-carnitine content has been reported in the failing heart [118]. L-carnitine may prevent myocardial fibrosis and HFpEF, through enhanced production of prostacyclin [119], and has been shown to promote weight loss, improve IR, and reduce appetite and food intake through a direct effect on the hypothalamus in obese adults [120]. In addition, administration of L-carnitine in animal with myocardial infarction shows effects in reducing oxidative stress and enhancing antioxidant enzyme activity through the inhibition of TNF-α and IL-1β [121]. Eighteen patients with HFpEF, presenting reduced L-carnitine at the baseline level, were supplemented with L-carnitine (300 mg daily) for 1 year (UMIN000011905) [122]. The study reported significant weight loss but no improvements in left ventricular (LV) diastolic function.
Vitamin D (VD) deficiency is associated with reduced functional capacity in patients with diastolic dysfunction or HFpEF [123]. Low VD levels are also associated with impaired glucose tolerance in nondiabetic hypertensive patients and may contribute to organ damage [124]. Serum 25-hydroxyvitamin D [25(OH)D] levels < 50 nmol/L have been associated with increased LV mass and LV hypertrophy in hypertensive patients [125]. VD appears to have cardiovascular protective effects by modulating inflammatory cytokines, reducing oxidative stress, and regulating the systemic renin–angiotensin–aldosterone system [126, 127]. VD supplementation improves glycaemic homeostasis and insulin sensitivity among adults at risk for T2D [128] and showed anti-inflammatory properties in a population of healthy Saudi males [129]. These effects point to potential positive effects on cardiometabolic health in patients with HFpEF. However, a 6-month VD supplementation (50,000 IU of vitamin D3 daily and calcium citrate 400 mg twice daily) in a mixed HF population (NCT01125436) showed no beneficial effects on aerobic capacity and physical performances [130].
Subtle differences in regulation of cortisol levels in hypertensive patients are associated with impaired glucose tolerance and IR [131], and minimal excess of cortisol in hypertensive patients contributes independently to LV hypertrophy and concentric remodeling, potentially contributing to LV diastolic dysfunction and HFpEF [132]. Supplementation of ω-3 PUFAs showed an association with lower cortisol levels and inflammation [133] and prevented fibrosis and diastolic dysfunction in transverse aortic constriction (TAC) animal models with pressure overload-induced cardiac hypertrophy, by activation of the cyclic guanosine monophosphate (GMP)/protein kinase G pathway in cardiac fibroblasts [134]. These findings suggest the clinical potential of ω-3 PUFAs supplementation. The MESA study [135] found an association between higher plasma EPA and lower risk of HF, including HFrEF and HFpEF. In addition, a retrospective study on 140 hospitalized decompensated HFpEF patients indicated that low DHA plasma levels were associated with an increase in all-cause death, suggesting a potential role of DHA for diagnosis and therapies in such patients [136]. The GISSI-HF trial in a mixed HF population (HFrEF and HFpEF), including 634 patients with HFpEF [90], revealed beneficial effects of treatment with ω-3 PUFAs towards reduced mortality and hospitalization [137]. The OCEAN trial [138] showed that supplemented EPA + DHA in a 2:1 ratio (four capsules of 400/200 EPA/DHA 500 mg per capsule daily) and EPA alone (four capsules of almost pure EPA 500 mg per capsule daily) for 12 weeks led to improved cognitive depressive symptoms related to HF. A clinically relevant improvement in physical function was also reported [138], given that the HF population was composed of 35% patients with HFpEF [90].
Protein supplementation (1.2 g/kg bodyweight per day) associated with low-intensity exercise in 23 obese HFpEF patients for 12 weeks showed benefits on physical and cardiovascular function [139]. In another RCT in a mixed HF population (NCT02240511), branched-chain amino acid (BCAA) supplementation (10 g daily) for 3 months was associated with resistance exercise (RE) [140]. BCAAs are supposed to have an anabolic effect in HF patients, acting as “fuel” during exercise and maintaining muscle mass metabolism [141]. The study did not find benefits from BCAA supplementation and beneficial effects in VO2 peak were attributed to resistance exercise program [140].
Dietary regimens
Dietary regimen studies tested the effectiveness of manipulation of foods and beverages composing the entire diet regimen.
The GOURMET-HF trial [142], including both HFrEF and HFpEF patients, demonstrated that the Dietary Approach to Stop Hypertension (DASH)/sodium-restricted (SDR) diet has a favorable trend in rehospitalization at 30 days. Other studies [143, 144] confirm the effectiveness of the DASH/SDR diet in treating hypertension, reducing 24-h systolic and diastolic BP, and improving diastolic LV relaxation, chamber stiffness, and ventricular-arterial coupling in HFpEF patients. The DASH-DHF 2 trial (NCT01942395) has been designed to confirm the findings of earlier studies in HFpEF patients with history of hypertension. Another clinical trial (NCT05236413) has been recently designed to evaluate the effects of the DASH diet combined with high-intensity interval training (HIIT).
The effects of a low-energy diet (LED) in reduction of myocardial steatosis and improving of diastolic filling in T2D are well known [145]. LED through a low-energy meal replacement plan (MRP) has been proposed as an alternative to achieve weight loss and improve cardiovascular outcomes. This dietary pattern comprises an average of approximately 810 kcal/day (30% protein, 50% carbohydrate, and 20% fat) [146]. Low-energy MRP leads to weight loss, improvement of diabetes-related cardiometabolic risk [147], and reverse of cardiovascular remodeling in obese adults with T2D [146]. The ALLEVIATE trial aims to evaluate the impact of low-energy MRP on symptomatology, physical activity, and QoL in patients with HFpEF and diabetes (NCT04173117). The AMEND trial (NCT05887271) is currently evaluating the results of low-calorie replacement plan in obese HFpEF adults.
The ketogenic diet (KD) is widely adopted to reach weight loss through increased lipolysis [148]. A recent study in a pre-clinical setting [149] showed that ketone supplementation can ameliorate the HFpEF phenotype in mice. Ketone body usage in HFrEF patients showed beneficial hemodynamic effects [150], and clinical studies in HFpEF are awaited. However, caution is needed because of evidenced detrimental effects of KD on cardiovascular health, raising circulating FA levels, which contribute to cardiac lipotoxicity and adversely modifies cardiac muscle energy metabolism [151]. An on-going RCT (NCT04235699) is designed to evaluate the effects of a low carbohydrate KD on exercise tolerance in patients with HFpEF. Another on-going trial (NCT06081543) is designed to evaluate the effects of a low carbohydrate KD versus a low-fat diet on exercise tolerance in participants with HFpEF and diabetes, pre-diabetes, or MetS, or obesity. A prospective pilot study (NCT04942548) aims to examine the impact of low carbohydrate KD on functional and clinical outcomes, and QoL in patients with HFpEF and related pulmonary hypertension HFpEF (PH-HFpEF).
The Mediterranean diet (MedDiet) indicates a dietary pattern including daily consumption of non-refined cereals, olive oil as the principal source of lipids, moderate intake of fish, poultry, potatoes, eggs, and sweets; monthly consumption of red meat, and regular physical activity [152]. The diet involves moderate consumption of alcohol with meals, preferably red wine [152]. Excess alcohol intake might contribute to development of HFpEF and hypertension related organ damage [153, 154]. MedDiet is composed of bioactive molecules, such as ω-3 PUFAs (e.g., EPA, DHA), MUFAs (e.g., oleate), and polyphenols, which confer cardioprotective effects [152]. The MEDIT-AHF trial observed that a greater adherence to MedDiet was associated with a significant reduction in HF hospitalizations following an admission for acute HF, although not with reduced long-term mortality [155]. The PREDIMED trial revealed the positive effects of MedDiet on systemic inflammation markers in patients with HF and MetS [156, 157]. The Hellenic Heart Failure Study, which included 38% of patients with HFpEF, confirmed these positive effects, opening new horizons about its potential benefits [158].
Other dietary regimens might have a positive impact on HFpEF. For instance, plant-based diets such as vegan, lacto-ovo vegetarian, and pesco-vegetarian offer positive effects on cardiometabolic health [159]. Vegetarian diets reduce BP, blood glucose, and lipids levels, with a positive impact on inflammation and body weight [159]. The effects of vegetarian diets in HFpEF should be further explored.
In summary, dietary management for HFpEF exhibits various effects on cardiovascular and metabolic health. Modulation of specific nutrients or manipulation of body composition with CR hold promise but require further validation to pave the way for tailored dietary interventions. Dietary supplementation and regiment studies in HFpEF have, to date, yielded mixed results. While partially dietary supplementation or regiments show promise, more targeted and extensive studies are required to establish their efficacy.
Physical activity in HFpEF
The American College of Cardiology (ACC)/American Heart Association (AHA) guidelines include a Class 1 recommendation (level of evidence A) for exercise training in patients with HF, without a distinction between HFpEF and HFrEF [160], although the association between physical activity and HFpEF is stronger than with other forms of HF [161]. Evidence suggests amelioration of diastolic function, CRF, exercise capacity, and quality of life (QOL) with exercise training in HFpEF [162,163,164]. Other studies reported reduction in the hospitalization [165] or fewer cardiac events [166] after exercise interventions in HFpEF. Importantly, a meta-analysis of 6 RCTs [167] reported no exercise-related major adverse events demonstrating the safety of exercise training.
Common indicators of CRF are VO2 peak (mL/kg/min) and 6MWD, which represents a valid practical alternative [168]. VO2 peak measures the ability to transport (cardiac output) and use (arteriovenous O2 difference) oxygen and is a strong predictor of patients’ functional capacity with significant prognostic value [169, 170]. HFpEF patients present a similar VO2 peak to that in age-matched patients with HFrEF, which is severely reduced (by around 30%) when compared with age-matched healthy individuals [171].
Exercise intolerance and skeletal muscle dysfunction
HFpEF-related-cardiometabolic alterations are linked to worse physical fitness [2]. Patients with HFpEF often exhibit exercise intolerance (EI) and exertional symptoms [172], which are linked to limited O2 transport and utilization due to central and peripheral mechanisms [173] and associated with concentric remodeling [174]. Central and peripheral alterations include cardiac (blunted stroke volume augmentation, chronotropic incompetence, exaggerated increase in filling pressures); pulmonary (pulmonary vascular remodeling, impaired gas exchange, pulmonary hypertension); vascular (central artery stiffness, reduced peripheral artery vasodilator response, microvascular dysfunction); and skeletal muscle (reduced mass, excess adipose infiltration, mitochondrial dysfunction) alterations [170]. The latter leads to reduced aerobic exercise capacity of patients with HFpEF, as assessed by 6MWD and VO2 peak. Moreover, compromised physical activity and HFpEF-related EI are associated with poor QoL and clinical outcomes and higher incidence of hospitalization [175].
HFpEF-related EI is partly attributed to skeletal muscle dysfunction. Skeletal muscle structure and function in HFpEF are involved in sarcopenic obesity (SO), which is defined as the coexistence of excessive BMI and low muscle mass with multiple comorbidities, excessive visceral adiposity, and heightened systemic inflammation [176]. SO exacerbates cardiometabolic risk, imposing a substantial burden on physical activity and poor QoL [177]. Stratifying HF patients by BMI and body composition could help identify those with SO, where targeted lifestyle interventions to maintain or increase lean mass might be clinically beneficial [178].
HFpEF-related skeletal muscle dysfunction is similar to what is described for HFrEF, and it is not merely a consequence of deconditioning since it develops even when levels of physical activity are maintained during HF development [179, 180]. The pattern of skeletal muscle abnormalities differs from deconditioning, especially as regards fiber-type shift [170]. Abnormal skeletal muscle mitochondrial function [181,182,183] linked to a perturbed MuSCs homeostasis, involving Hedgehog and apelin pathways signaling has been found [184]. Blunted overload-induced myofiber growth of skeletal muscle is reported in HFpEF despite adequate physical stimulation and ascribed in part to mitochondrial dysfunction [97]. Thus, patients with HFpEF show reduced mitochondrial content and skeletal muscle type I fiber. This contributes to a faster rate of high-energy phosphate depletion during exercise and impaired recovery afterward, as assessed by a study with phosphorous magnetic resonance spectroscopy [181].
Skeletal muscle metabolic abnormalities are linked to functional limitations of patients with HFpEF. On the other hand, evidence suggests that targeting skeletal muscle metabolism might be a promising approach to improving the EI of HFpEF patients [181]. Exercise training leads to peripheral adaptations, such as increased mitochondrial density and function, myoglobin content, capillary density, and blood flow redistribution [185]. Although no significant changes to central artery stiffness are reported, peripheral benefits are observed [94, 163, 186]. Given the high plasticity and predisposition in skeletal muscle [187], increased VO2 peak from exercise training results in increased diffusion capacity and oxygen extraction by the exercising muscles [185, 188]. In particular, aerobic training conducted alone or combined with strength training for 3 to 6 months resulted in a safe and effective therapy and enhanced aerobic capacity, endurance, and QoL in HFpEF patients [189].
Exercise interventions modalities and outcomes
A recent scientific statement from ACC/AHA analyzed data of the 11 latest RCTs on supervised exercise training (SET) for chronic HFpEF subjects [170]. Training approaches range from walking and stationary cycle ergometry to high-intensity interval training (HIIT), strength training, and dancing in both facility setting and home-based training [170]. SET generally occurred 3 sessions per week, from 1 to 8 months, with intensity from 40 to 90% of exercise capacity and individual sessions from 25 to 60 min [170]. SET significantly ameliorates 6MWD and baseline peak VO2 by 14%, compared to a reduction in baseline peak VO2 by 0.2% in the control group [170]. For comparison, an increase in peak VO2 of 6–7% is considered clinically meaningful in patients with HFrEF [190, 191]. However, effects on QoL have been mixed, with some studies concluding no benefits and others demonstrating improved QoL scores [170]. The same applies to cardiovascular and peripheral parameters, showing mixed data among RCTs. Improvements in diastolic function have been demonstrated in some studies, whereas no changes are reported by other investigations [170]. However, the authors conclude that the strength of currently available data on SET and the sparsity of effective therapies for HFpEF provide the rationale for increasing efforts to promote exercise-based therapies for patients [170].
HIIT has recently emerged as an alternative to moderate-intensity continuous training (MICT) in cardiac rehabilitation [192]. HIIT resulted as the best exercise modality in improving V̇O2 peak and QoL in a period of about 16 weeks, followed by low intensity training (LIT) with a low-calorie diet as regards effectiveness [193]. HIIT consists of repeated sessions of brief and intermittent exercise that induce ≥ 85% of V̇O2 peak, alternated by sessions of rest or LIT for recovery [194, 195]. However, LIT — continuous exercise at a gentle pace, such as walking, light cycling, or slow swimming — with a low-calorie diet resulted as the best lifestyle change in improving 6MWD [193]. Other studies reported the beneficial effects of HIIT in patients with cardiometabolic disorders and chronic diseases, suggesting its effectiveness in improving metabolic health [196,197,198]. However, the OptimEx-Clin study [199] found no statistically significant differences at 3 months in V̇O2 peak by comparing HIIT to moderate intensity continuous training (MICT). Besides this, the findings did not support either HIIT or MICT compared with guideline-based physical activity for patients with HFpEF [199].
In summary, exercise training is highly recommended for HFpEF patients, ameliorating diastolic dysfunction, CRF, exercise capacity, and QoL, while reducing hospitalizations and bringing peripheral beneficial effects, particularly in skeletal muscle. Patients with HFpEF often experience EI due to multiple systemic alterations. In this context, the aerobic capacity and endurance of patients may be enhanced with exercise training, utilizing HIIT as the most effective exercise modality.
Future perspectives
The relationship between lifestyle interventions and cardiometabolic HFpEF seems to be stronger than in other forms of HF. Given this, dietary interventions can/should be targeted on the metabolic profile of HFpEF patients for precision medicine approaches, to optimize dietary plans considering the unique metabolic disturbances of each patient. For example, as gut microbiome influence in HFpEF is increasingly recognized [200], future dietary interventions may include strategies to modify gut microbiome composition, to enhance its beneficial effects on systemic inflammation and altered metabolism.
The benefits of CR and intermittent fasting in improving metabolic health and inflammation are also emerging. Future studies may focus on optimizing protocols for patients with HFpEF, determining the most effective duration and frequency of fasting periods. Moreover, the nutraceutical properties of some food components reveal potential benefits, targeting specific pathophysiological mechanisms in HFpEF, such as oxidative stress and inflammation. In this regard, further studies on the effect of polyphenols in HFpEF are required, considering their anti-inflammatory and antioxidant properties [201, 202]. Similarly, further investigations should also focus on the effects of spermidine due to its anti-inflammatory and cardioprotective properties.
Combined aerobic and resistance training could provide synergistic effects for patients with HFpEF. Different exercise modalities may be integrated to target both cardiovascular and muscular health, enhancing both cardiometabolic and physical function. HIIT — which emerges as the most effective exercise modality — shows superior benefits in improving exercise capacity and QoL in patients with HFpEF. Future studies may refine HIIT protocols, including intensity and duration to maximize advantages and ensure safety for patients. Importantly, lifestyle intervention studies should consider and further investigate the long-term adherence of patients, which remains a challenge and may attenuate lifestyle intervention benefits.
Conclusion
Systemic inflammation and metabolic derangements are the main pathophysiological characteristics of HFpEF. Dietary and exercise interventions play a pivotal role in managing both features. Control of body weight, dietary plans, and regular physical activity can significantly improve clinical outcomes in patients with HFpEF. A better understanding of lifestyle intervention modalities will greatly help researchers and clinicians in the management of patients with HFpEF, considering the formulation of multidisciplinary treatment programs. In this perspective, the combination of lifestyle interventions with pharmacological therapies may plausibly show greater effects.
References
Borlaug BA, Sharma K, Shah SJ, Ho JE (2023) Heart failure with preserved ejection fraction: JACC scientific statement. J Am Coll Cardiol 81:1810–1834. https://doi.org/10.1016/j.jacc.2023.01.049
Roh J, Hill JA, Singh A, Valero-Muñoz M, Sam F (2022) Heart failure with preserved ejection fraction: heterogeneous syndrome, diverse preclinical models. Circ Res 130:1906–1925. https://doi.org/10.1161/CIRCRESAHA.122.320257
Dunlay SM, Roger VL, Redfield MM (2017) Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 14:591–602. https://doi.org/10.1038/nrcardio.2017.65
Shah SJ, Borlaug BA, Kitzman DW, McCulloch AD, Blaxall BC, Agarwal R, Chirinos JA, Collins S, Deo RC, Gladwin MT, Granzier H, Hummel SL, Kass DA, Redfield MM, Sam F, Wang TJ, Desvigne-Nickens P, Adhikari BB (2020) Research priorities for heart failure with preserved ejection fraction: national heart, lung, and blood institute working group summary. Circulation 141:1001–1026. https://doi.org/10.1161/CIRCULATIONAHA.119.041886
Borlaug BA, Redfield MM (2011) Diastolic and systolic heart failure are distinct phenotypes within the heart failure spectrum. Circulation 123:2006–2014. https://doi.org/10.1161/CIRCULATIONAHA.110.954388
Figtree GA, Rådholm K, Barrett TD, Perkovic V, Mahaffey KW, De Zeeuw D, Fulcher G, Matthews DR, Shaw W, Neal B (2019) Effects of canagliflozin on heart failure outcomes associated with preserved and reduced ejection fraction in type 2 diabetes mellitus: results from the CANVAS program. Circulation 139:2591–2593. https://doi.org/10.1161/CIRCULATIONAHA.119.040057
Dyck JRB, Sossalla S, Hamdani N, Coronel R, Weber NC, Light PE, Zuurbier CJ (2022) Cardiac mechanisms of the beneficial effects of SGLT2 inhibitors in heart failure: evidence for potential off-target effects. J Mol Cell Cardiol 167:17–31. https://doi.org/10.1016/j.yjmcc.2022.03.005
Capone F, Nambiar N, Schiattarella GG (2024) Beyond weight loss: the emerging role of incretin-based treatments in cardiometabolic HFpEF. Curr Opin Cardiol. https://doi.org/10.1097/HCO.0000000000001117
Borlaug BA (2020) Evaluation and management of heart failure with preserved ejection fraction. Nat Rev Cardiol 17:559–573. https://doi.org/10.1038/s41569-020-0363-2
Schiattarella GG, Hill JA (2021) Cardiometabolic HFpEF: mechanisms and therapies. Cardiometab Syndr J 1:117. https://doi.org/10.51789/cmsj.2021.1.e18
Lobstein T, Jackson-Leach R, Powis J, Brinsden H, Gray M (2023) World Obesity Atlas 2023. https://www.worldobesity.org/resources/resource-library/world-obesity-atlas-2023
Guo L, Liu X, Yu P, Zhu W (2022) The “obesity paradox” in patients with HFpEF with or without comorbid atrial fibrillation. Front Cardiovasc Med 8:743327. https://doi.org/10.3389/fcvm.2021.743327
Haass M, Kitzman DW, Anand IS, Miller A, Zile MR, Massie BM, Carson PE (2011) Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction: results from the irbesartan in heart failure with preserved ejection fraction (I-PRESERVE) trial. Circ: Heart Failure 4:324–331. https://doi.org/10.1161/CIRCHEARTFAILURE.110.959890
Schiattarella GG, Alcaide P, Condorelli G, Gillette TG, Heymans S, Jones EAV, Kallikourdis M, Lichtman A, Marelli-Berg F, Shah SJ, Thorp EB, Hill JA (2022) Immunometabolic mechanisms of heart failure with preserved ejection fraction. Nat Cardiovasc Res 1:211–222. https://doi.org/10.1038/s44161-022-00032-w
Shah MS, Brownlee M (2016) Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circ Res 118:1808–1829. https://doi.org/10.1161/CIRCRESAHA.116.306923
Carbone S, Mauro AG, Mezzaroma E, Kraskauskas D, Marchetti C, Buzzetti R, Van Tassell BW, Abbate A, Toldo S (2015) A high-sugar and high-fat diet impairs cardiac systolic and diastolic function in mice. Int J Cardiol 198:66–69. https://doi.org/10.1016/j.ijcard.2015.06.136
La Gerche A, Howden EJ, Haykowsky MJ, Lewis GD, Levine BD, Kovacic JC (2022) Heart failure with preserved ejection fraction as an exercise deficiency syndrome. J Am Coll Cardiol 80:1177–1191. https://doi.org/10.1016/j.jacc.2022.07.011
Capone F, Sotomayor-Flores C, Bode D, Wang R, Rodolico D, Strocchi S, Schiattarella GG (2023) Cardiac metabolism in HFpEF: from fuel to signalling. Cardiovasc Res 118:3556–3575. https://doi.org/10.1093/cvr/cvac166
American Heart Association Life’s Simple 7® Journey to HealthTM. https://www.heart.org/-/media/files/professional/workplace-health/detailed-overview-whs-with-ls7-journey-1218.pdf?la=en&hash=9D16F77814743A12695010D025065588CD1F9A44
Aggarwal M, Bozkurt B, Panjrath G, Aggarwal B, Ostfeld RJ, Barnard ND, Gaggin H, Freeman AM, Allen K, Madan S, Massera D, Litwin SE (2018) Lifestyle modifications for preventing and treating heart failure. J Am Coll Cardiol 72:2391–2405. https://doi.org/10.1016/j.jacc.2018.08.2160
Bohmke NJ, Billingsley HE, Kirkman DL, Carbone S (2022) Nonpharmacological strategies in heart failure with preserved ejection fraction. Cardiol Clin 40:491–506. https://doi.org/10.1016/j.ccl.2022.06.003
Larsson SC, Tektonidis TG, Gigante B, Åkesson A, Wolk A (2016) Healthy lifestyle and risk of heart failure: results from 2 prospective cohort studies. Circ: Heart Failure 9:e002855. https://doi.org/10.1161/CIRCHEARTFAILURE.115.002855
Lara KM, Levitan EB, Gutierrez OM, Shikany JM, Safford MM, Judd SE, Rosenson RS (2019) Dietary patterns and incident heart failure in U.S. adults without known coronary disease. J Am Coll Cardiol 73:2036–2045. https://doi.org/10.1016/j.jacc.2019.01.067
Folsom AR, Shah AM, Lutsey PL, Roetker NS, Alonso A, Avery CL, Miedema MD, Konety S, Chang PP, Solomon SD (2015) American Heart Association’s Life’s Simple 7: avoiding heart failure and preserving cardiac structure and function. Am J Med 128:970-976.e2. https://doi.org/10.1016/j.amjmed.2015.03.027
Carbone S, Canada JM, Buckley LF, Trankle CR, Dixon DL, Buzzetti R, Arena R, Van Tassell BW, Abbate A (2016) Obesity contributes to exercise intolerance in heart failure with preserved ejection fraction. J Am Coll Cardiol 68:2487–2488. https://doi.org/10.1016/j.jacc.2016.08.072
Levitan EB, Lewis CE, Tinker LF, Eaton CB, Ahmed A, Manson JE, Snetselaar LG, Martin LW, Trevisan M, Howard BV, Shikany JM (2013) Mediterranean and DASH diet scores and mortality in women with heart failure: the women’s health initiative. Circ: Heart Failure 6:1116–1123. https://doi.org/10.1161/CIRCHEARTFAILURE.113.000495
Carbone S, Canada JM, Buckley LF, Trankle CR, Billingsley HE, Dixon DL, Mauro AG, Dessie S, Kadariya D, Mezzaroma E, Buzzetti R, Arena R, Van Tassell BW, Toldo S, Abbate A (2017) Dietary fat, sugar consumption, and cardiorespiratory fitness in patients with heart failure with preserved ejection fraction. JACC: Basic to Translational Science 2:513–525. https://doi.org/10.1016/j.jacbts.2017.06.009
Kim MY, Pellot I, Bresee C, Nawaz A, Fournier M, Cho JH, Cingolani E (2023) Diet modification reverses diastolic dysfunction in rats with heart failure and preserved ejection fraction. J Molec Cell Cardiol Plus 3:100031. https://doi.org/10.1016/j.jmccpl.2023.100031
Shah SJ (2017) Sedentary lifestyle and the risk for HFpEF. J Am Coll Cardiol 69:1143–1146. https://doi.org/10.1016/j.jacc.2017.01.010
McGee SL, Hargreaves M (2020) Exercise adaptations: molecular mechanisms and potential targets for therapeutic benefit. Nat Rev Endocrinol 16:495–505. https://doi.org/10.1038/s41574-020-0377-1
Fiuza-Luces C, Santos-Lozano A, Joyner M, Carrera-Bastos P, Picazo O, Zugaza JL, Izquierdo M, Ruilope LM, Lucia A (2018) Exercise benefits in cardiovascular disease: beyond attenuation of traditional risk factors. Nat Rev Cardiol 15:731–743. https://doi.org/10.1038/s41569-018-0065-1
Nayor M, Shah RV, Miller PE, Blodgett JB, Tanguay M, Pico AR, Murthy VL, Malhotra R, Houstis NE, Deik A, Pierce KA, Bullock K, Dailey L, Velagaleti RS, Moore SA, Ho JE, Baggish AL, Clish CB, Larson MG, Vasan RS, Lewis GD (2020) Metabolic architecture of acute exercise response in middle-aged adults in the community. Circulation 142:1905–1924. https://doi.org/10.1161/CIRCULATIONAHA.120.050281
Tune JD, Goodwill AG, Sassoon DJ, Mather KJ (2017) Cardiovascular consequences of metabolic syndrome. Transl Res 183:57–70. https://doi.org/10.1016/j.trsl.2017.01.001
Gutiérrez-Cuevas J, Sandoval-Rodriguez A, Meza-Rios A, Monroy-Ramírez HC, Galicia-Moreno M, García-Bañuelos J, Santos A, Armendariz-Borunda J (2021) Molecular mechanisms of obesity-linked cardiac dysfunction: an up-date on current knowledge. Cells 10:629. https://doi.org/10.3390/cells10030629
Henry JA, Couch LS, Rider OJ (2024) Myocardial metabolism in heart failure with preserved ejection fraction. JCM 13:1195. https://doi.org/10.3390/jcm13051195
Hahn VS, Petucci C, Kim M-S, Bedi KC, Wang H, Mishra S, Koleini N, Yoo EJ, Margulies KB, Arany Z, Kelly DP, Kass DA, Sharma K (2023) Myocardial metabolomics of human heart failure with preserved ejection fraction. Circulation 147:1147–1161. https://doi.org/10.1161/CIRCULATIONAHA.122.061846
Murashige D, Jang C, Neinast M, Edwards JJ, Cowan A, Hyman MC, Rabinowitz JD, Frankel DS, Arany Z (2020) Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science 370:364–368. https://doi.org/10.1126/science.abc8861
Tong D, Schiattarella GG, Jiang N, Altamirano F, Szweda PA, Elnwasany A, Lee DI, Yoo H, Kass DA, Szweda LI, Lavandero S, Verdin E, Gillette TG, Hill JA (2021) NAD + repletion reverses heart failure with preserved ejection fraction. Circ Res 128:1629–1641. https://doi.org/10.1161/CIRCRESAHA.120.317046
Sun Q, Güven B, Wagg CS, Almeida De Oliveira A, Silver H, Zhang L, Chen B, Wei K, Ketema EB, Karwi QG, Persad KL, Vu J, Wang F, Dyck JRB, Oudit GY, Lopaschuk GD (2024) Mitochondrial fatty acid oxidation is the major source of cardiac adenosine triphosphate production in heart failure with preserved ejection fraction. Cardiovasc Res 120:360–371. https://doi.org/10.1093/cvr/cvae006
Rijzewijk LJ, Van Der Meer RW, Lamb HJ, De Jong HWAM, Lubberink M, Romijn JA, Bax JJ, De Roos A, Twisk JW, Heine RJ, Lammertsma AA, Smit JWA, Diamant M (2009) Altered myocardial substrate metabolism and decreased diastolic function in nonischemic human diabetic cardiomyopathy. J Am Coll Cardiol 54:1524–1532. https://doi.org/10.1016/j.jacc.2009.04.074
Barouch LA, Berkowitz DE, Harrison RW, O’Donnell CP, Hare JM (2003) Disruption of leptin signaling contributes to cardiac hypertrophy independently of body weight in mice. Circulation 108:754–759. https://doi.org/10.1161/01.CIR.0000083716.82622.FD
Zhang L, Jaswal JS, Ussher JR, Sankaralingam S, Wagg C, Zaugg M, Lopaschuk GD (2013) Cardiac insulin-resistance and decreased mitochondrial energy production precede the development of systolic heart failure after pressure-overload hypertrophy. Circ Heart Failure 6:1039–1048. https://doi.org/10.1161/CIRCHEARTFAILURE.112.000228
Mori J, Basu R, McLean BA, Das SK, Zhang L, Patel VB, Wagg CS, Kassiri Z, Lopaschuk GD, Oudit GY (2012) Agonist-induced hypertrophy and diastolic dysfunction are associated with selective reduction in glucose oxidation: a metabolic contribution to heart failure with normal ejection fraction. Circ Heart Failure 5:493–503. https://doi.org/10.1161/CIRCHEARTFAILURE.112.966705
Mori J, Alrob OA, Wagg CS, Harris RA, Lopaschuk GD, Oudit GY (2013) ANG II causes insulin resistance and induces cardiac metabolic switch and inefficiency: a critical role of PDK4. Am J Physiol-Heart Circ Physiol 304:H1103–H1113. https://doi.org/10.1152/ajpheart.00636.2012
Morigny P, Houssier M, Mouisel E, Langin D (2016) Adipocyte lipolysis and insulin resistance. Biochimie 125:259–266. https://doi.org/10.1016/j.biochi.2015.10.024
Leggat J, Bidault G, Vidal-Puig A (2021) Lipotoxicity: a driver of heart failure with preserved ejection fraction? Clin Sci 135:2265–2283. https://doi.org/10.1042/CS20210127
Neubauer S (2007) The failing heart—an engine out of fuel. N Engl J Med 356:1140–1151. https://doi.org/10.1056/NEJMra063052
Burrage MK, Lewis AJ, Miller JJJ (2023) Functional and metabolic imaging in heart failure with preserved ejection fraction: promises, challenges, and clinical utility. Cardiovasc Drugs Ther 37:379–399. https://doi.org/10.1007/s10557-022-07355-7
Karwi QG, Uddin GM, Ho KL, Lopaschuk GD (2018) Loss of metabolic flexibility in the failing heart. Front Cardiovasc Med 5:68. https://doi.org/10.3389/fcvm.2018.00068
Burrage MK, Hundertmark M, Valkovič L, Watson WD, Rayner J, Sabharwal N, Ferreira VM, Neubauer S, Miller JJ, Rider OJ, Lewis AJM (2021) Energetic basis for exercise-induced pulmonary congestion in heart failure with preserved ejection fraction. Circulation 144:1664–1678. https://doi.org/10.1161/CIRCULATIONAHA.121.054858
Mahmod M, Pal N, Rayner J, Holloway C, Raman B, Dass S, Levelt E, Ariga R, Ferreira V, Banerjee R, Schneider JE, Rodgers C, Francis JM, Karamitsos TD, Frenneaux M, Ashrafian H, Neubauer S, Rider O (2018) The interplay between metabolic alterations, diastolic strain rate and exercise capacity in mild heart failure with preserved ejection fraction: a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson 20:88. https://doi.org/10.1186/s12968-018-0511-6
Phan TT, Abozguia K, Nallur Shivu G, Mahadevan G, Ahmed I, Williams L, Dwivedi G, Patel K, Steendijk P, Ashrafian H, Henning A, Frenneaux M (2009) Heart failure with preserved ejection fraction is characterized by dynamic impairment of active relaxation and contraction of the left ventricle on exercise and associated with myocardial energy deficiency. J Am Coll Cardiol 54:402–409. https://doi.org/10.1016/j.jacc.2009.05.012
Beer M, Seyfarth T, Sandstede J, Landschütz W, Lipke C, Köstler H, Von Kienlin M, Harre K, Hahn D, Neubauer S (2002) Absolute concentrations of high-energy phosphate metabolites in normal, hypertrophied, and failing human myocardium measured noninvasively with 31P-SLOOP magnetic resonance spectroscopy. J Am Coll Cardiol 40:1267–1274. https://doi.org/10.1016/S0735-1097(02)02160-5
De Wit-Verheggen VHW, Schrauwen-Hinderling VB, Brouwers K, Jörgensen JA, Schaart G, Gemmink A, Nascimento EBM, Hesselink MKC, Wildberger JE, Segers P, Montaigne D, Staels B, Schrauwen P, Lindeboom L, Hoeks J, Van De Weijer T (2023) PCr/ATP ratios and mitochondrial function in the heart. a comparative study in humans. Sci Rep 13:8346. https://doi.org/10.1038/s41598-023-35041-7
Actis Dato V, Lange S, Cho Y (2024) Metabolic flexibility of the heart: the role of fatty acid metabolism in health, heart failure, and cardiometabolic diseases. IJMS 25:1211. https://doi.org/10.3390/ijms25021211
Wang R, Schiattarella GG (2024) Tackling metabolic defects in HFpEF. Eur Heart J 45:1494–1496. https://doi.org/10.1093/eurheartj/ehad884
Schiattarella GG, Rodolico D, Hill JA (2021) Metabolic inflammation in heart failure with preserved ejection fraction. Cardiovasc Res 117:423–434. https://doi.org/10.1093/cvr/cvaa217
Franssen C, Chen S, Unger A, Korkmaz HI, De Keulenaer GW, Tschöpe C, Leite-Moreira AF, Musters R, Niessen HWM, Linke WA, Paulus WJ, Hamdani N (2016) Myocardial microvascular inflammatory endothelial activation in heart failure with preserved ejection fraction. JACC Heart Failure 4:312–324. https://doi.org/10.1016/j.jchf.2015.10.007
Schiattarella GG, Altamirano F, Tong D, French KM, Villalobos E, Kim SY, Luo X, Jiang N, May HI, Wang ZV, Hill TM, Mammen PPA, Huang J, Lee DI, Hahn VS, Sharma K, Kass DA, Lavandero S, Gillette TG, Hill JA (2019) Nitrosative stress drives heart failure with preserved ejection fraction. Nature 568:351–356. https://doi.org/10.1038/s41586-019-1100-z
Matuschik L, Riabov V, Schmuttermaier C, Sevastyanova T, Weiss C, Klüter H, Kzhyshkowska J (2022) Hyperglycemia induces inflammatory response of human macrophages to cd163-mediated scavenging of hemoglobin-haptoglobin complexes. IJMS 23:1385. https://doi.org/10.3390/ijms23031385
Ouchi N, Parker JL, Lugus JJ, Walsh K (2011) Adipokines in inflammation and metabolic disease. Nat Rev Immunol 11:85–97. https://doi.org/10.1038/nri2921
Catena C, Colussi G, Martinis F, Pezzutto F, Sechi LA (2013) Plasma glucose levels and left ventricular diastolic function in nondiabetic hypertensive patients. Am J Hypertens 26:1353–1361. https://doi.org/10.1093/ajh/hpt114
Hulsmans M, Sager HB, Roh JD, Valero-Muñoz M, Houstis NE, Iwamoto Y, Sun Y, Wilson RM, Wojtkiewicz G, Tricot B, Osborne MT, Hung J, Vinegoni C, Naxerova K, Sosnovik DE, Zile MR, Bradshaw AD, Liao R, Tawakol A, Weissleder R, Rosenzweig A, Swirski FK, Sam F, Nahrendorf M (2018) Cardiac macrophages promote diastolic dysfunction. J Exp Med 215:423–440. https://doi.org/10.1084/jem.20171274
Mouton AJ, Li X, Hall ME, Hall JE (2020) Obesity, Hypertension, and cardiac dysfunction: novel roles of immunometabolism in macrophage activation and inflammation. Circ Res 126:789–806. https://doi.org/10.1161/CIRCRESAHA.119.312321
Morris E, Jebb SA, Oke J, Nickless A, Ahern A, Boyland E, Caterson ID, Halford J, Hauner H, Aveyard P (2021) Effect of weight loss on cardiometabolic risk: observational analysis of two randomised controlled trials of community weight-loss programmes. Br J Gen Pract 71:e312–e319. https://doi.org/10.3399/bjgp20X714113
Lin CH, Kurup S, Herrero P, Schechtman KB, Eagon JC, Klein S, Dávila-Román VG, Stein RI, Dorn-II GW, Gropler RJ, Waggoner AD, Peterson LR (2011) Myocardial oxygen consumption change predicts left ventricular relaxation improvement in obese humans after weight loss. Obesity 19:1804–1812. https://doi.org/10.1038/oby.2011.186
Madigan MJ, Racette SB, Coggan AR, Stein RI, McCue LM, Gropler RJ, Peterson LR (2019) Weight loss affects intramyocardial glucose metabolism in obese humans. Circ Cardiovascular Imaging 12:e009241. https://doi.org/10.1161/CIRCIMAGING.119.009241
Rider OJ, Francis JM, Tyler D, Byrne J, Clarke K, Neubauer S (2013) Effects of weight loss on myocardial energetics and diastolic function in obesity. Int J Cardiovasc Imaging 29:1043–1050. https://doi.org/10.1007/s10554-012-0174-6
Sarzani R, Landolfo M, Di Pentima C, Ortensi B, Falcioni P, Sabbatini L, Massacesi A, Rampino I, Spannella F, Giulietti F (2024) Adipocentric origin of the common cardiometabolic complications of obesity in the young up to the very old: pathophysiology and new therapeutic opportunities. Front Med 11:1365183. https://doi.org/10.3389/fmed.2024.1365183
Forsythe LK, Wallace JMW, Livingstone MBE (2008) Obesity and inflammation: the effects of weight loss. Nutr Res Rev 21:117–133. https://doi.org/10.1017/S0954422408138732
Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM (2003) Influence of weight reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension 42:878–884. https://doi.org/10.1161/01.HYP.0000094221.86888.AE
Colín Ramírez E, Castillo Martínez L, Orea Tejeda A, Rebollar González V, Narváez David R, Asensio Lafuente E (2004) Effects of a nutritional intervention on body composition, clinical status, and quality of life in patients with heart failure. Nutrition 20:890–895. https://doi.org/10.1016/j.nut.2004.06.010
Colin-Ramirez E, McAlister FA, Zheng Y, Sharma S, Armstrong PW, Ezekowitz JA (2015) The long-term effects of dietary sodium restriction on clinical outcomes in patients with heart failure. The SODIUM-HF (study of dietary intervention under 100 mmol in heart failure): a pilot study. Am Heart J 169:274-281.e1. https://doi.org/10.1016/j.ahj.2014.11.013
Philipson H, Ekman I, Swedberg K, Schaufelberger M (2010) A pilot study of salt and water restriction in patients with chronic heart failure. Scand Cardiovasc J 44:209–214. https://doi.org/10.3109/14017431003698523
Philipson H, Ekman I, Forslund HB, Swedberg K, Schaufelberger M (2013) Salt and fluid restriction is effective in patients with chronic heart failure. European J of Heart Fail 15:1304–1310. https://doi.org/10.1093/eurjhf/hft097
Machado d’Almeida KS, Rabelo-Silva ER, Souza GC, Trojahn MM, Santin Barilli SL, Aliti G, Rohde LE, Biolo A, Beck-da-Silva L (2018) Aggressive fluid and sodium restriction in decompensated heart failure with preserved ejection fraction: results from a randomized clinical trial. Nutrition 54:111–117. https://doi.org/10.1016/j.nut.2018.02.007
Li J, Zhen Z, Huang P, Dong Y-G, Liu C, Liang W (2022) Salt restriction and risk of adverse outcomes in heart failure with preserved ejection fraction. Heart 108:1377–1382. https://doi.org/10.1136/heartjnl-2022-321167
Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O’Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM (2014) Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 370:1383–1392. https://doi.org/10.1056/NEJMoa1313731
Brosolo G, Da Porto A, Marcante S, Picci A, Capilupi F, Capilupi P, Bertin N, Vivarelli C, Bulfone L, Vacca A, Catena C, Sechi LA (2023) Omega-3 fatty acids in arterial hypertension: is there any good news? IJMS 24:9520. https://doi.org/10.3390/ijms24119520
Chen X, Gu J, Huang Y (2023) High dietary intake of unsaturated fatty acids is associated with improved insulin resistance–a cross-sectional study based on the NHANES database. Lipids Health Dis 22:216. https://doi.org/10.1186/s12944-023-01982-1
Talbot NA, Wheeler-Jones CP, Cleasby ME (2014) Palmitoleic acid prevents palmitic acid-induced macrophage activation and consequent p38 MAPK-mediated skeletal muscle insulin resistance. Mol Cell Endocrinol 393:129–142. https://doi.org/10.1016/j.mce.2014.06.010
Sun Y, Wang J, Guo X, Zhu N, Niu L, Ding X, Xie Z, Chen X, Yang F (2021) Oleic acid and eicosapentaenoic acid reverse palmitic acid-induced insulin resistance in human HepG2 cells via the reactive oxygen species/JUN pathway. Genomics Proteomics Bioinforma 19:754–771. https://doi.org/10.1016/j.gpb.2019.06.005
Guriec N, Le Foll C, Delarue J (2023) Long-chain n-3 PUFA given before and throughout gestation and lactation in rats prevent high-fat diet-induced insulin resistance in male offspring in a tissue-specific manner. Br J Nutr 130:1121–1136. https://doi.org/10.1017/S000711452300017X
Cavaliere G, Trinchese G, Bergamo P, De Filippo C, Mattace Raso G, Gifuni G, Putti R, Moni BH, Canani RB, Meli R, Mollica MP (2016) Polyunsaturated fatty acids attenuate diet induced obesity and insulin resistance, modulating mitochondrial respiratory uncoupling in rat skeletal muscle. PLoS ONE 11:e0149033. https://doi.org/10.1371/journal.pone.0149033
Carbone S, Billingsley HE, Canada JM, Kadariya D, Medina De Chazal H, Rotelli B, Potere N, Paudel B, Markley R, Dixon DL, Trankle CR, Van Tassell BW, Celi FS, Abbate A (2019) Unsaturated fatty acids to improve cardiorespiratory fitness in patients with obesity and HFpEF. JACC: Basic Transl Sci 4:563–565. https://doi.org/10.1016/j.jacbts.2019.04.001
Kleissl-Muir S, Owen A, Rasmussen B, Zinn C, Driscoll A (2023) Effects of a low carbohydrate diet on heart failure symptoms and quality of life in patients with diabetic cardiomyopathy: a randomised controlled trial pilot study. Nutr Metab Cardiovasc Dis 33:2455–2463. https://doi.org/10.1016/j.numecd.2023.08.015
Kleissl-Muir S, Rasmussen B, Owen A, Zinn C, Driscoll A (2022) Low carbohydrate diets for diabetic cardiomyopathy: a hypothesis. Front Nutr 9:865489. https://doi.org/10.3389/fnut.2022.865489
Jonasson L, Guldbrand H, Lundberg AK, Nystrom FH (2014) Advice to follow a low-carbohydrate diet has a favourable impact on low-grade inflammation in type 2 diabetes compared with advice to follow a low-fat diet. Ann Med 46:182–187. https://doi.org/10.3109/07853890.2014.894286
González-Islas D, Orea-Tejeda A, Orea-Tejeda A, Castillo-Martínez L, Castillo-Martínez L, Olvera-Mayorga G, Olvera-Mayorga G, Rodríguez-García WD, Rodríguez-García WD, Santillán-Díaz C, Santillán-Díaz C, Keirnes-Davis C, Keirnes-Davis C, Vaquero-Barbosa N, Vaquero-Barbosa N (2017) The effects of a low-carbohydrate diet on oxygen saturation in heart failure patients: a randomized controlled clinical trial. Nutr Hosp 34. https://doi.org/10.20960/nh.784
Forsyth F, Mulrennan S, Burt J, Hartley P, Kuhn I, Lin H, Mant J, Tan S, Zhang R, Deaton C (2023) What dietary interventions have been tested in heart failure with preserved ejection fraction? A systematic scoping review. Eur J Cardiovasc Nurs 22:126–140. https://doi.org/10.1093/eurjcn/zvac062
Forte M, Rodolico D, Ameri P, Catalucci D, Chimenti C, Crotti L, Schirone L, Pingitore A, Torella D, Iacovone G, Valenti V, Schiattarella GG, Perrino C, Sciarretta S (2023) Molecular mechanisms underlying the beneficial effects of exercise and dietary interventions in the prevention of cardiometabolic diseases. J Cardiovasc Med 24:e3–e14. https://doi.org/10.2459/JCM.0000000000001397
Johnson ML, Distelmaier K, Lanza IR, Irving BA, Robinson MM, Konopka AR, Shulman GI, Nair KS (2016) Mechanism by which caloric restriction improves insulin sensitivity in sedentary obese adults. Diabetes 65:74–84. https://doi.org/10.2337/db15-0675
Park CY, Park S, Kim MS, Kim H-K, Han SN (2017) Effects of mild calorie restriction on lipid metabolism and inflammation in liver and adipose tissue. Biochem Biophys Res Commun 490:636–642. https://doi.org/10.1016/j.bbrc.2017.06.090
Kitzman DW, Brubaker P, Morgan T, Haykowsky M, Hundley G, Kraus WE, Eggebeen J, Nicklas BJ (2016) Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 315:36. https://doi.org/10.1001/jama.2015.17346
Cocco G, Chu D (2007) Weight reduction decreases NT-proBNP levels in obsese coronary patients with chronic diastolic heart failure. Arch Med Sci 3:112–116
Yang L, Licastro D, Cava E, Veronese N, Spelta F, Rizza W, Bertozzi B, Villareal DT, Hotamisligil GS, Holloszy JO, Fontana L (2016) Long-term calorie restriction enhances cellular quality-control processes in human skeletal muscle. Cell Rep 14:422–428. https://doi.org/10.1016/j.celrep.2015.12.042
Espino-Gonzalez E, Tickle PG, Altara R, Gallagher H, Cheng CW, Engman V, Wood N, Justo Da Silva GJ, Scalabrin M, Yu X, Zhong Z, Colman MA, Yuldasheva NY, Booz GW, Adams V, Pereira MG, Cataliotti A, Roberts LD, Egginton S, Bowen TS (2024) Caloric restriction rejuvenates skeletal muscle growth in heart failure with preserved ejection fraction. JACC: Basic to Translational Science 9:223–240. https://doi.org/10.1016/j.jacbts.2023.09.014
Moro T, Tinsley G, Bianco A, Marcolin G, Pacelli QF, Battaglia G, Palma A, Gentil P, Neri M, Paoli A (2016) Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J Transl Med 14:290. https://doi.org/10.1186/s12967-016-1044-0
Wilkinson MJ, Manoogian ENC, Zadourian A, Lo H, Fakhouri S, Shoghi A, Wang X, Fleischer JG, Navlakha S, Panda S, Taub PR (2020) Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab 31:92-104.e5. https://doi.org/10.1016/j.cmet.2019.11.004
Ulgherait M, Midoun AM, Park SJ, Gatto JA, Tener SJ, Siewert J, Klickstein N, Canman JC, Ja WW, Shirasu-Hiza M (2021) Circadian autophagy drives iTRF-mediated longevity. Nature 598:353–358. https://doi.org/10.1038/s41586-021-03934-0
Evangelista LS, Jose MM, Sallam H, Serag H, Golovko G, Khanipov K, Hamilton MA, Fonarow GC (2021) High-protein vs. standard-protein diets in overweight and obese patients with heart failure and diabetes mellitus: findings of the Pro-HEART trial. ESC Heart Failure 8:1342–1348. https://doi.org/10.1002/ehf2.13213
Tong D, Hill JA (2017) Spermidine promotes cardioprotective autophagy. Circ Res 120:1229–1231. https://doi.org/10.1161/CIRCRESAHA.117.310603
Minois N, Carmona-Gutierrez D, Bauer MA, Rockenfeller P, Eisenberg T, Brandhorst S, Sigrist SJ, Kroemer G, Madeo F (2012) Spermidine promotes stress resistance in Drosophila melanogaster through autophagy-dependent and -independent pathways. Cell Death Dis 3:e401–e401. https://doi.org/10.1038/cddis.2012.139
Wang J, Li S, Wang J, Wu F, Chen Y, Zhang H, Guo Y, Lin Y, Li L, Yu X, Liu T, Zhao Y (2020) Spermidine alleviates cardiac aging by improving mitochondrial biogenesis and function. Aging 12:650–671. https://doi.org/10.18632/aging.102647
Ni Y-Q, Liu Y-S (2021) New insights into the roles and mechanisms of spermidine in aging and age-related diseases. Aging and disease 12:1948. https://doi.org/10.14336/AD.2021.0603
Liu R, Li X, Ma H, Yang Q, Shang Q, Song L, Zheng Z, Zhang S, Pan Y, Huang P, Fang J, Li Y, Liu Z, Cao L, Feng C, Gong Z, Chen Y, Wang Y, Melino G, Shao C, Shi Y (2020) Spermidine endows macrophages anti-inflammatory properties by inducing mitochondrial superoxide-dependent AMPK activation, Hif-1α upregulation and autophagy. Free Radical Biol Med 161:339–350. https://doi.org/10.1016/j.freeradbiomed.2020.10.029
Lv F, Zhang J, Tao Y (2023) Efficacy and safety of inorganic nitrate/nitrite supplementary therapy in heart failure with preserved ejection fraction. Front Cardiovasc Med 10:1054666. https://doi.org/10.3389/fcvm.2023.1054666
Qin L, Wang S (2022) Protective roles of inorganic nitrate in health and diseases. Curr Med 1:4. https://doi.org/10.1007/s44194-022-00002-1
Borlaug BA, Koepp KE, Reddy YNV, Obokata M, Sorimachi H, Freund M, Haberman D, Sweere K, Weber KL, Overholt EA, Safe BA, Omote K, Omar M, Popovic D, Acker NG, Gladwin MT, Olson TP, Carter RE (2024) Inorganic nitrite to amplify the benefits and tolerability of exercise training in heart failure with preserved ejection fraction: the INABLE-training trial. Mayo Clin Proc 99:206–217. https://doi.org/10.1016/j.mayocp.2023.08.031
Selvaraj S, Bhatt DL, Steg PhG, Miller M, Brinton EA, Jacobson TA, Juliano RA, Jiao L, Tardif J, Ballantyne CM, the REDUCE‐IT Investigators (2022) Impact of icosapent ethyl on cardiovascular risk reduction in patients with heart failure in REDUCE‐IT. JAHA 11:e024999. https://doi.org/10.1161/JAHA.121.024999
Huynh K, Kiriazis H, Du X-J, Love JE, Jandeleit-Dahm KA, Forbes JM, McMullen JR, Ritchie RH (2012) Coenzyme Q10 attenuates diastolic dysfunction, cardiomyocyte hypertrophy and cardiac fibrosis in the db/db mouse model of type 2 diabetes. Diabetologia 55:1544–1553. https://doi.org/10.1007/s00125-012-2495-3
Farsi F, Mohammadshahi M, Alavinejad P, Rezazadeh A, Zarei M, Engali KA (2016) Functions of coenzyme Q10 supplementation on liver enzymes, markers of systemic inflammation, and adipokines in patients affected by nonalcoholic fatty liver disease: a double-blind, placebo-controlled, randomized clinical trial. J Am Coll Nutr 35:346–353. https://doi.org/10.1080/07315724.2015.1021057
Mortensen SA, Rosenfeldt F, Kumar A, Dolliner P, Filipiak KJ, Pella D, Alehagen U, Steurer G, Littarru GP (2014) The effect of coenzyme Q 10 on morbidity and mortality in chronic heart failure. JACC: Heart Failure 2:641–649. https://doi.org/10.1016/j.jchf.2014.06.008
Sobirin MA, Herry Y, Sofia SN, Uddin I, Rifqi S, Tsutsui H (2019) Effects of coenzyme Q10 supplementation on diastolic function in patients with heart failure with preserved ejection fraction. DD&T 13:38–46. https://doi.org/10.5582/ddt.2019.01004
Pierce JD, Shen Q, Mahoney DE, Rahman F, Krueger KJ, Diaz FJ, Clark L, Smith C, Vacek J, Hiebert JB (2022) Effects of ubiquinol and/or D-ribose in patients with heart failure with preserved ejection fraction. Am J Cardiol 176:79–88. https://doi.org/10.1016/j.amjcard.2022.04.031
Samuel TY, Hasin T, Gotsman I, Weitzman T, Ben Ivgi F, Dadon Z, Asher E, Amir O, Glikson M, Alcalai R, Leibowitz D (2022) Coenzyme Q10 in the treatment of heart failure with preserved ejection fraction: a prospective, randomized, double-blind, placebo-controlled trial. Drugs R D 22:25–33. https://doi.org/10.1007/s40268-021-00372-1
Vaz FM, Wanders RJA (2002) Carnitine biosynthesis in mammals. Biochem J 361:417–429. https://doi.org/10.1042/bj3610417
Soukoulis V, Dihu JB, Sole M, Anker SD, Cleland J, Fonarow GC, Metra M, Pasini E, Strzelczyk T, Taegtmeyer H, Gheorghiade M (2009) Micronutrient deficiencies: an unmet need in heart failure. J Am Coll Cardiol 54:1660–1673. https://doi.org/10.1016/j.jacc.2009.08.012
Omori Y, Mano T, Sakata Y, Ohtani T, Takeda Y, Tamaki S, Kamimura D, Tsukamoto Y, Aizawa Y, Ikeya Y, Miwa T, Soga T, Yamamoto K, Komuro I (2011) L-carnitine supplementation as treatment for cardiac fibrosis and heart failure with preserved ejection fraction. J Cardiac Fail 17:S154. https://doi.org/10.1016/j.cardfail.2011.06.504
Askarpour M, Hadi A, Miraghajani M, Symonds ME, Sheikhi A, Ghaedi E (2020) Beneficial effects of l-carnitine supplementation for weight management in overweight and obese adults: an updated systematic review and dose-response meta-analysis of randomized controlled trials. Pharmacol Res 151:104554. https://doi.org/10.1016/j.phrs.2019.104554
Emran T, Chowdhury NI, Sarker M, Bepari AK, Hossain M, Rahman GMS, Reza HM (2021) L-carnitine protects cardiac damage by reducing oxidative stress and inflammatory response via inhibition of tumor necrosis factor-alpha and interleukin-1beta against isoproterenol-induced myocardial infarction. Biomed Pharmacother 143:112139. https://doi.org/10.1016/j.biopha.2021.112139
Kinugasa Y, Sota T, Ishiga N, Nakamura K, Kamitani H, Hirai M, Yanagihara K, Kato M, Yamamoto K (2020) l-Carnitine supplementation in heart failure patients with preserved ejection fraction; a pilot study. Geriatrics Gerontology Int 20:1244–1245. https://doi.org/10.1111/ggi.14060
Nolte K, Herrmann-Lingen C, Platschek L, Holzendorf V, Pilz S, Tomaschitz A, Düngen H, Angermann CE, Hasenfuß G, Pieske B, Wachter R, Edelmann F (2019) Vitamin D deficiency in patients with diastolic dysfunction or heart failure with preserved ejection fraction. ESC Heart Failure 6:262–270. https://doi.org/10.1002/ehf2.12413
Brosolo G, Da Porto A, Bulfone L, Scandolin L, Vacca A, Bertin N, Vivarelli C, Sechi LA, Catena C (2022) Vitamin D deficiency is associated with glycometabolic changes in nondiabetic patients with arterial hypertension. Nutrients 14:311. https://doi.org/10.3390/nu14020311
Fallo F, Catena C, Camozzi V, Luisetto G, Cosma C, Plebani M, Lupia M, Tona F, Sechi LA (2012) Low serum 25-hydroxyvitamin D levels are associated with left ventricular hypertrophy in essential hypertension. Nutr Metab Cardiovasc Dis 22:871–876. https://doi.org/10.1016/j.numecd.2011.06.001
Lee JH, O’Keefe JH, Bell D, Hensrud DD, Holick MF (2008) Vitamin D deficiency. J Am Coll Cardiol 52:1949–1956. https://doi.org/10.1016/j.jacc.2008.08.050
Bouillon R, Carmeliet G, Verlinden L, Van Etten E, Verstuyf A, Luderer HF, Lieben L, Mathieu C, Demay M (2008) Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev 29:726–776. https://doi.org/10.1210/er.2008-0004
Mirhosseini N, Vatanparast H, Mazidi M, Kimball SM (2018) Vitamin D supplementation, glycemic control, and insulin resistance in prediabetics: a meta-analysis. Journal of the Endocrine Society 2:687–709. https://doi.org/10.1210/js.2017-00472
AlGhamdi SA, Enaibsi NN, Alsufiani HM, Alshaibi HF, Khoja SO, Carlberg C (2022) A single oral vitamin D3 bolus reduces inflammatory markers in healthy Saudi males. IJMS 23:11992. https://doi.org/10.3390/ijms231911992
Boxer RS, Kenny AM, Schmotzer BJ, Vest M, Fiutem JJ, Piña IL (2013) A randomized controlled trial of high-dose vitamin D 3 in patients with heart failure. JACC: Heart Failure 1:84–90. https://doi.org/10.1016/j.jchf.2012.11.003
Brosolo G, Da Porto A, Bulfone L, Vacca A, Bertin N, Catena C, Sechi LA (2023) Cortisol secretion and abnormalities of glucose metabolism in nondiabetic patients with hypertension. J Hypertens. https://doi.org/10.1097/HJH.0000000000003590
Brosolo G, Catena C, Da Porto A, Bulfone L, Vacca A, Verheyen ND, Sechi LA (2022) Differences in regulation of cortisol secretion contribute to left ventricular abnormalities in patients with essential hypertension. Hypertension 79:1435–1444. https://doi.org/10.1161/HYPERTENSIONAHA.122.19472
Thesing CS, Bot M, Milaneschi Y, Giltay EJ, Penninx BWJH (2018) Omega-3 polyunsaturated fatty acid levels and dysregulations in biological stress systems. Psychoneuroendocrinology 97:206–215. https://doi.org/10.1016/j.psyneuen.2018.07.002
Chen J, Shearer GC, Chen Q, Healy CL, Beyer AJ, Nareddy VB, Gerdes AM, Harris WS, O’Connell TD, Wang D (2011) Omega-3 fatty acids prevent pressure overload–induced cardiac fibrosis through activation of cyclic GMP/protein kinase g signaling in cardiac fibroblasts. Circulation 123:584–593. https://doi.org/10.1161/CIRCULATIONAHA.110.971853
Block RC, Liu L, Herrington DM, Huang S, Tsai MY, O’Connell TD, Shearer GC (2019) Predicting risk for incident heart failure with omega-3 fatty acids. JACC: Heart Failure 7:651–661. https://doi.org/10.1016/j.jchf.2019.03.008
Matsuo N, Miyoshi T, Takaishi A, Kishinoue T, Yasuhara K, Tanimoto M, Nakano Y, Onishi N, Ueeda M, Ito H (2021) High plasma docosahexaenoic acid associated to better prognoses of patients with acute decompensated heart failure with preserved ejection fraction. Nutrients 13:371. https://doi.org/10.3390/nu13020371
Tavazzi L, Maggioni AP, Marchioli R, Barlera S (2008) Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. The Lancet 372:1223–1230. https://doi.org/10.1016/S0140-6736(08)61239-8
Jiang W, Whellan DJ, Adams KF, Babyak MA, Boyle SH, Wilson JL, Patel CB, Rogers JG, Harris WS, O’Connor CM (2018) Long-chain omega-3 fatty acid supplements in depressed heart failure patients. JACC: Heart Failure 6:833–843. https://doi.org/10.1016/j.jchf.2018.03.011
Azhar G, Raza S, Pangle A, Coleman K, Dawson A, Schrader A, Wolfe RR, Wei JY (2020) Potential beneficial effects of dietary protein supplementation and exercise on functional capacity in a pilot study of individuals with heart failure with preserved ejection fraction. Gerontology and Geriatric Medicine 6:233372142098280. https://doi.org/10.1177/2333721420982808
Pineda-Juárez JA, Sánchez-Ortiz NA, Castillo-Martínez L, Orea-Tejeda A, Cervantes-Gaytán R, Keirns-Davis C, Pérez-Ocampo C, Quiroz-Bautista K, Tenorio-Dupont M, Ronquillo-Martínez A (2016) Changes in body composition in heart failure patients after a resistance exercise program and branched chain amino acid supplementation. Clin Nutr 35:41–47. https://doi.org/10.1016/j.clnu.2015.02.004
Rennie MJ, Bohé J, Smith K, Wackerhage H, Greenhaff P (2006) Branched-chain amino acids as fuels and anabolic signals in human muscle. J Nutr 136:264S-268S. https://doi.org/10.1093/jn/136.1.264S
Hummel SL, Karmally W, Gillespie BW, Helmke S, Teruya S, Wells J, Trumble E, Jimenez O, Marolt C, Wessler JD, Cornellier ML, Maurer MS (2018) Home-delivered meals postdischarge from heart failure hospitalization: the GOURMET-HF pilot study. Circ: Heart Failure 11:e004886. https://doi.org/10.1161/CIRCHEARTFAILURE.117.004886
Hummel SL, Seymour EM, Brook RD, Kolias TJ, Sheth SS, Rosenblum HR, Wells JM, Weder AB (2012) Low-sodium dietary approaches to stop hypertension diet reduces blood pressure, arterial stiffness, and oxidative stress in hypertensive heart failure with preserved ejection fraction. Hypertension 60:1200–1206. https://doi.org/10.1161/HYPERTENSIONAHA.112.202705
Hummel SL, Seymour EM, Brook RD, Sheth SS, Ghosh E, Zhu S, Weder AB, Kovács SJ, Kolias TJ (2013) Low-sodium DASH diet improves diastolic function and ventricular–arterial coupling in hypertensive heart failure with preserved ejection fraction. Circ: Heart Failure 6:1165–1171. https://doi.org/10.1161/CIRCHEARTFAILURE.113.000481
Hammer S, Snel M, Lamb HJ, Jazet IM, Van Der Meer RW, Pijl H, Meinders EA, Romijn JA, De Roos A, Smit JWA (2008) Prolonged caloric restriction in obese patients with type 2 diabetes mellitus decreases myocardial triglyceride content and improves myocardial function. J Am Coll Cardiol 52:1006–1012. https://doi.org/10.1016/j.jacc.2008.04.068
Gulsin GS, Swarbrick DJ, Athithan L, Brady EM, Henson J, Baldry E, Argyridou S, Jaicim NB, Squire G, Walters Y, Marsh A-M, McAdam J, Parke KS, Biglands JD, Yates T, Khunti K, Davies MJ, McCann GP (2020) Effects of low-energy diet or exercise on cardiovascular function in working-age adults with type 2 diabetes: a prospective, randomized, open-label, blinded end point trial. Diabetes Care 43:1300–1310. https://doi.org/10.2337/dc20-0129
Lean MEJ, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, Peters C, Zhyzhneuskaya S, Al-Mrabeh A, Hollingsworth KG, Rodrigues AM, Rehackova L, Adamson AJ, Sniehotta FF, Mathers JC, Ross HM, McIlvenna Y, Welsh P, Kean S, Ford I, McConnachie A, Messow C-M, Sattar N, Taylor R (2019) Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol 7:344–355. https://doi.org/10.1016/S2213-8587(19)30068-3
Nordmann AJ, Nordmann A, Briel M, Keller U, Yancy WS, Brehm BJ, Bucher HC (2006) Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: a meta-analysis of randomized controlled trials. Arch Intern Med 166:285. https://doi.org/10.1001/archinte.166.3.285
Deng Y, Xie M, Li Q, Xu X, Ou W, Zhang Y, Xiao H, Yu H, Zheng Y, Liang Y, Jiang C, Chen G, Du D, Zheng W, Wang S, Gong M, Chen Y, Tian R, Li T (2021) Targeting mitochondria-inflammation circuit by β-hydroxybutyrate mitigates HFpEF. Circ Res 128:232–245. https://doi.org/10.1161/CIRCRESAHA.120.317933
Nielsen R, Møller N, Gormsen LC, Tolbod LP, Hansson NH, Sorensen J, Harms HJ, Frøkiær J, Eiskjaer H, Jespersen NR, Mellemkjaer S, Lassen TR, Pryds K, Bøtker HE, Wiggers H (2019) Cardiovascular effects of treatment with the ketone body 3-hydroxybutyrate in chronic heart failure patients. Circulation 139:2129–2141. https://doi.org/10.1161/CIRCULATIONAHA.118.036459
Lopaschuk GD, Dyck JRB (2023) Ketones and the cardiovascular system. Nat Cardiovasc Res 2:425–437. https://doi.org/10.1038/s44161-023-00259-1
Finicelli M, Di Salle A, Galderisi U, Peluso G (2022) The Mediterranean diet: an update of the clinical trials. Nutrients 14:2956. https://doi.org/10.3390/nu14142956
Sanderson JE (2017) Alcohol, hypertension, and heart failure with preserved (or normal) ejection fraction. Eur Heart J Qual Care Clin Outcomes 3:93–93. https://doi.org/10.1093/ehjqcco/qcw042
Vacca A, Bulfone L, Cicco S, Brosolo G, Da Porto A, Soardo G, Catena C, Sechi LA (2023) Alcohol intake and arterial hypertension: retelling of a multifaceted story. Nutrients 15:958. https://doi.org/10.3390/nu15040958
Miró Ò, Estruch R, Martín-Sánchez FJ, Gil V, Jacob J, Herrero-Puente P, Herrera Mateo S, Aguirre A, Andueza JA, Llorens P, Alonso H, Fuentes M, Gil C, Pérez-Durá MJ, Salvo E, Escoda R, Xipell C, Sánchez C, Gaytan JM, Noval A, Torres JM, López-Grima ML, Juan MA, Valero A, Pedragosa MÀ, Alonso MI, Ruiz F, Romero R, Calvache R, Morante C, Lorca MT, Mecina AB, Tost J, De La Fuente Penco B, López Sánchez A, Sánchez S, Piñera P, Garate RT, Alquézar A, Rizzi MA, Richard F, Álvarez Pérez JM, López Diez MP, Lucas J, Roset Á, Rodríguez-Adrada E, Llopis García G, Garrido JM, Fernández-Cañadas JM, Marquina V, Jiménez I, Javaloyes P, Vázquez Alvarez J, Alonso Morilla A, Irimia A (2018) Adherence to Mediterranean diet and all-cause mortality after an episode of acute heart failure. JACC: Heart Failure 6:52–62. https://doi.org/10.1016/j.jchf.2017.09.020
Casas R, Sacanella E, Urpí-Sardà M, Chiva-Blanch G, Ros E, Martínez-González M-A, Covas M-I, Lamuela-Raventos RM, Salas-Salvadó J, Fiol M, Arós F, Estruch R (2014) The effects of the Mediterranean diet on biomarkers of vascular wall inflammation and plaque vulnerability in subjects with high risk for cardiovascular disease. A randomized trial PLoS ONE 9:e100084. https://doi.org/10.1371/journal.pone.0100084
Fitó M, Estruch R, Salas‐Salvadó J, Martínez‐Gonzalez MA, Arós F, Vila J, Corella D, Díaz O, Sáez G, De La Torre R, Mitjavila M, Muñoz MA, Lamuela‐Raventós R, Ruiz‐Gutierrez V, Fiol M, Gómez‐Gracia E, Lapetra J, Ros E, Serra‐Majem L, Covas M, on behalf of the PREDIMED Study Investigators (2014) Effect of the Mediterranean diet on heart failure biomarkers: a randomized sample from the PREDIMED trial. European J of Heart Fail 16:543–550. https://doi.org/10.1002/ejhf.61
Kouvari M, Chrysohoou C, Aggelopoulos P, Tsiamis E, Tsioufis K, Pitsavos C, Tousoulis D (2017) Mediterranean diet and prognosis of first-diagnosed acute coronary syndrome patients according to heart failure phenotype: hellenic heart failure study. Eur J Clin Nutr 71:1321–1328. https://doi.org/10.1038/ejcn.2017.122
Wang T, Masedunskas A, Willett WC, Fontana L (2023) Vegetarian and vegan diets: benefits and drawbacks. Eur Heart J 44:3423–3439. https://doi.org/10.1093/eurheartj/ehad436
Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, Fang JC, Fedson SE, Fonarow GC, Hayek SS, Hernandez AF, Khazanie P, Kittleson MM, Lee CS, Link MS, Milano CA, Nnacheta LC, Sandhu AT, Stevenson LW, Vardeny O, Vest AR, Yancy CW (2022) 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 145. https://doi.org/10.1161/CIR.0000000000001063
Pandey A, LaMonte M, Klein L, Ayers C, Psaty BM, Eaton CB, Allen NB, De Lemos JA, Carnethon M, Greenland P, Berry JD (2017) Relationship between physical activity, body mass index, and risk of heart failure. J Am Coll Cardiol 69:1129–1142. https://doi.org/10.1016/j.jacc.2016.11.081
Edelmann F, Gelbrich G, Düngen H-D, Fröhling S, Wachter R, Stahrenberg R, Binder L, Töpper A, Lashki DJ, Schwarz S, Herrmann-Lingen C, Löffler M, Hasenfuss G, Halle M, Pieske B (2011) Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction. J Am Coll Cardiol 58:1780–1791. https://doi.org/10.1016/j.jacc.2011.06.054
Angadi SS, Mookadam F, Lee CD, Tucker WJ, Haykowsky MJ, Gaesser GA (2015) High-intensity interval training vs. moderate-intensity continuous exercise training in heart failure with preserved ejection fraction: a pilot study. J Appl Physiol 119:753–758. https://doi.org/10.1152/japplphysiol.00518.2014
Donelli Da Silveira A, Beust De Lima J, Da Silva PD, Dos Santos MD, Zanini M, Nery R, Laukkanen JA, Stein R (2020) High-intensity interval training is effective and superior to moderate continuous training in patients with heart failure with preserved ejection fraction: a randomized clinical trial. Eur J Prev Cardiolog 27:1733–1743. https://doi.org/10.1177/2047487319901206
Lang CC, Smith K, Wingham J, Eyre V, Greaves CJ, Warren FC, Green C, Jolly K, Davis RC, Doherty PJ, Miles J, Britten N, Abraham C, Van Lingen R, Singh SJ, Paul K, Hillsdon M, Sadler S, Hayward C, Dalal HM, Taylor RS (2018) A randomised controlled trial of a facilitated home-based rehabilitation intervention in patients with heart failure with preserved ejection fraction and their caregivers: the REACH-HFpEF Pilot Study. BMJ Open 8:e019649. https://doi.org/10.1136/bmjopen-2017-019649
Andryukhin A, Frolova E, Vaes B, Degryse J (2010) The impact of a nurse-led care programme on events and physical and psychosocial parameters in patients with heart failure with preserved ejection fraction: a randomized clinical trial in primary care in Russia. European J Gen Pract 16:205–214. https://doi.org/10.3109/13814788.2010.527938
Pandey A, Parashar A, Kumbhani DJ, Agarwal S, Garg J, Kitzman D, Levine BD, Drazner M, Berry JD (2015) Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials. Circ: Heart Failure 8:33–40. https://doi.org/10.1161/CIRCHEARTFAILURE.114.001615
Guo Y, Xiao C, Zhao K, He Z, Liu S, Wu X, Shi S, Chen Z, Shi R (2022) Physical exercise modalities for the management of heart failure with preserved ejection fraction: a systematic review and meta-analysis. J Cardiovasc Pharmacol 79:698–710. https://doi.org/10.1097/FJC.0000000000001254
Shafiq A, Brawner CA, Aldred HA, Lewis B, Williams CT, Tita C, Schairer JR, Ehrman JK, Velez M, Selektor Y, Lanfear DE, Keteyian SJ (2016) Prognostic value of cardiopulmonary exercise testing in heart failure with preserved ejection fraction. The Henry Ford HospITal cardiopulmonary exercise testing (FIT-CPX) project. Am Heart J 174:167–172. https://doi.org/10.1016/j.ahj.2015.12.020
Sachdev V, Sharma K, Keteyian SJ, Alcain CF, Desvigne-Nickens P, Fleg JL, Florea VG, Franklin BA, Guglin M, Halle M, Leifer ES, Panjrath G, Tinsley EA, Wong RP, Kitzman DW, on behalf of the American Heart Association Heart Failure and Transplantation Committee of the Council on Clinical Cardiology; Council on Arteriosclerosis, Thrombosis and Vascular Biology; and American College of Cardiology (2023) Supervised Exercise Training for Chronic Heart Failure With Preserved Ejection Fraction: A Scientific Statement From the American Heart Association and American College of Cardiology. Circulation 147:. https://doi.org/10.1161/CIR.0000000000001122
Kitzman DW (2002) Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA 288:2144. https://doi.org/10.1001/jama.288.17.2144
Nayor M, Houstis NE, Namasivayam M, Rouvina J, Hardin C, Shah RV, Ho JE, Malhotra R, Lewis GD (2020) Impaired exercise tolerance in heart failure with preserved ejection fraction. JACC: Heart Failure 8:605–617. https://doi.org/10.1016/j.jchf.2020.03.008
Upadhya B, Kitzman DW (2023) Mechanisms of exercise intolerance in chronic heart failure with preserved ejection fraction. Chest 164:574–577. https://doi.org/10.1016/j.chest.2023.06.019
Lam CSP, Grewal J, Borlaug BA, Ommen SR, Kane GC, McCully RB, Pellikka PA (2010) Size, shape, and stamina: the impact of left ventricular geometry on exercise capacity. Hypertension 55:1143–1149. https://doi.org/10.1161/HYPERTENSIONAHA.109.146845
Salzano A, De Luca M, Israr MZ, Crisci G, Eltayeb M, Debiec R, Ranieri B, D’Assante R, Rega S, D’Agostino A, Mauro C, Squire IB, Suzuki T, Bossone E, Guazzi M, Marra AM (2021) Exercise intolerance in heart failure with preserved ejection fraction. Heart Fail Clin 17:397–413. https://doi.org/10.1016/j.hfc.2021.03.004
Chien S, Lo C, Lin C, Sung K, Tsai J, Huang W, Yun C, Hung T, Lin J, Liu C, Hou CJ, Tsai I, Su C, Yeh H, Hung C (2019) Malnutrition in acute heart failure with preserved ejection fraction: clinical correlates and prognostic implications. ESC Heart Failure 6:953–964. https://doi.org/10.1002/ehf2.12501
Mirzai S, Carbone S, Batsis JA, Kritchevsky SB, Kitzman DW, Shapiro MD (2024) Sarcopenic obesity and cardiovascular disease: an overlooked but high-risk syndrome. Curr Obes Rep. https://doi.org/10.1007/s13679-024-00571-2
Carbone S, Lavie CJ, Arena R (2017) Obesity and heart failure: focus on the obesity paradox. Mayo Clin Proc 92:266–279. https://doi.org/10.1016/j.mayocp.2016.11.001
Middlekauff HR (2010) Making the case for skeletal myopathy as the major limitation of exercise capacity in heart failure. Circ: Heart Failure 3:537–546. https://doi.org/10.1161/CIRCHEARTFAILURE.109.903773
Haykowsky MJ, Tomczak CR, Scott JM, Paterson DI, Kitzman DW (2015) Determinants of exercise intolerance in patients with heart failure and reduced or preserved ejection fraction. J Appl Physiol 119:739–744. https://doi.org/10.1152/japplphysiol.00049.2015
Weiss K, Schär M, Panjrath GS, Zhang Y, Sharma K, Bottomley PA, Golozar A, Steinberg A, Gerstenblith G, Russell SD, Weiss RG (2017) Fatigability, exercise intolerance, and abnormal skeletal muscle energetics in heart failure. Circ: Heart Failure 10:e004129. https://doi.org/10.1161/CIRCHEARTFAILURE.117.004129
Kumar AA, Kelly DP, Chirinos JA (2019) Mitochondrial dysfunction in heart failure with preserved ejection fraction. Circulation 139:1435–1450. https://doi.org/10.1161/CIRCULATIONAHA.118.036259
Scandalis L, Kitzman DW, Nicklas BJ, Lyles M, Brubaker P, Nelson MB, Gordon M, Stone J, Bergstrom J, Neufer PD, Gnaiger E, Molina AJA (2023) Skeletal muscle mitochondrial respiration and exercise intolerance in patients with heart failure with preserved ejection fraction. JAMA Cardiol 8:575. https://doi.org/10.1001/jamacardio.2023.0957
Zhang H, Ryu D, Wu Y, Gariani K, Wang X, Luan P, D’Amico D, Ropelle ER, Lutolf MP, Aebersold R, Schoonjans K, Menzies KJ, Auwerx J (2016) NAD + repletion improves mitochondrial and stem cell function and enhances life span in mice. Science 352:1436–1443. https://doi.org/10.1126/science.aaf2693
Tucker WJ, Angadi SS, Haykowsky MJ, Nelson MD, Sarma S, Tomczak CR (2020) Pathophysiology of exercise intolerance and its treatment with exercise-based cardiac rehabilitation in heart failure with preserved ejection fraction. J Cardiopulm Rehabil Prev 40:9–16. https://doi.org/10.1097/HCR.0000000000000481
Kitzman DW, Brubaker PH, Herrington DM, Morgan TM, Stewart KP, Hundley WG, Abdelhamed A, Haykowsky MJ (2013) Effect of endurance exercise training on endothelial function and arterial stiffness in older patients with heart failure and preserved ejection fraction. J Am Coll Cardiol 62:584–592. https://doi.org/10.1016/j.jacc.2013.04.033
Pandey A, Shah SJ, Butler J, Kellogg DL, Lewis GD, Forman DE, Mentz RJ, Borlaug BA, Simon MA, Chirinos JA, Fielding RA, Volpi E, Molina AJA, Haykowsky MJ, Sam F, Goodpaster BH, Bertoni AG, Justice JN, White JP, Ding J, Hummel SL, LeBrasseur NK, Taffet GE, Pipinos II, Kitzman D (2021) Exercise intolerance in older adults with heart failure with preserved ejection fraction. J Am Coll Cardiol 78:1166–1187. https://doi.org/10.1016/j.jacc.2021.07.014
Kitzman DW, Haykowsky MJ, Tomczak CR (2017) Making the case for skeletal muscle myopathy and its contribution to exercise intolerance in heart failure with preserved ejection fraction. circ: heart failure 10:e004281. https://doi.org/10.1161/circheartfailure.117.004281
Haykowsky M, Brubaker P, Kitzman D (2012) Role of physical training in heart failure with preserved ejection fraction. Curr Heart Fail Rep 9:101–106. https://doi.org/10.1007/s11897-012-0087-7
Pandey A, Kitzman DW (2021) Searching for the optimal exercise training regimen in heart failure with preserved ejection fraction. JAMA 325:537. https://doi.org/10.1001/jama.2020.26347
Keteyian SJ, Brawner CA, Ehrman JK, Ivanhoe R, Boehmer JP, Abraham WT (2010) Reproducibility of peak oxygen uptake and other cardiopulmonary exercise parameters. Chest 138:950–955. https://doi.org/10.1378/chest.09-2624
Dun Y, Smith JR, Liu S, Olson TP (2019) High-intensity interval training in cardiac rehabilitation. Clin Geriatr Med 35:469–487. https://doi.org/10.1016/j.cger.2019.07.011
Walters GWM, Yeo JL, Bilak JM, Pepper C, Gulsin GS, Freeman SC, Gray LJ, McCANN GP, Brady EM (2024) The effectiveness of lifestyle interventions in heart failure with preserved ejection fraction: a systematic review and network meta-analysis. Journal of Cardiac Failure S1071916424000460. https://doi.org/10.1016/j.cardfail.2024.01.015
Norton K, Norton L, Sadgrove D (2010) Position statement on physical activity and exercise intensity terminology. J Sci Med Sport 13:496–502. https://doi.org/10.1016/j.jsams.2009.09.008
Trapp EG, Chisholm DJ, Freund J, Boutcher SH (2008) The effects of high-intensity intermittent exercise training on fat loss and fasting insulin levels of young women. Int J Obes 32:684–691. https://doi.org/10.1038/sj.ijo.0803781
Jelleyman C, Yates T, O’Donovan G, Gray LJ, King JA, Khunti K, Davies MJ (2015) The effects of high-intensity interval training on glucose regulation and insulin resistance: a meta-analysis. Obes Rev 16:942–961. https://doi.org/10.1111/obr.12317
Anjos JM, Neto MG, Dos Santos FS, Almeida KDO, Bocchi EA, Lima Bitar YDS, Duraes AR (2022) The impact of high-intensity interval training on functioning and health-related quality of life in post-stroke patients: a systematic review with meta-analysis. Clin Rehabil 36:726–739. https://doi.org/10.1177/02692155221087082
Hwang C-L, Wu Y-T, Chou C-H (2011) Effect of aerobic interval training on exercise capacity and metabolic risk factors in people with cardiometabolic disorders: a meta-analysis. J Cardiopulm Rehabil Prev 31:378–385. https://doi.org/10.1097/HCR.0b013e31822f16cb
Mueller S, Winzer EB, Duvinage A, Gevaert AB, Edelmann F, Haller B, Pieske-Kraigher E, Beckers P, Bobenko A, Hommel J, Van De Heyning CM, Esefeld K, Von Korn P, Christle JW, Haykowsky MJ, Linke A, Wisløff U, Adams V, Pieske B, Van Craenenbroeck EM, Halle M, OptimEx-Clin Study Group (2021) Effect of high-intensity interval training, moderate continuous training, or guideline-based physical activity advice on peak oxygen consumption in patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 325:542. https://doi.org/10.1001/jama.2020.26812
Vacca A, Schiattarella GG (2024) From gut to heart: role of indole-3-propionic acid in HFpEF. Circ Res 134:390–392. https://doi.org/10.1161/CIRCRESAHA.123.323947
Da Porto A, Cavarape A, Colussi G, Casarsa V, Catena C, Sechi LA (2021) Polyphenols rich diets and risk of type 2 diabetes. Nutrients 13:1445. https://doi.org/10.3390/nu13051445
Hussain T, Tan B, Yin Y, Blachier F, Tossou MCB, Rahu N (2016) Oxidative stress and inflammation: what polyphenols can do for us? Oxid Med Cell Longev 2016:1–9. https://doi.org/10.1155/2016/7432797
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the following grants: DZHK (German Centre for Cardiovascular Research — 81X3100210); the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation — SFB-1470 — A02) and the European Research Council — ERC StG 101078307 to G.G.S. HI-TAC (Helmholtz Institute for Translational AngioCardiScience) Early Career Investigator Grant (7.11442HIEC2401 to R.W.). Scholarship from Heart Failure Association (HFA) of the European Society of Cardiology (ESC) — HFA Basic and Translational Research Grant to F.C. Rosa Luxemburg Foundation to C.F. Bruno Magnani scholarship from Società Italiana dell’Ipertensione Arteriosa (Italian Society of Hypertension, SIIA) to A.V.
Author information
Authors and Affiliations
Contributions
A.V., R.W., and G.G.S drafted and reviewed the manuscript. N.N., C.F., F.C., A.M., and L.A.S. provided critical input. The figure has been created with BioRender.com licensed to G.G.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vacca, A., Wang, R., Nambiar, N. et al. Lifestyle interventions in cardiometabolic HFpEF: dietary and exercise modalities. Heart Fail Rev (2024). https://doi.org/10.1007/s10741-024-10439-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s10741-024-10439-1