Abstract
In this study two types of marine algae: red algae (Callithamnion corymbosum – CC-RAB) and green algae (Ulva lactuca – UL-GAB), were used for the retention of Cu2+, Zn2+ and Co2+ ions from aqueous media, by biosorption. Both types of marine algae are abundant on the Romanian coast of the Black Sea and, since they have no uses, they represent a serious problem for the beach area. Therefore, their use as biosorbents for the recovery of some metal ions of strategic industrial importance (such as Cu2+, Zn2+ and Co2+ ions) may represent a way to valorise this biomass resource. In order to evaluate the biosorptive performances of the red algae biomass (CC-RAB) and green algae biomass (UL-GAB), batch experimental studies were carried out at different initial solution pH, biosorbent dose, initial metal ions concentration contact time and temperature. The optimal conditions (pH = 5.0; 2.0 g biosorbent L-1, 3 h, 25 ±1 °C) were then used to obtain kinetic curves and biosorption isotherms, which were modelled. The pseudo-second order kinetic model best fits the kinetic data, while the biosorption isotherms are described by the Langmuir model, for all studied metal ions on both biosorbents. The maximum biosorption capacity depends on the nature of algae biosorbent, and follows the order: Cu2+ (81.25 mg g-1) > Zn2+ (73.69 mg g-1) > Co2+ (27.89 mg g-1) in the case of CC-RAB, and Zn2+ (69.29 mg g-1) > Cu2+ (43.47 mg g-1) > Co2+ (26.15 mg g-1) in the case of UL-GAB. The thermodynamic parameters (∆G0, ∆H0 and ∆S0) were also evaluated, and the obtained values indicate that all biosorption processes are spontaneous and endothermic. In addition, desorption of metal ions is quantitative in acid media, but the biosorption capacities decrease significantly after the first cycle of use. All these aspects have important environmental implications, and may provide benchmarks in the design of a strategy for the valorisation of this biomass resource.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environment pollution, especially of water sources, is one of the problems that are becoming more significant and widespread, as industrial activities expand (Crini and Lichtfouse 2019; Nowicka 2022). This is because most industrial activities use significant amounts of water in production activities and generate large amounts of wastewater which represent a threat to the quality of the environment. Most of the time, wastewater contains significant amounts of contaminants (metal ions and/or persistent organic compounds) which, once they reach the environment, cause severe degradation of ecosystems. Therefore, it is necessary to remove such contaminants from wastewater before they are discharged into the environment and finding technologically and economically efficient methods to achieve this is still an open issue for research.
A particular category of contaminants found in wastewater are heavy metal ions. It is well known that heavy metal ions are non-biodegradable, persistent for a long period of time, are toxic (even at low concentrations) and tend to accumulate in plants and aquatic organisms (Ackova 2018; Briffa et al. 2020). In addition, due to their economic and industrial importance (e.g., mining industry, metal coating industry, textile industry, manufacturing of alloys, fertilizers, paints, etc.) (Aji et al. 2012; Edebali and Pehlivan 2016), these ions frequently appear in wastewater, from they must be removed to fulfil the quality conditions imposed by the legislation (Vareda et al. 2019).
Although numerous methods for removing heavy metal ions from aqueous media are reported in the literature (eg., precipitation, coagulation, membrane filtration processes, ion exchange, etc.), (Bashir et al. 2019; Varjani et al. 2020; Saleh et al. 2022), their practical utility is limited by important drawbacks. For example, precipitation and coagulation require a significant consumption of chemical reagents and energy, generating large amounts of sludge (secondary waste), which in turn, must be properly treated so as not to pollute the environment (Pohl 2020). On the other hand, membrane filtration processes and ion exchange involve high energy consumption and high operating costs, due to the materials required for their realization (Fabre et al. 2020; Xiang et al. 2022). In addition, each of these methods requires significant improvements in terms of increasing efficiency, selectivity and reducing working time.
Biosorption is considered an alternative to these methods for the removal of metal ions from aqueous media, mainly because it is ecological, inexpensive, simple to operate, and easy to adapt to various experimental conditions, which gives it great technological flexibility (Vijayaraghavan and Balasubramanian 2015). Biosorption involves the retention of heavy metal ions on the surface of materials of biological origin through electrostatic interactions (most often ion exchange type) (Crini and Lichtfouse 2019; Yaashikaa et al. 2021). Biosorption has two important advantages in the treatment of the industrial effluents. The first advantage is related to the possibility of quantitative recovery of retained heavy metal ions, through desorption using mineral acids solutions (Chatterjee and Abraham 2019). The solution obtained after desorption has a much higher concentration of heavy metal ions (since desorption is carried out with small volumes of desorption agent), is stable over time (due to the acid media that prevent the change of the speciation form of the metal ion) and can be reintroduced into the technological circuit to minimize costs. The second advantage is determined by the nature of the solid materials that can be used as biosorbents. Many studies (Crini et al. 2019; Yaashikaa et al. 2021) have shown that practically any natural material, agricultural or industrial waste (biomass), can retain significant amounts of heavy metal ions in well-established experimental conditions. Most of the time, such materials have no other practical applications and their use as biosorbents represents a simple and sustainable way of valorising them. Moreover, due to the low cost of preparation, in most cases, biosorbents are used in biosorption processes until exhaustion (Agarwal et al. 2020), and after the recovery of retained metal ions, they are incinerated to obtain thermal energy (Vasic et al. 2023). Such an approach is in line with the principles of circular economy and sustainable development (Karic et al. 2022), and makes biosorption a cheap, ecological and effective method of treating industrial effluents containing heavy metal ions. However, all these advantages depend on the choice of a suitable biosorbent, which can be easily purchased in large quantities.
In the last two decades the use of marine algal biomass as biosorbents has been intensively studied for the removal of heavy metal ions (Farooq et al. 2010; Anastopoulos and Kyzas 2015; Lee et al. 2022; Ordonez et al. 2023). Higher biosorption capacity for various heavy metals, absence of secondary waste generation, availability throughout the year in many region of the world, low cost and low energy consumption (Qulatein and Yilmaz 2023), are only a few reasons for the intensive use of these materials as biosorbents. To date, many studies on metal ions biosorption by marine algal biomass have focused on brown algae, while the use of green and red algae as biosorbents has been less evaluated (Romera et al. 2006). Unfortunately, on the Romanian coast of the Black Sea, green and red algae (especially Ulva lactuca and Callithamnion corymbosum) are much more abundant that brown ones (Taskin et al. 2020). Since these algae have no other applications and cause serious inconveniences to tourists and beach managers, their use as biosorbents for the retention of heavy metal ions may represent a solution to this problem.
In this study, the two types of marine algae biomass (Ulva lactuca (green algae – UL-GAB) and Callithamnion corymbosum (red algae – CC-RAB)) were used as biosorbents for the removal of Cu2+, Zn2+ and Co2+ ions from aqueous media. The selection of marine algae biomass was made taking into account their abundance in the Black Sea (in order to open up new possibilities for valorisation of this biomass resource), while the metal ions were selected considering their economic and industrial relevance. The biosorption studies were carried out in batch systems, for each type of biosorbent and metal ion, and aimed to establish the optimal experimental conditions for maximum metal ions retention efficiency, as well as modelling the experimentally obtained isotherms and kinetic curves. Based on these data and the structural characteristics of the biosorbents (obtained by FTIR spectrometry and SEM microscopy), the biosorption mechanism was developed. All the experimental results were discussed comparatively, in order to highlight the application potential of the two types of algae biomasses in the treatment of aqueous effluents, but also the environmental implications derived from them.
Materials and methods
Chemical reagents
650-700 mg M2+ L-1 (M2+ = Cu2+, Zn2+ and Co2+) were used as stock solutions in the biosorption experiments. These solutions were prepared from solid metal nitrate (purity > 99.5 %, Chemical Company, Iaşi, Romania) by dissolving in an appropriate volume of distilled water (APS 40 distillation system). The working solutions were obtained by fresh dilution of stock solutions. 10-2 N HNO3 and NaOH solutions were used to adjust the pH.
Marine algae preparation and characterization
Two types of marine algae: red algae Callithamnion corymbosum (CC-RAB) and green algae Ulva lactuca (UL-GAB) were used as biosorbents for the retention of the studied metal ions. Both algae were sampled from the Romanian coast of the Black Sea (Costineşti, Romania) in 2019 and visually identified. After sampling, the algae were washed with tap water to remove solid impurities (sand, shell fragments, etc.), then with distilled water to remove dissolved salts from the thalli, and air-dried at room temperature (3-4 days). The dry samples were then ground and sieved, and only fractions smaller than 1.0 mm were retained for the experimental studies. After preparation, the algae samples (CC-RAB and UL-GAB) were kept in desiccators at constant humidity so that their composition would not change.
For both types of algae biomass the surface morphology was examined using SEM microscopy (SEM S3000N, Hitachi, Japan (by graphitization, at 5 and 10 kV, 50 mm distance)), while FTIR spectrometry (Bio-Rad FTIR Spectrometer, USA) was used for the identification of surface functional groups. FTIR spectra were recorded between 4000 and 400 cm-1, with a resolution of 4 cm-1, by KBr pellet technique.
Biosorption/desorption experiments
All biosorption experiments were performed in batch systems (for each metal ions (Cu2+, Zn2+ and Co2+) and biosorbent (CC-RAB and UL-GAB)), by mixing 25 mL of metal ions solution with given amount of biosorbent in 100 mL conical flasks. To establish the optimal conditions, the experimental parameters were varied one by one in a given range (pH (2.0 – 6.0), biosorbent dose (2.0 – 20.0 g biosorbent L-1) and temperature (10, 25 and 50 °C)), for a metal ion concentration of 25 ± 1 mg M2+ L-1 and a contact time of 24 h. The kinetic experiments were performed by varying the contact time between 5 and 180 min at a constant initial M2+ ions concentration (25 ± 1 mg L-1), initial pH (5.0), biosorbent dose (2.0 g L-1) and temperature (25 ± 1°C). The experimental isotherms were obtained by altering the initial concentration of metal ions (13 – 180 mg L-1), while the other parameters were maintained constant (pH = 5.0; biosorbent dose = 2.0 g L-1; contact time = 3 h and temperature = 25 ± 1°C). After the biosorption experiments were completed the solid biosorbent was separated for the solution by filtration (0.45μm polypropylene filter (Whatman, USA)), and the concentration of metal ions in the filtered solutions was determined by Atomic Absorption Spectrometry (AAS NovAA 400P Spectrometer (Analytik Jena, Germany), air/acetylene flame).
The evaluation of the biosorption performance of CC-RAB and UL-GAB for the studied heavy metal ions (Cu2+, Zn2+ and Co2+) was done using the biosorption capacity (q, mg g-1) and the removal percent (R, %), calculated for the experimental data using the equations:
where: co is the initial concentration of metal ions in solution (mg L-1), c is the final concentration of metal ions in solution (mg L-1), V is volume of solution (L), and m is the mass of biosorbent used in the experiments (g).
The evaluation of the biosorption potential of the two biosorbents for the studied metal ions in the presence of other coexisting ions was done under optimal conditions (pH = 5.0, 2 g biosorbent L-1, contact time = 3 h, temperature = 25 ± 1 °C), using 25 mL of binary mixture of Cu2+, Zn2+ and Co2+ ions (50 ± 4 mg L-1) and coexisting ions: Ca2+, Mg2+, Na+ and K+ (0.01, 0.1 and 1 mol L-1). The other stages of the experimental methodology are similar to those presented above.
In desorption experiments, samples of each biosorbent (0.5 g) were mixed with 100 mL of each heavy metal ion solution (120 mg L-1) of pH 5.0, kept in contact for 3 h, then filtered and dried. The biosorbent samples loaded with heavy metal ions were then treated with 20 mL of 0.1 N HNO3, stirred for 3 h, filtered and analyzed (as mentioned above). The same sample of biosorbent was used in three successive biosorption/desorption cycles. The obtained results were used to calculate the recovery percent of heavy metal ions (% recovery) with the relation:
where: cd is the concentration of metal ions in the solutions obtained after desorption (mg L-1), q is the biosorption capacity of each biosorbent for each metal ion (mg g-1), and m is the mass of biosorbent loaded with metal ion (g).
Both biosorption and desorption experiments were performed in triplicate, and the average values were used to calculate the biosorption/desorption parameters. All experimental results were statistically analyzed by ANOVA, and p-values below 0.05 were considered significant.
Data modeling
The mathematical models used for the modeling of the experimental data are presented in Table 1. These models were selected taking into account the usefulness of the information they provide in describing biosorption processes. The kinetic data were analyzed using pseudo-first order model, pseudo-second order model and intra-diffusion particle model (Ho and McKay 1999; Tan and Hameed 2017; Wang and Guo 2022). The experimental equilibrium data were modeled using the Langmuir, Freundlich and Temkin models (Chong and Volesky 1995; Rangabhashiyam et al. 2014).
The characteristic parameters of each model were calculated from nonlinear equations, and the best model fitting the experimental results was selected based on the highest value of the regression coefficient (R2) and lowest value of the sum of squared errors (SSE).
Tests on real samples
Tap water samples were used as background for tests on real samples. For each water sample (250 mL), the amount of biosorbent (CC-RAB and UL-GAB), contact time and temperature were kept constant (0.5 g biosorbent, 3 h and 25 ± 1°C), while the pH and initial concentration of each metal ion were adjusted at 5.0 and 30 mg L-1, in mono-component system. After filtration, the concentration of metal ions was determined by AAS spectrometry (as was mentioned above). Other quality indicators of water samples (pH, CCO-Cr, TSS, chloride, hardness) were also analyzed using the standard methods (Fresenius et al. 1998).
Results
Structural characteristics of marine algae biosorbents
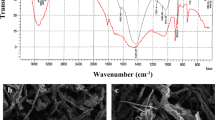
SEM images of CC-RAB and UL-GAB biosorbents are illustrated in Fig. 1a and b. Both biosorbents have a heterogeneous surface, where wrinkles and cracks can be observed, mostly consisting of cell walls. Fig. 1c presents the FTIR spectra recorded for CC-RAB and UL-GAB. In both FTIR spectra the following can be observed: (i) absorption bands corresponding to O–H and N–H stretching vibration in alcohols and amines (3421 cm-1 for CC-RAB and 3296 cm-1 for UL-GAB), (ii) absorption bands corresponding to C–H stretching vibration in hydrocarbon radicals (2954-2922 cm-1 for CC-RAB and 2939-2908 cm-1 for UL-GAB), (iii) absorption bands for C=O stretching vibrations in carbonyl and carboxylic compounds (1654 cm-1 for CC-RAB and 1656 cm-1 for UL-GAB), and (iv) absorption bands assigned to C–O–C stretching vibrations in carboxylic compounds and alcohols (1051-1035 cm-1 for CC-RAB and 1055-1031 cm-1 for UL-GAB).
The bands at 1419 cm-1 for CC-RAB and 1417 cm-1 for UL-GAB are due to the deformation vibrations of the –C–OH groups in the polysaccharide structure, while the band at 1338 cm-1 (for both types of algae) is due to the presence of C–O stretching of ether groups (Khan et al. 2016). The band at 1161 cm-1 corresponds to stretching vibrations of C–O bond in polysaccharides, while the bands at ~1535 cm-1 can be attributed to S=O sulfate esters of the polysaccharides (Fabre et al. 2020).
Effect of the experimental parameters
It is well known that the efficiency of a given biosorption process depends significantly on the experimental conditions under which it takes place. Although there is a relatively large number of parameters that can define the experimental conditions, in the case of biosorption processes, the initial pH, biosorbent dose and the temperature have the most significant contribution (Crini et al. 2019; Qulatein and Yilmaz 2023). For this reason, in order to establish the optimal conditions for the biosorption of Cu2+, Zn2+ and Co2+ ions on CC-RAB and UL-GAB, the influence of these parameters was analyzed, one by one. The obtained experimental results, for an initial concentration of metal ions of 25 ± 1.0 mg L-1 and contact time of 24 h, are illustrated in Fig. 2.
Fig. 2(a and b) shows the variation of the biosorption capacity of CC-RAB and UL-GAB at different values of initial solution pH. It should be noted that for both biosorbents, the biosorption capacity increases with increasing initial pH in the range 2.0 – 5.0, for all studied metal ions.
The biosorbent dose is the second experimental parameter which was detailed examined for the biosorption of Cu2+, Zn2+ and Co2+ ions biosorption on CC-RAB and UL-GAB, and the results are illustrated in Fig. 2(c and d). Regardless of the nature of the metal ion or the nature of algae biomass, the increase in the biosorbent dose in the range of 2.0 – 20.0 g L-1 (initial pH of 5.0) causes a decrease in the biosorption capacities.
The effect of temperature of Cu2+, Zn2+ and Co2+ ions biosorption on CC-RAB and UL-GAB is shown in Fig. 2e and f. The temperature increase in the range 10 – 50 °C causes a certain increase in the biosorption capacities for all metal ions and for both biosorbents. But as can be seen from Fig. 2e and f, an increase in temperature of 40 °C results an insignificant increase in biosorption capacity up to 2 mg g-1, regardless of the nature of the metal ions or the nature of the biosorbent.
The experimental results presented above show that the biosorption of Cu2+, Zn2+ and Co2+ ions on CC-RAB and UL-GAB occurs with maximum efficiency when the initial pH is 5.0, the biosorbent dose is 2.0 g L-1 and at ambient temperature (25 ± 1 °C). Under these conditions, for an initial concentration of metal ions of 25 ± 1 mg L-1 and a contact time of 24 hours, the removal percents obtained experimentally are in all cases higher than 65 %, which ensures a good efficiency of the biosorption processes.
Kinetics of metal ions biosorption on CC-RAB and UL-GAB
Figure 3 illustrates the variation of removal percent at different values of contact time for the biosorption of Cu2+, Zn2+ and Co2+ ions on CC-RAB and UL-GAB biosorbents. In the first 30 min, the biosorption processes are fast and a metal ion removal efficiency over 53 % is obtained in the case of both biosorbents (CC-RAB: Cu2+ (73 %) > Zn2+ (61 %) > Co2+ (54 %); UL-GAB: Cu2+ (83 %) > Zn2+ (66 %) > Co2+ (53 %)). After that, the biosorption rate gradually decreases and saturation is reached after 60 min in the case of UL-GAB, and after 120 min in the case of CC-RAB, for all metal ions. Under these conditions, a contact time of 180 min can be considered sufficient for the biosorption processes of Cu2+, Zn2+ and Co2+ ions on CC-RAB and UL-GAB to reach equilibrium.
Equilibrium isotherms of metal ions biosorption on CC-RAB and UL-GAB
The variation of biosorption capacity as a function of the initial metal ions concentration for the biosorption of Cu2+, Zn2+ and Co2+ ions on CC-RAB and UL-GAB is illustrated in Fig. 4.
The experimental data (Fig. 4) indicate that over the entire studied concentration range (13 – 180 mg L-1), the biosorption capacity of both biosorbents increases with the increase of the initial concentration of metal ions, for all studied metal ions. Moreover, the increase in biosorption capacity is almost linear in all cases, which shows that the saturation of the biosorbents is not reached and that both algae biomasses have enough superficial functional groups to retain metal ions even when their concentration is high.
On the other hand, the analysis of the results presented in Fig. 4 allows the highlighting of two observations, which have practical importance. The first observation is related to the biosorption capacity values, which are higher in the case of CC-RAB that in the case of UL-GAB, for all studied metal ions. Such a difference is well known in the literature (Crini et al. 2019; Syeda et al. 2022), and is due to the fact that red algae have a large number of functional groups on the surface (so they are more efficient in biosorption processes) than green algae, due to the color pigments in their composition.
The second observation considers the efficiency of metal ions biosorption on each biosorbent. Thus, in the case of CC-RAB, Cu2+ ions are retained most effectively, then those of Zn2+ and Co2+ (Fig. 4a), while in the case of UL-GAB, the efficiency of the biosorption processes follows the order Zn2+ > Cu2+ > Co2+ (Fig. 4b). These differences are mainly determined by the structural particularities of the metal ions and of the biosorbents. Therefore, it is necessary to model the experimental data to be able to evaluate the biosorption performances of the two algae biomasses.
Desorption of retained metal ions
The retention of Cu2+, Zn2+ and Co2+ ions on CC-RAB and UL-GAB predominantly by electrostatic interactions has two particularly important consequences from a practical point of view. The first is related to the fact that biosorption processes are reversible (as long as no other secondary processes occur due to the metal ions speciation). This means that the retention of the metal ions will not be fully achieved and only the equilibrium state will be reached. This observation, proved by the experimental data presented in the previous sections, explains the relatively modest efficiency of the studied biosorption processes.
The second consequence is related to desorption of the metal ions retained on the surface of the biosorbents, which allows their quantitative recovery. To evaluate the metal ion recovery efficiency, exact amounts of biosorbents (CC-RAB and UL-GAB) loaded with metal ions (Cu2+, Zn2+ and Co2+) were treated with 20 mL of 0.1 HNO3 solution in three biosorption/desorption cycles. The obtained results are illustrated in Fig. 5.
As seen from Fig. 5b and d, all metal ions are quantitatively desorbed (over 97 %) in all three cycles. After desorption, the metal ions are found in an acidic solution, being able to be easily reintroduced in the technological processes.
Unfortunately, the biosorption capacities of CC-RAB and UL-GAB decrease significantly in the biosorption cycles 2 and 3 (Fig. 5a and c). This means that when algae biomass is treated with HNO3 (0.1 N), an important number of the functional groups becomes unavailable to further interactions with metal ions in the aqueous solution (either biomass degradation or in intra-molecular bonds formation). Therefore, CC-RAB and UL-GAB can be successfully used to retain Cu2+, Zn2+ and Co2+ ions from aqueous solution, but only in one biosorption cycle. After biosorption, the metal ions can be quantitatively recovered, but other valorization alternatives must be found for the exhausted biosorbents. This will be the subject of another study.
Effect of coexisting ions
In real water samples, in addition to target metal ions (Cu2+, Zn2+ and Co2+, in this case), there are inevitable other metal ions (such as Ca2+, Mg2+, Na+, K+, etc.). Therefore, it is necessary to evaluate the biosorption performances of the two algae biomasses in such situations.
The competitive biosorption of Cu2+, Zn2+ and Co2+ on CC-RAB and UL-GAB in presence of different concentration of Ca2+, Mg2+, Na+, K+ ions (10-2 – 1 mol L-1), in mono-component batch system, was examined. The experimental conditions were maintained at optimal values (c0(M2+) = 50 ± 4 mg L-1, pH=5.0, biosorbent dose = 2.0 g L-1, contact time = 3 h, temperature = 25 ± 1 °C), and the results are presented in Fig. 6.
Real water samples tests
To highlight the practical applicability of these biosorbents in the removal processes of Cu2+, Zn2+ and Co2+ ions, tap water samples were used, in which the metal ions concentration was adjusted at 30 ± 2 mg L-1. The other experimental parameters were maintained at optimal values (pH=5.0, biosorbent dose = 2.0 g L-1, contact time = 3 h, temperature = 25 ± 1 °C), and after the completion of biosorption, in addition to the concentration of each metal ions, several water quality parameters (pH, COD, TSS, chloride, hardness) were determined.
Figure 7 shows the results for each biosorbent (CC-RAB and UL-GAB), noting that all experiments were performed in a mono-component system, and the quality parameters values (pH, COD, TSS, chloride, hardness) represent the average of three experimental values (obtained for each individual ion). All the experimental values were compared with the maximum permissible limits, according with Romanian legislation (NTPA 001/2005).
Discussion
Characterization of CC-RAB and UL-GAB
Heterogenic surface morphology of both biosorbents (Fig. 1a and b) is mainly due to the physical operations (drying, grinding) used for the preparation of the biosorbents, but it also represents an advantage from the biosorption process point of view, as it favors the increase in the number of superficial functional groups that can interact with metal ions in aqueous solution. However, the values determined for the specific surface area of the two biosorbents are quite close (54.02 m2 g-1 for CC-RAB and 52.28 m2 g-1 for UL-GAB) (Ciobanu et al. 2023), which means that the biosorptive performance of the two type of marine algae biomass will be mainly determined by the number and nature of superficial functional groups.
The detailed analysis of FTIR spectra (Fig. 1c) shows that both algae biosorbents have a large number of functional groups on their surface, such as hydroxyl, carbonyl, carboxyl, amino, sulfonate, etc., which under certain experimental conditions can dissociate, thus ensuring the presence of negative charges for the binding of metal ions from aqueous solution. So, the next step in evaluating the biosorptive performances of CC-RAB and UL-GAB is to establish the optimal experimental conditions under which the biosorption processes can occurs with maximum efficiency.
Optimal conditions of biosorption process
The influence of initial solution pH (Fig. 2a and b) shows that regardless of the nature of the biosorbent, each metal ion is retained with low efficiency at pH 2.0 (below 30 %), and this is mainly due to the low dissociation degree of functional groups and the completion of protons for the active center of the biosorbent. The increase in initial pH causes a continuous increase in the efficiency of biosorption processes up to pH 5.0, where the biosorption capacities reach maximum values, being higher in the case of CC-RAB (Zn2+ (12.51 mg g-1) > Cu2+ (10.01 mg g-1) > Co2+ (6.70 mg g-1)) than in the case of UL-GAB (Zn2+ (5.34 mg g-1) > Cu2+ (4.33 mg g-1) > Co2+ (3.17 mg g-1)). The continuous increase in the biosorption capacities in this pH range (2.0 – 5.0) is a consequence of the increase in the dissociation degree of superficial functional groups of the biosorbents, which facilitates the retention of metal ions through electrostatic interactions. At initial pH higher than 5.0, the biosorption capacities decrease in the case of UL-GAB (Fig. 2b), and remain approximately constant in the case of CC-RAB (Fig. 2a). Since this behavior can be observed for all studied metal ions (Cu2+, Zn2+ and Co2+), their precipitation is excluded.
Most likely, the decrease in biosorption capacities at pH > 5.0 is determined by some secondary processes (e.g. dissolution of some organic compounds (starch) from the biosorbent composition) (Romera et al. 2006), which most likely reduce the number of binding sites available for metal ions. Therefore, according to the experimental data, an initial pH of 5.0 can be considered optimal for the biosorption of Cu2+, Zn2+ and Co2+ ions on CC-RAB and UL-GAB, and this will be used in subsequent experiments. At this initial pH (5.0), the removal percents are higher than 65 % for both biosorbent (CC-RAB: Zn2+ (87.88 %) > Cu2+ (82.81 %) > Co2+ (65.89 %); UL-GAB: Zn2+ (82.87 %) > Cu2+ (78.92 %) > Co2+ (66.03 %)), and the biosorption processes can be considered effective.
The effect of biosorbent dose indicates that the biosorption capacities of metal ions on UL-GAB decrease the most (by almost 6 times), compared to the values obtained for CC-RAB (which decrease by about 4 times) (Fig. 2c and d). This variation is generally valid for biosorption processes of metal ions on biosorbents of biological origin (Syeda et al. 2022), and can be explained based on Eq. (1), which indicates an inversely proportional relationship between the values of m (biosorbent mass) and q (biosorption capacity). In addition, with increasing biosorbent dose, biomass particles get too close to each other, which allows weak physical interactions between superficial functional groups of different particles of the biosorbent. Consequently, the biosorbent particles agglomerate and some functional groups become unavailable for interactions with metal ions in the aqueous solution. On the other hand, the increase of the biosorbent dose by 10 times (2.0 – 20.0 g L-1), resulted in a relatively small increase in the removal percentage (up to 15 %), regardless of the nature of metal ion and the nature of the biosorbent (data not shown). Under these conditions, a biosorbent dose of 2.0 g L-1 can be considered optimal for the biosorption of Cu2+, Zn2+ and Co2+ ions on CC-RAB and UL-GAB, and this value will be used in all experiments. The selected value of the biosorbent dose ensures: (i) a low cost of biosorption processes, being advantageous from an economic point of view, (ii) the highest values of biosorption capacity for all metal ions and both biosorbents (see Fig. 2 c and d), and (iii) a high efficiency of biosorption processes, proved by the high values of the removal percents (CC-RAB: Zn2+ (89.47 %) > Cu2+ (83.12 %) > Co2+ (66.09 %); UL-GAB: Zn2+ (81.92 %) > Cu2+ (79.04 %) > Co2+ (66.32 %)).
On the other hand, the small improvement in the efficiency of biosorption processes (up to 4-5 %) does not justify the cost associated with maintaining aqueous solution at high temperatures (50 °C), even though experimental data clearly show that all biosorption processes are endothermic (Fig. 2e and f). Therefore, the ambient temperature (25 ± 1 °C) can be considered optimal for the biosorption of Cu2+, Zn2+ and Co2+ ions on CC-RAB and UL-GAB. In addition, the selection of this temperature for the further experiments has two advantages: (i) maintaining a low cost for the biosorption processes, and (ii) the temperature does not need to be strictly controlled, since the variation of a few units (expected under laboratory conditions) does not significantly affect the values of the biosorption parameters.
Kinetic modeling of metal ions biosorption on CC-RAB and UL-GAB
As can be seen from Fig. 3, the increase in the biosorption rate in two distinct stages, a rapid one (initial stage (up to 30 min)), and a much slower one (final stage (after 30 min)) can be explained by taking into account the availability of the superficial functional groups of the biosorbents. In the initial stage, most of the functional groups of biosorbent are free and can interact easily with the metal ions in the aqueous solution, and the biosorption rate is high. As these functional groups become occupied, the metal ions have a harder time finding free functional groups to interact with, and the biosorption rate decreases significantly. This similar behavior of the two biosorbents (as well as many other materials of biological origin) is a first indication that the retention of metal ions on their surface requires the existence of chemical interactions (most likely electrostatic, considering the effect of initial pH (Fig. 2a and b)). Therefore, kinetic modeling of the experimental data is necessary to highlight the elementary steps of the biosorption processes.
In this study, kinetic modeling of the experimental data was performed using the pseudo-first order model, the pseudo-second order model and the intra-particle diffusion model, because the first two models indicate the number of functional groups required for metal ions biosorption, while the last one provides information related to the contribution of elementary diffusion processes to the achievement of biosorption. The selection of the most appropriate model to describe the experimental data was made using the regression coefficients (R2) and the sum of squared errors (SSE), calculated for the statistical analysis.
The kinetic curves (theoretical and experimental) obtained for the biosorption of Cu2+, Zn2+ and Co2+ ions on CC-RAB and UL-GAB are shown in Fig. 8 and the kinetic parameters are summarized in Table 2.
Table 2 shows that all kinetic models describe the experimental results quite well, because the R2 values are higher than 0.9 in almost all cases. But, in the case of the pseudo-second order model: (i) R2 values are the highest, (ii) SSE values are the lowest, and (iii) the calculated qe values are close to the experimental ones (Table 2). This means that the pseudo-second order kinetic model best fits the experimental data obtained on the biosorption of Cu2+, Zn2+ and Co2+ ions on CC-RAB and UL-GAB. The very good concordance between the experimental data and the pseudo-second order kinetic model shows that the retention of metal ions on the two biosorbents (CC-RAB and UL-GAB) is achieved through chemical interactions (most likely electrostatic type). To achieve these interactions, metal ions need two superficial functional groups located at the appropriate distance (geometrically favorable). However, the high values of R2 obtained in the case of the pseudo-first order kinetic model (Table 2) indicate that the binding of the metal ions on the biosorbent surface is achieved by two such electrostatic interactions, which occur successively (one after the other). In the first moments, the metal ions interact with the most available functional groups on the surface of the biosorbent, and once their binding is achieved, the intermediate “complex” passes into a more stable form, through the second interactions with another functional group. This observation is supported by the rate constant values calculated for the two kinetic models (Table 2).
But, the metal ions reach the surface of the biosorbent through elementary diffusion steps. The linear representation of the intra-particle diffusion model (Fig. 9) shows that in neither case do the regression lines pass through the origin, therefore the diffusion steps are not the rate limiting steps in the studied biosorption processes.
In Fig. 9 two distinct regions can be observed for all metal ions and biosorbents and these regions correspond to: region 1 - the diffusion of metal ions inside of the biosorbent particles, and region 2 - attributed to the diffusion of metal ions from the bulk of the solution to the surface of the biosorbent (Wang and Guo 2022). The kinetic parameters calculated for the two regions (Table 2) show that the metal ions are easily transported to the surface of the biosorbent (kdiff2 – large, c2 – small), where they accumulate (kdiff1 – small, c1 – large), until find the functional groups with which to be able to interact.
It can be also observed from Table 2 that the values of the kinetic parameters of the pseudo-second order model depend on the nature of the metal ion and the nature of the biosorbent. Thus, the values of the rate constants (k2) and the calculated biosorption capacities (qe) follow the order: Cu2+ > Co2+ > Zn2+ for CC-RAB, and Zn2+ > Cu2+ > Co2+ for UL-GAB, respectively. This variation suggests that the efficiency of biosorption processes is determined by both the nature of the biosorbent and the structural characteristics (ex. electronegativity and geometric radius) of the metal ions in the aqueous solution.
Consequently, the retention of Cu2+, Zn2+ and Co2+ ions on the surface of CC-RAB and UL-GAB is not random, but occurs through directed interactions, such as chemical interactions.
Isotherm modeling of metal ions biosorption on CC-RAB and UL-GAB
The modeling of equilibrium data for the biosorption of Cu2+, Zn2+ and Co2+ ions on CC-RAB and UL-GAB was performed using Langmuir, Freundlich and Temkin models. The experimental and theoretical isotherms are illustrated in Fig. 10, while the characteristic parameters of these models are presented in Table 3.
The values of R2 and SSE indicate that the Langmuir model is most appropriate to accurately describe the biosorption of Cu2+, Zn2+ and Co2+ ions on these two biosorbents, although as in the case of Freundlich and Temkin models, the values of these statistical parameters are also quite good (Table 3). The very good agreement between the experimental data and the Langmuir model, and the good tie between the experimental data and the Freundlich model show that the retention of metal ions occurs on the biosorbent surface until a complete monolayer is formed (in agreement with Langmuir model). However, due to the heterogeneity of the biosorbents surface (Fig. 1a and b), the superficial functional groups used for binding the metal ions are, most likely, located in different geometric planes, which explains the concordance between the experimental data and the Freundlich model.
According to the Temkin model, these interactions are predominantly electrostatic (probably ion-exchange type, taking into account the initial pH of the aqueous solutions (5.0)), since the values of the biosorption energy (B, kJ mol-1) do not exceed 24 kJ mol-1 (Table 3). In addition, the values of the maximum biosorption capacities (qmax, calculated for the Langmuir model) are higher than those experimentally obtained (Table 4), which indicate that both biosorbents (CC-RAB and UL-GAB) have enough functional groups on their surface to retain metal ions even at high concentration, without reaching saturation (see Fig. 4).
Moreover, even in the range of high metal ions concentration, all biosorption processes are favorable (chemical interactions take place), which is proven by the values greater than unit of the n parameter, calculated from the Freundlich model (Table 3). As seen from Table 3, all these observations are valid regardless of the nature of the metal ion or the nature of the biosorbent, which means that all studied biosorption processes occur through similar mechanisms.
However, the efficiency of biosorption processes depends on both the nature of the biosorbent and the metal ion in the aqueous solution. The detailed analysis of the experimental results and those obtained by isotherm modeling (Fig. 10 and Table 3) shows that: (i) CC-RAB is more efficient in biosorption processes than UL-GAB, because the biosorption capacities are higher for all studied metal ions, and (ii) the removal efficiency of metal ions is different depending on the nature of the biosorbent. Thus, in the case of CC-RAB, the removal efficiency increases in the order: Zn2+ (78.39 %) > Cu2+ (62.57 %) > Co2+ (52.23 %), while for UL-GAB the order is: Cu2+ (89.75 %) > Zn2+ (64.72 %) > Co2+ (60.54 %). These differences can be attributed to the greater number of functional groups on the CC-RAB surface compared to UL-GAB, but also to the way metal ions bind to the biosorbent surface during the biosorption process.
The fact that red algal biomass has a great number of superficial functional groups available to interact with metal ions in aqueous solution is well known (Zeraatkar et al. 2016) and also proven in this case by FTIR spectra (Fig. 1c). This signifies that the metal ions interact much more effectively with the functional groups of CC-RAB than with those of UL-GAB, and this observation is supported by the values of the Langmuir constants (Table 3), which in the case of CC-RAB are an order of magnitude higher than those obtained in the case of UL-GAB.
On the other hand, the kinetic and isotherm modeling have revealed that the biosorption of metal ions occurs through two successive steps until the biosorbent surface is completely covered with a monolayer of metal ions. Under these conditions, the covalency index (IC = (XM2+)2⋅r; XM2+ - Pauling electronegativity, r – ionic radius) of metal ions are expected to play an important role in the biosorption processes. In the case of Cu2+, Zn2+ and Co2+ ions, their covalency index are quite close (Cu2+ – 498.2, Zn2+ – 386.6, Co2+ – 445.3 (Dean 1995)), which prove that all metal ions have an affinity for the functional groups (especially those with O donor atoms) of the biosorbents.
Thus, in the case of UL-GAB, where the number of functional groups is smaller (the functional groups are at a large distance from each other), the Zn2+ ions (which have the lowest covalency index) are most efficiently retained, since they have a low tendency to form covalent bonds and can successively participate in the two electrostatic interactions (according with the pseudo-second order kinetic model). In the case of CC-RAB, the number of functional groups is much larger (the distance between them is smaller), which favors the binding of metal ions through bonds with a higher degree of covalence. These conditions are more suitable for Cu2+ ions than for Zn2+ ions, which, due to the higher value of the covalency index, allow the two successive interactions in the biosorption process to be carried out much more easily. In the case of Co2+ ions, two aspects must be considered, namely: (i) the small size of these ions (ionic radius = 126 ppm), compared to the others (Cu2+ - 138 ppm, Zn2+ - 142 ppm) (Dean 1995), and the high affinity for functional groups with O donor atom (determined by the value of the covalency index). The concerted action of these two factors makes it difficult for Co2+ ions to participate in two successive interactions in the biosorption process. Most likely, Co2+ ions interact with a first functional group on the biosorbent surface (due to their small size), and if it does not encounter a second functional group, in a geometrically favorable position, it gathers water molecules around it (due to the high value of covalency index). Therefore, regardless of the nature of the biosorbent (CC-RAB or UL-GAB), the biosorption of Co2+ ions quickly reach the equilibrium, although its efficiency is low. A schematic illustration of the possible mechanism is presented in Fig. 11.
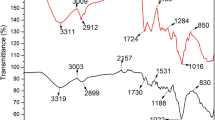
The realization of the biosorption process of Cu2+, Zn2+ and Co2+ on CC-RAB and UL-GAB through the two successive interactions (Fig. 11) is supported by the isotherm and kinetic modeling results, and their predominant electrostatic nature is also demonstrated by the FTIR spectra. The FTIR spectra recorded for each biosorbent (not show data) show that after the biosorption of metal ions, no new absorption bands were identified, but only shifts of the absorption maxima characteristic of the OH, C=O, COOH and C–O–C functional groups appear (Table 5). Furthermore, these shifts of the absorption maxima are quite small (± 30 cm-1) (Table 5), which suggests that the metal ions interact with these functional groups, but these interactions do not involve the formation of new covalent bonds.
The biosorption capacities obtained for the retention of Cu2+, Zn2+ and Co2+ ions on CC-RAB and UL-GAB (Table 4) are comparable (or even high) to those reported in the literature for other types of algae-based biosorbents, under similar experimental conditions (Romera et al. 2006; Zeraatkar et al. 2016; Ordonez et al. 2023). Therefore, CC-RAB and UL-GAB biosorbents, in addition to being readily available and inexpensive materials, have the potential to be used in the removal of metal ions from contaminated effluents.
Thermodynamic study
Evaluation of thermodynamic parameters (variation of free Gibbs energy (ΔG0), variation of enthalpy (ΔH0) and variation of entropy (ΔS0)) for the biosorption of Cu(II), Zn(II) and Co(II) ions on GAB and RAB was performed using the van’t Hoff equations (Sahmoune 2019; Lima et al. 2023).
where: R is the universal gas constant (8.314 J K-1 mol-1); T is the temperature (K); KL is the Langmuir constant (L g-1) and was calculated for the biosorption of each metal ion at three temperatures (10, 25 and 50 °C).
Table 6 summarizes the obtained values of the thermodynamic parameters for the biosorption of Cu2+, Zn2+ and Co2+ ions on CC-RAB and UL-GAB. The negative values of ΔG0 pointed out that the biosorption processes occurs spontaneously, regardless the nature of the metal ion or the nature of the biosorbent. In addition, the values of this parameter are close to each other for all metal ions and for both biosorbents, which indicates that the mechanism of biosorption processes is similar in all cases.
The positive ΔH0 values confirm that the retention of Cu2+, Zn2+ and Co2+ ions on CC-RAB and UL-GAB is an endothermic process, which is favoured by the increasing of the temperature. These results are consistent with those presented in previous sections (Fig. 2e and f), where it was shown that temperature increase leads to a rather modest increase in the efficiency of biosorption processes. Although the values of ΔH0 increase in the order: Co2+ < Cu2+ ≈ Zn2+ in the case of CC-RAB, and in the order: Co2+ < Zn2+ < Cu2+ in the case of UL-GAB, all are lower than 40 kJ mol-1 (Lima et al. 2023), which once again confirms that the retention of metal ions on these biosorbents is done predominantly through electrostatic interactions (probably ion exchange type). In the case of UL-GAB, where the number of functional groups is less, the ΔH0 values are somewhat higher than in the case of CC-RAB (where the abundance of functional groups is higher).
The retention of Cu2+, Zn2+ and Co2+ ions on CC-RAB and UL-GAB predominantly by electrostatic interactions is also supported by the values of ΔS0 (Table 6). The positive values of ΔS0 show that all metal ions have affinity for the functional groups of CC-RAB and UL-GAB, which makes the biosorption processes spontaneous. In addition, the rather small values of this parameter indicate that during of biosorption the degree of disorder at the aqueous solution/biosorbent interface varies rather little. Such small and positive values of ΔS0 are characteristic of ion exchange processes, in which the retention of the metal ion on the surface of the biosorbent occurs simultaneously with the release of a mobile ion (most likely Ca2+ ion) into the aqueous solution.
All these observations once again emphasize that the retention of metal ions (Cu2+, Zn2+ and Co2+) on CC-RAB and UL-GAB is a process that requires low cost and low energy consumption, and therefore can be considered environmental friendly.
Effect of coexisting ions and tests on real water samples
Analyzing the results in Fig. 6, three observations can be made, namely:
-
(i)
all coexisting ions influence the values of the biosorption capacity quite a bit. Even for the highest concentration (1 mol L-1), the biosorption capacity varies up to 15 %, regardless of the nature of the biosorbent. This can be explained taking into account the fact that both algae biomasses were sampled from seawater, where the concentration of such ions is very high.
-
(ii)
the decrease in biosorption capacities with increasing concentration of coexisting ions is more evident in the case of Ca2+ and Mg2+ ions than in the case of Na+ and K+ ions. This suggests that these divalent ions ca compete with Cu2+, Zn2+ and Co2+ ions for the functional groups of the biosorbents, which is an argument in favour of the proposed biosorption mechanism.
-
(iii)
among all studied biosorption processes, the most affected by the presence of coexisting ions is the retention of Co2+ ions on UL-GAB. This is because this biosorption process has the lowest efficiency (43 %) and therefore the presence of other ions (in a much higher concentration) results in an event greater decrease in the removal percent values (39.3 – 40.9 %).
All these observations show that, for an initial concentration of metal ions (Cu2+, Zn2+ and Co2+) of 50 ± 4 mg L-1, the biosorption capacities of CC-RAB and UL-GAB have comparative values (in the absence and presence of coexisting ions), which highlights the possibility of the use of these biosorbents in real effluent treatment processes.
The experimental results obtained in the case of tests on real water samples (Fig. 7) show that all metal ions are retained with significant efficiency (83 – 95 %) on both biosorbents (CC-RAB and UL-GAB). However, the concentration of Cu2+, Zn2+ and Co2+ ions after biosorption is much higher than the maximum permissible limits (in the case of CC-RAB: 10 times – Cu2+, 2 times – Zn2+ and 4 times – Co2+, while in the case of UL-GAB: 9 times – Cu2+, 2 times – Zn2+ and 5 times – Co2+). This means that the two biosorbents (CC-RAB and UL-GAB) can be successfully used in the treatment of aqueous effluents containing Cu2+, Zn2+ and Co2+ ions, with the mention that after the biosorption step it is necessary to use an additional step of advanced treatment of the resulting effluent, which allows the metal ions concentration to decrease bellow the maximum permissible limits. This is a consequence of the fact that biosorption processes are equilibrium processes, which means that at low concentrations of metal ions, their removal is not efficient.
The values of the other quality parameters of the water samples obtained after biosorption fall within the limits imposed by the maximum permissible values, with one exception, the pH. The values of this parameter is lower that the permissible value (6.5 – 8.5) (NTPA 001/2005) for both biosorbents (6.12 in the case of CC-RAB and 5.89 in the case of UL-RAB) (Fig. 7). This is most likely due to the fact that the initial pH value was adjusted to 5.0, in order to comply with the optimal conditions for biosorption of metal ions. Even if the final pH, measured after biosorption, is higher with one unit, the water samples are still weakly acidic and will require a neutralization step.
It should be also noted that after biosorption, the measured values for COD and TSS increase for both biosorbents (CC-RAB and UL-GAB) (Fig. 7). This increase shows that during the biosorption process, the organic compounds in the composition of the two algae biomasses are released into the solution, an aspect frequently mentioned in the literature (Romera et al. 2006; Lima et al. 2023). Moreover, the increase in the values of the two parameters (COD and TSS) is more significant in the case of CC-RAB (45 % and 13 %, respectively) than in the case of UL-GAB (16 % and 7 %, respectively) (Fig. 7). These negative consequences on the quality of water samples treated by biosorption can be significantly reduced if, in the preparation methodology, the algae biomasses are treated with an alkaline solution (ex. NaOH, 0.1 N). Such a treatment with alkaline solutions has the role of releasing easily soluble organic compounds from the composition of the two algae biomasses, even from the preparation stage, thus reducing the risk of loading the effluents resulting from biosorption with organic compounds.
Conclusions
In this study, two types of marine algae: red algae (Callithamnion corymbosum sp. – CC-RAB) and green algae (Ulva lactuca sp. – UL-GAB) were used for the biosorption of Cu2+, Zn2+ and Co2+ ions from aqueous media. To evaluate the biosorptive performances of the red algae biomass (CC-RAB) and green algae biomass (UL-GAB), batch experimental studies were carried out, in order to establish the optimal biosorption conditions. The maximum removal efficiency of studied metal ions was obtained at initial pH of 5.0, 2.0 g biosorbent L-1 and ambient temperature (22 ± 1°C). The pseudo-second order kinetic model best fits the kinetic data in all cases, while the biosorption isotherms are described by the Langmuir model. The maximum biosorption capacity depends on the nature of algae biosorbent, and follows the order: Cu2+ > Zn2+ > Co2+ in the case of CC-RAB, and Zn2+ > Cu2+ > Co2+ in the case of UL-GAB. The thermodynamic parameters (∆G0, ∆H0 and ∆S0) were also evaluated, and the obtained values indicate that all biosorption processes are spontaneous and endothermic. Desorption of metal ions is quantitative (> 97 %) in acid media (0.1 N HNO3), but the biosorption capacities decrease significantly after first cycle of use. All these aspects are important in the design of a strategy for the valorisation of this biomass resource.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ackova DG (2018) Heavy metals and their general toxicity on plants. Plant Sci Today 5:15–19
Agarwal A, Upadhyay U, Sreedhar I, Singh SA, Patel CM (2020) A review on valorization of biomass in heavy metal removal from wastewater. J Water Process Eng 38:101602
Aji BA, Yavuz Y, Koparal AS (2012) Electrocoagulation of heavy metals containing model wastewater using monopolar ion electronedes. Separat Purif Technol 86:248–254
Anastopoulos I, Kyzas GZ (2015) Progress in batch biosorption of heavy metals onto algae. J Molec Liquids 209:77–86
Bashir A, Malik LA, Ahad S, Manzoor T, Bhat MA, Dar GN, Pandith AH (2019) Removal of heavy metal ions from aqueous system by ion-exchange and biosorption methods. Environ Chem Lett 17:729–754
Briffa J, Sinagra E, Blundell R (2020) Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 6:e04691
Chatterjee A, Abraham J (2019) Desorption of heavy metals from metal loaded sorbents and e-wastes: a review. Biotechnol Lett 41:319–333
Chong KH, Volesky B (1995) Description of two-metal biosorption equilibria by Langmuir-type models. Biotechnol Bioeng 47:451–460
Ciobanu AA, Munteanu L, Vasile G, Bulgariu L (2023) Evaluation of the biosorption performance of marine green algae biomass (Ulva lactuca sp.) in the removal of inorganic pollutants. Bull Polytech Inst Iasi. 69:93–104
Crini G, Lichtfouse E (2019) Advantages and disadvantages of techniques used for wastewater treatment. Env Chem Lett 17:145–155
Crini G, Lichtfouse E, Wilson LD, Morin-Crini N (2019) Conventional and non-conventional adsorbents for wastewater treatment. Env Chem Lett 17:195–213
Dean JA (1995) Handbook of Analytical Chemistry. Mc-Graw Hill Inc., New York
Edebali S, Pehlivan E (2016) Evalution of chelate and cation exchange resins to remove copper ions. Powder Technol 301:520–525
Fabre E, Dias M, Costa M, Henriques B, Vale C, Lopes CB, PinheiroTorres J, Silva CM, Pereira E (2020) Negligible efect of potentially toxic elements and rare earth elements on mercury removal from contaminated waters by green, brown and red living marine macroalgae. Sci Total Environ 724:138133
Farooq U, Kozinski JA, Khan MA, Athat M (2010) Biosorption of heavy metal ions using wheat based biosorbents – A review of the recent literature. Bioresour Technol 101:5043–5053
Fresenius W, Quentin KE, Schneider W (1998) Water Analysis. A Practical Guide to Physico-Chemical, Chemical and Microbiological Water Examination and Quality Assurance. Springer, Berlin
Ho YS, McKay G (1999) Pseudo-second-order model for sorption processes. Process Biochem 34:451–465
Karic N, Maia AS, Teodorovic A, Atanasova N, Langergraberf G, Crini G, Ribeiro ARL, Dolich M (2022) Bio-waste valorisation: Agricultural wastes as biosorbents for removal of (in)organic pollutants in wastewater treatment. Chem Eng J Adv 9:100239
Khan TA, Mukhlif AA, Khan EA, Sharma DK (2016) Isotherm and kinetics modeling of Pb(II) and Cd(II) adsorptive uptake from aqueous solution by chemically modified green algal biomass. Model Earth Syst Environ 2:117
Lee XJ, Ong HC, Ooi J, Yu KL, Tham TC, Chen WH, Ok YS (2022) Engineered macroalgal and microalgal adsorbents: synthesis routes and adsorptive performance on hazardous water contaminants. J Hazard Mat 423:126921
Lima EC, Gomes AA, Tran HN (2023) Comparison of the nonlinear and linear forms of the van’t Hoff equation for calculation of adsorption thermodynamic parameters (ΔS° and ΔH°). J Molec Liq 311:113315
Nowicka B (2022) Heavy metal–induced stress in eukaryotic algae—mechanisms of heavy metal toxicity and tolerance with particular emphasis on oxidative stress in exposed cells and the role of antioxidant response. Env Sci Pollut Res 29:16860–16911
NTPA (001/2005). https://legislatie.just.ro/Public/DetaliiDocument/61585. Accessed 12 Aug 2024
Ordonez JI, Cortes S, Maluenda P, Soto I (2023) Biosorption of heavy metals with algae: critical review of its application in real effluents. Sustainability. 15:5521
Pohl A (2020) Removal of heavy metal ions from water and wastewaters by sulfur-containing precipitation agents. Water Air Soil Pollut 231:503
Qulatein HA, Yilmaz MS (2023) Preparation of low-cost and non-conventional macroalgae-based biosorbent for fast and effectively selective dye adsorption. Mat Chem Phys 303:127741
Rangabhashiyam S, Anu N, Nandagopal Giri MS, Selvaraju N (2014) Relevance of isotherm models in biosorption of pollutants by agricultural by-products. J Env Chem Eng 2:398–414
Romera E, González F, Ballester A, Blázquez ML, Munoz JA (2006) Biosorption with algae: a statistical review. Crit Rev Biotech 26:223–235
Sahmoune MN (2019) Evaluation of thermodynamic parameters for adsorption of heavy metals by green adsorbents. Env Chem Lett 17:697–704
Saleh TA, Mustaqeem M, Khaled M (2022) Water treatment technologies in removing heavy metal ions from wastewater: a review. Env Nanotech Monit Manage 17:100617
Syeda HI, Sultan I, Razavi KS, Yap PS (2022) Biosorption of heavy metals from aqueous solution by various chemically modified agricultural wastes: A review. J Water Process Eng 46:102446
Tan KL, Hameed BH (2017) Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J Taiwan Inst Chem Eng 74:25–48
Taskin E, Tan I, Minareci E, Minareci O, Cakir M, Polat-Beken C (2020) Ecological quality status of the Turkish coastal waters by using marine macrophytes (macroalgae and angiosperms). Ecol Indicat 112:106
Vareda JP, Valente AJM, Duraes L (2019) Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: a review. J Environ Manage 246:101–118
Varjani S, Joshi R, Srivastava VK, Ngo HH, Guo W (2020) Treatment of wastewater from petroleum industry: current practices and perspectives. Env Sci Pollut Res 27:27172–27180
Vasic V, Kukic D, Sciban M, Durisic-Mladenovic N, Velic N, Pajin B, Crespo J, Farre M, Seres Z (2023) Lignocellulose-based biosorbents for the removal of contaminants of emerging concern (CECs) from water: A review. Water 15:1853
Vijayaraghavan K, Balasubramanian R (2015) Is biosorption suitable for decontamination of metal-bearing wastewaters? A critical review on the state-of-the-art of biosorption processes and future directions. J Environ Manage 160:283–296
Wang J, Guo X (2022) Rethinking of the intraparticle diffusion adsorption kinetics model: Interpretation, solving methods and applications. Chemosphere. 309:136732
Xiang H, Min X, Tang CJ, Sillanpaa M, Zhao F (2022) Recent advances in membrane filtration for heavy metal removal from wastewater: A mini review. J Water Process Eng 49:103023
Yaashikaa PR, Senthil Kumar P, Saravanan A, Vo DVN (2021) Advances in biosorbents for removal of environmental pollutants: a review on pretreatment, removal mechanism and future outlook. J Hazard Mat 420:126596
Zeraatkar AK, Ahmadzadeh H, Talebi AF, Moheimani NR, McHenry MP (2016) Potential use of algae for heavy metal bioremediation, a critical review. J Environ Manage 181:817–831
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
A.A.C and A.R.L were obtained the experimental data and make the calculations. LB wrote the main manuscript text and prepared figures/tables. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ciobanu, AA., Lucaci, AR. & Bulgariu, L. Efficient metal ions biosorption on red and green algae biomass: Isotherm, kinetic and thermodynamic study. J Appl Phycol (2024). https://doi.org/10.1007/s10811-024-03332-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10811-024-03332-9