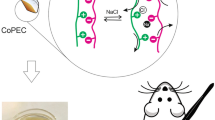
Abstract
Although allicin has potent antibiotic properties, its low stability, which is responsible for its persistent biological activity, has posed a significant challenge to its practical application in modern medicine. To harness the healing benefits of this phytochemical, known by humans for thousands of years, we propose a controlled in situ synthesis of allicin vapour near the site of infection. Considering the critical need for novel approaches to prevent pandemic scenarios caused by MDR bacteria, we suggest encapsulating and physically separating allicin precursors (substrate alliin and enzyme alliinase) in alginate-based films and spray-dried chitosan microparticles. The mechanical properties of the hydrogel films of various compositions were evaluated, as well as their ability to protect the encapsulated alliinase against thermal stress and control the overall rate of allicin release upon hydration. Furthermore, the non-contact antibacterial efficacy of free alliin/alliinase reaction mixture (aqueous solution) and three compartmentalised configurations, i.e. film-solution, film-particles, and double-film, were tested against selected bacterial strains, i.e. E. coli, S. epidermidis, and S. aureus. The results indicate that the formation of allicin vapour using the proposed compartmentalised systems addresses allicin’s stability issues and provides better control over the rate of allicin production. The observed antibacterial effect was comparable with directly formed allicin using higher initial amounts of both substances, which is given by diffusion limitations associated with encapsulation. These findings illustrate the potential of compartmentalised systems in developing nature-based wound dressings for infection prevention and promoting healing.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The skin is not only our largest organ, providing thermoregulation, sensation, and secretion of various fluids, but it also serves as a natural barrier that protects the human internal environment against external factors. The outermost layer of the skin also provides a habitat for resident microflora (mostly Gram-positive bacteria) that occupy the skin niche and help prevent colonisation by pathogenic microorganisms [1]. Skin infections are most often the result of skin breaches (e.g. cutaneous wounds, abrasions, and burns), enabling access of microorganisms (pathogenic or permanent skin residents) to normally sterile underlying tissues [2]. The healing process can usually stop a microbial invasion and repair acute wounds within a short period of time [3]. However, if the bacterial count becomes too high, the host’s immune system may be unable to control it, leading to infections [4]. Besides causing pain, swelling, fever, or other unpleasant symptoms, these infections can also impair the healing process, e.g. by altering the pH or restricting oxygen transport to the skin [5].
In some cases, wounds become chronic and highly susceptible to microbial contamination, leading to significantly delayed healing that can take up to several months or even years [6]. Chronic wounds are more frequent in elderly and ill individuals with underlying disorders or weakened immune systems [1]. Additionally, the growth of microorganisms as biofilms can make chronic wounds more challenging to treat due to their high tolerance towards systemic and topical antimicrobial agents and host defences compared to their planktonic counterparts [7, 8].
Wound dressings made from various sources such as animals, herbs, or synthetics have been shown to speed up the process of wound healing and reduce secondary infections. Modern wound dressings, including foams, films, hydrogels, hydrocolloids, and their combinations, play a vital role in healing. They protect the wound from outside contaminants and elements, reduce pain, and keep the wound moist through interaction with exudate, which aids in the debridement of necrotic tissue [9].
Biopolymers are commonly used in wound dressing manufacturing due to their biocompatibility, absorption capacity, or gas permeability. However, they often have poor mechanical properties. To address this issue, biopolymer composites and blends, which combine different materials to overcome this drawback, are utilised [10]. Therapeutic agents such as antibiotics, growth factors, vitamins, and minerals can also be incorporated into wound dressings to enhance their effectiveness [3]. However, the overuse of antimicrobial dressings has led to the rise of antimicrobial resistance (AMR) in many common bacterial pathogens. This is a significant global issue [11], which results in growing interest in plant-derived substances that have been used for centuries as a natural remedy for many ailments [12].
Herbal medicines were the primary form of treatment in the pharmacopoeia two centuries ago. Many cultures relied on garlic and various extracts of Allium species to inhibit the growth of planktonic bacteria and biofilms. For instance, a 1000-year-old remedy called Bald’s eyesalve, which included a blend of onion, garlic, wine, and bile salts, was utilised for this purpose [13]. The substance responsible for most of garlic’s beneficial properties is allicin, which plays a vital role as a natural self-defence system enzymatically formed from substrate alliin after injury to the garlic tissues. Allicin, one of the most bioactive compounds found in nature, is not only active in solution and as an aerosol but also via the gas phase. This can be of great importance for applications where access to the target site is limited or obscured or where direct contact with concentrated allicin solution may cause tissue irritation [14].
Allicin exhibits broad-spectrum antimicrobial activity against various Gram-positive and Gram-negative bacteria, including multidrug-resistant S. aureus [15]. Allicin’s antibacterial mechanisms are very diverse compared to other antibiotics, targeting multiple cellular pathways and structures: (i) disruption of cell membrane integrity leading to the loss of membrane potential and cellular lysis; (ii) allicin reacts with thiol-containing bacterial enzymes such as alcohol dehydrogenase and RNA polymerase, impairing their function; (iii) induction of oxidative stress by lowering of glutathione levels followed by the formation of ROS within bacterial cells; and (iv) disruption of quorum sensing reduces bacterial virulence and the ability to form protective biofilms [16, 17]. The non-specific nature of allicin’s antibacterial activity is the reason why bacterial resistance to allicin develops over 1000 times more slowly than to synthetic antibiotics [18]. Moreover, it has been reported that allicin forms transient pores in bacterial membranes, which may explain its synergistic effect with membrane-active antibiotics such as amphotericin B and polymyxin B [19]. Garlic compounds also enhance the immune system by stimulating certain cell types during infection [20].
In recent years, allicin has garnered significant interest due to its versatile properties, making it a valuable resource for addressing various challenges with minimal environmental impact. Allicin has been utilised as a catalyst [21], a capping agent in the green synthesis of nanoparticles [22, 23], a soil urease inhibitor [24], an agricultural biocide [25], an active food packaging material [26], a pollutant remover in wastewater treatment [27], and for environmental remediation [28]. However, the practical use of allicin as a therapeutic agent is currently limited due to its very low stability and high reactivity in body fluids [3]. Promising cytostatic [29] and antifungal [30] properties of in situ formed allicin were previously reported using alliinase conjugated to a monoclonal antibody. Upon alliin administration (in the rat model), allicin formation is strictly limited to the site where the alliinase conjugate is specifically attached. Thus, systemic exposure and side effects are virtually eliminated. However, such highly complex targeted-allicin treatment is not needed for topical application where the site of the application and action is identical.
This study aims to create a compartmentalised garlic system, where substrate alliin and enzyme alliinase are stabilised and physically separated in multiple layouts, i.e. film-solution, film-particles, and double-film. The antibacterial activity of in situ formed allicin vapour against selected bacterial strains was demonstrated using a custom film holder. This approach based on in situ synthesis of allicin from its encapsulated precursors addresses issues linked to its limited shelf-life (rapid conversion to various compounds, high reactivity, low thermal stability, and volatility) [3] and skin irritation caused by unrestrained allicin release [31]. The therapeutic potential of allicin presented in this study can be effectively broadened by employing a stable precursor (alliin), which can undergo enzymatic conversion to allicin at the required time and location using the variability of presented composite layouts.
Materials and Methods
Materials
Chitosan (Mw 150–250 kDa, 75–85% deacetylated), tricine, tryptone, yeast extract, agar, sodium alginate (Mw 12–40 kDa, M/G ratio of 1.56), gelatine from porcine skin (powder, gel strength–300 g Bloom, Type A), kanamycin, phosphate-buffered saline (tablet), pyridoxal-5′-phosphate hydrate (PLP), poly(ethylene glycol) (PEG) 6000, and ethylenediaminetetraacetic acid (EDTA; BioUltra; anhydrous; ≥ 99% purity) were obtained from Sigma-Aldrich. Acetic acid (98% p.a.), potassium chloride (p.a.), and sodium chloride (p.a.) were purchased from PENTA s.r.o. Calcium chloride (anhydrous) and glycerine anhydrous (p.a.) were purchased from Lach-Ner, fetal bovine serum was purchased from Thermo Fisher Scientific, BHI (brain–heart infusion broth) was purchased from Carl Roth, and Dulbecco’s modified eagle’s medium (DMEM) and minimum essential medium eagle (MEM) were purchased from Merck. NADH (disodium salt, purity approx. 98%) and L-lactate dehydrogenase (LDH from rabbit muscle) were purchased from Roche Diagnostic. PLA filament (1.75 mm) was obtained from Creality, and PETG filament (1.75 mm) was obtained from Prusa Research. Fresh garlic of Czech origin was purchased at a local market. Human fibroblasts (MRC-5) and human keratinocytes (HaCaT) for cytotoxicity study were provided by the Department of Biochemistry and Microbiology, UCT Prague. Escherichia coli (E. coli) K12 (EC43) and Staphylococcus epidermidis (S. epidermidis) DBM 3179 were provided by the Czech Academy of Sciences. Protein A positive Staphylococcus aureus (S. aureus) strain S11 was obtained from the German collection of microorganisms and cell cultures (DSM No. 20372).
Alliin synthesis and characterisation
Alliin was synthesised according to Stoll and Seebeck’s previous work [32]. It consists of two steps: alkylation of L-cysteine to form S-allyl-L-cysteine followed by oxidation with hydrogen peroxide. First, L-cysteine (50 g; 0.413 M) was alkylated with allyl bromide (75 g; 0.620 M) in an aqueous solution of ammonium hydroxide (2 M; 1.2L) at 0 °C. The reaction was terminated after 40 min, followed by: (i) volume reduction using a rotary evaporator; (ii) filtration and washing of raw product with ethanol; (iii) vacuum drying; and (iv) recrystallisation from 2:3 water/ethanol mixture. Afterwards, S-allyl-L-cysteine (53 g; 0.329 mM) was resuspended in water (530 mL; 25 °C) and mixed with hydrogen peroxide (30% w/w; 37 mL). After stirring for 2 days, a rotary evaporator reduced the reaction volume, 400 mL of acetone was added, and the mixture was stirred for the next 2 hours. Finally, the racemic mixture of alliin was filtered, washed (mixture of acetone:water ratio 5:1 v/v), and vacuum dried. The diastereomers were not further separated. The chemical structures of S-allyl-L-cysteine and alliin were confirmed by NMR. 1H NMR spectra of S-allyl-L-cysteine and (±)-L-alliin recorded on Agilent 400-MR DDR2 (1H: 400 MHz) are presented in the supporting information document (Figs. S1 and S2). All NMR data were processed using MestReNova software. The purity of synthetic alliin (≥ 95%) was determined by Agilent 1100 Series HPLC using commercially available ( ±)-L-alliin as standard (Fig. S3). The product was stored in the dark at − 20 °C before use.
Enzyme extraction and purification
Alliinase was isolated from garlic tissues at 4 °C to prevent excessive degradation. The isolation protocol described by Mallika et al. was followed with some minor modifications [33, 34]. Freshly peeled garlic cloves were homogenised by hand blender in ice-cold sodium phosphate buffer (pH 6.5, 0.02 M; glycerol 10% v/v; EDTA 5 mM; NaCl 5% w/v; 0.02-mM PLP; and garlic/buffer ratio 1:1.5 w/v). The crude mixture was filtered through cheesecloth, and fine solids were separated using vacuum filtration (pore size 100–160 µm). Afterwards, PEG 6000 was added gradually to the supernatant while stirring (25% w/w), and the mixture was left for an additional 30 min to ensure complete enzyme precipitation, followed by centrifugation (30 000 g; 30 min for all centrifugation cycles). The supernatant was separated, and the solid yellowish pellet was resuspended in PLP solution (0.02 mM; 50 mL) and centrifugated again. The resulting supernatant was lyophilised and stored as a dry powder in the dark at − 20 °C before use.
Preparation and characterisation of particles
The microparticles were prepared by spray-drying process following the method previously reported by Mašková et al. (2021) [35]. First, the alliinase/alliin solutions were prepared by dissolving 20 mg of alliin crystals or lyophilised alliinase powder in 2 mL of 200-mM Tricine-KOH buffer (pH 8). The carrier solution was prepared by dissolving 1 g of chitosan in 98 mL of acetic acid (1% v/v) and stirred overnight (1400 rpm, 60 °C) to ensure proper homogenisation. The feed solution was prepared by mixing 98 mL of the carrier solution with 2 mL of alliinase/alliin solution. The mixture was then spray-dried immediately after homogenisation (1400 rpm, 25 °C) to prevent excessive enzyme degradation in an acidic environment.
The feed solution was pumped (5 mL min−1) into BÜCHI Mini Spray Dryer B-290 and atomised by the ultrasonic nozzle (Sono-Tek). The hot air with an inlet temperature of 120 °C and volumetric flowrate of 31.5 m3 h−1 were used as a drying medium. The outlet air temperature did not exceed 65 °C, and the drying process typically took under 30 min. In the case of alliinase, the additional external cooling of the high-performance cyclone and collection vessel (12 °C, flow rate 300 mL min−1, tube diameter 4 mm) was used to prevent thermal degradation of the product. Afterwards, the collected particles (containing approx. 2% w/w of alliinase or alliin) were kept under reduced pressure in a desiccator for 24 h to remove residual moisture, and the dry powdered product was stored in the freezer at − 20 °C before use.
The particle size distribution (PSD) was determined by a static light scattering method (SLS, HORIBA Partica LA-950 S2). Before analysis, particles were sonicated in absolute ethanol to break apart electrostatically charged particles. The suspended sample was added into a quartz sample cell prefilled with absolute ethanol. All measurements were performed in triplicate. The surface morphology of microparticles was examined by scanning electron microscope (SEM) Jeol JCM-5700. Before measurement, the prepared microparticle samples were sputter-coated (Emitech K550X) by a thin layer of gold (5 nm). SEM images were taken at an acceleration voltage of 5 kV. Encapsulation efficiency (EE) of alliin encapsulated in the spray-dried particles was determined using the LDH assay described in Sect. 2.6.
Preparation and characterisation of films
The alginate–glycerine–gelatine films (AGG films) were prepared by modifying the method published by Ibrahim S. et al. [36]. AGG solution was prepared by dissolving 2 g of sodium alginate, 2 g of glycerine, and gelatine (1–3 g) in 100 mL of distilled water and stirring for 1 h and 45 min at 60 °C (sample composition is shown in Table 1). Afterwards, 15 mL of AGG solution was poured into each Petri dish (90 mm diameter). The dishes were then transferred to a levelled surface in a freezer at − 18 °C for 10 min to solidify. Afterwards, the films were crosslinked by immersion in 0.2 M CaCl2 solution for 5 min. Finally, the films were air-dried at 25 °C for 48 h, carefully detached from Petri dishes, and stored at room temperature. This protocol ensured that collected films were of uniform thickness and free of visible cracks or wrinkles.
Films containing alliin or alliinase were prepared by adding a specific amount of alliin (120, 60, 30, 15, or 7.5 mg) or alliinase (40 or 10 mg) into 100-mL solution homogenised for 90 min and stirred for another 15 min at 60 °C and 25 °C, respectively. Multi-layered films of different thicknesses were prepared by pouring 15 or 25 mL of AGG solution over already prepared alginate–alliin films, which dried for 24 h. The remaining preparation steps were identical to those for simple AGG films.
The weight (ma) of pure alliin (or alliinase) [mg] in the AGG disc sample was calculated using the following formula:
Here cAGG is the mass concentration of enzyme or substrate (ranging from 7.5 to 120 mg) per 100 mL of AGG solution, V0 is the volume of casted AGG solution (15 mL), ddisc is the diameter of film disc (12.8 mm), and ddish is the inner diameter of Petri dish used for film casting (84 mm).
Swelling ratio
Three squares of 20-mm edge length were cut from AGG films to measure the swelling ratio. The polymer film was immersed in phosphate-buffered saline (PBS) for 1 hour to rehydrate it for the test. The film samples were weighed in a dry state (w0) and after incubation in PBS buffer (wt), using filter paper to remove excess PBS from the surface. The swelling ratio (SR) was calculated as follows:
The film thickness
The measurement of film thickness in the dry and fully swollen state (after 1 hour of incubation in Tricine-KOH buffer) was done by optical microscopy (Olympus CKX41) using a customised holder that secured film samples in the vertical position during image acquisition. QuickPHOTO MICRO software was used to evaluate film thickness.
Tensile testing
The tensile testing of prepared AGG films was conducted using the CT3 Texture Analyser (Brookfield, USA) with a dual grip fixture (probe TA44). The test speed was set to 0.1 mm s−1. The film sample was cut into a rectangular shape (25 × 20 mm) and soaked in distilled water for 10 s before testing. The measurements were carried out in triplicate using AGG films that did not contain alliin or alliinase, as preliminary data showed no significant impact of their presence on film mechanical properties. AGG films used for antibacterial testing contained no more than 2.1% of alliin (120 mg/mL) and 0.6% of alliinase (40 mg/mL) by weight. Young’s modulus was determined as the slope of the linear part of the stress–strain curve. The stress was calculated by dividing the load by the cross-sectional area, and the strain was calculated as elongation divided by the original length.
Release of alliin and production of allicin—UV–Vis assay
Circle discs (18 mm diameter), precisely punched from single or multi-layered films, were used to assess the release of alliin from AGG films in an aqueous environment. These discs were positioned on the bottom of a beaker using double-sided tape, with the alliin layer facing downwards. One mL of 200-mM Tricine-KOH buffer was added to each beaker to soak the films and provide sink conditions for alliin dissolution. Aliquots of the solution were collected after 15, 30, 60, 90, 120 min, and 24 h, and the alliin content was determined using the LDH assay (as described below). Each data point of the alliin release curve represents a mean value ± SD of samples prepared from four individual films of the same type.
A stack of alliin and alliinase films was used to determine the rate of allicin production. Both films were prepared separately to prevent possible allicin production prior to testing (alliin concentration 120 mg/mL and alliinase concentration 40 mg/mL). The 18-mm-diameter discs covering the bottom of a beaker were punched from the films with the alliin film secured with double-sided tape to the bottom and the alliinase film placed over it. One mL of 200-mM Tricine-KOH buffer was added to each beaker, and aliquots collected after 5, 15, 30, 60 min, and 24 h were analysed using the LDH assay. Each data point of the allicin release curve represents a mean value ± SD of samples prepared from three sets of films.
The LDH assay is based on the reduction of pyruvic to lactic acid, coupled with the oxidation of nicotinamide adenine dinucleotide (NAD) from the reduced NADH to NAD+ (Fig. S4). As NADH oxidation is linked with a decrease in absorbance at 340 nm, the amount of pyruvic acid can be calculated from the amount of NADH transformed given the known stoichiometry (1:1). In our case, pyruvic acid is a side product of allicin formation with known stoichiometry towards alliin (1:1). This can be used for the determination of alliin concentration in its release assays or allicin concentration in the experiment with alliin and alliinase films. For accurate calculations, it was necessary to ensure that the concentrations of other compounds in the test, i.e. alliinase, PLP, NADH, or LDH, were not limiting the reaction rate. The reaction mixture of 100 µL typically contained: 0.8-mM NADH, 20-µM PLP, LDH (2.5 μL, 550 units/mg), and 0.125-mg/mL alliinase and alliin (to be determined). It was also necessary to have appropriately diluted samples so that the absorbance was higher than 0.3, and the alliin/pyruvate concentration was not high enough to trigger the substrate inhibition of the LDH enzyme. Samples were measured at 340 nm using a Tecan Infinite M200 PRO spectrophotometer with a transparent 96-well plate (Greiner).
Cytotoxicity evaluation of films
The cytotoxicity of AGG films with alliin and alliinase prepared in triplicate on the surface of 12-well plates (Greiner BIO-ONE) was evaluated. The highest concentrations of alliin (120 mg/mL) and alliinase (40 mg/mL) in the film were observed in all experiments. First, the samples were sterilised by two different methods either with UV germination lamp (15 min) used in flowbox for work with cell cultures or by using 70% (v/v) ethanol (absolute) in deionised water. After sterilisation, each sample was gently rinsed with phosphate-buffered saline (PBS, pH of 7.4) at sterile conditions. After the PBS was aspirated, 1 mL of Dulbecco’s modified eagle’s medium (DMEM) was added to each sample. AGG films in well plates were kept in a cell culture incubator at 37 °C, 5% CO2 in air, and 95% humidity for 24 and 72 h.
Subsequently, the leachates from the samples were used for cytotoxicity evaluation, which proceeded as follows: 104 human fibroblasts (MRC-5) or human keratinocytes (HaCaT) per well of 96-well plates were seeded in quadruplicate in 100 µL of DMEM and minimum essential medium eagle (MEM plus 1% of non-essential amino acids), respectively, with 10% of fetal bovine serum and cultivated for 24 h at standard cultivation conditions. Then, the cells were treated with 100 µL of AGG film leachates, which were added to the cells and incubated for 24 h. Cells treated only with cultivation medium and cells treated with kanamycin (1 mg mL−1) served as controls. After the 24-h treatment, cell viability was measured using WST-1 assay, i.e. the culture medium was discarded and changed for 100 µL of phenol red-free DMEM with 5 µL of WST-1 reagent in it. After 2-h incubation, the absorbance of the raised formazan was measured at 450 nm (reference 650 nm) by UV–Vis spectrometer (Bio-Rad Laboratories, USA). The data from individual replicates were averaged, and the standard deviation was calculated using Microsoft Excel.
Antibacterial testing of allicin vapour
Two bacterial strains, i.e. E. coli (Gram-negative) and S. epidermidis (Gram-positive), were selected to test the antibacterial activity of allicin vapour generated in situ. The previous studies reported that both strains had similar sensitivity to allicin, with S. epidermidis being slightly more susceptible, as shown by Janská et al. [34]. S. aureus (Gram-positive) was also tested as a high-risk pathogenic strain in a double-film layout.
Media preparation
Luria–Bertani medium (LB) used for E. coli cultivation was prepared as follows: 20 g of LB broth (10-g Tryptone, 5-g yeast extract, and 5-g NaCl) was dissolved in 1 L of demineralised water, and pH was adjusted to 7.2–7.4 with 1 M sodium hydroxide solution. Brain–heart infusion (BHI) medium suitable for S. epidermidis and S. aureus cultivation was prepared by dissolving 37 g of BHI broth in 1 L of distilled water. Solid agar plates were prepared by pouring 25 mL of LB or BHI with agar (1.5 g per 100 mL of medium), followed by inoculation of bacterial suspension (200 µL, OD600 = 0.5). All cultivation media were sterilised in an autoclave at 120 °C for 20 min.
Holder for testing antibacterial vapour
A 3D-printed sample holder was proposed to test the antibacterial activity of allicin vapour generated in situ from its precursors in their free, i.e. aqueous solution (20 µL) and encapsulated forms, i.e. particles in dry powder (10 mg), or film (disc diameter of 12.8 mm). The holder was printed from PLA or PETG filaments using the 3D printer (Prusa i3 MK3). The holder’s body is assembled from two parts, i.e. the lower part with the embedded reservoir accommodating solution or particles and the retaining ring holding the film in the position sealing the reservoir (Fig. 1A). Three different configurations were further tested, i.e. solution-film, particle-film, and double-film layouts (Fig. 2).
Three-dimensional-printed holder for testing of allicin vapour: a dimensions of holder parts and cross-section of assembled film holder; b 3D-printed sample holders (FH test) and paper disc (DD test) soaked with antibiotic placed on an agar plate; and c legend for the rating of observed antibacterial effect.
At the beginning of the incubation period (24 h, 37 °C), the sample holder was positioned upside down on an inoculated agar plate, creating a temporary seal allowing moisture uptake, followed by allicin enzymatic formation and release (Fig. 1B). After the incubation, the antibacterial activity (presence of clear zones) under the holder was evaluated by image analysis software Fiji (ImageJ) [37]. The antibacterial test using a film holder is denoted as the “FH test” in the results section. Kanamycin solution (20 µL, 50 mg/mL) soaked to filter paper (Whatman) was used as a reference antibiotic in all cases, denoted as disc diffusion “DD test”. The magnitude of the observed antibacterial effect was rated as none, low, moderate, and strong, as illustrated in Fig. 1C. The strong effect represents the complete inhibition of bacterial growth under the holder, whereas none symbolises no evidence of antibacterial activity.
Results and discussion
Particle characterisation
Chitosan microparticles were prepared by spray drying (Büchi B-290). The collected dry product was characterised using SLS and SEM to obtain information about the size and surface morphology. SEM measurements confirmed that the particles were spherical in shape and had a wrinkled surface topography (Fig. 3A), which is typical for particles made with chitosan as the carrier material [35]. The volume mean diameter was found to be 2.5 ± 0.8 μm, with relatively narrow PSD, as presented in Fig. 3B. The LDH assay was used to determine the encapsulation efficiency of alliin in carrier material, which was found to be nearly 100%.
Film characterisation
The effect of different amounts of gelatine (Table 1) on the properties of the film was investigated. The film properties were measured by maximum load, Young’s modulus, and swelling ratio. The results showed a significant difference in maximum load between samples with low (“L”) and medium (“M”) gelatine content. An increase in twofold in gelatine concentration resulted in almost three times higher values of maximum load (Fig. 4A). However, an additional increase in gelatine amount (sample high (“H”)) did not show any statistically significant difference (p < 0.05, ANOVA, Tukey’s test) from samples “M”. The maximum load for samples “L”, “M”, and “H” fluctuated around their time-averaged values, which were 1.7, 4.9, and 5.8 N, respectively.
Film characterisation as an effect of drying time and different gelatine contents (low/medium/high as defined in Table 1) on maximum load (a), Young’s modulus (b), and swelling ratio (c). Statistically significant differences (p < 0.05, ANOVA, Tukey’s test) between samples M and L (a), M and H (b), and H and L (c).
Figure 4B illustrates the impact of gelatine on Young’s modulus. Notably, sample “L” displayed a Young’s modulus that was more than two times lower than samples “M” and “H”. Furthermore, there was no significant difference in Young’s modulus between samples “M” and “H”. All samples demonstrated time invariance in maximum load and Young’s modulus, indicating the mechanical stability of casted films.
The study also investigated the correlation between the swelling ratio, drying time, and gelatine content. All film types dried for 2 days had the lowest swelling ratio due to incomplete drying. The highest swelling ratio values were achieved after 4 days, with no further improvement observed for longer drying times. While the relationship between gelatine content and swelling ratio is complex, each film demonstrated a high capacity for moisture uptake, with a swelling ratio of up to 8 for sample “M”. This feature is critical for film hydration and absorption of wound exudate in topical applications (Fig. 4C).
Based on these findings, sample “M” was deemed the most suitable for subsequent alliin/alliinase encapsulation and antibacterial activity testing. The average thickness of dry films for sample “M” was 160.3 ± 7.4 µm, which increased to 448.0 ± 29.1 µm after 1-hour incubation in Tricine-KOH buffer.
Release kinetics of alliin and allicin from films
Alliin, a small hydrophilic substance (Mw = 177.22 g mol−1), can be easily transported through swelled hydrogel membranes. However, sudden burst release of alliin combined with the fast enzymatic formation of allicin can lead to short-term antibacterial activity, excessive skin irritation or tissue burns. To address this issue, the use of a second AGG layer serving as an additional mass transport barrier was investigated.
Three different versions of alliin film were tested: simple alliin AGG film (containing 120 mg/mL of alliin) and alliin AGG films with an additional AGG layer (volume of 15 and 25 mL). These films were left to incubate in Tricine-KOH buffer for 24 h. The thickness of films with additional 15 and 25 mL of AGG solution was found to be 2 times and 2.7 times larger, respectively, than the simple alliin AGG film (thickness of 160 µm in a dry state). Figure 5A shows that the majority of alliin content (89%) was released from the AGG film with no additional layer within the 1st hour. However, the additional AGG layer resulted in slower alliin release. After an hour, the amount of released alliin was reduced to 80% and 61% with increasing film thickness due to a longer diffusion path. In contrast with simple AGG film, only 90% of the theoretical alliin content was recovered after 24 h, likely due to dissolved alliin remaining in the hydrated AGG film.
The time-dependent allicin release from a double-film direct layout was investigated using 120 mg/mL of alliin AGG film and 40 mg/mL of alliinase AGG film. By comparing the release kinetics of alliin and allicin, it can be concluded that the mass transport of product and substrate in the AGG film is the rate-limiting factor rather than the enzymatic conversion of alliin to allicin. This conclusion was drawn from the comparable release profiles of both compounds, i.e. alliin and allicin, in the double-film layout (see “alliin film + 15 mL AGG” in Fig. 5A and alliin–alliinase film in Fig. 5B). It means that the enzymatic conversion of alliin to allicin is rapid and does not affect release kinetics.
Cytotoxicity evaluation of films
Leachates from blank, 40-mg/100-mL alliinase and 120-mg/100-mL alliin AGG films, were examined for cytotoxicity against HaCaT and MRC-5 cell lines after 24 and 72 h of incubation (as shown in Fig. 6). The study also compared the effect of two methods of film sterilisation on cell viability. The results indicated that the simple AGG film (labelled as UV/EtOH-Blank in the graphs) and films containing either alliin or alliinase did not affect the viability of the two cell lines. However, a decrease in a relative number of live cells was detected in the case of double-film with both alliin and alliinase, which produces allicin (labelled as UV/EtOH-Alliin + Alliinase). This outcome was expected and is consistent with the previous studies that have reported allicin’s toxicity to mammalian cell lines and its anti-tumour activity [38, 39].
Encouragingly, the results showed that human skin keratinocytes (HaCaT) were less susceptible than human fibroblasts (MRC-5). The study also revealed minimal difference between leachate after 24 and 72 h of incubation, which is in line with release kinetics data (as shown in Fig. 5), indicating that release is completed within a 24-h period. Both methods of sterilisation were comparable, with insignificant variations between the samples.
Although a significant toxic effect of allicin on the studied cell lines was observed, the experimental conditions were intentionally set to evaluate the worst-case scenario. This includes using AGG films with the highest concentrations of both compounds, testing leachate containing all allicin gradually formed during 24 and 72 h, having no existing air gap between the AGG compartment and cells, and dissolving allicin in the liquid phase only. However, the main application potential and novelty of this study lies in the antibacterial activity of films and in situ produced allicin vapours. In our future research, the goal is to verify the hypothesis that AGG films can release allicin vapours gradually, which can be safely applied to human skin. This will be achieved through tests on air–liquid interface (ALI) skin cell cultures that replicate the typical skin microenvironment. Additionally, we plan to develop wearable AGG patches and evaluate their efficacy through volunteer trials as an alternative approach.
Antibacterial activity of allicin
The antibacterial effects of in situ formed allicin and kanamycin were compared using two different tests: a standard disc diffusion test (DD) and a proposed film holder test (FH). The DD test confirmed that both solutions, i.e. kanamycin and alliin–alliinase mixture, were effective against E. coli, as evidenced by clear inhibition zones around the discs with the applied solutions (Fig. 7). However, a significant difference was observed when the same volume of solutions was applied to a holder’s reservoir covered with AGG film. Only the alliin–alliinase mixture produced an inhibition zone identical to the holder’s footprint. This suggests that enzymatic formation and transport of allicin vapour occurred across the film barrier and air gap to the surface of the agar plate. In contrast, the kanamycin solution did not exhibit any antibacterial activity in the FH test due to its non-volatile nature and retention in the AGG film. In the following experiments, the allicin vapours will be tested in the FH type of test, and kanamycin in the form of the DD test will only serve as a positive control in all cases.
Antibacterial activity against E. coli—comparison of DD test (top half) and FH test (bottom half), and kanamycin antibiotic on the left half and allicin produced from 10-µL alliin (100 mM) + 10-µL alliinase solutions (0.6 mg/mL) mixture on the right half of the Petri dish (theoretical allicin content 0.5 µmol).
Thermal stability of immobilised alliinase in films
Enzyme immobilisation in AGG films is practical for several reasons, such as protection of alliinase against external factors, prolonged shelf-life, and ease of handling and recovery, compared to an aqueous solution. It is crucial to ensure proper film preparation protocol and storage conditions to preserve most of the alliinase activity. Therefore, the activity of alliinase embedded in dry AGG films (40 mg per 100 mL) was assessed by FH test after 4 days of storage at various temperatures, including freezer (− 18 °C), fridge (5 °C), room temperature (25 °C), incubator (35 °C), and oven (60 °C). For the testing, the holder reservoir was filled with 20 µL of an alliin solution (100-mM, theoretical allicin content 1 µmol) and then covered by an AGG alliinase film. FH tests revealed that the growth of S. epidermidis was entirely inhibited in all cases except for films stored at 60 °C, as shown in Fig. 8. Here, no inhibition zone was observed as a result of alliinase denaturation, which starts at 42 °C, as described by Lagunas et al. [40]. To minimise thermal degradation over time, reducing moisture content and relative humidity during storage can be helpful [34]. Additionally, the use of additives such as maltodextrin in the final formulation can effectively preserve the native conformation of alliinase in a solid state, even at very high temperatures exceeding 100 °C [35].
Composite layout—alliinase film and alliin solution/particles
In the first composite layout, an alliinase film was tested with alliin in two forms: dissolved in aqueous solution and encapsulated in spray-dried chitosan microparticles. The antibacterial activity of two different alliinase concentrations in AGG film, 10 and 40 mg/100 mL, was tested against E. coli. As shown in Fig. 9A, the sample containing 10 mg of alliin particles (2% w/w) did not exhibit any antibacterial effect when combined with 10-mg/100-mL alliinase film. On the other hand, the same amount of alliin added as a solution was notably more effective (low to moderate effect). A fourfold increase in alliinase amount resulted in complete inhibition for alliin solution and a moderate effect for alliin particles (Fig. 9B). These results demonstrate that the mass transport of alliin is a limiting factor in various arrangements since a simple aqueous solution is more effective compared to particles.
Antibacterial activity of composite layouts of alliinase film with alliin solution and alliin particles against E. coli (FH test). The tests were conducted using 0.2 mg of alliin in all cases; corresponding to the theoretical allicin content 0.3 µmol. Alliinase films of two concentrations were tested—a 10 mg/100 mL and b 40 mg/100 mL; kanamycin was used as a positive control (DD test).
Composite layout—alliin film and alliinase solution/particles
Here, the inverse arrangement from the previous results was tested against E. coli, with alliin encapsulated in the AGG film and alliinase in both forms, i.e. the aqueous solution and particles in dry powder form.
It was found that an alliinase solution containing 0.2 mg of pure alliinase effectively inhibited bacterial growth when combined with all prepared alliin films (7.5, 15, 30, 60, and 120 mg/100 mL), as shown in Fig. 10 left. The equivalent amount of alliinase in chitosan particles was significantly less effective (Fig. 10 middle and right) due to the encapsulation via spray drying, which enhanced its stability but lowered overall activity [41, 42]. Alliin films of 7.5 and 15 mg/100 mL did not show any effect, while 30 mg/100 mL showed low inhibition (Fig. 10 middle). In contrast, 60 and 120 mg/100 mL resulted in complete inhibition (Fig. 10 right). Although the freshly prepared alliinase solution was found to be the most effective, its limited stability in its free form makes it unsuitable for extended storage, repeated use, or recovery. On the other hand, the particle form with encapsulated alliinase is more stable, but higher concentrations need to be used to obtain the antibacterial effect.
Antibacterial activity against E. coli (FH test) of different alliin films (7.5, 15, 30, 60, and 120 mg/100 mL; corresponding theoretical allicin content 0.07, 0.1, 0.3, 0.6, and 1.2 µmol) with alliinase solution (left) and alliinase particles (middle and right). Alliinase content was 0.2 mg for both cases; kanamycin was used as a positive control (DD test).
Composite layout—double alliin–alliinase film
In the final composite layout, two distinct AGG films, containing alliin and alliinase, were tested for their antibacterial activity against S. epidermidis. The experiment employed two arrangements—the “direct layout”, where the alliinase layer was closer to the agar plate, and the “reversed layout”, with the alliin layer in closer proximity (as schematically shown in Fig. 2). The concentration of alliinase in all films was kept constant at 40 mg/100 mL, while the alliin film was tested at concentrations of 15, 30, and 60 mg per 100 mL (corresponding theoretical allicin content 0.1, 0.3, and 0.6 µmol).
The direct layout showed high antibacterial activity for 30- and 60-mg/100-mL alliin films, but no effect for the lowest concentration of 15 mg/100 mL. On the other hand, the reversed layout showed no activity for 15 and 30 mg/100 mL and moderate activity for 60 mg/100 mL (Fig. 11). As the order of both films was the only variable in this test, the lower antibacterial activity observed in the reversed layout can be explained by the longer diffusion path of alliin and allicin, which was formed relatively far from the agar plate. Therefore, the overall antibacterial effect is governed by the rate of molecular transport rather than enzymatic reaction, which was also confirmed by the release kinetics of alliin and allicin from the AGG films (as shown in Fig. 5 and described in Sect. 3.3).
The antibacterial activity of double alliin–alliinase film with direct layout (alliin 60 mg per 100 ml, theoretical allicin content 0.6 µmol, and alliinase 40 mg per 100 ml) was also tested against S. aureus, which is a clinically important pathogen that causes a wide range of human infections. The results showed no antibacterial activity for alliin or alliinase films alone. However, when both types of AGG films were stacked together (double composite film), high antibacterial activity comparable to S. epidermidis was observed (Fig. 12). From all antibacterial tests, it can be concluded that allicin vapour is effective for both types of bacterial strains (Gram-positive and Gram-negative) and more resilient bacteria such as S. aureus.
Overview of allicin effect with various layouts
The results of the antibacterial testing for various composite layouts, together with calculated amounts of both components, are summarised in Table 2. The freshly prepared solutions of alliin and alliinase consistently exhibited superior antibacterial activity compared to encapsulated counterparts (particles and films). However, the long-term stability of free alliinase is insufficient and prohibits its practical use. On the other hand, encapsulation of alliinase and alliin in spray-dried microparticles or casted films significantly improves the stability of both substances but introduces transport limitations. This diffusion limitation was mainly observed when the direct and reversed layout of the double-film configuration was compared. In such cases, the antibacterial effect can be achieved, but higher amounts of encapsulated alliin and alliinase are needed. We believe that this trade-off is justifiable due to the long-term stability, improved control over allicin production, and potential application in topical treatments based on natural phytochemicals.
Conclusion
The study investigated the effectiveness of in situ formed allicin as an antimicrobial agent. Allicin vapour, which is formed enzymatically from alliin in the presence of an alliinase enzyme, was tested against both Gram-negative E. coli and Gram-positive S. epidermidis and S. aureus. Using a specially designed film holder, the allicin vapour showed a strong bactericidal effect, which has not been observed for any other synthetic antibiotic. It is important to note that the allicin vapour is the sole contributor to the observed antibacterial effect, as the testing holder is designed to be contactless.
Various alliin–alliinase forms, including aqueous solutions and encapsulated in AGG films or chitosan particles, were tested and compared for their antibacterial effect. The study showed that the transport and diffusion of alliin to the enzyme active site is a rate-limiting step in allicin formation. This allows for the enzymatic reaction to be controlled and the antibacterial effect to be prolonged by varying the thickness of the composite film materials. The prepared composite materials in the form of double-film or particle-film patches may find their applications in the long-term healing of open wounds or skin-related infections, either alone or in conjunction with traditional antibiotics.
Data and code availability
The data presented in this study are available on request from the corresponding author.
References
Shirtliff M, Leid JG, Shirtliff M (2009) The role of biofilms in device-related infections, vol 2. Springer, Berlin
Jia B et al (2023) Recent progress of antibacterial hydrogels in wound dressings. Mater Today Bio 19:100582
Fujisawa H et al (2008) Biological and chemical stability of garlic-derived allicin. J Agric Food Chem 56(11):4229–4235
Canchy L et al (2023) Wound healing and microbiome, an unexpected relationship. J Eur Acad Dermatol Venereol 37:7–15
Barie PS, Eachempati SR 2008 Infections of skin and soft tissue. In: Surgery. Springer. p 237-257
Las Heras K et al (2020) Chronic wounds: current status, available strategies and emerging therapeutic solutions. J Control Release 328:532–550
Jamaledin R et al (2020) Advances in antimicrobial microneedle patches for combating infections. Adv Mater 32(33):2002129
Roy R et al (2018) Strategies for combating bacterial biofilms: a focus on anti-biofilm agents and their mechanisms of action. Virulence 9(1):522–554
RezvaniGhomi E et al (2019) Wound dressings: current advances and future directions. J Appl Polym Sci 136(27):47738
Sadasivuni KK et al (2020) Recent advances in mechanical properties of biopolymer composites: a review. Polym Compos 41(1):32–59
Bjarnsholt, T., et al. 2020 The role of non-medicated dressings for the management of wound infection. In: 6th World Union of Wound Healing Societies Congress 2020: Global Healing-Changing Lives. Wounds International
Bandyopadhyay D (2021) Topical antibacterials in dermatology. Indian J Dermatol 66(2):117–125
Furner-Pardoe J et al (2020) Anti-biofilm efficacy of a medieval treatment for bacterial infection requires the combination of multiple ingredients. Sci Rep 10(1):12687
Schier C et al (2022) Allicin as a volatile or nebulisable antimycotic for the treatment of pulmonary mycoses: in vitro studies using a lung flow test rig. Int J Mol Sci 23(12):6607
Ankri S, Mirelman D (1999) Antimicrobial properties of allicin from garlic. Microbes Infect 1(2):125–129
Xu Z et al (2019) Allicin inhibits Pseudomonas aeruginosa virulence by suppressing the rhl and pqs quorum-sensing systems. Can J Microbiol 65(8):563–574
Marchese A et al (2016) Antifungal and antibacterial activities of allicin: a review. Trends Food Sci Technol 52:49–56
Gupta K, Viswanathan R (1955) Combined action of streptomycin and chloramphenicol with plant antibiotics against Tubercle Bacilli part i: streptomycin and chloramphenicol with cepharanthine part ii: streptomycin and allicin. Antibiot Chemother 5(1):24–27
Gruhlke MC et al (2015) The defense substance allicin from garlic permeabilizes membranes of Beta vulgaris, Rhoeo discolor, Chara corallina and artificial lipid bilayers. Biochim Biophys Acta 1850(4):602–611
Arreola R et al (2015) Immunomodulation and anti-inflammatory effects of garlic compounds. J immunol res 2015:1–13
Koca FD et al (2020) Green synthesis of allicin based hybrid nanoflowers with evaluation of their catalytic and antimicrobial activities. Biotech Lett 42:1683–1690
Ariyanta HA, Ivandini TA, Yulizar Y (2021) A novel way of the synthesis of three-dimensional (3D) MoS2 cauliflowers using allicin. Chem Phys Lett 767:138345
Chanani J, Buazar F, Nikpour Y (2023) Promoted photocatalytic activity of green titanium oxide-clay nanocomposite toward polychlorinated biphenyl degradation in actual samples. Water Air Soil Pollut 234(6):364
Mathialagan R et al (2017) Evaluation of allicin as soil urease inhibitor. Procedia eng 184:449–459
Hayat S et al (2022) Garlic, from medicinal herb to possible plant bioprotectant: A review. Sci Hortic 304:111296
Sharma S, et al. 2024 Natural antimicrobials from fruits and plant extract for food packaging and preservation. In: Food Packaging and Preservation, Elsevier, p 133-152
Alvarino LAdS et al (2023) Antibacterial potential of activated carbon impregnated with garlic extract. Processes 11(10):2948
Ashraf MA et al (2023) Allicin decreases phytotxic effects of petroleum hydrocarbons by regulating oxidative defense and detoxification of cytotoxic compounds in wheat. J Plant Growth Regul 42(6):3632–3649
Miron T et al (2003) Inhibition of tumor growth by a novel approach: in situ allicin generation using targeted alliinase delivery. Mol Cancer Ther 2(12):1295–1301
Appel E et al (2010) Therapy of murine pulmonary aspergillosis with antibody-alliinase conjugates and alliin. Antimicrob Agents Chemother 54(2):898–906
Jappe U et al (1999) Garlic-related dermatoses: case report and review of the literature. Am J Contact Dermat 10(1):37–39
Stoll A, Seebeck E (1949) About the specificity of aliinase and the synthesis of several compounds related to alliin. Helv Chim Acta 32(3):866–876
Mallika T, Omer E, Lianfu Z (2014) Separation and purification of alliinase and alliin from garlic (Allium sativum). J Acad Ind Res 2(11):599–605
Janská P et al (2021) Effect of physicochemical parameters on the stability and activity of garlic alliinase and its use for in-situ allicin synthesis. PLoS ONE 16(3):e0248878
Mašková L et al (2021) Development of compartmentalized antibacterial systems based on encapsulated alliinase. Adv Powder Technol 32(8):2720–2732
bt Ibrahim SF, Azam NANM, Amin KAM 2019 Sodium alginate film: The effect of crosslinker on physical and mechanical properties. In: IOP Conference Series: Materials Science and Engineering. IOP Publishing
Schindelin J et al (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9(7):676–682
Ossama M et al (2019) Enhanced allicin cytotoxicity on HEPG-2 cells using glycyrrhetinic acid surface-decorated gelatin nanoparticles. ACS Omega 4(6):11293–11300
Gruhlke MC et al (2016) The effects of allicin, a reactive sulfur species from garlic, on a selection of mammalian cell lines. Antioxidants 6(1):1
Lagunas LLM, Castaigne F (2008) Effect of temperature cycling on allinase activity in garlic. Food Chem 111(1):56–60
Garcia-Galan C et al (2011) Potential of different enzyme immobilization strategies to improve enzyme performance. Adv Synth Catal 353(16):2885–2904
Clark DS (1994) Can immobilization be exploited to modify enzyme activity? Trends Biotechnol 12(11):439–443
Acknowledgements
This work was financially supported by the Czech Science Foundation (GAČR), project No. 23-07356S, and the grant of Specific university research (A1_FCHI_2023_005), and by the Ministry of Education, Youth, and Sports of the Czech Republic, project Talking microbes-understanding microbial interactions within One Health framework (CZ.02.01.01/00/22_008/0004597).
Funding
Open access publishing supported by the National Technical Library in Prague. Grantová Agentura České Republiky,23-07356S,Ondřej Kašpar, Vysoká Škola Chemicko-technologická v Praze,A1_FCHI_2023_005, Lucie Mašková, Ministerstvo Školství, Mládeže a Tělovýchovy,CZ.02.01.01/00/22_008/0004597,Viola Tokárová.
Author information
Authors and Affiliations
Contributions
Lucie Mašková and Lenka Závišová contributed equally to this work. LM and LZ conducted experiments, data curation, formal analysis, and investigation. ZK contributed to data curation, analysis, and methodology for antibacterial testing. SR contributed to data curation, analysis, and methodology for cytotoxicity testing. OK and VT contributed to conceptualisation, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, and writing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts to disclose.
Ethical approval
Not applicable.
Additional information
Handling Editor: Annela M. Seddon.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mašková, L., Závišová, L., Kašpar, O. et al. Design and evaluation of composite films for in situ synthesis and antibacterial activity of allicin vapour. J Mater Sci 59, 13614–13631 (2024). https://doi.org/10.1007/s10853-024-09990-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-024-09990-x