Abstract
The aim of potato breeding is to release cultivars that exhibit high and stable performance across the target population of environments. The objective of this research was therefore to investigate the use of various methods (site regression [SREG], coefficient of variation and regression deviation [σ2δ]) for determining the adaptability and stability of productive and quality traits in the Nordic region of Europe. The multi-environment trials included 256 breeding clones and released cultivars grown by EU farmers at three distinct testing sites over two years in Sweden. There was significant (P < 0.001) variation in tuber yield, starch percentage and reducing sugars in the tuber flesh among the breeding clones and cultivars, testing environments and the genotype by environment interaction (GEI). The environments were very diverse, as revealed by the SREG biplots and particularly for the GEI patterns noted in terms of their productive and quality characteristics. The percentage of stable high-tuber yielding germplasm was greater for breeding clones (23%) than for released European cultivars (2%), thus revealing the advantage of potato breeding in the target population of environments. SLU 1415001 and SLU 1314015 were the most promising breeding clones due to their stable high tuber yield. This characteristic was best for the starch potato cultivars, although none of them exhibited a significant different σ2δ. ‘Talent’ shows an almost stable good performance among low reducing sugar cultivars and breeding clones, which are often unstable in terms of their scoring across environments. Neither a breeding clone nor a cultivar was at the top for stable tuber yield, tuber flesh starch or reducing sugars in the tuber flesh, which shows the challenge faced by potato breeding while addressing the needs of different markets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The testing of potato breeding clones along with released cultivars in various environments (e.g., sites, years, cropping seasons) generates trial data for the various characteristics measured therein. This dataset results from combining the effects of both genetic (G) and environmental (E) factors, as well as their interaction (GEI), which reduces the association between genotype and phenotype, thereby affecting selection effectiveness. Hence, the main goal of testing across sites and over several years is to obtain an accurate estimate of the performance of breeding clones or cultivars by minimizing the variance in their mean while considering both costs and time. Reducing the variance increases the chance of obtaining significant differences among breeding clones or cultivars, which facilitates the selection of promising germplasm that perform regularly across the target population of environments where they will be released for farming. Such stability should be a key characteristic of a cultivar.
There are several ways of either determining the adaptability or measuring the stability of a potato cultivar (or soon to be). According to Chloupek and Hrstkova [1], crops that respond positively to changes in agricultural conditions exhibit adaptability, while Tai and Young [2] indicated that potato cultivars with average genotypic stability and above-average mean performance generally exhibit adaptability. Lin et al. [3] defined stability groups according to whether they refer to deviations from the average genotype effect or to the GEI and whether they include a regression model on an environmental index. Furthermore, these stability measurements are related to classifying germplasm as stable if their among-environment variance is small (Type 1), if their response to the environment parallels that of the mean response of the trial (Type 2), or if they have a small residual mean square of the regression model on the environmental index (Type 3). The coefficient of variation (CV), as defined by Francis and Kannenberg [4], is Type 1, while Type 3 includes the regression approach improved by Eberhart and Russell [5]. According to Lin et al. [3], Type 3 splits the variability of a breeding clone or cultivar into a predictable performance related to a regression and an unpredictable performance corresponding to the deviation from the residual mean square (σ2δ). On the other hand, Type 1 is related to homeostasis, that is, the ability of a crop to remain stable despite changes in its surroundings [6], which allows the use of this concept as a criterion for determining stability.
Potato breeding clones should have a predictable performance for key characteristic(s) showing little GEI if they are meant to be released as cultivars in the target population of the environment. Hence, stability measurements are useful for identifying such a promising bred germplasm. According to Eberhardt and Russell [5], a stable cultivar has a regression coefficient (β) and σ2δ equal to 1 and 0, respectively. Cultivars with β above 1 and σ2δ equal to 0 perform well in high-yielding environments, while those with β values less than 1 and showing nonsignificant deviations are suitable for low-yielding environments. Any cultivar with a significant σ2δ is regarded as unstable. This stability analysis of potato plants has been used for interpreting the results of multiple environmental trials and for selecting superior breeding clones and suitable cultivars, for example, in Brazil [7], Canada [2], Ethiopia [8], India [9], Kenya [10, 11], Latvia [12], Perú [13], Türkiye [14], Uganda [15] and the USA [16, 17]; moreover, the CV facilitated the identification of promising potato bred germplasm in Iran under water deficit and potassium humate [18] or in Kenya [19] and in Poland [20].
The additive main effect multiplicative interaction (AMMI) model was proposed by Gauch [21] to determine the GEI and structure the interactions between the tested germplasm and the environments where the field trials were conducted. The AMMI model first uses analysis of variance to determine the main effects due to both genotypes and environments and the GEI and thereafter partitions the GEI into components through principal component analysis. Likewise, AMMI modeling facilitates the delineation of mega environments and improves trial accuracy. AMMI has been used for analyzing multi-environment potato yield trials in Brazil [22], Croatia [23], Ethiopia [8, 24], Iran [25, 26], Kenya [11], Korea [27], Rwanda [28], and Uganda [29]. Its modeling, particularly when using the ensuing biplots, led to the identification of breeding clones for further cultivar registration and suitable cultivars for respective sites in the target population of the environment. Cornelius [30], Crossa and Cornelius [31] and Crossa et al. [32] proposed site regression analysis (SREG) as an alternative to the AMMI. SREG uses a bilinear model to remove environmental effects and provides the answer solely as a function of the effects of the genotype and GEI; thus, this approach is most suitable when the environment accounts for more variation than does the genotype and GEI [33]. Moreover, GEI can be detected through the crossover effect resulting from ranking changes in genotypes across environments [34]. This approach was used by Gedif and Yigzaw [35] along with the GGE biplot [34] to visualize patterns and interactions without environmental effects and to identify stable potato cultivars for tuber yield in Ethiopia. Similarly, Sood et al. [36] used the GGE biplot for the analysis of potato multi-environment trials in subtropical India.
There are gaps in the assessment of the adaptability and stability of potato germplasm in Scandinavia. Hence, the objective of this research was to determine the adaptability and stability of productivity and quality traits using SREG, CV and regression approaches for analyzing the multi-environment traits of potato breeding clones and cultivars in Sweden, as well as to dissect and visualize GEI patterns that assist in defining whether the testing sites belong to the same target population of environments.
2 Materials and methods
The multi-environment trials included up to 256 breeding clones and released cultivars grown by EU farmers (Supplementary Table 1; https://hdl.handle.net/11529/10548617). The cultivars ‘Bintje’, ‘Carolus’, ‘Connect’, ‘King Edward’ and ‘Solist’ were the checks used by the Svenska Potatisförädling run by the Swedish University of Agricultural Sciences (SLU, Alnarp, Sweden). SLU also provided 47 advanced breeding clones (36 for table whose parents were breeding clones and released cultivars, 8 for crisps and 3 for tables but derived from using crop wild relatives as grandparents). The 209 released cultivars were mostly ware (190) or starch (9) potatoes.
The testing sites were at the Skåne (Helgegården and Mosslunda) and Norrland (Umeå) regions of Sweden, where trials were run in 2020 and 2021 using simple lattice designs with two replications of 10-plant plots. Helgegården and Mosslunda are potato-producing sites near Kristianstad (56°01′46″N 14°09′24″E) that experience long days (hours with sun above 120 w/m2: 192–308; Supplementary Table 2) and relatively warm temperatures (monthly average: 23.1–32.6 °C; Supplementary Table 2) during the cropping season, which took between 110 and 120 days, while Umeå (63°49′30″ N 20°15′50″ E) has very long days (hours with sun above 120 w/m2: 110–399; Supplementary Table 2) and cool temperatures (monthly average: 18.3–28.2 °C; Supplementary Table 2) during the 90– to 100–day cropping season.
The soils used for trials in Skåne were disturbed IVA with pH 5.9 in 2020 and class V with pH 7.7 in 2021, while the soils for trials in Umeå were III or IVA with pH 5.8 and 5.9 respectively. The crop husbandry practices used were the same as those used in potato farming at each site. There were fungicide sprays against the oomycete Phytophthora infestans only in Helgegården to avoid late blight throughout the growing season and to allow for the achievement of tuber yield potential at this testing site. The characteristics evaluated were total tuber yield in a 10-plant plot (kg), tuber flesh starch content, which was calculated after determining the specific gravity at harvest [37] and reducing sugars in the tuber flesh using potato glucose strip tests [38].
2.1 Statistical data handling
The analysis of variance was used to determine the significance of G, E and the GEI in the multiple environment trials. The general additive linear model was as follows:
where \(\mu\) is the mean, \(L\) denotes the \({i}{th}\) location \((i=\text{1,2},\dots\), \(J\) denotes the \({j}{th}\) environment (\(j=\text{1,2},\dots\)), and \(e\sim N\left(0,{\sigma }_{e}^{2}\right)\) is the residual error.
The effects of the GEI on each breeding clone or cultivar were analyzed using the CV [4], regression [5], and AMMI [21] models. Analysis of the trials in each and across environments was performed with GEA-R [39]. The CV [4] was defined as follows:
where \({S}_{i}\) and \(BLUE\) are the simple standard deviations of the mean performance (for tuber yield, tuber flesh starch or reducing sugars in the tuber flesh) across environments and the best linear unbiased estimator across environments, respectively. The environmental variance \({{S}_{i}}^{2}\) was therefore estimated as follows:
in which \({R}_{ij}\), \({m}_{i}\), and \(e\) are the observed genotype performance in the jth environment, the genotype mean across environments, and the number of environments, respectively. \(BLUE\) is an estimator with the smallest variance among those unbiased and linear in the observed output. The Eberhardt and Russell regression approach [5] considers the regression coefficient (\({b}_{i}\)) and its deviations (\({{\upsigma }^{2}\updelta }_{i}\)), which are defined as follows:
and
where \({x}_{ij}\), \({\mu }_{.j}\), \({\mu }_{i}.\), and \({\mu }_{..}\) are the mean of the ith genotype in the jth environment, mean of the jth environment, mean of genotypes across environments, and total, respectively, whereas the number of genotypes is \(g\).
The SREG considers this linear-bilinear model:
where Yij refers to the performance of the ith genotype in the jth environment; \(\mu\) is the grand mean; ej is the environmental deviation from the grand mean; \({\lambda }_{n}\) denotes the eigenvalue of the nth principal component axis; \({\xi }_{in}\) and \({\eta }_{jn}\) are the genotypic and environmental principal component scores for axis \(n\), respectively; and \({\upvarepsilon }_{ij}\sim N\left(0,{\sigma }_{\varepsilon }^{2}\right)\) is the random term or residual. Singular value decomposition (SVD) of the first two principal components (PCs) was subsequently applied to fit the biplot model:
where \({e}_{j}\) is the main effect of the jth environment; \(\mu +{e}_{j}\) is the mean performance across all the genotypes in the jth environment; \({\lambda }_{1}\) and \({\lambda }_{2}\) are the singular values for PC1 and PC2, respectively; \({\xi }_{i1}\) and \({\xi }_{i2}\) are the eigenvectors of the ith genotype for PC1 and PC2, respectively; \({\eta }_{j1}\) and \({\eta }_{j2}\) are the eigenvectors of the jth environment for PC1 and PC2, respectively; and \({\upvarepsilon }_{ij}\) is the residual associated with the ith genotype in the jth environment.
2.2 Biplot graphs
Biplots were used to discriminate high- and low-yield breeding clones and cultivars as well as favorable and unfavorable environments [32]. The SREG1 biplot uses the X axis for the BLUEs for each productivity or quality trait and the Y axis for factor 1 (PC1), whereas the SREG2 biplot has factor 1 along the X axis and factor 2 (PC2) following the Y axis. A vertical line in the mid-section of the performance for the respective biplots of each trait and a horizontal line at PC = 0 were drawn to group the evaluated bred germplasm for performance, adaptability and stability. Among the SREG1 biplots, the breeding clones or cultivars and environments on the right had above average performance, while the breeding clones or cultivars and environments in the center of the SREG2 biplot had the minimum genotype × environment interaction, thereby being better than the other breeding clones or cultivars and environments because they did not have a significant GEI. Furthermore, breeding clones or cultivars adjacent to an environment were those specifically adaptive to such a related environment, while those breeding clones or cultivars near the component axes had relative adaptability. The X-axis represents the BLUE for each productivity or quality trait, and the Y-axis represents the CV; for the regression approach, the X-axis represents \({{s}^{2}d}_{i}\), and \(b\) represents the Y-axis. Vertical and horizontal lines in the mid-sections of the respective CV biplots were drawn to group the evaluated bred germplasm as follows: [1] high yield and small variation (low CV) in the right lower quadrant; [2] high yield and large variation (large CV) in the right upper quadrant; [3] low yield and small variation in the left lower quadrant; and [4] low yield and large variation in the left upper quadrant. In the regression biplots, the vertical line was drawn at \({{s}^{2}d}_{i}\) = 0, and the horizontal line was drawn at \(b\) = 1 to identify adaptable or stable breeding clones and cultivars.
3 Results
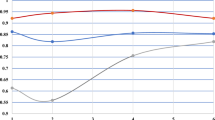
Late blight was not observed (or was not visible) at high latitudes in Umeå. However, in both locations in Skåne, late blight was prevalent and had a high incidence and severity. In the Helgegården trial, pesticides were employed to manage late blight, thereby enabling the cultivars and breeding clones to express their tuber yield potential. There was significant (P < 0.001) variation in tuber yield, starch percentage and reducing sugars in the tuber flesh among the breeding clones and cultivars (hereafter, together denoted as g), the environment and the GEI (Table 1). The \(e\), g and GEI effects accounted for approximately 42%, 33% and 25%, respectively, of the tuber yield variation, while \(e\) described 72%, g 20% and GEI effects on approximately 8% of the tuber flesh starch variability. Approximately 43%, 31% and 26% of the tuber flesh starch variability was due to \(e\), g and GEI, respectively, for reducing sugars in the tuber flesh. The linear components of the regression were significantly greater than the pooled deviations. Similarly, the \({{\upsigma }^{2}\updelta }_{i}\) from the regression was significant, thereby indicating some degree of nonlinearity in the GEI. This could arise from either specific GEIs or changes in GEI expression according to the testing environment, which highlights that the GEI is a key factor in potato cultivar trials that may affect the ability to detect differences among breeding clones and cultivars. The first five interaction principal components (PC1–PC5) were significant (P < 0.05) for the productivity and quality traits evaluated according to the SREG analysis (Table 1). PC1 accounted for more than 50% of these traits, while the SREG 2 biplot captured ca. 70% of the tuber yield GEI, more than ¾ of the GEI for tuber flesh starch, and more than 69% of the GEI for reducing sugars in the tuber flesh.
The red circle in Fig. 1 includes 9 (of 39 or approximately 23%) high-yield table breeding clones and 4 other European released cultivars (of 209 or ca. 2%) (namely, ‘Galactica’ [34], ‘Papageno’ [179], ‘Connect’ [194] and ‘Taisiya’ [211]) in Nordic testing environments. The outstanding high-yielding SLU 1415001 [221] exhibited little interaction across testing sites over time, as noted in the biplot, and SLU 1314015 [237] exhibited the least interaction with the testing environments among those included in the ellipse. The outstanding breeding clones according to their CV were SLU 1415001 [221], 1,402,003 [216], 2-IV-6 [244] and 1,314,015 [237] (Fig. 2). Together, these data were obtained with the high-yield release cultivar ‘Galactica’ [34] in the lower right quadrant of the graph. Svensk Potatisförädling SLU 1452001 [143] and SLU 1438004 [232], as well as the starch cultivar ‘Avenue’ [144], were adaptable (red numbers), while the table cultivars ‘Red Emmalie’ [137] (having reddy-pink skin and matching flesh rich in anthocyanins) and the white skin and creamy flesh ‘Valor’ [142] were adaptable and stable (green numbers) according to the regression approach (Fig. 2). However, none of them were among the high-yielding bred-germplasm or released cultivars.
Stability graph for total tuber yield in a 10-plant plot (X axis) as determined by the coefficient of variation (CV) on the Y axis. The numbers in red indicate the stable breeding clones and released cultivars with above-average yields (left). The right graph shows the adaptability determined by the regression coefficient (Y axis) and its deviations (s2d) on the X axis
In both years, highest tuber yield was obtained from the site in Helgegården, and Mosslunda (also in Skåne), though exhibiting less tuber yield, was the closest of the other two sites in both years (Fig. 1). The least yielding Umeå showed similar GEI patterns over time, while the other two sites in Skåne did not.
3.1 Tuber flesh starch
The red ellipse in Fig. 3 includes four starch release cultivars grown in Europe (namely, ‘Quadriga’ [147], ‘All Star’ [254], ‘Nofy’ [255] and ‘Serum Star’ [256]) along with the old floury cultivar ‘Odenwälder Blaue’ [45], the crisp cultivar ‘Verdi’ [187], which may sometimes be used for starch production and dehydration of potatoes, and the crisp Svenska potatisförädling clone SLU 131 [12]. ‘Avenue’ [144], ‘Kuras’ [146], ‘Saprodi’ [148] and ‘Tarzan’ |185] are other four potato cultivars released in Europe for starch production, while ‘Golden Wonder’ [35] is an old, crisp cultivar used in organic farming. Figure 4 shows that within the right bottom quadrant (towards the right), the cultivars and breeding clones had the highest and most stable starch content in the tuber flesh (SLU 1452001 [143], ‘Red Emmalie’ [137], ‘Colomba’ [71], ‘Lady Christl’ [126], ‘Rosara’ [99] and ‘Blaue St. Galler’ [26]). The starch cultivars used were ‘Serum Star’ [256], ‘All Star’ [254], ‘Tarzan’ [185], ‘Avenue’ [144], ‘Quadriga’ [147], and ‘Saprodi’ [148]. Similarly, in this group, the crisp cultivar Verdi [187]; the old mealy or floury potato cultivars ‘Odenwälder Blaue’ [45], ‘Golden Wonder’ [35], and ‘Eigenheimer’ [31]; and Svenska Potatisförädling SLU 107 [9] and SLU 131 [12]. Neither a breeding clone nor a released cultivar had adaptability or stability for tuber flesh starch according to \({{\upsigma }^{2}\updelta }_{i}\) and \(b\) (Fig. 4).
Stability graph for the tuber flesh starch percentage (X axis) as determined by the coefficient of variation (CV) on the Y axis. The numbers in red indicate the stable breeding clones and released cultivars with above-average yields (left). The right graph shows the adaptability determined by the regression coefficient (Y axis) and its deviations (s2d) on the X axis
The site with the highest tuber flesh starch was Helgegården, and the worst was Umeå (particularly in 2021), as shown by the SREG1 data in Fig. 3. Neither Helgegården 2020 nor Umeå 2021 were included in the same GEI clustering in the SREG2 biplot as was the case for the other four testing environments. The best starch cultivars (‘Arran Victory’ [113] and SLU 127 [11]) were nearby Helgegården 2020 and they had specific adaptation to this testing environment.
3.2 Reducing sugars in the tuber flesh
Figure 5 shows two European released cultivars for chips, namely, ‘Talent’ [55] and ‘Charlotte’ [192], two diploid Solanum tuberosum Gp. Phureja with yellow tuber flesh [’Mayan Gold (131) and its offspring ‘Inca Bella’ (123)], and Svenska potatisförädling SLU 1410004 [218]. According to the other stability statistics, only the cultivar ‘Talent’ [55] exhibited almost stable good performance among the cultivars and breeding clones with low reducing sugars (> 1). The other cultivars were unstable in their scoring across environments (Fig. 6).
Stability graph for reducing sugars in the tuber flesh (X axis) as determined by the coefficient of variation (CV) on the Y axis. The numbers in red indicate the stable breeding clones and released cultivars with above-average yields (left). The right graph shows the adaptability determined by the regression coefficient (Y axis) and its deviations (s2d) on the X axis
Umeå 2021 and Helgegården 2021 were the best testing environments for this trait because both were able to differentiate the genotypes, but their GEIs were different according to the SREG biplots (Fig. 6). The GEI was very similar for both testing sites in Skåne in 2021.
4 Discussion
In plant breeding, the terms "wide adaptation" and "narrow adaptation" refer to the range of environmental conditions under which a particular crop or cultivar performs well [40, 41]. A breeding clone or cultivar with wide adaptability performs well across a broad range of environmental conditions because it can thrive in various climates, soils, and management practices [42]. Wide adaptability is desirable for crops intended for cultivation in diverse geographic locations. Moreover, these systems provide resilience to unpredictable environmental changes and increase the likelihood of successful cultivation in different regions with variable temperatures, pathogens, pests and soil types. A breeding clone or cultivar with narrow adaptation is specialized for a specific set of environmental conditions because it performs optimally only under a limited range of factors, such as climate, soil type, or management practices. Narrowly adapted cultivars are often optimized for specific environments, resulting in higher yields or quality under those specific conditions [43]. A cultivar may therefore be developed specifically for a particular region with a unique set of climatic and soil conditions. The choice between wide and narrow adaptation in potato plants depends on the specific breeding goals, the target environment for cultivation, and the balance between resilience and optimization. In some cases, potato breeders may aim to compromise upon this approach by seeking cultivars that exhibit a balance between adaptability across different conditions and optimized performance under specific, well-defined conditions.
The testing environments were very diverse, as revealed by the SREG biplots and, in particular, by the GEI patterns for both productive and quality characteristics. These populations represent different target populations of environments in Scandinavia: those with high yield potential for avoiding pathogens with the use of pesticides, those with late blight proneness and potential for organic farming, and those with a short, cool cropping season under very long days. The significant GEI across testing sites and over several years noted in multi-environment trials indicates the further need to determine the adaptability and stability of breeding clones before they are released as new cultivars in the Nordic region of Europe. As indicated by Cotes et al. [43], stable and sustainable tuber yields under varying environments should be pursued while breeding new potato cultivars.
It was clear that the tuber yield of the potato bred-germplasm and released cultivars across the three testing sites over two years was affected by a crossover GEI, as previously noted among the same testing sites with a small set of released cultivars [44]. Statistical analysis using appropriate methods, as described in this research article, allows the identification of promising germplasm with stable high tuber yields, for example, SLU 1415001 and SLU 1314015. The breeding clone SLU 1314015 also had a high level of host plant resistance to the oomycete Phytophthora infestans [45] and a high genomic value for this characteristic as well as for tuber yield [46]. The greater percentage of high- and stable-yielding breeding clones (23%) than European-released cultivars (2%) confirms that potato breeding should be within the target population because foreign cultivars bred elsewhere are not always very suitable for Scandinavia. Consequently, SLU 1314015 was chosen for subsequent cultivar release, as detailed in Ortiz et al. [47]. Currently, SLU's Svensk Potatisförädling is in the registration process for inclusion in Svenska Sortlistan. This compilation of cultivar information serves as a prerequisite for certifying planting materials within the European Union. The upcoming launch of Svalöf represents a noteworthy achievement, as the initial potato cultivar was exclusively cultivated in Sweden in the mid-1990s.
The testing environment had a significant effect on the amount of tuber flesh starch. This result was not surprising because this multigenic characteristic [48] seems to be influenced by the farming site. As indicated by Flis et al. [49], neither temperature nor precipitation explain the site effect on tuber flesh starch. Although the released starch cultivars performed on average well in terms of these characteristics in multiple environment trials, none of the strains had a low \({{\upsigma }^{2}\updelta }_{i}\), which agrees with the findings of Flis et al. [50], who noted a tendency toward instability in bred germplasm with high starch content. Furthermore, they also indicated that a Type 1 measurement such as the CV should be used with caution when considering it for breeding processing potatoes with stable starch content. In view of these findings, various stability analyses should be used for obtaining multi-environment trial data for tuber flesh starch. In this regard, after using various stability measurements, Lenartowicz et al. [51] identified ‘Kuras’ as a stable starch cultivar. Notably, Naeem and Caliskan [19] reported that stability statistics depend on the method used for determining dry matter in tubers.
Reducing sugars in tuber flesh are the most difficult characteristic for identifying stable or adaptable potato germplasm through multi-environment testing. Umeå 2021 and Helgegården 2021 emerged as optimal testing environments for low reducing sugars influenced by the GEI. This outcome may be attributed to the potato plants achieving full chemical maturity (ensuring sugar stability through natural senescence) without succumbing to defoliation caused by late blight. The impact of late blight on reducing sugars is likely more significant than the variation attributed to different sites. Typically, genotypes that reach full maturity exhibit relatively low GEIs. According to Mackenzie et al. [16], who used chip color ratings after frying, a great amount of experimental error (or the residual in the ANOVA) affects the assessment of this characteristic.
Although Flis et al. [49] indicated that a stable tuber yield was often associated with stable starch yield and content after testing a set of 21 cultivars bred in Hungary, Poland and Spain, neither a breeding clone nor a cultivar was at the top in terms of the rankings for tuber yield, tuber flesh starch and reducing sugars in the tuber flesh in our multi-environment trials. This result was similar to that of Tai and Young [2], who were unable to detect a cultivar combining both high marketable tuber yield and specific gravity after a decade of variety trials in New Brunswick (Canada). These findings also show the challenges faced by potato breeding, which must consider developing cultivars for each market segment, such as those eaten after baking, boiling, frying, mashing or roasting and for salads in the home tables; used as fingerlings and petit potatoes by chefs and as chips in fast food restaurants; and for both crisp and starch processing by the industry.
5 Conclusion
The adaptability and stability of breeding clones are crucial considerations before their release as new cultivars in the Nordic region of Europe. This is due to the significant genotype-by-environment interaction that impact productivity and quality traits. These interactions necessitate a thorough evaluation of breeding clones within the specific environmental conditions of the target populations to ensure their suitability. In this context, the breeding clones SLU 1314015 and 1,415,001 emerged as particularly promising candidates. Their stability in producing high tuber yields under various conditions highlights their potential for successful cultivation in the region. Specifically, clone SLU 1314015 has been selected for further development and eventual release in southern Scandinavia. This decision underscores the approach of potato breeding suitable to the local environment, thus avoiding the introduction of foreign cultivars that may not perform optimally in high-latitude climates.
Despite these advancements, challenges remain. Notably, identifying breeding clones or cultivars that combine high productivity with desirable characteristics such as high dry matter content and low levels of reducing sugars in the tuber flesh proved elusive. This indicates a gap in the current breeding approach and suggests the necessity of employing a selection index during population improvement efforts within the reference breeding germplasm. Such an index could facilitate the targeted selection of traits that are essential for both productivity and quality, addressing the limitations observed in the current breeding pool. In summary, this study emphasizes the importance of tailored breeding approaches in the Nordic region, focusing on the adaptation of cultivars to specific environments.
Data Availability
The datasets generated and analyzed during the current study are available at https://hdl.handle.net/11529/10548617
References
Chloupek O, Hrstkova P. Adaptation of crops to environment. Theor Appl Genet. 2005;111:1316–21. https://doi.org/10.1007/s00122-005-0060-x.
Tai GCC, Young DA. Genotypic stability analysis of eight potato varieties tested in a series of ten trials. Am Potato J. 1972;49:138–50.
Lin CS, Binns MR, Lefkovitch LP. Stability analysis: where do we stand. Crop Sci. 1986;26:894–900.
Francis TR, Kannenberg LW. Yield stability studies in short season maize. 1. A descriptive method for grouping genotypes. Can J Plant Sci. 1978;58:1029–34.
Eberhart SA, Russell WA. Stability parameters for comparing varieties. Crop Sci. 1966;6:36–40.
Lerner M. Genetic homeostasis. New York: Wiley; 1954. (Oliver & Boyd, London, United Kingdom).
de Souza LE, Pereira Pinto CAB, de Menezes CB. Potato improvement for tropical conditions. I. Analysis of stability. Crop Breed Appl Biotechnol. 2006;6:129–35.
Fufa TW, Fufa AN. Yield stability analysis of elite Irish potato (Solanum tuberosum L.) varieties in western Ethiopia. Int J Biochem Biophys Mol Biol. 2021;6:6–10. https://doi.org/10.11648/j.ijbbmb.20210601.13.
Raja S, Verma MR, Sathpathy PC, Yadav LM, Kumar R, Ullah Z, Khaiwal R, Dubey RK, Kumar S, Singh D, Deshmukh MR, Verma D, Govindakrishnan PM. Genotype by environment interaction and yield stability of potato cultivars under tropical conditions. J Agric Sci Technol. 2018;20:583–95.
Lung’aho C, Ojiambo PS, Kidanemariam HM. Yield stability analysis of promising potato clones in mid and high altitude regions of Kenya. Afr Crop Sci J. 1998;6:137–42. https://doi.org/10.4314/acsj.v6i2.27809.
Naawe EKGenotype × environment interaction and stability analysis of potato breeding lines. MSc Thesis. Niğde Ömer Halisdemir University, Merkez/Niğde, Turkiye. 2020.
Skrabule I, Vaivode A, Piliksere D, Liniņa A, Mūrniece I, Krūma Z. Evaluation of traits stability for selection purposes in potato breeding programme. In: Proceedings 25th NJF Congress “Nordic View to Sustainable Rural Development”, Riga, Latvia, 16–18 June 2015. Nordic Association of Agricultural Scientists, Stockholm, Sweden, 2015; pp 154–155
Ortiz R, Freyre R, Peloquin SJ, Iwanaga M. Adaptation to day length and yield stability of families from 4x x 2x crosses in potato. Euphytica. 1991;56:187–98.
Pehluvan M, Kaya C, Kumlay AM, Tozlu E, Dizkisa D, Okçu M, Taçoğly M (2006) Patates klonlarinda stabilite analyzi. In: IV Ulusal Patates Kongresi. 6–8 July 2006. Bildirber Kitabi, Nidğe, Turkiye, pp 53–58
Kakuhenzire R, Hakiza JJ, Adipala E, Wagoire W, Lemaga B. Yield stability and acceptability of two new potato Solanum potato varieties in Uganda. Uganda J Agric Sci. 2004;9:718–22.
Mackenzie DR, Mills WR, Watts JO. Predicting yield and processing quality of potato breeding selections. Amer Potato J. 1976;53:87–98.
Darmo E, Peloquin SJ. Performance and stability of nine 4x clones crosses and four commercial cultivars from 4x–2x crosses and four commercial cultivars. Potato Res. 1990;33:357–64.
Hassanpanah D, Gurbanov E, Gadimov A, Shahriari R. Determination of yield stability in advanced potato cultivars as affected by water deficit and potassium humate in Ardabil Region. Iran Pakistan J Biol Sci. 2008;11:1354–9.
Naeem M, Caliskan ME. Comparison of methods for dry matter content determination in potato using multienvironments field data and stability statistic. Turkish J Field Crops. 2020;25:197–207.
Przystalski M, Lenartowicz T. Yielding stability of early maturing potato varieties: Bayesian analysis. J Agric Sci. 2020;158:564–73. https://doi.org/10.1017/S0021859620000945.
Gauch HG Jr. Model selection and validation for yield trials with interaction. Biometrics. 1988;44:705–15.
Queiroz de Souza V, da Silva Pereira VA, da Silva GO, Fritsche Neto RA, Costa de Oliveira RA. Consistency of two stability analysis methods in potatoes. Ciência Rural. 2007;37:656–61.
Mijić Z, Kozumplik V, Šarčević H, Meglić V, Varnica I, Čupić T. Stability analysis of tuber yield using unbalanced data from potato variety trials. Genetika. 2019;51:1151–64. https://doi.org/10.2298/GENSR1903151M.
Worku A, Mulugeta G, Berhun B, Abebe T, Giorgis GW, Chindie A, Kebede G. Performance and yield stability analysis of potato genotypes in Ethiopia. Adv Crop Sci Technol. 2018;6:1. https://doi.org/10.4172/2329-8863.1000336.
Hassanpanah D, Azimi J. Yield stability analysis of potato cultivars in spring cultivation and after barley harvest cultivation. Ame-Euras J Agric Env Sci. 2010;9:140–4.
Mohammadnia S, Asghari A, Hassanpanah D, Karimizadeh R, Shokouhian AA. Determining the most stable potato genotypes using AMMI yield stability analysis method. J Agric Sci (Tarim Bilimleri Dergisi). 2021;27:146–54.
Kim SJ, Sohn HB, Lee YY, Park MW, Chang DC, Kwon OK, Park YE, Hong SY, Suh JT, Nam JH, Jeong JC, Koo BC, Kim YH. Genotype x environment interaction and stability analysis for potato performance and glycoalkaloid content in Korea. Korean J Crop Sci. 2017;62:333–45.
Placide R, Theophile N, Vandamme E, Nshimiyimana JC, Mendes T. Yield performance, adaptability and processing qualities of prerelease potato clones under different Rwandan agro-ecologies. CABI Agric Biosci. 2022;3:40. https://doi.org/10.1186/s43170-022-00105-7.
Mulema JMK, Adipala E, Olanya OM, Wagoire W. Yield stability analysis of late blight resistant potato selections. Exp Agric. 2008;44:145–55. https://doi.org/10.1017/S0014479708006133.
Cornelius PL. Statistical test and retention of terms in the additive main effects and multiplicative interaction model for cultivar trials. Crop Sci. 1993;33:1186–93.
Crossa J, Cornelius PL. Sites regression and shifted multiplicative model clustering of cultivar trial sites under heterogeneity of error variances. Crop Sci. 1997;37:406–15. https://doi.org/10.2135/cropsci1997.0011183X003700020017x.
Crossa J, Cornelius PL, Yan W. Biplots of linear-bilinear models for studying crossover genotype–environment interactions. Crop Sci. 2002;42:619–33.
Balzarini M, Bruno C, Arroyo A. Análisis de ensayos agrícolas multiambientales: Ejemplos con Info-Gen. Córdoba: Facultad de Ciencias Agropecuarias Universidad Nacional de Córdoba; 2005. p. 141.
Yan W, Hunt LA, Sheng Q, Szlavnics Z. Cultivar evaluation and mega-environmental investigation based on the GGE biplot. Crop Sci. 2000;40:597–605.
Gedif M, Yigzaw D. Genotype by environment interaction analysis for tuber yield of potato (Solanum tuberosum l.) using a GGE biplot method in Amhara region, Ethiopia. Agric Sci. 2014;5:239–49. https://doi.org/10.4236/as.2014.54027.
Sood S, Bhardwaj V, Kumar V, Gupta VK. BLUP and stability analysis of multienvironment trials of potato varieties in subtropical Indian conditions. Heliyon. 2020;6: e05525. https://doi.org/10.1016/j.heliyon.2020.e05525.
Schippers PA. The relationship between specific gravity and percentage dry matter in potato tubers. Am Potato J. 1976;53:111–22.
Mann DJ, Lammerink JP, Coles GD. Predicting potato crisp darkening: two methods for analysis of glucose. N Z J Crop Hort Sci. 1991;19:199–201. https://doi.org/10.1080/01140671.1991.10421799.
Pacheco Á, Vargas M, Alvarado G, Rodríguez F, Crossa J, Burgueño J. GEA-R (Genotype × Environment Analysis with R for Windows) v4.1. https://hdl.handle.net/11529/10203 CIMMYT Research Data & Software Repository Network, v16. Centro Internacional de Mejoramiento de Maíz y Trigo, Texcoco, México. 2015. https://data.cimmyt.org/dataset.xhtml?persistentId=hdl:11529/10203. Accessed 12 Sept 2024.
Braun HJ, Rajaram S, van Ginkel M. CIMMYT’s approach to breeding for wide adaptation. Euphytica. 1996;92:175–83. https://doi.org/10.1007/BF00022843.
Ceccarelli S. Wide adaptation: how wide? Euphytica. 1989;40:197–205. https://doi.org/10.1007/BF00024512.
Ewing PM, Runck BC, Kono TYJ, Kantar MB. The home field advantage of modern plant breeding. PLoS ONE. 2019;26: e0227079. https://doi.org/10.1371/journal.pone.0227079.
Cotes JM, Ñustez CE, Martinez R, Estrada N. Analyzing genotype by environment interaction in potato using yield-stability index. Am J Potato Res. 2002;79:211–8. https://doi.org/10.1007/BF02871937.
Ortiz R, Selga C, Reslow F, Carlson-Nilsson U. Svensk potatisförädling: breeding the new table and crisp potatoes. Sveriges Utsädesförenings Tidskrift. 2020;1–2020:16–26.
Reslow F, Carlson-Nilsson U, Crossa J, Cuevas JD, Ortiz R. Public potato breeding progress for the Nordic Region of Europe: evidence from multisite testing of selected breeding clones and available released cultivars. Acta Agric Scand Sect B Soil Plant Sci. 2022;72:553–62.
Ortiz R, Crossa J, Reslow F, Pérez-Rodríguez P, Cuevas JD. Genome-based genotype × environment prediction enhances potato (Solanum tuberosum L.) improvement using pseudodiploid and polysomic tetraploid modeling. Front Plant Sci. 2022;13:785196. https://doi.org/10.3389/fpls.2022.785196.
Ortiz R, Reslow F, Carlson-Nilsson U. Svalöf: a high yielding potato with resistance to late blight in Nordic latitudes. Am J Potato Res. 2023;100:399–406. https://doi.org/10.1007/s12230-023-09926-2.
Schäfer-Pregl R, Ritter E, Concilio L, Hesselbach J, Lovatti L, Walkemeier B, Thelen H, Salamini F, Gebhardt C. Analysis of quantitative trait loci (QTLs) and quantitative trait alleles (QTAs) for potato tuber yield and starch content. Theor Appl Genet. 1998;97:834–45.
Flis B, Domański L, Zimnoch-Guzowska E, Polgar Z, Pousa FÁ, Pawlak A. Stability analysis of agronomic traits in potato cultivars of different origin. Amer J Potato Res. 2014;91:404–13. https://doi.org/10.1007/s12230-013-9364-6.
Flis B, Tatarowska B, Milczarek D, Plich J. Effect of location on starch content and tuber texture characteristics in potato breeding lines and cultivars. Acta Agric Scand Sect B-Soil Plant Sci. 2017;67:453–61. https://doi.org/10.1080/09064710.2017.1299792.
Lenartowicz T, Piepho HP, Przystalski M. Stability analysis of tuber yield and starch yield in mid-late and late maturing starch cultivars of potato (Solanum tuberosum). Potato Res. 2020;63:179–97. https://doi.org/10.1007/s11540-019-09434-z.
Acknowledgements
We thank Boel Sandström and other staff of SLU in Umeå; and Hushallningssallskapet staff at both Helgegården and Mosslunda for planting and managing the multisite trials.
Funding
Open access funding provided by Swedish University of Agricultural Sciences. Swedish University of Agricultural Sciences (SLU) and the Swedish Research Council Formas through the project (2019–2022) “Genomisk prediktion i kombination med högkapacitetsfenotypning för att öka potatisens knölskörd i ett föränderligt klimat”.
Author information
Authors and Affiliations
Contributions
Rodomiro Ortiz contributed to the study conception and design. Material preparation, data collection and analysis were performed by Fredrik Reslow, José Huicho, José Crossa and Rodomiro Ortiz. Ramesh Vetukuri contributed with data analysis interpretation and text editing. The first draft of the manuscript was written by Rodomiro Ortiz, and all the authors commented on previous versions of the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Potato breeding clones and cultivars were included in the field trials following the research exception of the Plant Variety Protection (PVP) that allow to use them for such a germplasm testing. The access to these was according to the International Treaty on Plant Genetic Resources for Food and Agriculture, which is harmony with the Convention of Biological Diversity (IT-PGRFA). The aims of the IT-PGRFA are to facilitate the conservation exchange and sustainable use of world’s plant genetic resources for food and agriculture, as well as the fair and equitable benefit sharing arising from their use.
Competing Interests
The authors have no relevant financial or nonfinancial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ortiz, R., Reslow, F., Huicho, J. et al. Adaptability, stability, and productivity of potato breeding clones and cultivars at high latitudes in Europe. Discov Life 54, 13 (2024). https://doi.org/10.1007/s11084-024-09658-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11084-024-09658-1