Abstract
Background
Despite developing a polypharmacy core outcome set (COS) in primary care, it is not clear how these outcomes should be measured.
Aim
To select outcome measurement instruments (OMIs) for a COS targeting appropriate polypharmacy in older patients in primary care.
Method
Following the Consensus-based Standards for the selection of health Measurement Instruments (COSMIN) guideline, OMIs were identified from a Cochrane review focusing on appropriate polypharmacy. The quality of OMIs was assessed using a published checklist. Subsequently, two rounds of Delphi questionnaires were conducted via the SoGoSurvey® platform, engaging stakeholders (researchers, clinicians and journal editors specialising in geriatric primary care) to achieve consensus on OMIs using a scale encompassing “agree”, “disagree”, or “unsure”. Consensus was achieved if 70% or more participants chose “agree” and 15% or fewer chose “disagree.”
Results
The quality of 20 OMIs identified from the Cochrane review was evaluated. Seven OMIs were selected based on meeting the COSMIN guideline’s minimum requirements. Out of 188 potential participants, 57 (30.3%) consented to participate. Rounds 1 and 2 of Delphi exercises were completed by 50 respondents, achieving agreement on three OMIs: ‘number of serious adverse drug reactions (ADRs)’ (98%), ‘number of deaths’ (76%), and ‘number of patients who fell’ (70%) for measuring ‘serious ADRs,’ ‘mortality,’ and ‘falls,’ respectively. No agreement was reached for ‘medication appropriateness,’ ‘medication side-effects,’ ‘quality of life,’ and ‘medication regimen complexity.’
Conclusion
OMIs were selected for a limited number of outcomes in the polypharmacy COS. Future research should identify suitable OMIs for the remaining four outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Impact statements
-
The research demonstrates the practical applicability of following the Consensus-based Standards for the selection of health Measurement Instruments (COSMIN) initiative’s guidance for selecting outcome measurement instruments (OMIs) for a core outcome set (COS) focusing on polypharmacy in older adults in primary care.
-
The three identified OMIs, ‘number of serious ADRs,’ ‘number of deaths,’ and ‘number of patients who fell’ provide methods to evaluate the impact of polypharmacy interventions on older people in primary care.
-
Using these OMIs, together with the polypharmacy COS in primary care settings, can help in measuring and comparing the effects of interventions that are tailored towards improving appropriate polypharmacy.
-
These OMIs could be directly applied in clinical practice to monitor and improve patient outcomes, thereby improving the safety and efficacy of polypharmacy management
Introduction
Polypharmacy is increasingly recognised as beneficial, especially for managing multimorbidity [1, 2]. However, balancing appropriate and inappropriate polypharmacy remains challenging [3]. Inconsistent reporting in polypharmacy studies complicates drawing conclusions about intervention effectiveness [4]. To address this, the Core Outcome Measures for Effectiveness Trials (COMET) Initiative promotes developing core outcome sets (COS) [5]. A COS comprises standardised, agreed-upon outcomes important to measure and report as a minimum in all trials within a particular health area [6]. A COS for evaluating appropriate polypharmacy interventions in older people in primary care has been developed [4], aiding in trial comparisons and identifying effective interventions.
Despite the development of a number of COSs, little research has focused on selecting Outcome Measurement Instruments (OMIs) for studies [5]. An OMI represents a tool or approach that dictates ‘how to’ measure a specific construct in terms of quality and quantity [7]. Multiple instruments for the same outcome can lead to variations and data synthesis challenges in systematic reviews [8, 9]. The COMET and Consensus-based Standards for the selection of health Measurement Instruments (COSMIN) initiatives aim to establish standards for selecting OMIs for COS outcomes by identifying existing OMIs from systematic reviews or other sources, evaluating their quality, selecting one OMI per outcome, and using stakeholder consensus [7]. Although a COS exists for trials targeting appropriate polypharmacy in older adults in primary care [4], no research has determined which OMIs to use with it.
Aim
This study aimed to reach consensus on OMIs for outcomes included in the polypharmacy COS for trials targeting appropriate polypharmacy in primary care for older patients.
Ethics approval
The study was approved by Ethics Committee of the Queen’s University Belfast (Ref: MHLS21_85; date of approval: July 28, 2021) in accordance with the Declaration of Helsinki. All participants provided written informed consent.
Method
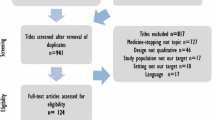
This study adhered to the COSMIN Initiative’s for selecting OMIs for a COS which encompasses four phases (Fig. 1).
Phase 1: Identifying outcomes for the OMIs
The target population was older patients (≥ 65 years) in primary care. We identified outcomes from the previously developed polypharmacy COS [4]. Among 16 outcomes in the COS, the seven highest ranking outcomes were prioritised based on published guidance [4]. Measuring more than seven outcomes could be impractical [10]. The seven highest ranking outcomes in the COS in primary care were serious adverse drug reactions (ADRs), medication appropriateness, falls, medication regimen complexity, quality of life (QoL), mortality, and medication side effects.
Phase 2: Finding existing OMIs
Systematic reviews are recommended by COSMIN guidance to identify existing OMIs [7]. This study extracted OMIs from randomised clinical trials (RCTs) included in our Cochrane review [11], which focused on interventions targeting appropriate polypharmacy in primary care.
Phase 3: Quality assessment of OMIs
The COSMIN checklist was used to evaluate the methodological quality of studies that developed OMIs relevant to the COS [7]. OMIs identified from the Cochrane review were categorised into ‘objective’ or ‘subjective’ based on their nature. Objective OMIs were evaluated for both content validity and feasibility aspects, whereas subjective OMIs were assessed for content validity, feasibility aspects and if applicable, other measurement properties. Two researchers independently evaluated the quality of OMIs, with discrepancies reviewed by a third researcher. The overall quality of OMIs was rated depending on the results of the assessments of their measurement properties and if applicable, their feasibility aspects [7].
Phase 4a: Selection of OMIs for outcomes included in the COS
Following the quality evaluation in Phase 3, one OMI was chosen for every outcome in the polypharmacy COS that fulfilled the best minimum requirements for selection and which was feasible to use [7, 8].
Phase 4b: Consensus procedure for OMIs
The Delphi approach was employed to achieve consensus on OMIs to use with the COS. The Delphi approach involves distributing a questionnaire to key stakeholders consisting of a series of items which are scored over a number of sequential rounds to reach consensus [12, 13].
Delphi questionnaire development
For the online Delphi exercise, the research team created an electronic questionnaire. This comprised a list of OMIs for the polypharmacy COS based on work undertaken in Phases 1–3, together with their definitions and illustrative examples (Supplementary Figure S1). Two versions of the questionnaire, one for researchers/academics and one for the public, were created and distributed using the SoGoSurvey® platform. Before distribution, the questionnaires were piloted with twelve members of the Primary Care Research Group at the School of Pharmacy, Queen’s University Belfast, and no modifications were required. Estimated completion time was 10 min.
Recruitment of Delphi panel members
Eligible participants included researchers or clinicians specialising in the care or prescribing for older adults in primary care, journal editors in geriatric medicine, or representatives of older patient interests (such as charities). There are no guidelines for the ideal Delphi panel size, with previous studies ranging from 12 to 311 participants [5]. Previous Delphi studies typically achieved a 30–40% recruitment rate [14, 15]. Expecting 45–60 participants (30–40%), we aimed to invite at least 150 potential participants to achieve this rate. International recruitment efforts were made to ensure the selection of OMIs had international relevance.
Based on the study team’s knowledge of primary healthcare, researchers’ publication records, and professional activities, a list of potential participants was compiled. The study team also contacted the Patient and Client Council, which represents the interests of people who use health and social care services in Northern Ireland, to recruit participants from this group. Invitation emails were sent to potential participants, who were asked to complete a consent form, which was included in the first questionnaire.
Delphi process
To reach consensus on OMIs for the polypharmacy COS, two rounds of the two versions of the Delphi surveys were distributed via email to participants.
Delphi Round 1 (21st June 2022–5th July 2022)
Participants provided demographic information at the start of the survey. Stakeholders indicated their agreement with using the selected OMI to measure the associated outcome by choosing from ‘agree’, ‘disagree’, or ‘unsure’. If ‘unsure’ or ‘disagree’ was chosen, a text box prompted the participant to provide a brief explanation. Participants were encouraged to suggest other OMIs for outcomes not presented in Round 1.
Following Round 1, participants’ response rate to the survey and the percentage of respondents who selected ‘agree’, ‘disagree’ and ‘unsure’ for OMIs were calculated. Any additional OMIs reported by participants were checked to ensure there was no overlap with OMIs previously presented in Round 1.
Delphi Round 2 (11th July 2022–14th August 2022)
Round 2 proceeded in the same manner as Round 1. Every participant received their Round 1 responses for each OMI along with the Round 2 email. Additionally, the Round 2 questionnaire included a summary of all Round 1 respondents' collective responses for each OMI to enable comparison. All OMIs from Round 1 were included in Round 2. The response rate and distribution of scores were determined similarly as in Round 1.
Data analysis
The Delphi questionnaire employed three scoring categories (agree, disagree, and unsure), a method previously used by Delphi questionnaires in the selection of OMIs for COSs [14,15,16]. Consensus for inclusion of an OMI to measure the polypharmacy COS was achieved if at least 70% of the respondents selected ‘agree’ and 15% or less selected ‘disagree’. Thereafter, OMIs were classified as ‘consensus in’ or ‘no consensus’. If an OMI was deemed ‘consensus in,’ it was included in the list of OMIs for the polypharmacy COS.
Results
In Phase 1, the seven highest ranking outcomes in the polypharmacy COS were identified: medication appropriateness, serious ADRs, QoL, medication regimen complexity, falls, medication side-effects and mortality [4].
In Phase 2, OMIs were extracted from trials conducted in primary care that were included in the Cochrane review [11]. Thirty-six studies were involved in the evaluation of OMI quality. The research team compiled a list of these OMIs, which included 20 OMIs to measure outcomes pertaining to the outcomes in the polypharmacy COS (Table 1).
In Phase 3, the COSMIN checklist was used to assess the methodological quality of studies that developed the OMIs as the first step in the quality evaluation of the instruments [7]. Overall quality was considered “doubtful” for one study, while 22 studies were considered “inadequate” in terms of overall quality (Supplementary Table S1). Subsequently, OMIs were then evaluated and categorised as either “objective” or “subjective” OMIs. Following the COSMIN guidance for evaluating the quality of OMIs, the quality of all objective OMIs was deemed to be high (Supplementary Table S2). In contrast, all subjective OMIs were classified as low to very low in terms of quality (Supplementary Table S3).
In Phase 4, the research team chose one OMI for every outcome based on the results of the quality evaluation (Table 1). This selection was in accordance with which instrument best met the COSMIN guideline minimum requirements to include an OMI to measure an outcome: good content validity, being feasible to use, and having the highest rating for other measurement properties [7]. The seven selected OMIs were included in the Delphi questionnaire.
Demographics of participants
Initially, 188 emails inviting potential participants to take part were distributed. A total of 57 potential participants (30.3% recruitment rate) consented to participate in the study. Fifty participants completed the questionnaires [n = 28 (56%) males and n = 22 (44%) females]. The median age was 51 years. The majority of respondents (n = 27; 54%) lived in Europe (outside the UK), followed by the UK (n = 12; 24%), North America (n = 6; 12%), Oceania (n = 4; 8%) and Asia (n = 1; 2%). The majority of respondents were classified as researchers. None of the questionnaires was completed by members of the public (Table 2).
Round 1 questionnaire
Fifty of the 57 participants who agreed to participate in the study completed Round 1 (response rate: 87.7%). Following completion of this round, 90% (n = 45) of the participants were in agreement that the OMI ‘number of serious ADRs’ should be used to measure ‘serious ADRs’ and consensus was reached for this OMI. No other OMIs in Round 1 achieved consensus (Table 3). Participant comments provided in Round 1 responses were checked. No additional OMIs were added to Round 2 as those suggested by respondents in Round 1 did not differ from those already included.
Round 2 questionnaire
The Round 2 questionnaire was distributed to only those who had completed Round 1 (n = 50). It was completed by all respondents who took part in Round 1 (response rate: 100%). Following Round 2, the percentage of agreement had increased from Round 1 for the OMI ‘number of serious ADRs’ (98%) to measure ‘serious ADRs’. Two additional OMIs—‘number of deaths’ (76%) and ‘number of patients who fell’ (70%) to measure ‘mortality’ and ‘falls’ respectively, also reached consensus (Table 4). There was no agreement on the remaining OMIs to measure “medication appropriateness,” “medication side-effects,” “QoL,” and “medication regimen complexity”. Therefore, these outcomes were stated as ‘OMI not available’.
Discussion
Statement of key findings
The COSMIN Initiative’s guidelines for selecting OMIs for a COS were utilised to choose OMIs for the polypharmacy COS [7]. Using the COSMIN checklist, 36 studies were evaluated for OMI quality, with most being judged as ‘inadequate’. According to the COSMIN guideline, the lowest rating of any standard in the COSMIN checklist is utilised to determine the overall study’s quality [7]. Subsequently, 20 OMIs related to the polypharmacy COS were identified and evaluated for quality. All OMIs for measuring ‘serious ADRs’, ‘falls’, ‘mortality’, and ‘medication side-effects’ were categorised as ‘objective’. For ‘medication regimen complexity’, three OMIs were identified, with two (‘total number of prescriptions’ and ‘number of single doses/day’) classified as ‘objective’ and one [‘Medication Regimen Complexity Index (MRCI)’] as ‘subjective’ [7]. All OMIs used to measure ‘QoL’ and ‘medication appropriateness’ were considered ‘subjective’. Objective OMIs were assessed for content validity and feasibility aspects, while subjective OMIs were assessed for content validity, feasibility aspects and other measurement properties (where applicable). Overall, all objective OMIs were assessed as being high in quality, whereas subjective OMIs was deemed low or very low. This may be attributed to subjective OMIs being dependent on assessors’ judgment. In contrast, objective OMIs were independent from assessors’ judgment and calculated as quantitative data.
Strengths and weaknesses
This research has several strengths. It is the first study to select OMIs for outcomes included in the polypharmacy COS by adhering to COMET and COSMIN guidelines. Every OMI was extracted from RCTs included in a Cochrane review [11]. The consensus process involved a Delphi panel from various countries and professional backgrounds. The response rates were high for both Delphi rounds.
There are a number of limitations. Despite achieving the expected number of respondents (n = 50), a low recruitment rate of 30.3% was recorded. COVID-19 restrictions prevented a recommended plenary discussion and voting during a face-to-face meeting for consensus taking place, prompting the use of an online Delphi procedure, which has been successful in previous studies [4, 15]. Efforts to involve public representatives faced challenges due to the perceived complexity of the study, potentially hindering recruitment from advocacy groups for older people.
Interpretation of findings
Despite their common use, OMIs for measuring ‘medication appropriateness’ and ‘QoL’ outcomes did not meet COSMIN criteria for good measurement properties. However, the MAI and EQ-5D were the most highly rated in terms of quality evaluation amongst all OMIs for measuring ‘medication appropriateness’ and ‘QoL’, respectively. Hence, they were included in the Delphi survey as the associated outcomes ‘medication appropriateness’ and ‘QoL’ had been highly ranked in the polypharmacy COS [4]. Three OMIs measured medication regimen complexity, with the MRCI deemed subjective and excluded from the polypharmacy COS as it did not meet the COSMIN criteria for good measurement properties. The other two OMIs, ‘total number of prescriptions’ and ‘number of single doses/day’, were classified as objective and met COSMIN criteria. To select one suitable OMI for measuring medication regimen complexity from these two OMIs, ‘total number of prescriptions’ was chosen based on evaluation of feasibility.
The seven OMIs that were supported by the best quality assessments were selected for the Delphi exercise. Fifty participants completed the Delphi surveys via two rounds. In the Delphi questionnaire, the recruitment rate was low (30.3%), however, it was consistent with similar Delphi studies (30–40%) [14, 15]. This study recorded high response rates (Round 1: 87.7%; Round 2: 100%), exceeding those of previous studies (Round 1: 44%; Round 2: 41%) [14], (Round 1: 86.8%; Round 2: 91.5%) [15], and ranging from 40 to 60% in a four-round study [17]. Public participants declined participation, which has also been observed in previous studies as well [14, 15].
Following the Delphi exercise, consensus was reached on three OMIs to measure three outcomes included in the polypharmacy COS, namely ‘number of serious ADRs’, ‘number of deaths’ and ‘number of patients who fell’. All these selected OMIs were objective, i.e. independent from assessor judgments, quantitative and easy to understand [18, 19]. No consensus was reached for other OMIs.
Despite no OMIs being selected for ‘medication appropriateness’, ‘QoL’, ‘medication regimen complexity’ and ‘medication side-effects’ in this study, previous studies used OMIs that are potentially applicable to the polypharmacy COS. For example, the Assessment of Underutilization of Medication (AOU) tool [20], Assessing Care of Vulnerable Elderly (ACOVE) indicators [21], the Meds75 + database [22], the Rationalization of Home Medication by an Adjusted STOPP in Older Patients (RASP) list [23] and Fit fOR The Aged (FORTA) criteria [24] used in literature to measure ‘medication appropriateness’. A study reported that a combination of implicit and explicit tools can measure medication appropriateness [25]. However, they were excluded from this study as these tools were not utilised in primary care-based trials included in the Cochrane review [11]. Similarly, a range of tools used in studies to measure QoL, for instance, medication-related QoL (MRQoL) [26, 27], the QUALIDEM [28] and ICECAP-O [29]. Again, these latter instruments were not included in this study as they were not used in primary care-based studies. For ‘medication regimen complexity’, five tools were reported in a systematic review as reliable for measuring medication regimen complexity [30]. They were: the MRCI [31], the Medication Complexity Index (MCI) [32], the Epilepsy Medication Treatment Complexity Index (EMTCI) [33], Antiretroviral Regimen Complexity (ARC) [34] and the Antiretroviral Medication Complexity Index (AMCI) [35]. The MRCI, however, was excluded in this present research arising from methodological and quality issues; for example, the study that developed the MRCI had a sample size lower than 30 [31], which is interpreted by the COSMIN checklist as inadequate [7]. The other four tools could measure medication regimen complexity, although some are disease-specific instruments, for instance, the AMCI and ARC are used for HIV, and the EMTCI scale is used for epilepsy. As noted above, ‘number of single doses/day’ tool met the COSMIN criteria for good measurement properties, thus, it could be used to measure medication regimen complexity. Regarding ‘medication side-effects’, questionnaires such as the Patient Reported Adverse Drug Event Questionnaire [36] and the Maudsley Side-Effects (MSE) tool [37] were used in previous studies to measure medication side-effects in older people. However, the reliability and validity of these tools require testing to identify the suitable one that can measure this outcome in older people in primary care.
Further research
Consensus was reached for the OMIs ‘number of serious ADRs’, ‘number of deaths’, and ‘number of patients who fell’ for measuring ‘serious ADRs’, ‘mortality’, and ‘falls’, respectively. These three selected OMIs can be used in future studies to measure and compare the effects of interventions targeting the appropriateness of polypharmacy in older patients.
As no consensus was reached for OMIs to measure ‘medication appropriateness’, ‘medication side effects’, ‘QoL’ and ‘medication regimen complexity’, further research may develop new OMIs for these four remaining outcomes in the polypharmacy COS. Future studies may also examine and use such OMIs that used in other health settings (hospital, care home) for measuring these four remaining outcomes.
Conclusion
The research has culminated in agreement on OMIs for some outcomes in the polypharmacy COS recommended for use in trials targeting appropriate polypharmacy. Following the COSMIN guidelines, ‘number of serious ADRs’, ‘number of deaths’ and ‘number of patients who fell’ OMIs were chosen for measuring ‘serious ADRs’, ‘mortality’ and ‘falls’, respectively.
References
Masnoon N, Shakib S, Kalisch-Ellett L, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230. https://doi.org/10.1186/s12877-017-0621-2.
Hughes CM, Cadogan CA, Patton D, et al. Pharmaceutical strategies towards optimising polypharmacy in older people. Int J Pharm. 2016;512:360–5. https://doi.org/10.1016/j.ijpharm.2016.02.035.
Cadogan CA, Ryan C, Hughes CM. Appropriate polypharmacy and medicine safety: when many is not too many. Drug Saf. 2016;39:109–16. https://doi.org/10.1007/s40264-015-0378-5.
Rankin A, Cadogan CA, Ryan C, et al. Core outcome set for trials aimed at improving the appropriateness of polypharmacy in older people in primary care. J Am Geriatr Soc. 2018;66:1206–12. https://doi.org/10.1111/jgs.15245.
Williamson P, Altman D, Bagley H, et al. The COMET handbook: version 1.0. Trials. 2017;18:1–50. https://doi.org/10.1186/s13063-017-1978-4.
Williamson P, Altman D, Blazeby J, et al. Developing core outcome sets for clinical trials: issues to consider. Trials. 2012;13:132. https://doi.org/10.1186/1745-6215-13-132.
Prinsen CA, Vohra S, Rose MR et al. Guideline for selecting outcome measurement instruments for outcomes included in a Core Outcome Set. 2016. https://cosmin.nl/wp-content/uploads/COSMIN-guideline-selecting-outcome-measurement-COS.pdf. Accessed 31 Mar 2022.
Prinsen CA, Vohra S, Rose MR, et al. How to select outcome measurement instruments for outcomes included in a “Core Outcome Set”–a practical guideline. Trials. 2016;17:449. https://doi.org/10.1186/s13063-016-1555-2.
Gorst SL, Prinsen C, Salcher-Konrad M, et al. Methods used in the selection of instruments for outcomes included in core outcome sets have improved since the publication of the COSMIN/COMET guideline. J Clin Epidemiol. 2020;125:64–75. https://doi.org/10.1016/j.jclinepi.2020.05.021.
Marcum Z, Steinman M. Developing a core outcome set for trials to improve medication use: guidelines or guidance? J Am Geriatr Soc. 2018;66:1058–9. https://doi.org/10.1111/jgs.15269.
Cole J, Gonçalves-Bradley DC, Alqahtani M, et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev. 2023;10:CD008165. https://doi.org/10.1002/14651858.CD008165.pub5.
Okoli C, Pawlowski S. The Delphi method as a research tool: an example, design considerations and applications. Inf Manag. 2004;2:15–29. https://doi.org/10.1016/j.im.2003.11.002.
Villiers M, Villiers P, Kent A. The Delphi technique in health sciences education research. Med Teach. 2005;27:639–43. https://doi.org/10.1080/13611260500069947.
Chiarotto A, Boers M, Deyo R, et al. Core outcome measurement instruments for clinical trials in nonspecific low back pain. Pain. 2018;159:481–95. https://doi.org/10.1097/j.pain.0000000000001117.
Nguyen H, Bradley D, Tunney M, et al. Development of a core outcome set for clinical trials aimed at improving antimicrobial stewardship in care homes. Antimicrob Resist Infect Control. 2021;10:52. https://doi.org/10.1186/s13756-021-00925-8.
Spuls PI, Gerbens LA, Simpson E, et al. Patient-Oriented Eczema Measure (POEM), a core instrument to measure symptoms in clinical trials: a Harmonising Outcome Measures for Eczema (HOME) statement. Br J Dermatol. 2017;176:979–84. https://doi.org/10.1111/bjd.15179.
Canning ML, Barras M, McDougall R, et al. Defining quality indicators, pharmaceutical care bundles and outcomes of clinical pharmacy service delivery using a Delphi consensus approach. Int J Clin Pharm. 2024;46:451–62. https://doi.org/10.1007/s11096-023-01681-y.
Velentgas P, Dreyer NA, Wu AW. Outcome definition and measurement. In: Velentgas P, Dreyer NA, Nourjah P, Smith SR, Torchia MM, editors. Developing a protocol for observational comparative effectiveness research: a user’s guide. Rockville: AHRQ; 2013. p. 71–92.
Moustgaard H, Bello S, Miller FG, et al. Subjective and objective outcomes in randomized clinical trials: definitions differed in methods publications and were often absent from trial reports. J Clin Epidemiol. 2014;67:1327–34. https://doi.org/10.1016/j.jclinepi.2014.06.020.
Jeffery S, Ruby CM, Hanlon JT, et al. The impact of an interdisciplinary team on suboptimal prescribing in a long term care facility. Consult Pharm. 1999;14:1386–9.
Wenger NS, Shekelle PG. Assessing care of vulnerable elders: ACOVE project overview. Ann Intern Med. 2001;135:642–6. https://doi.org/10.7326/0003-4819-135-8_part_2-200110161-00002.
Auvinen KJ, Räisänen J, Voutilainen A, et al. Interprofessional medication assessment has effects on the quality of medication among home care patients: randomized controlled intervention study. J Am Med Dir Assoc. 2021;22:74–81. https://doi.org/10.1016/j.jamda.2020.07.007.
Van der Linden L, Decoutere L, Walgraeve K, et al. Combined use of the Rationalization of home medication by an Adjusted STOPP in older Patients (RASP) list and a pharmacist-led medication review in very old inpatients: impact on quality of prescribing and clinical outcome. Drugs Aging. 2017;34:123–33. https://doi.org/10.1007/s40266-016-0424-8.
Kuhn-Thiel AM, Weiss C, Wehling M. Consensus validation of the FORTA (Fit fOR the aged) list: a clinical tool for increasing the appropriateness of pharmacotherapy in the elderly. Drugs Aging. 2014;31:131–40. https://doi.org/10.1007/s40266-013-0146-0.
Basger BJ, Moles RJ, Chen TF. Impact of an enhanced pharmacy discharge service on prescribing appropriateness criteria: a randomised controlled trial. Int J Clin Pharm. 2015;37:1194–205. https://doi.org/10.1007/s11096-015-0186-0.
Tegegn HG, Erku DA, Sebsibe G, et al. Medication-related quality of life among Ethiopian elderly patients with polypharmacy: a cross-sectional study in an Ethiopia university hospital. PLoS ONE. 2019;14:e0214191. https://doi.org/10.1371/journal.pone.0214191.
Jennings EL, O’Mahony D, Gallagher PF. Medication-related quality of life (MRQoL) in ambulatory older adults with multi-morbidity and polypharmacy. Eur Geriatr Med. 2022;13:579–83. https://doi.org/10.1007/s41999-021-00573-6.
Ettema TP, Droes RM, de Lange J, et al. QUALIDEM: development and evaluation of a dementia-specific quality of life instrument—scalability, reliability and internal structure. Int J Geriatr Psychiatry. 2007;22:549–56. https://doi.org/10.1002/gps.1713.
Coast J, Flynn TN, Natarajan L, et al. Valuing the ICECAP capability index for older people. Soc Sci Med. 2008;67:874–82. https://doi.org/10.1016/j.socscimed.2008.05.015.
Paquin AM, Zimmerman KM, Kostas TR, et al. Complexity perplexity: a systematic review to describe the measurement of medication regimen complexity. Expert Opin Drug Saf. 2013;12:829–40. https://doi.org/10.1517/14740338.2013.823944.
George J, Phun YT, Bailey MJ, et al. Development and validation of the medication regimen complexity index. Ann Pharmacother. 2004;38:1369–76. https://doi.org/10.1345/aph.1D479.
Kelley SO. Measurement of the complexity of medication regimens of the elderly. University of Missouri-Columbia (1988)
DiIorio C, Yeager K, Shafer PO, et al. The epilepsy medication and treatment complexity index: reliability and validity testing. J Neurosci Nurs. 2003;35:155–62. https://doi.org/10.1097/01376517-200306000-00005.
Martin S, Wolters PL, Calabrese SK, et al. The antiretroviral regimen complexity index: a novel method of quantifying regimen complexity. J Acquir Immune Defic Syndr. 2007;45:535–44. https://doi.org/10.1097/QAI.0b013e31811ed1f1.
DiIorio C, McDonnell M, McCarty F, et al. Initial testing of the antiretroviral medication complexity index. J Assoc Nurses AIDS Care. 2006;17:26–36. https://doi.org/10.1016/j.jana.2005.11.003.
De Vries ST, Mol PG, de Zeeuw D, et al. Development and initial validation of a patient-reported adverse drug event questionnaire. Drug Saf. 2013;36:765–77. https://doi.org/10.1007/s40264-013-0036-8.
Wykes T, Evans J, Paton C, et al. What side effects are problematic for patients prescribed antipsychotic medication? The Maudsley Side Effects (MSE) measure for antipsychotic medication. Psychol Med. 2017;47:2369–78. https://doi.org/10.1017/S0033291717000903.
Acknowledgements
We wish to thank all the participants who contributed to this research.
Funding
This work is part of a PhD project and is being undertaken at the School of Pharmacy, Queen’s University Belfast. The PhD project is being funded by the Saudi Cultural Bureau, London.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alqahtani, M.N., Barry, H.E. & Hughes, C.M. Selection of outcome measurement instruments for a core outcome set for trials aimed at improving appropriate polypharmacy in older people in primary care: a Delphi consensus study. Int J Clin Pharm (2024). https://doi.org/10.1007/s11096-024-01780-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11096-024-01780-4