Abstract
We report the generation and characterization of the K5: CAT bigenic mouse in which the constitutively activated form of β-catenin (ΔN89 β-catenin) is conditionally expressed in cytokeratin-5 (K5) positive epidermal keratinocytes. Following short-term doxycycline intake during the telogen resting phase, the adult K5: CAT bigenic develops enlarged pilosebaceous units that expand deep into the dermis, an expansion usually observed during the anagen growth phase. Prolonged doxycycline treatment results in significant thickening and folding of the K5: CAT epidermis. During this persistent induction period, there is clear evidence of increased keratinocyte proliferation, particularly in the epidermal basal cell layer and the outer root sheath of the hair follicle. This unscheduled increase in cellular proliferation likely explains the decrease in hair density observed in the K5: CAT mouse following persistent doxycycline intake. Numerous hyperplastic endometrioid cysts, which display cornification toward their lumens, are also observed during this treatment period. Remarkably, de-induction of ΔN89 β-catenin expression through doxycycline withdrawal results in a marked reversal of the skin phenotype, suggesting that these morphological changes are dependent on continued signaling by β-catenin and/or its downstream molecular mediators. Joining a small group of mouse models for conditional β-catenin signaling, our K5: CAT mouse model will be particularly useful in identifying those molecular mediators of β-catenin that are responsible for initiating and maintaining these phenotypic responses in the K5: CAT skin. Such studies are predicted to shed more light on β-catenin signaling in epidermal epithelial morphogenesis, hair follicle cycling, and hair growth pathologies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Through α-catenin binding, β-catenin connects E-cadherin to the actin cytoskeleton at adherens junctions to effect intercellular adhesion, cell motility, and cell surface signaling (Huelsken et al. 2001; Kemler 1993; Takeichi 1991). Non-junctional β-catenin is rapidly targeted for ubiquitin-mediated destruction by glycogen synthase kinase-3β (GSK-3β) as part of a destruction complex containing adenomatous polyposis coli (APC) and axin (Aberle et al. 1997; Behrens et al. 1998; Liu et al. 2002; Marikawa and Elinson 1998; Salomon et al. 1997). The GSK-3β kinase phosphorylates conserved serine and threonine residues within the N-terminus of β-catenin, which post-translationally modifies β-catenin for proteolysis by the ubiquitin–proteasome system (Aberle et al. 1997; Liu et al. 2002).
Binding their cognate frizzled and low-density lipoprotein-related protein receptor complexes on the cell surface, Wnts inhibit GSK-3β activity leading to an increased cytoplasmic pool of stabilized β-catenin, which translocates to the nucleus (Clevers 2006; Clevers and van de Wetering 1997; Nelson and Nusse 2004; Willert and Jones 2006). Nuclear β-catenin interacts with members of the T-cell factor/lymphoid enhancer factor (Tcf/Lef) family of transcription factors that together transactivate a broad spectrum of target genes required for normal embryonic and postnatal tissue development and function (Clevers 2006; Clevers and van de Wetering 1997; Nelson and Nusse 2004; Willert and Jones 2006) (http://www.stanford.edu/~rnusse/pathways/targets.html). Inactivating mutations in APC or axin as well as activating mutations that remove N-terminal phosphorylation sites in β-catenin lead to abnormal accumulation of stabilized β-catenin, which constitutively transactivates Wnt-target genes in a Wnt-independent manner (Clevers 2006; Clevers and van de Wetering 1997; Nelson and Nusse 2004; Willert and Jones 2006). Accordingly, aberrant β-catenin signaling results in myriad embryonic and postnatal developmental abnormalities, including numerous cancer types (Polakis 1999, 2000).
Previous mouse knockout and transgenic studies demonstrated a critical role for canonical Wnt signaling in the development and homeostasis of the epidermis and its appendages (Baker et al. 2010; Gat et al. 1998; Grigoryan et al. 2008; Huelsken et al. 2001; Lo Celso et al. 2004; Suzuki et al. 2009; Van Mater et al. 2003; Zhang et al. 2008). In particular, mouse transgenics that conditionally express gain-of-function β-catenin mutants, which lack N-terminal phosphorylation sites responsible for protein turnover, have significantly expanded our understanding of β-catenin signaling in the biology and pathobiology of epidermal keratinocytes as well as specific cell-types (including progenitor/stem cells) that comprise the pilosebaceous unit (Grigoryan et al. 2008). An epidermal invagination, the pilosebaceous unit comprises the hair shaft or fiber within the hair follicle, the arrector pili muscle, and the sebaceous gland (Panteleyev et al. 2001).
The majority of mouse transgenics to date, which are designed for the temporal and cell-type specific expression of constitutively active β-catenin, harbor a transgene consisting of a cytokeratin promoter to target expression to epidermal keratinocytes. The cytokeratin promoter is followed by a cDNA encoding a version of a constitutively active β-catenin fused in-frame at its C-terminus with the mutated ligand binding domain (LBD) of the murine estrogen receptor-α (ER-α or ESR1) (Baker et al. 2010; Lo Celso et al. 2004; Van Mater et al. 2003), which expresses an inactive β-catenin-ER-α fusion protein (Baker et al. 2010; Lo Celso et al. 2004; Van Mater et al. 2003). In these transgenics, the β-catenin-ER fusion protein can be activated in a defined area of depilated skin by the topical application of 4-hydroxytamoxifen (4-OHT) in acetone; 4-OHT is a potent agonist ligand for the mutated version of the LBD of ER-α (Littlewood et al. 1995).
Here, we provide a brief technical report that describes the generation (and first line characterization) of a novel bigenic mouse (K5: CAT) in which temporal expression of a constitutively active β-catenin (ΔN89 β-catenin) in cytokeratin-5 (K5) positive cells is achieved through use of the TET-ON system (Lewandoski 2001). We believe this new mouse model will complement existing conditional mouse models that target constitutively active β-catenin within epidermal keratinocytes in a temporally controlled manner. Our mouse model will be particularly useful for those investigators who are interested in studying the effects of timed induction and reversal of β-catenin signaling in epidermal keratinocytes in the same animal.
Results and discussion
Generation of the K5: CAT bigenic mouse
Crossing our TetO-ΔN89β-Catenin transgenic (Mukherjee et al. 2011) with the K5-rtTA transgenic (Hai et al. 2019; Raimondi et al. 2006) generated the K5rtTA: β-CATΔN89 bigenic (termed K5: CAT here (Fig. 1a)). Briefly, the TetO-ΔN89β-Catenin transgenic carries a responder transgene containing the cytomegalovirus minimal promoter with a tandem repeat of seven Tet operator (TetO) sequences followed by a cDNA encoding the Xenopus-derived ΔN89β-catenin protein, which is myc epitope-tagged in frame at the N-terminus (Mukherjee et al. 2011). The Xenopus and human β-catenin protein sequences share 98% homology (Imbert et al. 2001; McCrea et al. 1991); lacking the first N-terminal 89 amino acids, the ΔN89β-catenin protein is constitutively active (Imbert et al. 2001; Mukherjee et al. 2011). A strong Simian virus 40-derived polyadenylation cassette terminates the expression from the ΔN89β-catenin cDNA in the responder transgene. The cytokeratin 5-reverse tetracycline transactivator (K5-rtTA) effector transgenic harbors the bovine keratin 5 (KRT5) promoter (Ramirez et al. 1994), which drives expression of the cDNA encoding the rtTA containing its own nuclear localization signal (Raimondi et al. 2006). Therefore, the K5-rtTA transgenic conditionally targets responder transgene expression to K5 positive cells following doxycycline administration (Fig. 1a) (Hai et al. 2019; Raimondi et al. 2006; Ramirez et al. 1994). Both the effector and responder transgenics are in the FVB/N genetic background.
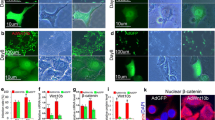
Doxycycline induction of ΔN89 β-catenin expression in the K5: CAT epidermis. a Left schematic summarizes the doxycycline induction of ΔN89 β-catenin protein in K5: CAT keratinocytes. The right schematic shows the experimental design in which K5: CAT adult (8 weeks old) mice were either treated with doxycycline (+ Dox) or left untreated (-Dox) for 72 h before molecular and histological analyses. For all experiments, mice (+ Dox or –Dox) were siblings or age-matched. b Compared with age-matched untreated K5: CAT mice (-Dox), qRT-PCR analysis shows a significant induction for β-catenin transcripts in the skin of K5: CAT mice when treated with doxycycline (+ Dox) for 3 days (n = 3 per treatment group). c In addition to detection of endogenous β-catenin, Western analysis shows a significant induction of transgene-derived ΔN89 β-catenin protein in the K5: CAT skin in response to 3 days of doxycycline administration (+ Dox), which is confirmed by the induction of transgene-derived myc epitope-tagged protein (+ Dox); β-actin serves as a loading control. Note: skin protein extracts from four mice per treatment group (-Dox and + Dox) were pooled per lane of the polyacrylamide gel. d A parasagittal skin section from an untreated K5: CAT mouse (-Dox (representative of N = 11 mice)); white arrowhead indicates the follicle bulb. Although the tissue section was immunohistochemically stained for the myc epitope-tag, immunopositivity was not observed, as expected. e A parasagittal section of skin tissue derived from a K5: CAT mouse treated with doxycycline (+ Dox) for 3 days (representative of N = 8 mice). Note the significant immunopositivity for the myc epitope-tag in the outer keratinocytes of the follicle bulb while the inner cells of the matrix (M) are negative for the myc epitope-tag. Along the outer root sheath (ORS (black arrowhead)), there is sparse immunopositivity for the myc epitope-tag in the basal keratinocyte layer (black arrowhead). f Higher power magnification of the bulb region in the 3-day doxycycline-treated K5: CAT skin shows the location of K5 positive cells in the outer layer of the follicle bulb (B); the inner matrix (M) cells are negative for K5 positivity. g The doxycycline-treated K5: CAT follicle bulb immunostained for the myc epitope-tag shows immunopositivity for the epitope-tag in the outer keratinocytes of the follicle bulb, with no immunopositivity detected in the matrix (M) cells; DP denotes dermal papilla. Scale bar in panels (d) and (f) apply to panels (e) and (g) respectively. Note: the significant difference in follicular bulb size between the + Dox and –Dox treated K5: CAT mouse groups is shown in Fig. S2
Doxycycline-induction of ΔN89 β-catenin expression in the K5: CAT bigenic mouse
By seven weeks-of-age, two sequential waves of hair growth (anagen) have occurred in mice before entering the telogen resting phase (Muller-Rover et al. 2001). Therefore, K5: CAT mice were treated with doxycycline (+ Dox) at 8 weeks-of-age to avoid potential complications of active growth of the hair follicle that normally occurs during the anagen period (Fig. 1a). Control mice for these experiments were age-matched K5: CAT mice that were maintained on chow and water without doxycycline (-Dox). Similar to wild type mice, it’s important to note that the hair follicle of K5: CAT mice undergoes normal anagen growth at 4 weeks-of-age in the absence of doxycycline (Fig. S1). Quantitative real-time PCR (qRT-PCR) analysis showed that transcript levels of transgene-derived ΔN89 β-catenin were significantly induced by three days of doxycycline treatment in the skin of K5: CAT mice when compared with the skin of K5: CAT mice that did not receive doxycycline (Fig. 1b). Western immunoblotting confirmed the qRT-PCR results by showing the induction of transgene-derived ΔN89 β-catenin (75 kDa) with its in-frame myc epitope-tag in the dorsal skin of K5: CAT mice, which were treated with doxycycline for three days (Fig. 1c). Western immunoblotting also showed an increase in endogenous β-catenin (Fig. 1c), which we speculate may result from ΔN89 β-catenin-mediated reprogramming of cutaneous keratinocytes that leads, in part, to diminished turnover of endogenous β-catenin protein (92 kDa). As expected, the myc epitope-tagged ΔN89 β-catenin was not detected by immunoblot when using skin protein isolates derived from age-matched K5: CAT mice on regular food and water (Fig. 1c). Immunohistochemistry confirmed that myc epitope-tagged ΔN89 β-catenin was not expressed in K5: CAT dorsal skin in the absence of doxycycline (Fig. 1d). Following the short-term doxycycline treatment regimen (Fig. 1a), however, myc epitope-tagged ΔN89 β-catenin was detected in keratinocytes of the outer epithelial cell layer of the follicle bulb while the inner undifferentiated matrix cells score negative for myc epitope expression (Fig. 1e). During this short-term induction period, the myc epitope-tagged ΔN89 β-catenin immunopositivity is detected in only a few keratinocytes of the outer root sheath of the hair follicle (Fig. 1e). Interestingly, the hair follicle bulb is discernibly larger in the K5: CAT skin (Fig. S2), which has extended deeper into the dermis after only 72 h of doxycycline exposure (compare Fig. 1e with Fig. 1d). Comparison of K5 and myc epitope-tagged ΔN89 β-catenin expression patterns in the K5: CAT skin treated with doxycycline show that similar outer keratinocyte cells of the bulb are immunopositive for both markers (compare Fig. 1f with Fig. 1g); K5 expression has been previously reported to be expressed in bulb keratinocytes (Baker et al. 2010). We observed similar results for both male and female mice (male data not shown); therefore, only data on doxycycline-treated K5: CAT female mice are described for the studies hereon.
Persistent myc epitope-tagged ΔN89 β-catenin expression causes significant histological changes in the K5: CAT epidermis
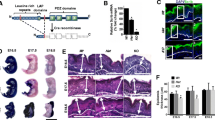
With prolonged doxycycline administration, prominent thickening of the epidermis along with the appearance of large surface folds are evident in the K5: CAT mouse skin (Fig. 2a-f). Elasticity of the K5: CAT skin is significantly diminished and accompanied by the presence of greasy hair (Fig. S3), which presumably is due to an increase in epicutaneous sebum levels derived from enlarged pilosebaceous units. Hair density is also reduced in the abdominal and dorsal truncal skin of the 21-day doxycycline-treated K5: CAT mouse (Fig. 2a-f). While hematoxylin and eosin (H &E) staining of a parasagittal skin section from the K5: CAT mouse without doxycycline treatment shows the expected spacing and depth of hair follicles within the epidermis in telogen (Fig. 1b), the epidermal histological architecture is markedly perturbed in the K5: CAT mouse following 21 days of doxycycline treatment (Fig. 2c (bracketed structures)). Skin sections stained with H&E show the striking appearance of abnormally enlarged pilosebaceous units that have now significantly extended deep into the dermal region (compare Fig. 2b with Fig. 2c). Furthermore, prolonged doxycycline activation results in a marked increase in the number of perifollicular keratinocytes (Fig. 2c), which generates a multi-cell layered outer root sheath that contributes to the abnormally enlarged size of the K5: CAT pilosebaceous unit. The skin of the prolonged doxycycline treated K5: CAT mouse also contained numerous epidermoid cyst-like structures (Fig. 2d), consisting of multiple concentric layers of squamous epithelial cells, which cornified toward the lumen. Despite evidence of a large number of these abnormal pilosebaceous units (Fig. 2f), many of these histological features did not produce hair fibers, explaining the lower hair density observed in the K5: CAT mouse when treated with doxycycline for 21 days.
Hyperkeratosis induced by doxycycline in the K5: CAT epidermis. a Top left panel (i) shows the side view of the K5: CAT mouse at day 0 of doxycycline treatment. The top second and third panels (ii and iii respectively) show ventral views of the mouse whereas the fourth top panel (iv) represents a higher power magnification image of the third panel (iii). At this treatment time, the K5: CAT mouse is a phenocopy of wild type (data not shown). The bottom panels (v-viii) are the same view perspectives as the top panels (i-iv) of the same mouse which has been treated with doxycycline for 21 days. Note the clear thickening of the epidermis, which is accompanied by numerous thick ridges and a reduction in hair density. b Hematoxylin & eosin (H&E) stained parasagittal section of K5: CAT back skin on day 0 of doxycycline treatment. Note the presence of hair follicles with hair shaft (HS); the interfollicular interval region, the epidermis and the lower white adipocyte cell layer are indicated. c A parasagittal section of H&E stained skin tissue from a K5: CAT mouse treated with doxycycline for 21 days. Note the striking enlargement of the epidermis accompanied by the presence of numerous enlarged pilosebaceous units (bracketed) that extend deep into the underlying dermis; horn pearl-like structures of multi concentric layers of squamous cell layers with increasing keratinization toward the center are also evident (asterisk). Scale bar in panel (b) applies to panel (c). (d) A higher power magnification of a horn pearl-like structure that features prominently in the epidermis of the K5: CAT mouse treated with doxycycline for 21 days. Note the extensive internal cornification of keratinocytes (bracket) that is reminiscent of the cornification of the stratum corneum. The histological data are representative of six age-matched K5: CAT mice per treatment condition. Quantification of epidermal thickness (μm) and the number of hair fibers per 500 μm are displayed in panels (e) and (f) respectively
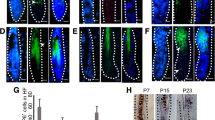
Consistent with the telogen resting phase (Muller-Rover et al. 2001), immunohistochemical staining for 5-bromo-2’-deoxyuridine (BrdU) incorporation showed that only a small number of scattered epidermal keratinocytes located mainly along the outer root sheath were detected in the epidermis of the K5: CAT mouse in the absence of doxycycline (Fig. 3a, b). Following 21 days of doxycycline administration, however, there was a significant increase in the number of keratinocytes in S-phase (Fig. 3c, d and Fig. S4), particularly in the basal cells of keratinizing pearl-like cysts, located deep in the dermis (Fig. 3e, f). This ring of proliferating keratinocytes lies within the concentric layers of cells that are positive for both K5 and the myc epitope-tag (Fig. 3c, f). Unlike previous reports on other transgenic mice in which expression of constitutively active β-catenin in the epidermis led to tumors (Baker et al. 2010; Lo Celso et al. 2004), histological evidence of benign tumors resembling human pilomatricomas and trichofolliculomas was not observed. Determining whether inducing ΔN89 β-catenin during the earlier anagen growth phase at 4 weeks of age elicits these benign skin tumors represents a future objective. Furthermore, 21-day doxycycline-treated K5: CAT mice did not show obvious signs of morbidity, which has been reported for other mouse models that experience prolonged activation of β-catenin signaling (Baker et al. 2010; Lo Celso et al. 2004). Because the rtTA is driven by the K5 promoter (Ramirez et al. 1994), morphological and cellular changes in response to induced β-catenin signaling in other K5 positive tissues were observed following this doxycycline treatment regimen. In the case of the female, extensive epithelial ductal side-branching and alveologenesis were observed in the mammary gland of the female K5: CAT mice following 21-day doxycycline treatment (Fig. S5). However, other than the presence of numerous dysplastic alveolar nodules along with extensive epithelial ductal side-branching, overt epithelial neoplasia was not observed during this treatment period.
A hyperplastic epidermis is induced in the K5: CAT mouse by doxycycline administration for 21 days. a A parasagittal skin section from an adult K5: CAT mouse without doxycycline induction stained for the transgene-derived myc epitope-tag. Note the absence of immunopositivity for the myc epitope-tag and the presence of normally spaced hair follicles (black arrowheads); hair shafts (HS) are indicated. b A parasagittal skin section from a K5: CAT mouse without doxycycline intake stained for BrdU incorporation. Note the presence of a few keratinocytes scoring positive for BrdU incorporation, mainly along the outer root sheath (ORS (black arrowhead)). c A parasagittal skin section from a K5: CAT mouse treated with doxycycline for 21 days. Immunohistochemical staining for the myc epitope-tag shows a significant induction of immunopositivity for the myc epitope-tag (black arrowhead). d Immunohistochemical detection of BrdU incorporation in parasagittal skin sections from the K5: CAT treated with doxycycline for 21 days shows a marked increase in the number of BrdU positive keratinocytes both in the basal cell layer of the epidermis (white arrowhead) and in the outer keratinocyte layer of large pilosebaceous units (black arrowhead); multilayered pearls of squamous cell layers with cornified keratin in their lumens is also observed (asterisk). Quantitation of the average number BrdU positive epithelial cells in K5: CAT skin per treatment (-Dox or + Dox) at day 0 and day 21 are shown in Fig. S4. e Immunohistochemistry reveals that transgene-derived myc tag-epitope expression is present in similar basal keratinocyte layers that display a hyperproliferative response phenotype shown in panel ((d) black arrow). f Immunohistochemical detection reveals that endogenous K5 protein is expressed in similarly located keratinocytes that score positive for myc epitope-tag positivity as shown in panel (e). Scale bar in panel (a) applies to all panels. The immunohistochemical data are representative of six age-matched K5: CAT mice per group
The doxycycline-induced K5: CAT epidermal phenotype is reversible
To determine whether the striking K5: CAT skin phenotype induced by 21-days of doxycycline treatment is reversible, we removed doxycycline from the food and water (Fig. 4). Following 30 days off doxycycline, the skin showed obvious phenotypic changes toward wild type. These changes included an increase in hair density and a marked reduction in the number of prominent skin ridges and folds that were observed in the K5: CAT mouse on doxycycline for 21-days (Fig. 3); skin elasticity returned to normal (data not shown). By 90 days off doxycycline (Fig. 4), the skin phenotype of K5: CAT mice was restored to normal (Fig. 4 and videos S1-3). Immunohistochemical analysis of parasagittal skin sections from K5: CAT mice, which were off doxycycline for 90 days, showed the absence of transgene-derived myc epitope-tag immunopositivity, with a distribution and number of BrdU positive epidermal keratinocytes that are consistent with the telogen resting phase in the wild type mouse (Fig. 5 and Fig. S6). Future investigations will confirm whether the return to the wild type skin phenotype in the de-induced K5: CAT mouse is due to apoptosis-driven hair follicle involution, which usually occurs during the catagen regression period of the hair cycle (Lindner et al. 1997).
The skin histopathology of the K5: CAT mouse treated with 21 days of doxycycline is reversible. Top three horizontal panels show both the side and ventral views of a K5: CAT mouse at day 0 of doxycycline (Dox) treatment. The second three horizontal panels show the same mouse after 21 days of doxycycline treatment; at the gross level, note the characteristic thickening of skin elicited by induction of β-catenin signaling. The third set of horizontal panels shows the same mouse that is 30 days off doxycycline. Note the decrease in the severity of the skin phenotype compared with that shown in the second set of horizontal panels. The bottom set of panels shows the same mouse following 90 days off doxycycline. At this stage of doxycycline de-induction of β-catenin signaling, the skin phenotype is nearly nonexistent. Black arrow in the left panels indicates the same ear tag (#319) throughout the study. The result is representative of similar doxycycline de-induction assays applied to four different mice (see also supplementary video file folder). The histological data are representative of six age-match mice per group
Myc epitope-tag expression and hyperkeratosis are not observed in the K5: CAT epidermis following a 21 days ON followed by a 90 days OFF doxycycline treatment regimen. a A H&E stained parasagittal section of skin from a K5: CAT mouse that was not treated with doxycycline (-Dox) showing a typical hair follicle, with hair shaft (HS). b A H&E stained parasagittal skin section from a K5: CAT mouse, which was previously treated with doxycycline for 21 days and subsequently left without doxycycline for 90 days; hair shaft is denoted by HS. Note the absence of the typical skin histopathology in the skin section following 3-months of de-induction of the transgene. c Immunohistochemical detection of BrdU incorporation shows only a small number of keratinocytes along the ORS that are positive for BrdU staining in the K5: CAT skin in the absence of doxycycline treatment. d Immunohistochemical staining for BrdU positivity in parasagittal skin sections from the K5: CAT mouse induced with doxycycline for 21 days and then de-induced following 90 days off doxycycline (Off Dox) shows a histomorphology that is indistinguishable from that shown for skin sections obtained from K5: CAT mice that were not treated with doxycycline (panel (c) -Dox). Quantitation of the number of BrdU positive cells per mouse group is shown in Fig. S6. e Immunohistochemistry shows the typical absence of expression of the myc epitope-tag in the skin of the K5: CAT mouse that is not treated with doxycycline (-Dox). f The expression of the transgene-derived myc epitope-tag in the skin of the K5: CAT mouse, which is induced with doxycycline for 21 days and then left without doxycycline for 90 days, is not present. Scale bar in panel (a) applies to all panels. The immunohistochemical data are representative of six age-matched K5: CAT mice per treatment group
Conclusions
Conventional and conditional transgenics have been used to furnish major insights into the role of β-catenin signaling in epidermal epithelial morphogenesis (Baker et al. 2010; Diamond et al. 2000; Gat et al. 1998; Wang et al. 2022). In addition, doxycycline-dependent transgenics have also been developed as in vivo tools in this field of study (Diamond et al. 2000). Although a conditional mouse model generated for the TET-ON system has been described in the study of β-catenin signaling in the skin (Zhang et al. 2008), this model is a trigenic system rather than a more simplified and affordable bigenic design. Using a simpler bigenic model, therefore, we show that doxycycline induction of ΔN89 β-catenin in K5: CAT keratinocytes results in striking changes in the cutaneous epithelial architecture, most notably the generation of numerous and enlarged pilosebaceous units that progress deep into the dermis. Strikingly, these epidermal morphological changes can be reversed following doxycycline de-induction. These results suggest that this exaggerated “anagen-type” epidermal epithelial morphogenesis and growth is tightly dependent on continued activation of β-catenin and/or its downstream molecular mediators. In the future, the K5: CAT bigenic mouse model will be particularly useful in concert with single cell and spatial transcriptomic analyses in identifying these downstream signals, which govern the induction and maintenance of the K5: CAT epidermal phenotype. Such future advancements are predicted to provide essential insights into not only hair follicle morphogenesis and cycling but also into hair growth pathologies, such as alopecia, anagen effluvium, hirsutism, hypertrichosis, and telogen effluvium (Schneider et al. 2009).
Materials and methods
Transgenic mice and doxycycline treatment.
The TetO-ΔN89 β-catenin responder transgenic mouse was generated in our laboratory and previously described (Mukherjee et al. 2011). The K5-rtTA effector transgenic was purchased from The Jackson Laboratory, Bar Harbor, ME (JAX Mice Stock number: 017519; allele type: Tg(KRT5-rtTA)T2D6Sgkd/J) and has been previously described (Raimondi et al. 2006; Ramirez et al. 1994; Vitale-Cross et al. 2004). To induce ΔN89β-catenin transgene expression, K5: CAT mice were provided rodent food pellets containing doxycycline at 200 mg/Kg (Bio-Serv, Flemington, NJ; cat: #S3888), made visually distinct by the inclusion of green food coloring. Containing 0.2% doxycycline (Takara Bio Inc., Mountain View, CA), deionized water was provided ad libitum from light protected amber bottles. To mitigate test aversion, water containing doxycycline was supplemented with 5% sucrose; doxycycline treated water was changed every three days to maintain induction potency. For the majority of these studies, the control group comprised K5: CAT mice that were not treated with doxycycline; K5: CAT mice that are not treated with doxycycline exhibit a wild type (WT) phenotype (data not shown).
Mouse husbandry and care
Mice for experiments were housed in the Baylor College of Medicine vivarium, which is accredited by the American Association for Accreditation of Laboratory Animal Care (AAALAC). When not treated with doxycycline, mice were fed irradiated Teklad global soy protein-free extruded rodent diet (Harlan Laboratories Inc., Indianapolis, IN) with free access to regular water. Mouse rooms operated at a temperature-controlled setting of 22 ± 2 °C and with a photocycle of 12-h lights on and 12-h lights off. Mouse experiments were conducted in accordance with the guidelines detailed in the Guide for the Care and Use of Laboratory Animals (“The Guide” (Eighth Edition 2011)), published by the National Research Council of the National Academies, Washington, D.C. The Institutional Animal Care and Use Committee (IACUC) at Baylor College of Medicine prospectively approved all animal protocols used in these experiments.
Histological analysis
Following 4% paraformaldehyde fixation overnight, processed tissues were embedded in paraffin as previously described (Mukherjee et al. 2011). For immunohistochemical staining, tissue Sections. (5μm) were placed on Superfrost Plus glass slides (ThermoFisher Inc., Waltham, MA). Sections were sequentially de-paraffinized, rehydrated, and incubated with an antigen unmasking solution before staining. Following an antigen-blocking step, tissue sections were incubated with the required primary antibody overnight at 4 °C. Primary antibodies used for immunohistochemistry were: the rabbit polyclonal anti-β-catenin (carboxyl-terminal antigen (cat: #9587)) and the rabbit monoclonal anti myc epitope-tag (71D10 (cat: #22,780)), both purchased from Cell Signaling Technology Inc., Danvers MA. After incubation with the primary antibody, tissue sections were incubated with the relevant secondary antibody conjugated with horseradish peroxidase (Vector Laboratories Inc., Burlingame, CA) for 1 h at room temperature. Tissue sections were subsequently incubated with the R.T. Vectastain Universal ABC reagent (Vector Laboratories Inc., (cat: # PK-6200)) for 30 min at room temperature. Incubation with 3, 3’-diaminobenzidine (DAB, Vector Laboratories Inc., (cat: #SK-4100)) enabled visualization of immunopositivity; tissue sections were lightly counter-stained with hematoxylin for contrast. Following a step-wise dehydration treatment, coverslips were mounted using permount solution (ThermoFisher Inc.) For BrdU immunohistochemical detection, mice were intraperitoneally injected with BrdU (10 mg/ml; Amersham Biosciences Corporation, Piscataway N.J. (1 mg BrdU/20 mg body weight)) 2 h before euthanasia. Mammary gland whole-mount staining has been previously described (Mukherjee et al. 2011). A color chilled AxioCam MRc5 digital camera affixed to a Carl Zeiss AxioImager A1 upright microscope (Zeiss, Jena, Germany) captured digital images of immunostained tissue. Captured images were digitally collated and annotated using Adobe Creative Cloud 2024 software (Adobe Systems Inc., San Jose CA). Using photomicrographs digitally captured at 200 × magnification, hair follicle bulb area (μm2) was measured using ImageJ software (Schroeder et al. 2021). With the ImageJ area selection tool, each hair follicle bulb was digitally demarcated by a perimeter before the area was quantitated using the Analyze tool within Image J. The average bulb area per mouse was obtained by quantifying the area of follicle bulbs within four 15 mm length dorsal skin parasagittal sections that were previously stained with H&E. The numbers of K5: CAT mice per treatment are indicated in Fig. S2. Using photomicrographs captured at 100 × magnification, epidermal thickness (μm) of H&E stained parasagittal skin sections was measured by digitally tracing a parallel line between the basal epithelial and the cornified cell layers. At least five measurements per image per mouse was recorded and then averaged. The numbers of K5: CAT mice per treatment used in this analysis are indicated in Fig. 2e. Using photomicrographs captured at 50 × magnification, the number of hair fibers along a 500 μm scale bar per field was counted. For these analyses, only hair fibers that traversed the epidermis to the outside were included in the count. Six fields per mouse were counted; the number of K5: CAT mice per treatment used in this study are detailed in Fig. 2f. Counting epithelial cells in S-phase was followed according to our recent publication (Hai et al. 2024). Briefly, BrdU + cells were counted within a field of 300 epithelial cells to obtain the average number of immunopositive cells per 300 cells counted. For the majority of studies, at least 3–4 fields of 300 cells were counted per mouse. The number of mice used in these analyses is indicated in Figs. S4 and S6.
Quantitative real-time PCR analysis
Total RNA was isolated from epidermal keratinocytes using the TRIzol reagent solution (ThermoFisher Inc., (cat: #15,596,026)); further RNA purification was performed using the RNeasy Plus Mini Kit (Qiagen Inc., Germantown road, MD (cat: #74,134)). Using the Superscript IV VILO Master Mix (ThermoFisher Inc., (cat: #11,756,050)), purified RNA was reverse transcribed into cDNA before qRT-PCR amplification with the Applied Biosystems Step One Plus Real Time PCR desktop System (ThermoFisher Inc., (cat: #4,376,374)). The TaqMan gene expression assays used in these studies were: the Xenopus tropicalis β-catenin (Ctnnb1; Xt03701931_m1 (ThermoFisher Scientific Inc., (cat: #4,351,372)) and 18S ribosomal RNA (Applied Biosystems Inc., Waltham MA, (cat: #4,352,655)).
Western immunoblot analysis
Small Sects. (2–3 mm2) of dorsal skin from mice were flash-frozen in liquid nitrogen before storage at − 80 °C to be homogenized later in lysis buffer: 50 mM Tris at pH7.5, 120 mM NaCl, 1 mM EDTA, 1% NP-40, 10% glycerol, and a Roche Complete Mini Protease Inhibitor Tablet (Sigma-Aldrich Inc., St. Louis, MO, (cat: 11,836,153,001)). Protein from keratinocytes was isolated and Western immunoblotting was conducted as previously reported (Mukherjee et al. 2011). Individual proteins from the protein isolate (20 mg/lane) were separated by electrophoresis on a 4–5% gradient sodium dodecyl sulphate–polyacrylamide gel prior to transfer to polyvinylidene difluoride membranes (Bio-Rad Laboratories, Hercules, CA). At 1:1000 dilution (5% BSA in TBS-T), the following primary antibodies were used: the rabbit polyclonal anti β-catenin (carboxyl-terminal antigen (cat: #9587)) and the rabbit monoclonal anti myc-tag-epitope (71D10 (cat: #22,780)) Cell Signaling Technology Inc.; the mouse monoclonal anti β-actin antibody was purchased from GenScript Inc., Piscataway, NJ (cat: #A00702S). Following incubation with the required secondary antibody (goat anti-rabbit IgG; Thermo Fisher Scientific Inc., cat: #A27036), immunopositive bands were detected using the Super Signal West Pico Chemiluminescent Substrate kit (Thermo Fisher Scientific Inc., (cat: #A38555)).
Statistical analyses
When required, results are displayed as means ± standard error (s.e.m). Statistical significance of differences between experimental groups was determined by two-tailed Student’s t test using GraphPad Prism (GraphPad software, La Jolla, CA). For most experimental analysis, a minimum of three independent replicates was used. A p-value < 0.05 was considered statistically significant; asterisks above bars in histograms signify the level of significance: *p < 0.05; **p < 0.01; and ***p < 0.001.
Data Availability
No datasets were generated or analysed during the current study.
References
Aberle H, Bauer A, Stappert J, Kispert A, Kemler R (1997) beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J 16:3797–3804. https://doi.org/10.1093/emboj/16.13.3797
Baker CM, Verstuyf A, Jensen KB, Watt FM (2010) Differential sensitivity of epidermal cell subpopulations to beta-catenin-induced ectopic hair follicle formation. Dev Biol 343:40–50. https://doi.org/10.1016/j.ydbio.2010.04.005
Behrens J, Jerchow BA, Wurtele M, Grimm J, Asbrand C, Wirtz R, Kuhl M, Wedlich D, Birchmeier W (1998) Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science 280:596–599. https://doi.org/10.1126/science.280.5363.596
Clevers H (2006) Wnt/beta-catenin signaling in development and disease. Cell 127:469–480. https://doi.org/10.1016/j.cell.2006.10.018
Clevers H, van de Wetering M (1997) TCF/LEF factor earn their wings. Trends Genet 13:485–489. https://doi.org/10.1016/s0168-9525(97)01305-x
Diamond I, Owolabi T, Marco M, Lam C, Glick A (2000) Conditional gene expression in the epidermis of transgenic mice using the tetracycline-regulated transactivators tTA and rTA linked to the keratin 5 promoter. J Invest Dermatol 115:788–794. https://doi.org/10.1046/j.1523-1747.2000.00144.x
Gat U, DasGupta R, Degenstein L, Fuchs E (1998) De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell 95:605–614. https://doi.org/10.1016/s0092-8674(00)81631-1
Grigoryan T, Wend P, Klaus A, Birchmeier W (2008) Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes Dev 22:2308–2341. https://doi.org/10.1101/gad.1686208
Hai L, Maurya VK, DeMayo FJ, Lydon JP (2024) Establishment of murine pregnancy requires the promyelocytic leukemia zinc finger transcription factor. Int J Mol Sci 25(6):3451. https://doi.org/10.3390/ijms25063451
Hai L, Szwarc MM, Lonard DM, Rajapakshe K, Perera D, Coarfa C, Ittmann M, Fernandez-Valdivia R, Lydon JP (2019) Short-term RANKL exposure initiates a neoplastic transcriptional program in the basal epithelium of the murine salivary gland. Cytokine 123:154745. https://doi.org/10.1016/j.cyto.2019.154745
Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W (2001) beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 105:533–545. https://doi.org/10.1016/s0092-8674(01)00336-1
Imbert A, Eelkema R, Jordan S, Feiner H, Cowin P (2001) Delta N89 beta-catenin induces precocious development, differentiation, and neoplasia in mammary gland. J Cell Biol 153:555–568. https://doi.org/10.1083/jcb.153.3.555
Kemler R (1993) From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet 9:317–321. https://doi.org/10.1016/0168-9525(93)90250-l
Lewandoski M (2001) Conditional control of gene expression in the mouse. Nat Rev Genet 2:743–755. https://doi.org/10.1038/35093537
Lindner G, Botchkarev VA, Botchkareva NV, Ling G, van der Veen C, Paus R (1997) Analysis of apoptosis during hair follicle regression (catagen). Am J Pathol 151:1601–1617
Littlewood TD, Hancock DC, Danielian PS, Parker MG, Evan GI (1995) A modified oestrogen receptor ligand-binding domain as an improved switch for the regulation of heterologous proteins. Nucleic Acids Res 23:1686–1690. https://doi.org/10.1093/nar/23.10.1686
Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X (2002) Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108:837–847. https://doi.org/10.1016/s0092-8674(02)00685-2
Lo Celso C, Prowse DM, Watt FM (2004) Transient activation of beta-catenin signalling in adult mouse epidermis is sufficient to induce new hair follicles but continuous activation is required to maintain hair follicle tumours. Development 131:1787–1799. https://doi.org/10.1242/dev.01052
Marikawa Y, Elinson RP (1998) beta-TrCP is a negative regulator of Wnt/beta-catenin signaling pathway and dorsal axis formation in Xenopus embryos. Mech Dev 77:75–80. https://doi.org/10.1016/s0925-4773(98)00134-8
McCrea PD, Turck CW, Gumbiner B (1991) A homolog of the armadillo protein in Drosophila (plakoglobin) associated with E-cadherin. Science 254:1359–1361. https://doi.org/10.1126/science.1962194
Mukherjee A, Soyal SM, Li J, Ying Y, Szwarc MM, He B, Kommagani R, Hodgson MC, Hiremath M, Cowin P et al (2011) A mouse transgenic approach to induce beta-catenin signaling in a temporally controlled manner. Transgenic Res 20:827–840. https://doi.org/10.1007/s11248-010-9466-6
Muller-Rover S, Handjiski B, van der Veen C, Eichmuller S, Foitzik K, McKay IA, Stenn KS, Paus R (2001) A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol 117:3–15. https://doi.org/10.1046/j.0022-202x.2001.01377.x
Nelson WJ, Nusse R (2004) Convergence of Wnt, beta-catenin, and cadherin pathways. Science 303:1483–1487. https://doi.org/10.1126/science.1094291
Panteleyev AA, Jahoda CA, Christiano AM (2001) Hair follicle predetermination. J Cell Sci 114:3419–3431
Polakis P (1999) The oncogenic activation of beta-catenin. Curr Opin Genet Dev 9:15–21. https://doi.org/10.1016/s0959-437x(99)80003-3
Polakis P (2000) Wnt signaling and cancer. Genes Dev 14:1837–1851
Raimondi AR, Vitale-Cross L, Amornphimoltham P, Gutkind JS, Molinolo A (2006) Rapid development of salivary gland carcinomas upon conditional expression of K-ras driven by the cytokeratin 5 promoter. Am J Pathol 168:1654–1665. https://doi.org/10.2353/ajpath.2006.050847
Ramirez A, Bravo A, Jorcano JL, Vidal M (1994) Sequences 5’ of the bovine keratin 5 gene direct tissue- and cell-type-specific expression of a lacZ gene in the adult and during development. Differentiation 58:53–64. https://doi.org/10.1046/j.1432-0436.1994.5810053.x
Salomon D, Sacco PA, Roy SG, Simcha I, Johnson KR, Wheelock MJ, Ben-Ze’ev A (1997) Regulation of beta-catenin levels and localization by overexpression of plakoglobin and inhibition of the ubiquitin-proteasome system. J Cell Biol 139:1325–1335. https://doi.org/10.1083/jcb.139.5.1325
Schneider MR, Schmidt-Ullrich R, Paus R (2009) The hair follicle as a dynamic miniorgan. Curr Biol 19:R132-142. https://doi.org/10.1016/j.cub.2008.12.005
Schroeder AB, Dobson ETA, Rueden CT, Tomancak P, Jug F, Eliceiri KW (2021) The Imagej ecosystem: open-source software for image visualization, processing, and analysis. Protein Sci 30:234–249. https://doi.org/10.1002/pro.3993
Suzuki K, Yamaguchi Y, Villacorte M, Mihara K, Akiyama M, Shimizu H, Taketo MM, Nakagata N, Tsukiyama T, Yamaguchi TP et al (2009) Embryonic hair follicle fate change by augmented beta-catenin through Shh and Bmp signaling. Development 136:367–372. https://doi.org/10.1242/dev.021295
Takeichi M (1991) Cadherin cell adhesion receptors as a morphogenetic regulator. Science 251:1451–1455. https://doi.org/10.1126/science.2006419
Van Mater D, Kolligs FT, Dlugosz AA, Fearon ER (2003) Transient activation of beta -catenin signaling in cutaneous keratinocytes is sufficient to trigger the active growth phase of the hair cycle in mice. Genes Dev 17:1219–1224. https://doi.org/10.1101/gad.1076103
Vitale-Cross L, Amornphimoltham P, Fisher G, Molinolo AA, Gutkind JS (2004) Conditional expression of K-ras in an epithelial compartment that includes the stem cells is sufficient to promote squamous cell carcinogenesis. Cancer Res 64:8804–8807. https://doi.org/10.1158/0008-5472.CAN-04-2623
Wang J, Cui K, Hua G, Han D, Yang Z, Li T, Yang X, Zhang Y, Cai G, Deng X et al (2022) Skin-specific transgenic overexpression of ovine beta-catenin in mice. Front Genet 13:1059913. https://doi.org/10.3389/fgene.2022.1059913
Willert K, Jones KA (2006) Wnt signaling: is the party in the nucleus? Genes Dev 20:1394–1404. https://doi.org/10.1101/gad.1424006
Zhang Y, Andl T, Yang SH, Teta M, Liu F, Seykora JT, Tobias JW, Piccolo S, Schmidt-Ullrich R, Nagy A et al (2008) Activation of beta-catenin signaling programs embryonic epidermis to hair follicle fate. Development 135:2161–2172. https://doi.org/10.1242/dev.017459
Acknowledgements
The authors thank Jie Li and Rong Zhao for their excellent technical assistance.
Funding
These studies were funded in part by the National Institutes of Health (NIH)/National Institute of Child Health and Human Development (NICHD) R01 HD042311 (J.P.L.).
Author information
Authors and Affiliations
Contributions
Vineet Maurya and Yan Ying contributed to the design, generation and phenotypic analysis of the K5: CAT conditional mouse model. Vineet Maurya, Yan Ying and John Lydon prepared and wrote the manuscript. All studies were planned and supervised by John P. Lydon.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (MOV 154967 KB)
Supplementary file3 (MOV 9955 KB)
Supplementary file4 (MOV 24166 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Maurya, V.K., Ying, Y. & Lydon, J.P. A Mouse Model for Conditional Expression of Activated β-Catenin in Epidermal Keratinocytes. Transgenic Res (2024). https://doi.org/10.1007/s11248-024-00402-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11248-024-00402-z