Abstract
Purpose
In light of the reported association between REM-related obstructive sleep apnoea (OSA) and heightened cardiovascular risk, this study aims to compare cardiac autonomic function in patients with REM-OSA and OSA independent of sleep stage. We hypothesized that REM-OSA patients would exhibit higher sympathetic cardiac modulation based on heart rate variability (HRV) profiles.
Methods
HRV was compared between the OSA group (AHI ≥ 5 events/h, n = 252) and the REM-OSA group (AHI ≥ 5 events/h, AHIREM:AHINREM ≥ 2, n = 137). Time- and frequency-domain measures of HRV were analysed during N2 and REM sleep.
Results
Clinical characteristics between the two test groups differed significantly, 45% of REM-OSA patients were female, with mild OSA (median, interquartile range (IQR)) AHI of 10 (7) events/h. Only 26% of the OSA cohort were female with moderate OSA (AHI = 17 (20) events/h, p < 0.001). Compared with the OSA group, the low frequency to high frequency ratio (LF:HF) and LF power were lower and HF power was higher in the REM-OSA group during N2 (LF:HF, p = 0.012; LF; p = 0.013; HF, p = 0.007) and in REM sleep (LF:HF, p = 0.002; LF, p = 0.004; HF, p < 0.001). Patient sex and OSA severity had a significant combined effect on average N to N interval, LF power, and LF:HF ratio during N2 and REM sleep (all p < 0.001).
Conclusion
Contrary to our hypothesis, REM-OSA patients demonstrated consistently higher cardiac vagal modulation, reflecting better cardiac autonomic adaptation. These results were attributed to differences in OSA severity and sex in these two groups, both independently affecting HRV. This study emphasises the need for future research into the underlying pathophysiology of REM-OSA and the potential implications of sex and OSA severity on cardiovascular risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnoea (OSA), a highly prevalent and chronic sleep disorder, confers a heightened susceptibility to the development of hypertension, cardiovascular disease, and stroke. Repeated upper airway collapse results in intermittent hypoxia, negative intrathoracic pressure swings and sleep fragmentation [1, 2], causing adverse cardiovascular effects, primarily through sympathetic activation [3].
While respiratory events may occur in all stages of sleep, muscle hypotonia during rapid eye movement (REM) sleep is believed to play a role in the heightened frequency of upper airway collapse during that sleep stage [4]. This, in turn, leads to longer duration of respiratory events accompanied by greater oxygen desaturation and increased sympathetic activity [4, 5]. Therefore, OSA during REM sleep may have a significant effect on cardiovascular risk, even when the apnoea–hypopnoea index (AHI) is low [6].
There is evidence that OSA during REM sleep may be associated with cardiovascular changes that may confer increased cardiovascular risk. However, the mechanisms underpinning this association are not well characterised. Previous studies have focused on a single clinical outcome rather than investigating the physiological mechanisms underlying the increased cardiovascular risk associated with OSA during REM sleep. While some studies show an association between REM-related OSA (REM-OSA) and markers of cardiovascular risk, namely, hypertension and nocturnal blood pressure dipping [7], other studies show no association with clinical features [8]. Moreover, research suggests potential sex-based differences in cardiac autonomic function in patients with OSA [9], warranting further investigation into the role of sex in cardiovascular outcomes in this population.
The sympathetic and parasympathetic nervous system has a profound influence on heart rate and blood pressure control [4]. Heart rate variability (HRV) serves as a non-invasive assessment of beat–to–beat changes in cardiac autonomic control, providing an intermediatory marker of cardiovascular risk. HRV analysis is particularly valuable for assessing the balance between sympathetic and parasympathetic inputs to the cardiovascular system. A large body of evidence has shown that OSA is associated with a reduced HRV, indicative of cardiac sympathetic predominance [10], which is significantly linked to a two-fold rise in cardiovascular morbidity, acting as an independent cardiovascular risk factor [10, 11]. In this study we aimed to compare HRV profiles between REM-OSA and patients with upper airway obstruction independent of sleep stage. We hypothesised increased cardiovascular risk during sleep in REM-OSA compared to OSA patients, reflected by heightened cardiac sympathetic function and vagal withdrawal.
Methods
Participants
We retrospectively reviewed polysomnograms (PSG) recorded in the Sydney Sleep Biobank, a database of prospective data collected from sleep clinic patients across 3 hospitals sleep clinics in Sydney, Australia (Fig. 1) [12]. As a part of their contribution to the Biobank, patients complete a subjective questionnaire responding to questions regarding ethnicity, comorbidities, and, medication use, and validated questionnaires including the Epworth Sleepiness Score (ESS), and Functional Outcomes of Sleep Questionnaire (FOSQ-10). The patients’ blood pressure was also taken pre- and post- polysomnography. Patients included in this study underwent nocturnal polysomnography at the Royal North Shore Hospital, Royal Prince Alfred Hospital and Westmead Hospital between 2018–2021. Patients required greater than 30 min of REM sleep to be included in the study. REM-related OSA (REM-OSA) can be sub-classified into two groups; (1) REM isolated OSA, defined as, AHIREM > 5/ hour, AHINREM < 5/ hour, with Total AHI > 5/ hour; and (2) REM-predominant OSA, defined as AHIREM:AHINREM ≥ 2, with Total AHI > 5/ hour. While these definitions are distinct from one another they result in overlapping patient records, and therefore patient records pertaining to the latter, more broad definition of REM- predominant OSA was used as inclusion criteria for the REM-related OSA group (REM-OSA). The OSA group was defined as a Total AHI > 5 events/hour with exclusion of patients meeting our REM-OSA definition.
Study flow. Polysomnograms available from the Sydney Sleep Biobank were conducted at three collection sites located in Sydney, Australia: RNS (Royal North Shore Hospital), RPA (Royal Prince Alfred Hospital), and WES (Westmead Hospital). The REM-related OSA group (REM-OSA) included 137 patient records, while 252 patient records were included in the OSA independent of stage group (OSA)
Polysomnography
During full night PSG, standard channels were utilized to record electroencephalography (EEG), electrooculography (EOG), chin electromyography (EMG), nasal airflow pressure via nasal cannula, thoracic and abdominal respiratory effort, finger pulse oximetry to measure oxygen saturation (SpO2%), body position, and leg electromyography. Trained sleep scientists scored each recording according to the guidelines set by the American Academy of Sleep Medicine [1] and analyzed the data using the Compumedics PSG4 V4.1 software developed by Compumedics, Australia.
Heart rate variability
The electrocardiogram was recorded as a part of in-clinic polysomnography and saved as a European data format (EDF). These recordings were imported into commercially available Sentinel software (Spacelabs Healthcare, Issaquah, WA, U.S.A.). The Sentinel software then processed the electrocardiogram by applying an automated R wave and electrocardiogram artefact detection algorithms and then exported an extensible markup language (xml) file containing the QRS times of heart-beats (with a 1ms resolution) and associated heart-beat labels (normal or non-normal beat/rhythm). Sentinel beat analysis files were imported into MATLAB software as xml files.
As part of in-laboratory PSG, electrocardiogram data was collected using three electrodes placed in the standard lead II configuration. The ECG signals were extracted from the polysomnograms and analysed using commercially available Holter software (Sentinel Holter Data Management System v11.5.1, Spacelabs Healthcare, Issaquah, WA, U.S.A.) for QRS detection, ectopic beat detection, and labeling. QRS detection was performed with a resolution of 1ms. An in-house algorithm was used to form the RR series [13]. The normal-normal (NN) beat series was derived from the RR series by using an accepted adaptive RR interval filtering algorithm method to remove RR intervals associated with non-sinus beats [14]. Time domain measures were calculated according to standard guidelines [15]. To calculate frequency-domain HRV parameters the Lomb periodogram was applied to the preprocessed data. The Lomb periodogram is based upon the same fundamental theory as the Discrete Fourier Transform but is superior as it does not require an evenly sampled data set – it allows for the inherent variability of the RR interval data and hence the tachogram can be transformed directly without an intervening approximation stage [16]. Unlike the Discrete Fourier Transform, the Lomb method also allows for the exclusion of ectopic beats. Time and frequency domain HRV measures used in this study are outlined in Table 1. HRV parameters were calculated using 2 min blocks, shifted by 30 s, across the entire ECG signal, and then averaged across sleep stage N2 and REM (Supplementary material Fig. 1 details the number of blocks of data analysed for each sleep stage).
Statistical analysis
Clinical data were summarised across the test groups. The Shapiro–Wilk test was used to assess normality. All outcomes were non-parametric and presented as median (interquartile ranges, IQR). Categorical variables were expressed as count (percentage, %) and examined using Chi Square tests, presented with Chi square test statistic, χ2, and degrees of freedom (df). The Mann–Whitney U test compared clinical, polysomnographic, and HRV measures between the OSA and REM-OSA groups.
Linear regression models were employed to investigate the effect of OSA severity, patient sex, and test groups on HRV measures during N2 and REM sleep. Each HRV measure was considered a dependent variable, and the predictors for each model are as follows: Model 1 included AHI; Model 2 included sex and AHI; and Model 3 also incorporated grouping, i.e., OSA or REM-related OSA. Since continuous variables exhibited non-normal distributions, HRV markers were logarithmically transformed using Log10 (HRV + 1). + 1 was included in the logarithm parameters to permit analysis of zero values [17].
The p-value was adjusted for multiple comparisons (which included average NN interval, RMSSD, pNN50, TP, absolute LF and HF power, normalised LF and HF power, and LF:HF ratio) using Bonferroni correction. Therefore, significance is indicated by p-values less than 0.005. All data analyses were conducted using SPSS software (version 24; SPSS Inc., Chicago, IL).
Results
Comparison of phenotypic characteristics between OSA groups
There were no significant differences in age, body mass index (BMI), blood pressure, ESS, or FOSQ-10 scores observed across the three study groups (Table 2). Females comprised approximately half of the REM-OSA group (45%), a proportion significantly higher than the OSA group, which consisted of 26% females (p < 0.001). Roughly half of the participants in each group self-identified as White (p = 0.031). In the REM-OSA group, there was a trend towards a higher proportion of participants identifying as North/North East Asian (p = 0.057). Subjective reports of hypertension were comparable across groups, with around one-third of participants reporting this condition (OSA, 41%; REM-OSA 29%; p = 0.042), as well as hypercholesterolaemia (OSA, 38%; REM-OSA 31%; p = 0.245). Notably, a larger proportion of participants in the OSA group reported using β-blockers (p = 0.013) and lipid-modifying agents (p = 0.029).
Comparison of polysomnographic characteristics
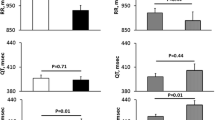
The REM sleep stage was longer in the REM-OSA group (p = 0.008) (Table 3). By design, the AHI varied significantly between test groups, where the REM-OSA group had an AHI of 10 (7) events/h, compared with 17 (20) events/h in the OSA group (p < 0.001). The AHINREM was significantly higher in the OSA group (p < 0.001), whereas the AHIREM was significantly higher in the REM-OSA group (p < 0.001). There were no differences in SpO2 nadir; however, SpO2Nadir NREM was highest in the REM-OSA group (p < 0.001).
Cardiac autonomic activity across sleep stages
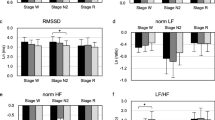
Compared with the OSA group, LF power (p = 0.013), and the LF:HF ratio (p = 0.012) was lower while HF power (p = 0.007) was higher in REM-OSA during N2 sleep (Table 4). This relationship continued into REM sleep and was significantly different between both groups (LF, p = 0.002; LF:HF ratio, p = 0.004; HF, p < 0.001) (Fig. 2).
Bar Graphs Illustrating Selected HRV Measures in OSA (n = 235, black bars) and REM-OSA Patients (n = 137, white bars). Wilcoxon-Mann–Whitney test was employed for statistical analysis. Data are presented as median and interquartile range (IQR) along with the test statistic (U). P values were adjusted for multiple comparisons using Bonferroni correction. Significance is indicated by for p < 0.005. a, p = 0.012; b, p = 0.002; c, p = 0.013; d, p = 0.004; e, p = 0.007; f, p < 0.001. Abbreviations: LF—low-frequency power; HF—high-frequency power; LF: HF—low-frequency: high-frequency ratio.; nu—normalised units
The effect of covariates on HRV
Linear regression models included OSA severity, sex, and test group as predictors. While no significant linear relationship was observed with AHI and HRV markers, the model explained 3% of the variance in normalised HF power during REM sleep (p = 0.003) (Table 5). When patient sex was introduced, significant linear relationships were identified. The combined model accounted for approximately 5–6% of the variance in absolute LF, LF:HF ratio, and normalised LF (all p < 0.001), as well as HF power during both N2 (p = 0.002) and REM sleep (p = 0.007). The combination of AHI, sex, and test group did not appear to have an effect on HRV measures, except for its contribution to 7% of the variance in LF:HF ratio during REM sleep (p < 0.001).
Discussion
This study explored nocturnal cardiac autonomic profiles of patients with REM-OSA to a group with OSA independent of sleep stage. The primary finding in this study is that compared with REM-OSA, OSA patients’ have a predominance of sympathetic cardiac modulation during both N2 and REM sleep stages. There was a significantly higher percentage of females with REM-OSA (45%) when compared with the OSA group (26%). We also demonstrated that while OSA severity has a limited effect of HRV measures, the combination of AHI and patient sex accounts for up to 6% in variance of sympathetically mediated HRV markers in both sleep stages.
We hypothesised increased sympathetic and reduced parasympathetic cardiac autonomic function in patients with REM-OSA, as a marker of increased cardiovascular risk. Our results, while not supporting our hypothesis, are consistent with available literature [18]. Of the 3 similar studies undertaken, only one study has reported that apnoea during REM sleep is associated with sympathetic predominance. The authors link higher OSA severity during REM sleep to increased low frequency power and LF:HF ratio in wakefulness [17]. Earlier work shows a dose–response relationship between OSA severity and absolute LF power in men and women during REM sleep and wakefulness. Interestingly, BMI inversely affects low frequency and total power during REM sleep (and no other sleep stage) [19]. Only one study provides an assessment of HRV in patients with REM-OSA [18], and found no evidence of increased cardiovascular risk in patients with REM-OSA compared with matched control and OSA groups. In fact, their results show significantly greater SDNN, a marker for global HRV and therefore lower cardiovascular risk [15], in REM-OSA patients when compared with an OSA group. Our results show that compared with OSA patients, REM-OSA have higher absolute HF power and lower LF:HF ratio during sleep. This is associated with greater cardiac vagal modulation and ultimately reduced cardiovascular risk [15]. Not to mention, the greater use of ACEI, ARB and lipid modifying agents in the OSA group, which also effect HRV [20]. Taken together, these studies provide evidence of distinct cardiac autonomic function profiles in patients with REM-OSA, though there remains disagreement on the direction of sympathovagal balance and therefore its impact on cardiovascular risk.
Altered cardiac autonomic control in sleep is affected by the degree of OSA severity. When shifting from NREM to REM sleep, HRV profiles of patients with moderate OSA are characterised by significantly greater cardiac sympathetic activity (increased LF and LF:HF components) coupled with reduced cardiac vagal activity (reduced HF spectral components) [21]. The genesis of this being related to sympathoexcitation which peaks towards the end of the respiratory event and is followed by a surge in blood pressure and heart rate repeatedly experienced by OSA patients throughout the duration of sleep. This ultimately contributes to the ‘non-dipping’ nocturnal blood pressure profiles commonly exhibited in OSA resulting in susceptibility to hypertension. In fact increased bursts of muscle sympathetic nerve activity have been reported during REM sleep of patients with OSA potentially contributing to cardiovascular sequalae [4]. In both of our OSA groups the SDNN falls below the clinically relevant threshold of > 50ms, which is strongly associated with cardiovascular mortality in patients with pre-existing cardiovascular disease [22].
The observed reduction in cardiac sympathetic activity among our REM-OSA group can, in part, be attributed to variations in OSA severity between the test groups. Notably, our REM-OSA cohort had a higher proportion of patients with mild OSA, which aligns with findings in existing literature. Studies have shown that about 70% of sleep studies with an overall AHI < 15 events/h (indicating either no OSA or mild OSA) are affected by REM-OSA, defined as REM AHI ≥ 15 events/h [23]. While no linear relationships were identified between AHI and HRV markers, our modelling did reveal that OSA severity explained approximately 3% of the variance in HF power during REM sleep. This likely contributed to a shift in the sympathovagal balance towards vagal predominance in the REM-OSA group.
The impact of sex on cardiovascular risk in patients with OSA also requires attention due to its potential implications for understanding the underlying pathophysiological consequences of OSA. Generally speaking, there are fewer HRV studies conducted in women than there are in men. Kesek and colleagues studied the relationship between OSA severity and HRV in 387 women and showed an inverse relationship between AHI and sympathetic HRV markers during REM sleep [9]. More recent work has confirmed a marked difference in OSA prevalence between women pre and post menopause, suggesting that comparing patients simply by sex does not accurately characterise an OSA phenotype [24]. The role that reproductive hormones play in OSA remains an underexplored area of study. Lower levels of progesterone, oestradiol, and 17-OH progesterone is noted in post-menopausal women with an AHI > 10 events/h, after matching for age, and menstrual time-point [25]. In fact, hormone replacement therapy in post-menopausal women appears to lower AHI significantly when compared with women not on therapy, though the absolute difference in AHI remains low (∼ 2 events/h) [24]. Our study showed that the combination of patient sex and OSA severity contributed to heightened cardiac vagal activity in REM-OSA patients during N2 sleep. Furthermore, when we factored in patient sex, we found that it explained a greater portion (up to 6%) of variance in global and sympathetically mediated HRV measures during REM sleep. This aligns with findings in related studies [9, 18], suggesting that the diminished cardiovascular risk observed in REM-OSA may be attributed to a dual effect: lower overall AHI severity and a higher proportion of female patients. Given the older average age of the test group it would have been beneficial to study the impact of reproductive hormones on the presence and severity of REM-related OSA. This is a major limitation to this study, which we believe will be important to consider in future investigations.
Nevertheless, our study found that one third of all OSA patients, irrespective of test group, were diagnosed with hypertension (OSA, 41%; REM-OSA, 29%) and hypercholesterolaemia (OSA, 38%; REM-OSA, 31%). This is in line with the literature, where severe REM-OSA is independently associated with the prevalence of hypertension [6], incidence of a composite cardiovascular endpoints [26], and impairment of glucose metabolism [23, 27]. Inconsistency within the literature is also likely related to the emphasis on clinical outcomes rather than understanding the underlying physiological differences in REM-OSA.
The major strength of our study is the quantitative analysis of the effect of autonomic dysfunction, a potential mechanism underpinning cardiovascular risk in patients with OSA. We controlled for patient sex and OSA severity, statistically different between test groups, which enabled us to highlight the importance in considering these factors in this clinical subtype. We used a conservative HRV methodology which excluded epochs with mixed sleep stages and ECG sections associated with respiratory events or cardiac arrhythmia and entire epochs were excluded if the total exclusion period exceeded 12 s (10% of epoch length). We also included only patients with a minimum REM duration of 30 min to ensure that the count of studied epochs was comparable across test groups. The study has a number of limitations. Firstly, the imbalanced sample sizes between the test groups potentially limited the statistical power to detect significant differences or associations between variables. Secondly, patients were only studied during sleep due to a lack of wakefulness data. Comparison with daytime wakeful HRV measures would have allowed us to deduce the “spillover” effect of different nocturnal HRV profiles into daytime cardiac autonomic function.
Conclusion
This comparative analysis of nocturnal HRV in REM-OSA and OSA independent of sleep stage found that REM-OSA patients consistently showed increased cardiac vagal modulation, indicating enhanced autonomic adaptability. Notably, differences in sex and OSA severity between groups independently influenced HRV profiles. Recognising and accounting for these factors is imperative when evaluating HRV in OSA patients. This study emphasises that, beyond OSA severity, sex can also play a significant role in influencing cardiac autonomic adaptability.
Data availability
Access to the datasets for this study may be available if permissible by the Sydney Sleep Biobank. To view generated analyses and request access to datasets please confer with corresponding author.
References
Kohler M, Stradling JR (2010) Mechanisms of vascular damage in obstructive sleep apnea. Nat Rev Cardiol 7(12):677–685
Veasey SC, Rosen IM (2019) Obstructive sleep apnea in adults. N Engl J Med 380(15):1442–1449
Esquinas C et al (2013) Rationale and methodology of the impact of continuous positive airway pressure on patients with ACS and nonsleepy OSA: the ISAACC Trial. Clin Cardiol 36(9):495–501
Somers VK et al (1995) Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Investig 96(4):1897–1904
Somers VK, Dyken ME, Mark AL, Abboud FM (1993) Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med 328(5):303–307
Mokhlesi B et al (2014) Obstructive sleep apnea during REM sleep and hypertension. Results of the Wisconsin Sleep Cohort. Am J Res Crit Care Med 190(10):1158–1167
Mokhlesi B et al (2015) Obstructive sleep apnoea during REM sleep and incident non-dipping of nocturnal blood pressure: a longitudinal analysis of the Wisconsin Sleep Cohort. Thorax 70(11):1062–1069
Marcus CL, Greene MG, Carroll JL (1998) Blood pressure in children with obstructive sleep apnea. Am J Respir Crit Care Med 157(4):1098–1103
Kesek M et al (2009) Heart rate variability during sleep and sleep apnoea in a population based study of 387 women. Clin Physiol Funct Imaging 29(4):309–315
Dissanayake HU (2021) Yu Sun Bin, Seren Ucak, Philip de Chazal, Kate Sutherland, and Peter A. Cistulli, Association between autonomic function and obstructive sleep apnea: a systematic review. Sleep Med Rev 57:101470
Goldenberg I et al (2019) Heart rate variability for risk assessment of myocardial ischemia in patients without known coronary artery disease: The HRV-DETECT (heart rate variability for the detection of myocardial ischemia) study. J Am Heart Assoc 8(24):e014540
Sutherland K, Sarkissian N, Amis TC, Bennett C, Chan ASL, Chan M, Chow CM, de Chazal P, Cohen G, Kairaitis K, Lambert S (2019) Sydney sleep biobank (SSB): development of a research resource. J Sleep Res 28
Ucak S, Dissanayake HU, de Chazal P, Bin YS, Sutherland K, Setionago B, Tong B, Yee BJ, Kairaitis K, Wheatley JR, Piper AJ (2024) Heart rate variability analysis in obstructive sleep apnea patients with daytime sleepiness. Sleep 47(6)
Wessel N, Schumann A, Schirdewan A, Voss A, Kurths J (2000) Entropy measures in heart rate variability data. In: Medical data analysis: first international symposium, ISMDA 2000 Frankfurt, Germany, September 29–30, 2000 Proceedings 1. Springer Berlin Heidelberg, pp 78–87
Malik M (1996) Heart rate variability: Standards of measurement, physiological interpretation, and clinical use: Task force of the European Society of Cardiology and the North American Society for Pacing and Electrophysiology. Ann Noninvasive Electrocardiol 1(2):151–181
Ruf T (1999) The Lomb-Scargle periodogram in biological rhythm research: analysis of incomplete and unequally spaced time-series. Biol Rhythm Res 30(2):178–201
Huang W, Zhang X, Wang X, Zhou T, Zhao X, Xu H, Li X, Guan J, Yi H, Yin S (2023) Effects of obstructive sleep apnea during rapid eye movement sleep on cardiac autonomic dysfunction: results from the Shanghai sleep health study cohort. J Sleep Res 32(5):e13904
Oh SM et al (2019) The association between obstructive sleep apnea during REM sleep and autonomic dysfunction as measured by heart rate variability. Sleep Breathing 23:865–871
Reynolds EB et al (2007) Autonomic function in sleep apnea patients: increased heart rate variability except during REM sleep in obese patients. Sleep Breathing 11:53–60
Maciorowska M, Krzesiński P, Wierzbowski R, Gielerak G (2020) Heart rate variability in patients with hypertension: the effect of metabolic syndrome and antihypertensive treatment. Cardiovasc Ther 2020(1):8563135
Jurysta F et al (2006) The link between cardiac autonomic activity and sleep delta power is altered in men with sleep apnea-hypopnea syndrome. Am J Physiol-Reg, Integ Comparative Physiol 291(4):R1165–R1171
Kleiger RE et al (1987) Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol 59(4):256–262
Chami HA et al (2010) Sleepiness, quality of life, and sleep maintenance in REM versus non-REM sleep-disordered breathing. Am J Respir Crit Care Med 181(9):997–1002
Heinzer R et al (2018) Impact of sex and menopausal status on the prevalence, clinical presentation, and comorbidities of sleep-disordered breathing. Sleep Med 51:29–36
Netzer NC, Eliasson AH, Strohl KP (2003) Women with sleep apnea have lower levels of sex hormones. Sleep Breathing 7(01):025–030
Aurora RN et al (2018) Obstructive sleep apnea during REM sleep and cardiovascular disease. Am J Respir Crit Care Med 197(5):653–660
Grimaldi D et al (2014) Association of obstructive sleep apnea in rapid eye movement sleep with reduced glycemic control in type 2 diabetes: therapeutic implications. Diabetes Care 37(2):355–363
Acknowledgements
*Sydney Sleep Biobank Investigators:
University of Sydney: Peter Cistulli, Philip de Chazal, Kate Sutherland, Nina Sarkissian, Yu Sun Bin, Chin Moi Chow
Royal North Shore Hospital: Andrew Chan, Aimee Lowth, Jacob Graham, William Wood, Gary Cohen, Callum Bennett, Mohammad Ahmadi
Westmead Hospital: John Wheatley, Kristina Kairaitis, Stephen Lambert, Rita Ginn, Tracey Burns
Royal Prince Alfred: Brendon Yee, Amanda Piper, Keith Wong, Kerri Melehan, Margaret Chan, David Wang, Gislaine Gauthier
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. No funding was received for this research.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Sydney Local Health District) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
PAC has an appointment to an endowed academic Chair at the University of Sydney that was established from ResMed funding, has received research support from ResMed and SomnoMed, and is a consultant to ResMed, SomnoMed, Signifier Medical Technologies, Bayer, and Sunrise Medical. All other authors (S.U., H.D., K.S., B.J.Y., K.K., J.R.W., A.P. and, P.dC.) certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers' bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ucak, S., Dissanayake, H.U., Sutherland, K. et al. Cardiac autonomic function in REM-related obstructive sleep apnoea: insights from nocturnal heart rate variability profiles. Sleep Breath (2024). https://doi.org/10.1007/s11325-024-03091-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11325-024-03091-4