Abstract
On December 7, 2022, China switched from dynamic zeroing strategy against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to reopening. A nationwide SARS-CoV-2 epidemic emerged rapidly. The effect of smoking on SARS-CoV-2 infection remains unclear. We aimed to retrospectively investigate the relationship between smoking and coronavirus disease 2019 (COVID-19) using a community-based cohort of smokers and non-smokers. We included participants from a pre-pandemic cohort with a prolonged follow-up period. Data on smoking status, body mass index, and history of other diseases were collected from health examination and consultation clinic records. Cox regression analysis was used to identify the relationship between groups and SARS-CoV-2 infection over time. We analysed 218 male patients with varied smoking statuses (46.3% current or ex-smokers; average age 68.63 ± 9.81 years). Two peaks in the epidemic were observed following the December 2022 outbreak. At the end of the second peak, non-smokers, current smokers, and ex-smokers had primary infection rates increase to 88.0%, 65.1%, and 81.0%, respectively, with a significant difference between the groups. Current smoking significantly protected against SARS-CoV-2 infection (HR 0.625, 95% CI 0.402–0.970, p = 0.036). Further analyses showed that the prevalence of pneumonia in the unvaccinated, older, diabetic, and non-smoking groups was significantly higher than that in the other groups (p < 0.05). Our study suggests a potential association between smoking and a reduced risk of SARS-CoV-2 infection and pneumonia. This indicates that nicotine and ACE2 play important roles in preventing COVID-19 and its progression. We suggest smokers use nicotine replacement therapy during hospitalization for COVID-19.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Until December 31, 2023, there have been 773,449,299 confirmed cases of coronavirus disease 2019 (COVID-19), with 6,991,842 deaths reported globally to the WHO [1]. The COVID-19 pandemic began in late 2019 and considerably impacted China. Since then, China has effectively controlled the pandemic, achieving a state of “dynamic zero” cases over 3 years. Factors such as widespread use of vaccines, expansion of medical resources, and weakened virulence of the virus have contributed to reducing deaths and harm caused by COVID-19 [2,3,4]. On December 7, 2022, China changed its management of COVID-19, switching from a dynamic zeroing stage approach to reopening. A nationwide epidemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged within a month. According to a report from the Chinese Center for Disease Control and Prevention, the number of hospitalized patients with COVID-19 in China reached a peak of 1.625 million on January 5, 2023, and has since declined. As of February 13, 2023, the number has decreased to 26,000, a 98.4% reduction from the peak [5]. This kind of outbreak on the basis of zero to go hand in hand, resulting in the number of infections peaked in a very short time, provides an excellent opportunity to observe the risk and protective factors associated with infection.
Compared to the well-established adverse effects of smoking on cancer, cardiovascular and cerebrovascular diseases, as well as chronic obstructive pulmonary disease, the risk of developing COVID-19 in patients with different smoking status remains paradoxical. Studies have reported mixed findings regarding the relationship between smoking and COVID-19 during the pandemic. A spatial analysis of 175 countries showed that the percentage of smokers was significantly and inversely associated with COVID-19 in Asian, Arab, and Pacific countries [6]. Similarly, an ecological study found a statistically significant negative association (p = 0.001) between smoking prevalence and the prevalence of COVID-19 in 38 European nations [7]. According to primary care records in the UK, it was found that current smoking was associated with an 11% lower chance of COVID-19 related death among 17.3 million adults [8]. However, several studies aimed to demonstrate that smoking increases the risk of COVID-19 and its progression [9,10,11,12,13,14,15,16,17]. In a meta-analysis of 22,939 patients with COVID-19, 2914 (12.7%) were current and former smokers. Among them, 33.5% of smokers experienced disease progression compared to 21.9% of non-smokers [17]. The evidence on the effects of smoking on COVID-19 infection and severe complications comes from three main sources: macroepidemiology, patient cohorts infected with COVID-19, and systematic reviews. During the pandemic, the latter two categories collected and assessed data from patients with COVID-19 and did not accurately represent uninfected populations. To date, no study has accurately represented the prevalence of SARS-CoV-2 infections, as well as non-infections, among smokers in general population. Therefore, this study used a community-based follow-up cohort of smokers and non-smokers established in a health care clinic prior to the pandemic to retrospectively observe the distribution characteristics of primary infection of SARS-CoV-2 among middle-aged and older community residents in China after three years of silence. Our aim was to investigate the potential association between smoking and a decreased risk of SARS-CoV-2 infection and pneumonia, as well as to explore possible preventive and therapeutic mechanisms.
Materials and methods
Ethical considerations
This study was approved by the Ethics Committee. The institutional review board waived the requirement for informed consent because the data were anonymous and this study was retrospective. This study was conducted in accordance with the principles of the Declaration of Helsinki.
Study setting
This retrospective cohort study investigated the association between smoking status and COVID-19. Participants were divided into three groups: non-smokers, current smokers, and ex-smokers. The observation period was from December 7, 2022, to June 30, 2023.
Study population
Eligible smokers and non-smokers who visited the pulmonary health consultation clinic between July 1, 2023, and December 1, 2023 were consecutively enrolled. Each patient had assessed for the presence of initial COVID-19 infection, smoking status, and COVID-19 vaccination history. The inclusion criteria were as follows: (1) male subjects aged > 50 years, (2) regularly followed up at the health care centre for more than 1 year, (3) and undergone at least one comprehensive health examination with formal history collection and chest computed tomography (CT) at the health care centre within the past 3 years. The exclusion criteria were as follows: (1) prior SARS-CoV-2 infection before December 2022, (2) history of immunodeficiency, including diagnosed haematological and autoimmune diseases, radiotherapy and chemotherapy for malignant tumours, and immunosuppressive therapy, (3) unstable conditions during the peri-observation period, such as progressive tumours, acute myocardial infarction, chronic active infections, and acute exacerbation of chronic diseases, and (4) patients with unstable or progressive respiratory system disease during the peri-observation period, including asthma with unstable symptoms, high-risk of exacerbation of chronic obstructive pulmonary disease (ECOPD), acute pulmonary embolism, interstitial lung disease (ILD) requiring antifibrotic or immunosuppressive therapy, diffuse bronchiectasis, or other lesions. A flowchart of the enrolment and exclusion process of the study population is shown in Fig. 1.
Data collection and assessment
Data on smoking status, body mass index (BMI), and history of other diseases such as diabetes and hypertension were collected from health screening information reports. COVID-19 history was collected from the records of the pulmonary health consultation clinic.
Non-smokers were defined as those who had not smoked in their lifetime and were not smoking at the time of consultation. Current smokers were defined as those who smoked regularly at the time of consultation. Ex-smokers were those who had smoked regularly in their lifetime, but not at the beginning of the observation period (December 7, 2022) for at least 6 months.
The basic pulmonary conditions of the enrolled patients were divided into mildly abnormal and normal groups according to CT findings. Mild abnormalities included local tubular bronchiectasis, pulmonary nodules after wedge resection, obsolete lesions after curing tuberculosis or other pulmonary injuries, and mild stable interstitial changes that did not reach a diagnosis of ILD.
The identification of SARS-CoV-2 infection required simultaneous respiratory and systemic symptoms with evidence of antigen or nucleic acid positivity. COVID-19 pneumonia was defined as new lung shadows appearing within 3 weeks of SARS-CoV-2 infection that was not attributable to other pulmonary infections. Therefore, occult infections or asymptomatic pneumonia may have existed among uninfected patients or non-pneumonia COVID-19 identified in this study. Primary infection refers to an initial encounter with SARS-CoV-2. Our study exclusively recorded and analysed primary infections among the participants.
Statistical analysis
The relationship between smoking status and COVID-19 infection was analysed using SPSS Modeller (V18.4, IBM, Arming, NY, USA). Normally distributed quantitative variables are shown as means ± SD, and the categorical data are presented as frequencies and percentages. Analysis of variance was used to compare measured data, and the χ2 test was used for categorical count data. Differences were considered statistically significant at p < 0.05. Cox regression analysis was used to identify the relationship between the variables and SARS-CoV-2 infection with prolonged exposure times. Survival time in the Cox regression analysis was defined as the duration of remaining uninfected, from the onset of the pandemic on December 7, 2022, to the participant's initial COVID-19 diagnosis.
Results
Basic characteristics based on smoking status
A total of 218 patients with different smoking status were included. There were 117 (53.7%) participants in the non-smoking group, 43 (19.7%) in the current smoking group, and 58 (26.6%) in the ex-smoking group. The average age was 68.63 ± 9.81 years, ranging from 51 to 94 years. Twelve (5.5%) patients had no SARS-CoV-2 vaccination, 201 (92.2%) had one to three doses, and five (2.3%) had four doses. Mean BMI was 26.00 for smokers, 25.85 for non-smokers, and 26.19 for ex-smokers. Seventy-four (33.9%) participants had mildly abnormal lung conditions, whereas 144 (66.1%) had normal lung conditions. In total, 110 (50.5%) and 64 (29.4%) patients had hypertension and diabetes, respectively. The distribution of the basic characteristics among the different smoking groups is shown in Table 1.
Prevalence of primary COVID-19 in China from December 2022 to June 2023
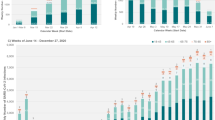
As per official forecasts, China experienced two peaks in COVID-19 since the outbreak in December 2022. The first peak occurred from December 2022 to January 2023, and the second peak emerged around May 2023, encompassing both primary and secondary COVID-19 infections. This study showed that at the end of the first peak, the primary infection rates of the current smokers, ex-smokers, non-smokers, and entire population were 58.1%, 69.0%, 74.4%, and 69.7%, respectively. At the end of the second peak, the primary infection rates in the above groups reached 65.1%, 81.0%, 88.0%, and 81.7%, respectively, and the difference among the groups was significant (p = 0.004). The changes in the new primary infection rates over time among the different groups are shown in Fig. 2.
Cox survival analysis of factors associated with overall uninfected
Univariate and multivariate Cox regression analyses were performed to determine the factors associated with SARS-CoV-2 infection over time. Only current smoking status was confirmed as an independent negative prognostic factor for SARS-CoV-2 infection (p < 0.05; Table 2). The proportion of uninfected individuals and the risk of infection over time between the different smoking groups are shown in Fig. 3.
Distribution of COVID-19 pneumonia in groups with different variables
We further analysed the distribution of the incidence of COVID-19 pneumonia among the different variable groups (Table 3). Among the participants, 18 (8.3%) were diagnosed with COVID-19 pneumonia. By comparing different variables, we found that the prevalence of pneumonia in current smokers (0%) was significantly lower than that in non-smokers (p = 0.047). Among various age groups, the prevalence of pneumonia in the ≥ 75 year old group (19%) was significantly higher than that in younger age groups (p = 0.002). Among different vaccination groups, the prevalence of pneumonia in the non-vaccinated group (33.3%) was significantly higher than that in the other two groups (p = 0.004). The prevalence of pneumonia in patients with diabetes (14.1%) was significantly higher than that in patients without diabetes (p = 0.045).
Discussion
Smoking poses a significant threat to public health and places a substantial economic burden on society. In 2019, China had 341 million smokers, constituting 24% of its population and representing one-third of global consumption. Smoking prevalence was notably high, reaching 49.7% in males and 3.54% in females [18]. In this study, the prevalence of smoking in males (46.3%) and hypertension in older individuals (50.5%) was consistent with the average levels in China [19]. However, the effect of smoking on COVID-19 remained unknown. This study therefore focused on the relationship between smoking and susceptibility to COVID-19 in relatively healthy male individuals older than 50 years, and suggests a potential association between smoking and a reduced risk of SARS-CoV-2 infection and pneumonia.
During the initial phase of the COVID-19 outbreak, a low prevalence of current smoking (6.5%, 95% CI 4.9–8.2%) was observed among patients with COVID-19 in Wuhan, China, constituting approximately one-fourth of the population smoking prevalence [20]. A systematic review of published articles indicated a lower prevalence of smoking in patients with COVID-19 than the regional average [21]. Globally, a spatial analysis involving 175 countries [6] and an ecological study across 38 European nations [7] found a significant negative association between smoking and COVID-19. However, based on the long-standing reputation that smoking is harmful to human health, some researchers have questioned the positive effects of smoking on protection against COVID-19 and disease progression [16]. Our study suggested that current smoking significantly protects against SARS-CoV-2 infection (HR 0.625, 95%CI 0.402–0.970, p = 0.036). Even ex-smoking status showed a trend of effect on protection against COVID-19 (HR 0.882, 95%CI 0.619–1.255, p = 0.485). The participants in this study were from a follow-up cohort of health care clinics. Detailed smoking history, underlying diseases, and COVID-19 vaccination status were collected during the long-term follow-up period, effectively eliminating potential unreliable factors in the context of emergency infections.
SARS-CoV-2 activates the innate immune system, leading to the release of numerous cytokines, including IL-6. This cytokine surge can increase vascular permeability, causing fluid and blood cells to migrate into the alveoli, resulting in dyspnoea and respiratory failure [22]. Nicotine has anti-inflammatory and immunomodulatory effects. It is an agonist of the cholinergic anti-inflammatory pathway that regulates host immune and inflammatory responses [23, 24]. Nicotine inhibits the production of pro-inflammatory cytokines such as TNFα, IL-1, and IL-6, without inhibiting the production of anti-inflammatory cytokines such as IL-10 [23]. Farsalinos et al. propose a hypothesis that COVID-19 appears to become a disease of the nicotinic cholinergic system. The extensive replication of the virus will disrupt the cholinergic anti-inflammatory pathway, leading to the development of a cytokine storm, with acute lung injury resulting in ARDS, coagulation disturbances, and multiorgan failure. Nicotine could potentially restore the function of the cholinergic anti-inflammatory system and prevent the cytokine storm [25]. Subsequent in silico studies have revealed a range of complexes with cholinergic agonists that may have the potential to inhibit the binding of the SARS-CoV-2 Spike glycoprotein to nicotinic acetylcholine receptors (nAChRs), thus averting dysregulation of the Nicotinic Cholinergic System [26, 27]. Based on the results of this study and the effective anti-inflammatory mechanism of nicotine, we suggest that current smokers should use nicotine replacement therapy during hospitalization due to COVID-19 to avoid further damage caused by the storm of inflammatory factors.
In smokers, the recently observed angiotensin-converting enzyme 2 (ACE2) upregulation may be an important and beneficial defence mechanism [20, 28]. ACE2 serves as a cellular entry receptor for SARS-CoV-2 [29]. Active cigarette smoking and COPD upregulate ACE2 expression in the lower airways [28]. Increased ACE2 expression and activity has also been observed in the serum of smokers [30]. ACE2 has indirect anti-inflammatory and anti-oxidative effects and may improve outcomes of critically ill patients [20, 31]. Lutchman et al. discussed the relationship among nicotine, ACE2, and SARS-CoV-2 and concluded that soluble ACE2 may even serve as a bait to neutralize the spike protein on the surface of SARS-CoV-2, offering an effective treatment for COVID-19 [32, 33]. However, this idea contradicts the well-known theory that downregulation of ACE2 reduces susceptibility to SARS-CoV-2 infection [34]. The low prevalence of infection in current smokers in this study indicates that although ACE2 is a pathway for viral entry, upregulation of the receptor in the serum or lungs may also play an important role in defence against the virus. This could explain the protective effect in both current smokers and ex-smokers, as ex-smokers may retain upregulated ACE2 in the serum or lungs.
SARS-CoV-2 mainly invades through the respiratory tract, and the innate immunity of the lung is the first recognition and defence barrier [35]. However, few studies have examined the correlation between mild lung abnormalities, including minor interstitial or parenchymal lung structure changes, and COVID-19 prognosis. The participants included in this study with a long-term follow-up period allowed us to assess this relationship. In total, 74 (33.9%) participants in our study exhibited mild lung abnormalities: 36 (48.6%) exhibited parenchymal lung structural changes and 38 (51.4%) exhibited mild stable interstitial changes. Our analyses found that these mild lung lesions did not significantly increase the risk of SARS-CoV-2 infection and pneumonia.
SARS-CoV-2 vaccines played a crucial role in controlling the COVID-19 pandemic in China [4]. The COVID-19 vaccine is efficient in preventing the development of severe and critical COVID-19 pneumonia [36]. The rate of protection against severe illness was 90.15% in patients over 60 years old [37]. Our study findings suggest a significant protective effect of vaccines in preventing pneumonia, which aligns with the previous findings. Increasing age, obesity, and general co-morbidities are associated with intensive care unit admission, need for invasive ventilation, and mortality [38,39,40]. Our study further analysed the differences in the prevalence of COVID-19 pneumonia among different groups for each variable. The prevalence of pneumonia in the unvaccinated (p = 0.004), older (p = 0.002), diabetic (p = 0.045), and non-smoking (p = 0.047) groups was significantly higher than that in the other groups.
This study had several limitations. First, because the data were collected from patients who visited the clinic for regular follow-up within a certain period, selection bias may have occurred. Additionally, the study’s limitation to male participants may impact the generalizability of the findings to females. Second, data collection was limited to a single centre, ensuring quality control but greatly limiting the study’s scale. Unaccounted confounding factors like socioeconomic status and lifestyle linked to smoking may affect outcomes. However, due to the study’s retrospective nature, unrecorded information couldn’t be retrieved for analysis. Third, subjective bias in data evaluation and collection was challenging to eliminate in this retrospective study. To mitigate bias, we opted to sample from the existing follow-up cohort and avoided relying on a single doctor for data evaluation. This study has two strengths. First, it accurately depicts the primary infection rates of the two epidemic peaks in the Chinese since the end of 2022. Second, detailed community-level data were used to demonstrate the protective effect of smoking against SARS-CoV-2 infection.
Conclusion
Our study findings suggest a potential association between smoking and a reduced risk of SARS-CoV-2 infection and pneumonia among middle-aged and older individuals in the general population during the COVID-19 pandemic in China. Two potential mechanisms were proposed to explain these observations. Firstly, the immunomodulatory effects of nicotine may have a protective impact against the virus. Additionally, the defence or neutralizing effect of ACE2 in serum and lungs may play a role in preventing viral invasion. It is important to note that while these findings are intriguing, they should be interpreted with caution due to the well-established health risks associated with smoking. We therefore suggest that smokers use nicotine replacement therapy during hospitalization for COVID-19. Furthermore, we recommend additional investigation into whether ACE2 can effectively neutralize SARS-CoV-2 at the laboratory level. Simultaneously, we anticipate prospectively including more comprehensive sociodemographic confounders and observing the long-term performance of our smoking cohort in later COVID-19 prevalence.
Data availability
The data presented in this study are available on request from the corresponding author.
References
WHO COVID-19 Dashboard (2020). Geneva: World Health Organization. https://covid19.who.int/ Accessed 31 Dec 2023
Iuliano AD et al (2022) Trends in disease severity and health care utilization during the early omicron variant period compared with previous SARS-CoV-2 high transmission periods - United States, december 2020-january 2022. MMWR Morb Mortal Wkly Rep 71(4):146–152
Cai J et al (2022) Modeling transmission of SARS-CoV-2 omicron in China. Nat Med 28(7):1468–1475
Altmann DM, Boyton RJ (2022) COVID-19 vaccination: the road ahead. Science 375(6585):1127–1132
Ge J (2023) The COVID-19 pandemic in China: from dynamic zero-COVID to current policy. Herz 48(3):226–228
Iyanda AE et al (2020) A retrospective cross-national examination of COVID-19 outbreak in 175 countries: a multiscale geographically weighted regression analysis (January 11-June 28, 2020). J Infect Public Health 13(10):1438–1445
Tsigaris P, Teixeira da Silva JA (2020) Smoking prevalence and COVID-19 in Europe. Nicotine Tob Res 22(9):1646–1649
Williamson EJ et al (2020) Factors associated with COVID-19-related death using openSAFELY. Nature 584(7821):430–436
Jackson SE et al (2021) COVID-19, smoking and inequalities: a study of 53 002 adults in the UK. Tob Control 30(e2):e111–e121
Liu W et al (2020) Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl) 133(9):1032–1038
Alqahtani JS et al (2020) Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PLoS ONE 15(5):e0233147
Kumar R et al (2021) Accumulating impact of smoking and co-morbidities on severity and mortality of COVID-19 infection: a systematic review and meta-analysis. Curr Genomics 22(5):339–352
Dessie ZG, Zewotir T (2021) Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect Dis 21(1):855
Birhanu MY, Jemberie SS (2023) Mortality rate and predictors of COVID-19 inpatients in Ethiopia: a systematic review and meta-analysis. Front Med (Lausanne) 10:1213077
Grundy EJ et al (2020) Smoking, SARS-CoV-2 and COVID-19: a review of reviews considering implications for public health policy and practice. Tob Induc Dis 18:58
Saadatian-Elahi M et al (2021) Tobacco smoking and severity of COVID-19: experience from a hospital-based prospective cohort study in Lyon. France J Med Virol 93(12):6822–6827
Patanavanich R, Glantz SA (2021) Smoking is associated with worse outcomes of COVID-19 particularly among younger adults: a systematic review and meta-analysis. BMC Public Health 21(1):1554
Collaborators GBDRF (2020) Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet 396(10258):1223–1249
Sheng CS et al (2013) Prevalence, awareness, treatment and control of hypertension in elderly Chinese. Hypertens Res 36(9):824–828
Farsalinos K, Barbouni A, Niaura R (2020) Systematic review of the prevalence of current smoking among hospitalized COVID-19 patients in China: could nicotine be a therapeutic option? Intern Emerg Med 15(5):845–852
Tajlil A et al (2020) Nicotine and smoking in the COVID-19 era. J Cardiovasc Thorac Res 12(2):136–139
Zhang C et al (2020) Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents 55(5):105954
Wang H et al (2003) Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421(6921):384–388
van Westerloo DJ et al (2005) The cholinergic anti-inflammatory pathway regulates the host response during septic peritonitis. J Infect Dis 191(12):2138–2148
Farsalinos K et al (2020) Editorial: nicotine and SARS-CoV-2: COVID-19 may be a disease of the nicotinic cholinergic system. Toxicol Rep 7:658–663
Lagoumintzis G et al (2021) Nicotinic cholinergic system and COVID-19: In silico identification of interactions between alpha7 nicotinic acetylcholine receptor and the cryptic epitopes of SARS-Co-V and SARS-CoV-2 Spike glycoproteins. Food Chem Toxicol 149:112009
Alexandris N et al (2021) Nicotinic cholinergic system and COVID-19: In silico evaluation of nicotinic acetylcholine receptor agonists as potential therapeutic interventions. Toxicol Rep 8:73–83
Leung JM et al (2020) ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Respir J 55(5):2000688
Zhou P et al (2020) A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579(7798):270–273
Nath D, Shivasekar M (2022) Role of cigarette smoking on serum angiotensin-converting enzyme and its association with inflammation and lipid peroxidation. Cureus 14(8):e27857
Garcia B et al (2023) The alternative renin-angiotensin system in critically ill patients: pathophysiology and therapeutic implications. Crit Care 27(1):453
Lutchman D (2020) Could the smoking gun in the fight against COVID-19 be the (rh)ACE-2? Eur Respir J 56(1):2001560
Batlle D, Wysocki J, Satchell K (2020) Soluble angiotensin-converting enzyme 2: a potential approach for coronavirus infection therapy? Clin Sci (Lond) 134(5):543–545
Brevini T et al (2023) FXR inhibition may protect from SARS-CoV-2 infection by reducing ACE2. Nature 615(7950):134–142
Merad M et al (2022) The immunology and immunopathology of COVID-19. Science 375(6585):1122–1127
McMenamin ME et al (2022) Vaccine effectiveness of one, two, and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong: a population-based observational study. Lancet Infect Dis 22(10):1435–1443
Fu Z et al (2023) Host protection against Omicron BA.2.2 sublineages by prior vaccination in spring 2022 COVID-19 outbreak in Shanghai. Front Med 17(3):562–575
Zhou F et al (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395(10229):1054–1062
Flook M et al (2021) Informing the public health response to COVID-19: a systematic review of risk factors for disease, severity, and mortality. BMC Infect Dis 21(1):342
Tadayon Najafabadi B et al (2023) Obesity as an independent risk factor for COVID-19 severity and mortality. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD015201
Funding
This research was supported by the National High Level Hospital Clinical Research Funding of China [2022-PUMCH-B-133].
Author information
Authors and Affiliations
Contributions
X.H. designed the study, reviewed, analysed data, and wrote the manuscript. Findings of COVID‐19 pneumonia and pre‐existing lung conditions in the lung CT scans were reviewed by X.H. (pulmonologist) and F.Z. (radiologist). F.Z. and L.L. collected and verified data and contributed to data analysis. X.N. and Z.W. contributed equally, designed the project, edited the manuscript, and supervised the study. All authors have approved the final version of this paper.
Corresponding authors
Ethics declarations
Conflict of interest
All the authors have no association that might pose a conflict of interest.
Ethical approval
This study was approved by the Ethics Committee of Peking Union Medical College Hospital (I-23PJ234).
Informed consent
Obtaining informed consent was waived by the Institutional Review Board of Peking Union Medical College Hospital because the data were anonymous and the study was retrospective.
Human and animal rights
All procedures performed in the present study were in accordance with the principles outlined in the 1964 Helsinki Declaration and its later amendments. The study was approved by the Ethics Committee of Peking Union Medical College Hospital (I-23PJ234).
Consent for publication
Obtaining informed consent was waived by the ethics/ IRB committee because the data were anonymous and this study was retrospective.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hou, X., Zheng, F., Lu, L. et al. Protecting effects of smoking against COVID-19: a community-based retrospective cohort study in middle- and older-aged adults. Intern Emerg Med (2024). https://doi.org/10.1007/s11739-024-03713-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11739-024-03713-5