Abstract
Prunus serotina is one of the most widespread alien tree species in temperate European forests. Although numerous studies revealed both negative and positive impacts on native ecosystems, only a few assessed the quantitative impacts along a species abundance gradient, based on aboveground biomass of per capita effects. Here we studied how alpha diversity of forest understory changes with increasing aboveground biomass of P. serotina. In Wielkopolska National Park (W Poland) we established 92 plots in both nutrient-rich and poor Pinus sylvestris stands. We assessed the effects of P. serotina proportion on understory species composition using non-metric multidimensional scaling (NMDS). We also assessed the effects of P. serotina proportion on alpha diversity metrics using generalized mixed-effects linear models, accounting also for stand age, soil C:N ratio, and light availability. P. serotina biomass ranged from 0.0 to 34.29 Mg ha−1 (with an average of 5.70 ± 0.83 Mg ha−1), comprising 0% to 17.26% (with an average of 2.93 ± 0.43%) of total woody species biomass. NMDS revealed a lack of P. serotina proportion effects on understory species composition. We found small positive impacts of P. serotina proportion on total species richness (+ 8.8 species along a gradient with a 10% increase of P. serotina biomass proportion) and functional richness (+ 0.08). The positive impacts of P. serotina were mostly related to the confounding effect of two factors: an increase in light availability facilitates both the development of understory plants as well as the growth of P. serotina.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive tree species are one of the major threats to forest ecosystems (Mack et al. 2000; Richardson 2008; Brondízio et al. 2019). This matter is not improved by the fact that we live in the age of global climate change that is likely to accelerate the expansion of many alien species and simultaneously contribute to the reduction of some native tree ranges (Dukes and Mooney 1999; Brondízio et al. 2019). Alien tree species can positively or negatively affect some ecosystem services, according to the CICES framework (Haines-Yong and Potschin 2012; Milanović et al. 2020) like regulation of ecosystem services e.g. climate regulation, water regulation, protection against erosion, provisioning ecosystem services e.g. timber production, cultural ecosystem services e.g. scientific interests and inspiration (Castro-Diez et al. 2019). They can also enhance some services, especially increasing total primary production of ecosystems, as invasive tree species usually grow faster than native ones (Tecco et al. 2010; Grotkopp et al. 2010). However, they can reduce other ecosystem services or provide disservices, e.g. causing allergies in humans (Castro-Diez et al. 2019). Invasive species can also reduce native species biodiversity (Parker et al. 1999; Woziwoda et al. 2019; Wohlgemuth et al. 2022; García et al. 2023).
The success of invasive species depends on many factors e.g. species invasiveness (Alpert et al. 2000; Pyšek et al. 2014) connected with the biology and ecology of the studied species, ecosystem resistance/habitat invasibility (Knight et al. 2008), and propagule pressure (Lonsdale 1999; Vanhellemont et al. 2009). Invasive plants can modify the environment (Richardson et al. 2000; Corenblit et al. 2014; Aerts et al. 2017) to a lesser or greater degree through changes in microclimate, soil properties and decomposition rate, or limitation of light availability (García et al. 2023). Therefore, we can hypothesize that higher biomass of invaders should intensify that transformation, environmental resource uptake should happen faster and interactions, especially competition with native species, would escalate (Kumschick et al. 2015). For that reason, finding compromise, including determining the quantitative impact of invasive species, is particularly important in nature conservation and forest management planning and gives guidance to managers on where and under what conditions invasive species stock should be limited (Sapsford et al. 2020).
Very often forest managers decide to eradicate invasive species by harvesting or chemical means, but the results are not as passable as they intended or may even be counterproductive (Namura-Ochalska and Borowa 2015; Nyssen and Vanhellemont 2016). For the management and conservation of forest ecosystems, assessment of the threshold abundance of invasive species, i.e., level when species start negative impacts on conservation or management targets, it is necessary to design management plans. We can quantify the impacts of invasive species in terms of biomass, cover, or number of individuals. If we convert the presented indicators to a per unit of area basis, we will obtain an impact per capita, and in the context of the impacts on ecosystems or the economy, we refer to this phenomenon as the per capita effect (Parker et al. 1999; Pearse et al. 2019; Sapsford et al. 2020). This allows to ascertain when the impacts of invaders are unexpected and may be more or less negative. In many cases, preliminary treatment at a site with low invader density is cost-effective. It should be considered that some species, distinguished by the ability to form stump and root suckers, achieve even greater strengthening after conducting a treatment, which can additionally contribute to decreasing biodiversity. The impact of the removal of invasive species on post-treatment density is therefore non-linear, and its effects may vary (Sapsford et al. 2020).
Assessment of per capita effects of biological invasion expressed as cover, density, or biomass can give us an answer regarding what quantity of invasive tree specimens negatively affect the forest, and emphasize which target indicators are mostly threatened by a particular invasive species (Parker et al. 1999; Schooler et al. 2006). Most of the previous studies were based on species cover (e.g. Chabrerie et al. 2008; Czortek et al. 2023; Wiatrowska et al. 2023), that does not always well reflect the quantity of the species in an ecosystem and its impacts (Younginger et al. 2017). For P. serotina Chabrerie et al. (2008, 2010a) revealed scaling of its impacts along a cover gradient while Vanhellemunt et al. (2009) revealed that low density and basal area of P. serotina decreases its spread rate. However, only a few studies assessed per capita effects for invasive trees and shrubs based on biomass or stand basal area (Dickie et al. 2011; García et al. 2023). Biomass is a measure of species quantity based on amount of occupied space and resources utilized for construction of tissues (Younginger et al. 2017). Therefore, it is a more complex metric than cover, which only informs about a two-dimensional projection of the plant canopy, without accounting for layers of canopy that transmit light to the forest floor. Density also does not scale with impact, as smaller plants occupy less space than larger ones, which have higher impacts by producing more leaves. Other metrics like basal area also do not account for differences in resource utilization, related with e.g. wood density, which also makes comparison less effective. Therefore, we expect that higher biomass of P. serotina will be connected with higher foliage mass, affecting nutrient cycling and light interception. As the studied species is not an overstory tree, these effects can be not as large in magnitude as in such species.
One of the most frequent invasive trees in European temperate forests is Prunus serotina Ehrh., commonly named wild black cherry or rum cherry (Muys et al. 1992; Starfinger et al. 2003; Campagnaro et al. 2018; Wohlgemuth et al. 2022). Introduced in the 17th century (Muys et al. 1992; Starfinger et al. 2003), it was planted on a large scale by foresters in the 19th and 20th centuries, and is still increasing its range. The natural area of occurrence for this species is the eastern and southern parts of North America. There are four varieties of Prunus serotina, including P. serotina var. salicifolia (Kunth) Koehne, invasive in South America, and Prunus serotina var. serotina, invasive in Europe. Artificially planted P. serotina in European countries was supposed to fulfill productive, phytomeliorative, and biocenotic roles, but over time it showed its invasive character (Muys et al. 1992; Starfinger et al. 2003; Godefroid et al. 2005). The reason for the success of this species is a small number of natural enemies, ease of root and stump sucker creation, the abundance of flowering and fruiting, ornithochory (Starfinger et al. 2003; Deckers et al. 2008), and relatively fast growth. P. serotina can grow in both fertile and poor sites, and also exists well in different light and water conditions (Starfinger et al. 2003; Otręba 2016).
Over the years, the approach to P. serotina population management in the forests of Western Europe, e.g., in the Netherlands, has changed. Various strategies for managing the populations of this species were proposed by (Nyssen and Vanhellemont 2016; Nyssen et al. 2019). According to them, management procedures should be adapted to specific stands, depending on their characteristics like e.g., species composition, age, and stand density. In the Netherlands, P. serotina can reach sizes and quality that are satisfactory from the point of view of forest management. It is difficult to find similar references to Poland, as these countries differ in annual sum of precipitation and mean annual temperature (Metzger et al. 2005). The historical background and management of forests is also different. As a rule, the possibilities of using P. serotina in Poland for timber production are low, and it is used as fuelwood. This might be related to lack of silvicultural treatments designed for the species (Jaworski 2011).
Effects of P. serotina on native ecosystems have been widely studied previously, revealing no impacts (Verheyen et al. 2007; Gentili et al. 2019) and negative effects (Godefroid et al. 2005) of P. serotina on alpha diversity of plants (Wohlgemuth et al. 2022). A recent review by Wohlgemuth et al. (2022) summarized 16 studies of P. serotina effects on biodiversity or soil chemistry. However, none of them accounted for invasive species quantity, but instead made comparisons between invaded and non-invaded plots. Therefore, we aimed to assess the relationships among P. serotina abundance metrics and per capita effects of Prunus serotina, based on proportion of stand biomass, on temperate forest understory alpha diversity at three levels: taxonomic, phylogenetic, and functional. Taxonomic diversity is the best recognized among these three diversity facets (Olden et al. 2018). Significantly less attention has been paid to phylogenetic and functional diversity. However, phylogenetic diversity of evolutionary lineages, as an indicator of biodiversity, is becoming increasingly important (Tucker et al. 2017). The higher number of evolutionarily and functionally distinct species in a community is related to their higher resistance to natural disturbances (Swenson and Enquist 2009). We might therefore expect that this resilience can also apply to the ability of ecosystems to resist invasions. High phylogenetic diversity also tells us about the uniqueness of individual communities and about the evolutionary potential, and is a deeper description of the concept of biological diversity by entering into relationships between individual species (Swenson and Enquist 2009). Impact of P. serotina on functional diversity is also less recognized (Chabrerie et al. 2010b), while this biodiversity facet explains variability of functional traits, i.e. characteristics describing physiology, morphology and ecology of species (Violle et al. 2007) and can be used to conclude about the prevailing processes shaping community species composition.
As species cover does not contain information about the quantity of foliage, that is related to biomass via allometric power laws (Enquist and Niklas 2002; Poorter et al. 2015), we assume that quantity of invasive species based on biomass would be a good predictor of its impact on Scots pine forests. We expect that a higher proportion of P. serotina in stand biomass should intensify environmental changes, based on previous studies describing the impacts on various factors. Aerts et al. (2017) proved that P. serotina changes biogeochemical cycles, especially the contents of C, N, and P in soil and litter. On the other hand, (Horodecki et al. 2019) revealed very high decomposition rates of P. serotina leaves. Several studies have also reported that the decline in biodiversity is related to the reduction of light availability by P. serotina (Starfinger et al. 2003; Chabrerie et al. 2010b; Jagodziński et al. 2019). As these effects depend on leaf mass (amount of litterfall) and area (shading), and leaf quantity increases with biomass (Enquist and Niklas 2002; Poorter et al. 2015), we can expect that higher P. serotina biomass will reflect higher leaf mass and area, and therefore have higher effects on soil nutrients, shaping understory vegetation, excluding species typical of nutrient-poor habitats. For that reason we also expect that the negative effects will depend on the fertility of the habitat and probably will be more harmful on nutrient-poor sites (Chmura 2004; Halarewicz 2011). For that reason, we expect that a higher proportion of P. serotina can lead to a decrease of acidophilous species, which are functionally and phylogenetically distinct, and in turn, to a decrease in alpha diversity of understory vegetation. Light conditions on the forest floor, which are created by the presence and abundance of black cherry will therefore determine plant species composition. In contrast, we can also expect that P. serotina invasion is a passenger rather than a driver of past disturbances or natural stand dynamics, leading to increase in canopy openness (Chabrerie et al. 2008; Jagodziński et al. 2019), resulting from the confounding effect of light availability.
Materials and methods
Study area
Wielkopolska National Park is a Polish national park with an area of 7584.93 ha, located by the Warta River, 15 km from the city of Poznań in Western Poland. The terrain of the park was closely related to the activity of continental glaciers, so specific elements like lakes, moraines, and eskers are visible. Forests are the dominant ecosystems in the park, mainly Galio-Carpinetum Oberd. 1957, Calamagrostio arundinaceae-Quercetum (Hartm. 1934) Scam. Et Pass. 1959, Querco roboris-Pinetum (W. Mat. 1981) J. Mat 1988 and Leucobryo-Pinetum (W. Mat. 1962) W. Mat. Et J. Mat. 1973 (Balcerkiewicz and Pawlak 2001). Wielkopolska NP has one of the biggest problems with biological invasions (Purcel 2009) of all National Parks in Poland (Bomanowska et al. 2014), which makes it a perfect place for these studies. Although there are no data about time since P. serotina introduction in Wielkopolska NP, in management plans the oldest trees are estimated to be 70 years old, and the oldest recorded Prunus serotina specimens in Wielkopolska National Park are also approximately 84 years old (as of 2013, www.bdl.lasy.gov.pl). However, older and thicker specimens occur singly or in small groups in stands in the subcanopy layer and their density is not high enough to significantly affect the share of this species in the total stand biomass. Additionally, age is not always correlated with the thickness and height of individual P. serotina specimens, as they grow in different habitat and light conditions. Determining the exact length of exposure of individual stands to invasion is usually estimated based on forest management plans, developed for 10 or 20 years, and therefore not very precise. However, since the establishment of the national park in 1957 alien tree species had been no longer introduced but spread spontaneously (Purcel 2009). Abundance of alien tree species in Wielkopolska NP is also modified by their removal, as part of active protection. In Wielkopolska National Park, Prunus serotina has been removed as part of the management plan, however, for P. serotina its effectiveness is low, and the species is abundant in numerous stands.
Data collection
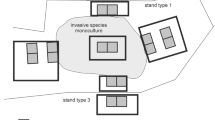
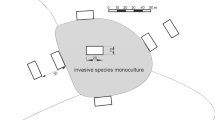
In this study, we analyzed the subset of data analyzed by Dyderski and Jagodziński (2021), covering 92 study plots in Scots pine-dominated stands without (control) and with P. serotina cover > 50%. All plots were established in a systematic design within 21 blocks of 18 plots, with a central plot, and eight other plots distributed at the edge of the invaded stand and 30 m from the edge, along cardinal directions (N, E, S, and W; Fig. 1). For this study we used only plots with Scots pine dominance, excluding plots where other alien tree species had more than 5% of total stand biomass. Based on dominant vegetation and soil type we divided Scots pine forests into two types: nutrient-poor, occupying potential habitats of subcontinental Scots pine forests mainly on rustic soils, and nutrient-rich – secondary communities of Scots pine, planted on fertile habitats, typical of mesic and rich oak forests, mainly on luvisols and cambisols.
Scheme of the study plot blocks (Dyderski and Jagodziński 2021). The central plot was established in the center of a stand with P. serotina dominance in the shrub layer, the closer set of four plots were established at the edge of invaded stand, and the further set – 30 m from the edge, to capture variability in invasive species biomass
Within each study plot we recorded vascular plant species and cover using a modified 9-degree Braun-Blanquet scale (Barkman et al. 1964) in July of each study year (2015–2018) and we averaged species cover at the study plot level. We standardized the taxonomic nomenclature following GBIF (2019), as suggested by Seebens et al (2020). We also obtained information about stand age from national park forest management plans. In August 2015 we measured diffusive non-interceptance (DIFN) within each plot, as a measure of light availability (Machado and Reich 1999) and we collected topsoil samples to obtain soil C:N ratio (Dyderski and Jagodziński 2021).
Assessment of invasion gradient
For study plots we also assessed stand structures. We measured the diameter at breast height (DBH) of each individual of each woody species including the bark. Individuals with a DBH greater than or equal to 5 cm were directly measured. However, for individuals with a DBH less than 5 cm, we recorded the total number of individuals present and then we assumed 2.5 cm as their DBH (Dyderski and Jagodziński 2021). We calculated all tree and shrub species biomass using previously published allometric models (Dyderski and Jagodziński 2021). From these measurements we calculated the proportion of P. serotina in total aboveground biomass, as a measure of invasive species abundance. We also measured P. serotina biomass, density (number of individuals per hectare), leaf area index (m2 m-2) and we estimated cover of this species with 5% accuracy. For leaf area index measurements, we used allometric models and values of specific leaf area presented by Dyderski and Jagodziński (2021).
This work investigated the impact on forest ecosystems dominated by Pinus sylvestris in fertile and poor habitats. Therefore, we chose compact stands, avoiding gaps in the stand, so that individual areas could be compared. We were not interested in the gaps in the tree stand, in which P. serotina reaches the largest size. All the above premises had a significant impact on the fact that the invasion gradient obtained by us, defined as the share of P. serotina biomass in relation to the total stand biomass, is low, i.e., max. 17.3% (with an average of 3%) (Fig. S1). In addition, it suggests that this complex is difficult to study due to anthropogenic disturbances. It is easier to draw unequivocal conclusions in natural forests, where human interference was minimal. However, in terms of planning protective measures in the future, the inclusion of this forest complex is an interesting enrichment of knowledge.
Calculation of diversity indices
In each plot we calculated total species richness, Shannon’s diversity index, alien species richness (sum of archaeophytes and neophytes), and ancient forest indicator species (AFIS). We distinguished ancient forest indicator species (AFIS), as a guild of forest specialists (Peterken 1974; Orczewska 2009) using a checklist provided by (Dzwonko and Loster 2001). Among 212 recorded species, 33 (15.6%) were AFIS while 41 were alien species (19.3%). To calculate phylogenetic and functional diversity we acquired a phylogenetic tree of recorded plant species from the V.phylo.maker package (Jin and Qian 2022). We acquired functional traits (Table 1) from trait databases: LEDA (Kleyer et al. 2008), BIEN (Maitner et al. 2018), BiolFlor (Klotz et al. 2002), and Pladias (Wild et al. 2019), while ecological indicator values came from Ellenberg and Leuschner (2010). Due to incomplete trait data, we decided to impute missing data rather than omit them in analyses (see Pyšek et al. 2015) by random forest-based imputation (Penone et al. 2014), implemented in the missForest package (Stekhoven and Buhlmann 2012), supported by phylogenetic eigenvectors (Diniz-Filho et al. 1998), obtained using the PVR package (Santos 2018).
To characterize phylogenetic diversity we used Faith’s phylogenetic diversity (PD, the sum of phylogenetic tree branch lengths, representing all species present in the community) and mean pairwise phylogenetic distance (MPD), calculated using the PhyloMeasures package (Tsirogiannis and Sandel 2016). For functional diversity we calculated functional richness (FRic), expressing the quantity of plant functional types present in a community and functional dispersion (FDis), expressing the size of a community species traits hypervolume within the functional trait space (Mason et al. 2005; Laliberté and Legendre 2010; Pla et al. 2011). We calculated them using the FD package (Laliberté et al. 2014). Details of calculations were published by Dyderski and Jagodziński (2021).
Data analysis
We conducted all analyses using R (R Core Team 2023) and R Studio software. We assessed relationships between each pair of abundance characteristics using linear regression. We used non-metric multidimensional scaling (NMDS) ordination with the Bray-Curtis matrix to analyze species composition. We developed NMDS using the vegan package (Oksanen et al. 2018). We used Wisconsin double-standardized and square-root transformation of species cover in NMDS. We ran particular iterations of NMDS as the best result reached a convergence. To assess how species composition gradients are related to forest characteristics and biodiversity indices we passively fit these variables as vectors on the ordination using the envfit (Oksanen et al. 2018) function from the vegan package (Oksanen et al. 2018). This function fits environmental vectors onto an ordination, aiming to maximize the correlation of site scores with corresponding environmental variables. For continuous variables this is equal to fitting a linear trend surface (plane in 2D), presented by showing its gradient (direction of steepest increase) using an arrow (Oksanen et al. 2018).
We used generalized linear mixed-effects models (GLMMs) to assess the effects of P. serotina proportion on biodiversity indices, accounting also for light availability (DIFN), stand age, soil C:N, and forest type as covariates that could affect per capita effects. To account for dependency within blocks of plots we assigned block ID as a random intercept. For functional FDis, FRic, PD, MPD, and Shannon’s index we assumed Gaussian distributions, while for count data (species richness, AFIS richness, alien species richness) we assumed Poisson distributions. We ensured lack of collinearity among predictors using a correlation matrix (Fig. S2). After that, we assessed Akaike’s Information Criterion, corrected for small sample size (AICc) of full and reduced models, to select the model with the lowest AICc as the final model. We used the MuMIn package to perform that selection using the dredge (Bartoń 2017) function (Bartoń 2017). We also reported AICc0, i.e. AICc of the null (intercept and random effect only) model, as well as marginal (R2m) and conditional (R2c) coefficients of determination, indicating the amount of variance explained by fixed effects-only and both random and fixed effects, respectively (Nakagawa and Schielzeth 2013). To isolate relationships between the dependent variable and single predictors we used marginal responses, i.e. predictions accounting for a constant (mean) value of all remaining predictors, implemented in the ggeffects package (Lüdecke 2018). That way marginal response to P. serotina proportion serves as quantification of per capita effects.
Results
Relationships between metrics of P. serotina abundance
Prunus serotina occurred in 64 of 92 study plots, and its biomass ranged from 0.0 to 34.29 Mg ha-1 (with an average of 5.70 ± 0.83 Mg ha-1), comprising 0% to 17.26% (with an average of 2.93 ± 0.43%) of total stand biomass (Fig. S1). Density of this species ranged from 0 to 7560 ind. ha-1, with an average of 1075 ± 165 ind. ha-1, LAI from 0.00 to 13.82 m2 m-2 (average 1.55 ± 0.26 m2 m-2), and cover from 0 to 90% (average 18.9 ± 2.5%). Among abundance metrics, P. serotina biomass and proportion of biomass were the most correlated with other abundance metrics (Fig. 2), in contrast to the cover and density of P. serotina.
Effect of P. serotina on understory species composition
NMDS revealed a lack of difference in understory species composition between nutrient-poor and rich P. sylvestris forests (Fig. 3), as red and blue points representing the two groups were grouped similarly with overlapping ranges. The main axis of differentiation is related to a diagonal of ordination space, indicating plots with high soil C:N ratio, older stands, and high functional dispersion (low values of NMDS1 and high values of NMDS2), opposite to sites with a high light availability (DIFN), positively correlated to P. serotina proportion, alien, AFIS, and overall species richness, and functional richness. Vectors representing MPD and Faith’s PD are perpendicular to the other characteristics on the graph, indicating a lack of correlation with other variables. According to the permutation test for fitness of environmental variables to non-metric multidimensional scaling (NMDS) axes we found significant results only in the case of species richness (p = 0.001), functional richness (p = 0.019) and alien species richness (p = 0.039) (Table 2). It must be highlighted that the NMDS ordination has a high stress index (stress = 0.20), close to the upper limit of values typical of meaningful ordination, therefore we should be careful about drawing conclusions.
Non-metric multidimensional scaling (NMDS) analysis, based on Wisconsin double-standardized and square-root-transformed species composition on study plots (n = 92), with passively fit dependent and independent variables (grey arrows). Stress = 0.20, RMSE = 0.02, max residual = 0.19. Red dots represent nutrient-rich P. sylvestris forests, while blue – nutrient-poor. Gray vectors represent passively fitted variables (for abbreviations and goodness of fit see Table 2)
Effect of P. serotina on understory diversity
For Shannon’s diversity index, FDis, and MPD, the best fit GLMMs were null models (Fig. 4; Table 3). We found the small effects of P. serotina proportion on AFIS, alien, and overall species richness, as well as on Fric. Species richness depended on P. serotina proportion (p < 0.0001), light availability (DIFN; p = 0.0097), C:N ratio (p = 0.0109), and stand age (p = 0.0678). An increase of P. serotina proportion by 10% increased species richness by 9 species. An increase of DIFN by 0.1 increased species richness by 3, an increase of stand age from 40 to 140 years old by 7, while an increase in C:N ratio by 10 decreased species richness by 4.5. AFIS richness increased with increasing P. serotina proportion (by 1.4 for a 10% increase) and decreased with increasing C:N ratio (by 1.3 species for an increase of 10 units). Although the effect of P. serotina proportion was statistically insignificant (p = 0.054), the effect size was biologically relevant. Similarly, alien species richness increased by 1.4 with a P. serotina proportion increase by 10% (p = 0.063) and decreased with an increase of 10 C:N ratio units by 1.5 species (p = 0.086). FRic increased by 0.08 with a P. serotina proportion increase by 10% and by 0.6 with a DIFN increase by 0.1. Both Faith’s PD and MPD decreased by 0.3 with a DIFN increase of 0.1, however, this result is both statistically and ecologically insignificant.
Scatterplots for dependent and independent variables, by forest type (red dots for nutrient-rich and blue dots for nutrient-poor sites). Lines indicate the marginal response curves for statistically significant relationships (Table 3). Grey ribbons indicate 95% confidence intervals of prediction
Discussion
Assessment of different metrics of P. serotina abundance revealed positive correlations among all of them, but with different strengths of this relationship. Leaf area index, related to light interception, was more explained by biomass and proportion of P. serotina in stand biomass than by density or cover, with higher dispersion of residuals. This can be connected with the two-dimensional assessment of cover, that does not account for the number of effective leaf layers. Our study revealed small per capita effects of an invasive P. serotina quantity gradient on understory alpha diversity. Calculated effects were positive for AFIS, alien, and overall species richness, as well as for functional richness, which is partially in line with our previous assessment of invaded and non-invaded stands (Dyderski and Jagodziński 2021). Effects for alien and AFIS richness were statistically insignificant, but effect sizes were biologically relevant. In this assessment, we did not confirm the effects on phylogenetic diversity indices, Shannon’s index, and functional dispersion. In the previous study we found a positive effect on species richness on nutrient-rich sites and no impact on nutrient-poor sites, but in this study, considering the quantitative gradient – described by the proportion of biomass, this effect turns out to be more ambiguous and more difficult to interpret. Most of our study plots were characterized by a low ratio of the invader to total biomass, due to the biology of P. serotina, reaching a shrub form in Central European forests. This should be considered when interpreting the results. Within 75% of study plots, P. serotina comprised < 5% of total stand aboveground biomass (30% of plots were uninvaded), and the maximum value was 17.26% (34.29 Mg ha-1). Our results are in line with the study on Pinus nigra in New Zealand, which positively affected plant species richness up to the quantity of approximately 100 Mg ha-1 (Dickie et al. 2011), however, over that threshold its impact was negative. Similarly, for Kunzea ericoides, at small abundance the plant species richness increased significantly and the decrease at a higher abundance was not intensive (Dickie et al. 2011). P. nigra contributed to an increase in non-native plant species from 28 to 35% and K. ericoides did not affect this feature in any way (Dickie et al. 2011; Sapsford et al. 2020; Catford et al. 2022). In the Patagonian Araucaria araucana forest, invasive Pinus contorta decreased species richness and abundance of both native and non-native species over a gradient from 0 to 156 Mg ha-1 (García et al. 2023).
Differences in effect sizes and directions between our study and the abovementioned sources resulted from accounting for different parts of the invader abundance gradient. Catford et al. (2022) explained that studies covering low and high ranges of the same independent variable can lead to opposite conclusions, due to context-dependence. This is the case for our study, resulting from the biology of P. serotina in Europe. This species is usually low in height and acts as a shrub, usually with multiple thin stems (Starfinger et al. 2003; Urban et al. 2009), which is reflected in weak correlations between cover and density with its biomass. For that reason, P. serotina in Poland is unable to comprise a significant proportion of stand biomass, despite the large cover. This strategy makes the assessment of the per capita effects of P. serotina more difficult. Another crucial point is the confounding effects of P. serotina invasion and light availability. Prunus serotina benefits from high light availability (Closset-Kopp et al. 2011; Jagodziński et al. 2019), simultaneously decreasing light transmittance into the forest floor. For that reason, our results do not allow for separating the effects of P. serotina invasion from canopy openness, due to the observational nature of our study. We can suspect that in our study, the level of light limitation by P. serotina was not enough to reduce understory alpha diversity. The positive impacts of DIFN on both species and functional richness support that claim. Possibly, P. serotina has not been present long enough for its impacts to be fully developed, and negative influences will occur in the future (Strayer et al. 2006). Although the slope of regression line could be affected by a single outlier with DIFN > 0.2, accounting for block as random effect can decrease its effect on the total estimate.
The effect of P. serotina density on understory vegetation was previously studied in the forests of northern France by Chabrerie et al. (2008, 2010). The study of Chabrerie et al. (2010) investigated the influence of P. serotina on the functional diversity of understory plant communities in forests with Pinus sylvestris, Fagus sylvatica Quercus robur, and Q. petraea. The authors found significant variations in functional diversity of plant compositions between invaded and non-invaded stands. On uninvaded stands there were more specialist species, but on invaded stands P. serotina contributed to the increase in the abundance of specialists. Environmental conditions of the understory connected with P. serotina presence benefit specific species, and they could outgrow generalists. The authors also found that the impacts of light availability and soil properties were independent of those from the invasion process. Two years earlier, Chabrerie et al. (2008) revealed that stands devoid of P. serotina created conditions in which the generalists and specialists co-existed with each other. They were also more resistant to the dominance of individual specialist species in communities. It must be emphasized that the P. serotina crown cover (%) used by Chabrerie et al. (2008) used as an abundance indicator is different from the percentage share of P. serotina in the total aboveground biomass (including stem, branches, and leaves) as in our work. In our case, considering the crown cover alone, the biomass range would be greater, because there were a lot of plots with a relatively high numbers of very thin individuals. In Wielkopolska National Park, where individuals of different ages and sizes grow on individual plots, considering the aboveground biomass best reflects the variability of the gradient and, consequently, the invader effects on the compared plots.
In Wielkopolska National Park, there are often various disturbances, both natural and anthropogenic. Disturbances cause co-consequent changes in the amount/biomass of the invasive species, as well as understory species (Chabrerie et al. 2008; Jagodziński et al. 2019; Oshima and Takahashi 2020). The problem is to estimate which factor – disturbances or the mere presence of the invader – has more significant effects on biodiversity, or in other words, to what extent the invasions have intensified the disturbance. Moreover, it is important how large the area affected by the disturbance was, what is the ecology of the invader and what was the understory species composition before the disturbance. As we mentioned in the materials and methods, to the extent possible we avoided plots in stands with visible disturbances. However, it was difficult to avoid small-scale, single-tree disturbances, such as single snags and windthrows.
Many previous studies confirm that not all habitats are equally susceptible to the entry and expansion of invasive species (Godefroid et al. 2005; Halarewicz 2011). Individual places differ in biotic resistance. Native species occurring for a long time in given conditions are well adapted to them and compete more effectively with invasive species for environmental resources. The high species richness of native species positively affects such resilience, occupying potential niches for alien species, according to the biotic resistance hypothesis (Elton 1958; Iannone et al. 2016). Biotic resistance (Elton 1958) is also related to the propagule pressure to some extent (Colautti and MacIsaac 2004). High propagule pressure can overcome biotic resistance systems, especially if the invasive species has features that help it to quickly occupy new niches. A large variety of native species can therefore effectively regulate competition with invaders and limit their spread even at high propagule pressure (Richardson et al. 2000).
The contrasting concept, the biotic acceptance hypothesis, assumes that sites suitable for hosting numerous native species will be also colonized by alien species (Stohlgren et al. 2006). In our study we found a positive effect of P. serotina proportion in stand biomass on the richness of all species, alien species, and forest specialists (AFIS). This might suggest that within this ecosystem P. serotina acts as a passenger rather than a driver of changes, taking advantage of canopy openness. Similarly, P. serotina established successfully in sites with higher understory species richness (Knight et al. 2008). Another study revealed a positive relationship between invasive Rhamnus cathartica and forest understory biodiversity in Minnesota, USA (Knight and Reich 2005). These authors found a positive relationship between R. cathartica cover and native plant richness in oak forests. However, R. cathartica had a negative impact on native species cover. The positive relationship between P. serotina proportion and alien species richness is in line with previous studies (Halarewicz and Żołnierz 2014) and also supports the biotic acceptance hypothesis. One of the explanations is the fact that P. serotina transformed the environment, e.g. enriching the soil with nitrogen (Aerts et al. 2017; Horodecki et al. 2019), which was beneficial for other alien species. Perhaps it is also the effect of resource availability favoring both P. serotina and other invasive species.
Another important element in the context of understory vegetation is soil fertility (Chmura 2004; Halarewicz 2011). In optimal and undisturbed coniferous forest habitats, there are mainly species that like the acidic pH of the soil and litter. In the case of P. serotina, with quickly decomposing leaves (Horodecki et al. 2019) providing a large amount of nitrogen into the soil which strongly modifies local trophic conditions, can lead to the retreat of native acidophilous species. Moreover, this modification supports the regeneration of P. serotina, as a species with a wide range of ecological requirements, adapted to both acidic and neutral soils. In fertile habitats with a large number of native species, the presence of an invader often does not reduce biodiversity and may even enrich it. In this study, the changes in the soil nutrient content associated with P. serotina presence were more consequential on the poor sites than on the fertile sites. P. serotina enriches the soil with phosphorus and nitrogen. This transformation is stressful for the understory and the plants typical for acidic habitats can retreat. The regional species pool in the areas surrounding the studied stands can also affect the results of our study. In Wielkopolska National Park, Scots pine stands are usually dispersed across the landscape mosaic, surrounded by other forest types, rich in understory species, which can migrate into pine stands and increase the understory species richness. For that reason, in study plots we found species typical of both acidophilous and fertile broadleaved forests, meadows, roadsides, as well as ruderal plants. Our previous work revealed that in poor habitats with pine, alpha diversity decreased, while in fertile habitats with pine, such a strong relationship was not shown. In the same work, we also showed negative effects on beta functional and phylogenetic diversity of pine coniferous forests. In fertile pine habitats, P. serotina reduced all three types of diversity studied (Dyderski and Jagodziński 2021). The studies of other authors also showed larger negative impacts of P. serotina on poor than on fertile sites (Godefroid et al. 2005; Halarewicz 2011; Halarewicz and Żołnierz 2014). This claim can also be supported by trends for AFIS richness, as these species are mostly typical of mesic and fertile broadleaved forests, and the list also covers forest generalists not occurring in Scots pine forests (Dzwonko and Loster 2001). The potential retreat of acidophilous AFIS (e.g. Vaccinium myrtillus) could be observed in the case of light limitation (Woziwoda et al. 2019), but in our case we did not observe it, which supports our claims about lack of light availability limitation.
Conclusions
In this study, we showed small per capita effects of P. serotina, based on its proportion in stand aboveground biomass. We revealed that abundance metrics of P. serotina are correlated. Due to its lifeform (shrub or subcanopy tree), the studied species revealed a relatively short invasion gradient. However, we found small positive effects on taxonomic and functional richness. These effects are confounding, as they might reflect either the effect of P. serotina presence or conditions required for its growth, i.e. increased light availability, which undermines the concluding power of our study. Light conditions, invasion level and understory compositions were linked with the history of stand development and disturbances. Knowledge about the relationship between the quantity/biomass of invaders and understory biodiversity is crucial for nature conservation and forest management in invaded sites. For that reason, monitoring and decision-making should account for a wider spectrum of invasive species effects, and future studies should also account for a wider gradient of invasive species abundance, as well as various environmental contexts. Moreover, assessment of the invasion gradient needs to account for local differences in the studied species life history, e.g., maximal size, which affects its impacts and local management sensitivity thresholds.
Data Availability
Data supporting the results are available as a supplementary materials to the article.
References
Aerts R, Ewald M, Nicolas M, et al (2017) Invasion by the Alien Tree Prunus serotina Alters Ecosystem Functions in a Temperate Deciduous Forest. Front Plant Sci 8:. https://doi.org/10.3389/fpls.2017.00179
Alpert P, Bone E, Holzapfel C (2000) Invasiveness, invasibility and the role of environmental stress in the spread of non-native plants. Perspect Plant Ecol Evol Syst 3:52–66. https://doi.org/10.1078/1433-8319-00004
Balcerkiewicz S, Pawlak G (2001) Szata roślinna Wielkopolskiego Parku Narodowego. In: Wojterska M (ed) Szata roślinna Wielkopolski i Pojezierza Południowopomorskiego. Przewodnik sesji terenowych 52. Zjazdu Polskiego Towarzystwa Botanicznego. 24–28 września 2001. Bogucki Wydawnictwo Naukowe, Poznań, pp 250–254
Barkman JJ, Doing H, Segal S (1964) Kritische Bemerkungen und Vorschláge zur quantitativen Vegetationsanalyse. Acta Bot Neerlandica 13:394–419. https://doi.org/10.1111/j.1438-8677.1964.tb00164.x
Bartoń K (2017) MuMIn: multi-model inference. https://doi.org/10.32614/CRAN.package.MuMIn
Bomanowska A, Kirpluk I, Adamowski W, et al (2014) Problem inwazji roślin obcego pochodzenia w polskich parkach narodowych. In: Otręba A, Michalska-Hejduk D (eds) Inwazyjne gatunki roślin w Kampinoskim Parku Narodowym. Kampinoski Park Narodowy, Izabelin, pp 9–14
Brondízio ES, Settele J, Díaz S, Ngo HT (eds) (2019) The global assessment report of the intergovernmental science-policy platform on biodiversity and ecosystem services. Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (IPBES), Bonn
Campagnaro T, Brundu G, Sitzia T (2018) Five major invasive alien tree species in European Union forest habitat types of the Alpine and Continental biogeographical regions. J Nat Conserv 43:227–238. https://doi.org/10.1016/j.jnc.2017.07.007
Castro-Diez P, Vaz AS, Silva JS et al (2019) Global effects of non-native tree species on multiple ecosystem services. Biol Revievs 94:1477–1501. https://doi.org/10.1111/brv.12511
Catford JA, Wilson JRU, Pyšek P et al (2022) Addressing context dependence in ecology. Trends Ecol Evol 37:158–170. https://doi.org/10.1016/j.tree.2021.09.007
Chabrerie O, Verheyen K, Saguez R, Decocq G (2008) Disentangling relationships between habitat conditions, disturbance history, plant diversity, and American black cherry (Prunus serotina Ehrh.) invasion in a European temperate forest. Divers Distrib 14:204–212. https://doi.org/10.1111/j.1472-4642.2007.00453.x
Chabrerie O, Loinard J, Perrin S et al (2010) Impact of Prunus serotina invasion on understory functional diversity in a European temperate forest. Biol Invasions 12:1891–1907. https://doi.org/10.1007/s10530-009-9599-9
Chmura D (2004) Penetration and naturalisation of invasive alien plant species (neophytes) in woodlands of the Silesian Upland (Southern Poland). Nat Conserv 60:3–11
Closset-Kopp D, Saguez R, Decocq G (2011) Differential growth patterns and fitness may explain contrasted performances of the invasive Prunus serotina in its exotic range. Biol Invasions 13:1341–1355. https://doi.org/10.1007/s10530-010-9893-6
Colautti RI, MacIsaac HJ (2004) A neutral terminology to define ‘invasive’ species: Defining invasive species. Divers Distrib 10:135–141. https://doi.org/10.1111/j.1366-9516.2004.00061.x
Corenblit D, Steiger J, Tabacchi E et al (2014) Ecosystem engineers modulate exotic invasions in riparian plant communities by modifying hydrogeomorphic connectivity: ecosystem engineers modulate exotic invasions. River Res Appl 30:45–59. https://doi.org/10.1002/rra.2618
Czortek P, Królak E, Borkowska L, Bielecka A (2023) Effects of surrounding landscape on the performance of Solidago canadensis L. and plant functional diversity on heavily invaded post-agricultural wastelands. Biol Invasions. https://doi.org/10.1007/s10530-023-03050-2
Deckers B, Verheyen K, Vanhellemont M et al (2008) Impact of avian frugivores on dispersal and recruitment of the invasive Prunus serotina in an agricultural landscape. Biol Invasions 10:717–727. https://doi.org/10.1007/s10530-007-9164-3
Dickie IA, Yates GW, John MGSt, et al (2011) Ecosystem service and biodiversity trade-offs in two woody successions. J Appl Ecol 48:926–934. https://doi.org/10.1111/j.1365-2664.2011.01980.x
Diniz-Filho JAF, de Sant’Ana CER, Bini LM (1998) An eigenvector method for estimating phylogenetic inertia. Evolution 52:1247–1262. https://doi.org/10.1111/j.1558-5646.1998.tb02006.x
Dukes JS, Mooney HA (1999) Does global change increase the success of biological invaders? Trends Ecol Evol 14:135–139. https://doi.org/10.1016/S0169-5347(98)01554-7
Dyderski MK, Jagodziński AM (2021) Impacts of invasive trees on alpha and beta diversity of temperate forest understories. Biol Invasions 23:235–252. https://doi.org/10.1007/s10530-020-02367-6
Dzwonko Z, Loster S (2001) Wskaźnikowe gatunki roślin starych lasów i ich znaczenie dla ochrony przyrody i kartografii roślinności. Prace Geograficzne 178:119–132
Ellenberg H, Leuschner C (2010) Vegetation Mitteleuropas mit den Alpen in ökologischer, dynamischer und historischer Sicht. UTB, Stuttgart
Elton CS (1958) The Ecology of Invasions by Animals and Plants. Springer, US, Boston, MA
Enquist BJ, Niklas KJ (2002) Global allocation rules for patterns of biomass partitioning in seed plants. Science 295:1517–1520. https://doi.org/10.1126/science.1066360
García RA, Fuentes-Lillo E, Cavieres L, et al (2023) Pinus contorta Alters Microenvironmental Conditions and Reduces Plant Diversity in Patagonian Ecosystems. Diversity 15:. https://doi.org/10.3390/d15030320
GBIF (2019) Global Biodiversity Information Facility. http://www.gbif.org/
Gentili, Ferrè, Cardarelli, et al (2019) Comparing Negative Impacts of Prunus serotina, Quercus rubra and Robinia pseudoacacia on Native Forest Ecosystems. Forests 10:842. https://doi.org/10.3390/f10100842
Godefroid S, Phartyal SS, Weyembergh G, Koedam N (2005) Ecological factors controlling the abundance of non-native invasive black cherry (Prunus serotina) in deciduous forest understory in Belgium. For Ecol Manag 210:91–105. https://doi.org/10.1016/j.foreco.2005.02.024
Grotkopp E, Erskine-Ogden J, Rejmánek M (2010) Assessing potential invasiveness of woody horticultural plant species using seedling growth rate traits: Seedling RGR and woody species invasiveness. J Appl Ecol 47:1320–1328. https://doi.org/10.1111/j.1365-2664.2010.01878.x
Haines-Yong R, Potschin M (2012) CICES Version 4: Response to Consultation. University of Nottingham, Nottingham, Centre for Environmental Management
Halarewicz A (2011) The reasons underlying the invasion of forest communities by black cherry, Prunus serotina and its subsequent consequences. For Res Pap 72:267–272. https://doi.org/10.2478/v10111-011-0026-5
Halarewicz A, Żołnierz L (2014) Changes in the understorey of mixed coniferous forest plant communities dominated by the American black cherry (Prunus serotina Ehrh.). For Ecol Manag 313:91–97. https://doi.org/10.1023/A:1023208215796
Horodecki P, Nowiński M, Jagodziński AM (2019) Advantages of mixed tree stands in restoration of upper soil layers on postmining sites: A five-year leaf litter decomposition experiment. Land Degrad Dev 30:3–13. https://doi.org/10.1002/ldr.3194
Iannone BV, Potter KM, Hamil K-AD et al (2016) Evidence of biotic resistance to invasions in forests of the Eastern USA. Landsc Ecol 31:85–99. https://doi.org/10.1007/s10980-015-0280-7
Jagodziński AM, Dyderski MK, Horodecki P et al (2019) Light and propagule pressure affect invasion intensity of Prunus serotina in a 14-tree species forest common garden experiment. NeoBiota 46:1–21. https://doi.org/10.3897/neobiota.46.30413
Jaworski A (2011) Hodowla lasu. III. Charakterystyka hodowlana drzew i krzewów leśnych. Powszechne Wydawnictwo Rolnicze i Leśne, Warszawa
Jin Y, Qian H (2022) V.PhyloMaker2: An updated and enlarged R package that can generate very large phylogenies for vascular plants. Plant Divers 44:335–339. https://doi.org/10.1016/j.pld.2022.05.005
Kleyer M, Bekker RM, Knevel IC et al (2008) The LEDA Traitbase: a database of life-history traits of the Northwest European flora. J Ecol 96:1266–1274. https://doi.org/10.1111/j.1365-2745.2008.01430.x
Klotz S, Kühn I, Durka W (2002) BIOLFLOR – Eine Datenbank zu biologisch-ökologischen Merkmalen der Gefäßpflanzen in Deutschland. Schriftenreihe Für Veg. Bundesamt Für Naturschutz Bonn
Knight KS, Reich PB (2005) Opposite relationships between invasibility and native species richness at patch versus landscape scales. Oikos 109:81–88. https://doi.org/10.1111/j.0030-1299.2005.13639.x
Knight KS, Oleksyn J, Jagodzinski AM et al (2008) Overstorey tree species regulate colonization by native and exotic plants: a source of positive relationships between understorey diversity and invasibility: Tree species, diversity, and invasibility. Divers Distrib 14:666–675. https://doi.org/10.1111/j.1472-4642.2008.00468.x
Kumschick S, Gaertner M, Vilà M et al (2015) Ecological Impacts of Alien Species: Quantification, Scope, Caveats, and Recommendations. Bioscience 65:55–63. https://doi.org/10.1093/biosci/biu193
Laliberté E, Legendre P (2010) A distance-based framework for measuring functional diversity from multiple traits. Ecology 91:299–305. https://doi.org/10.1890/08-2244.1
Laliberté E, Legendre P, Shipley B (2014) FD: measuring functional diversity (FD) from multiple traits, and other tools for functional ecology https://doi.org/10.32614/CRAN.package.FD
Lonsdale WM (1999) Global patterns of plant invasions and the concept of invasibility. Ecology 80:1522–1536. https://doi.org/10.1890/0012-9658(1999)080[1522:GPOPIA]2.0.CO;2
Lüdecke D (2018) ggeffects: Tidy Data Frames of Marginal Effects from Regression Models. J Open Source Softw 3:772. https://doi.org/10.21105/joss.00772
Machado J-L, Reich PB (1999) Evaluation of several measures of canopy openness as predictors of photosynthetic photon flux density in deeply shaded conifer-dominated forest understory. Can J for Res 29:1438–1444. https://doi.org/10.1139/x99-102
Mack RN, Simberloff D, Mark Lonsdale W et al (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10:689–710. https://doi.org/10.1890/1051-0761(2000)010[0689:BICEGC]2.0.CO;2
Maitner BS, Boyle B, Casler N et al (2018) The bien r package: A tool to access the Botanical Information and Ecology Network (BIEN) database. Methods Ecol Evol 9:373–379. https://doi.org/10.1111/2041-210X.12861
Mason NWH, Mouillot D, Lee WG, Wilson JB (2005) Functional richness, functional evenness and functional divergence: the primary components of functional diversity. Oikos 111:112–118. https://doi.org/10.1111/j.0030-1299.2005.13886.x
Metzger MJ, Bunce RGH, Jongman RHG et al (2005) A climatic stratification of the environment of Europe: A climatic stratification of the European environment. Glob Ecol Biogeogr 14:549–563. https://doi.org/10.1111/j.1466-822X.2005.00190.x
Milanović M, Knapp S, Pyšek P, Kühn I (2020) Linking traits of invasive plants with ecosystem services and disservices. Ecosyst Serv 42:101072. https://doi.org/10.1016/j.ecoser.2020.101072
Muys B, Maddelein D, Lust N (1992) Ecology, practice and policy of black cherry (Prunus serotina Ehrh.) management in Belgium. Silva Gandav 57:. https://doi.org/10.21825/sg.v57i0.885
Nakagawa S, Schielzeth H (2013) A general and simple method for obtainingR2fromgeneralized linear mixed-effects models. Methods Ecol Evol 4:133–142. https://doi.org/10.1111/j.2041-210x.2012.00261.x
Namura-Ochalska A, Borowa B (2015) Walka z czeremchą amerykańską Padus serotina (Ehrh.) Borkh. w leśnictwie Rózin w Kampinoskim Parku Narodowym; ocena skuteczności wybranych metod. In: Krzysztofiak L, Krzysztofiak A (eds) Inwazyjne gatunki obcego pochodzenia zagrożeniem dla rodzimej przyrody red. Stowarzyszenie “Człowiek i Przyroda,” Krzywe, pp 127–142
Nyssen B, Koopmans G, Ouden J (2019) Beslisboom Amerikaanse vogelkers. Wageningen, Bosgroenpern
Nyssen B, Vanhellemont M (2016) 5.6 Integrating black cherry in forest management in the Netherlands and Belgium. In: Krumm F, Vítková L (eds) Introduced tree species in European forests: opportunities and challenges. European Forest Institute, Freiburg, pp 362–372
Oksanen J, Blanchet F, Kindt R (2018) “vegan” 2.3.3. - Community Ecology Package. https://doi.org/10.32614/CRAN.package.vegan
Olden JD, Comte L, Giam X (2018) The Homogocene: a research prospectus for the study of biotic homogenisation. NeoBiota 37:23–36. https://doi.org/10.3897/neobiota.37.22552
Orczewska A (2009) The impact of former agriculture on habitat conditions and distribution patterns of ancient woodland plant species in recent black alder (Alnus glutinosa (L.) Gaertn.) woods in south-western Poland. For Ecol Manag 258:794–803. https://doi.org/10.1016/j.foreco.2009.05.021
Oshima K, Takahashi K (2020) Forest disturbances promote invasion of alien herbaceous plants: a comparison of abundance and plant traits between alien and native species in thinned and unthinned stands. Biol Invasions. https://doi.org/10.1007/s10530-020-02283-9
Otręba A (2016) Czeremcha amerykańska Padus serotina (Ehrh.) Borkh. In: Obidziński A, Kołaczkowska E, Otręba A (eds) Metody zwalczania obcych gatunków roślin występujących na terenie Puszczy Kampinowskiej. Kampinowski Park Narodowy, Izabelin, pp 73–88
Parker IM, Simberloff D, Lonsdale WM et al (1999) Impact: toward a framework for understanding the ecological effects of invaders. Biol Invasions 1:3–19. https://doi.org/10.1023/A:1010034312781
Pearse IS, Sofaer HR, Zaya DN, Spyreas G (2019) Non-native plants have greater impacts because of differingper-capita effects and nonlinear abundance–impact curves. Ecol Lett 22:1214–1220. https://doi.org/10.1111/ele.13284
Penone C, Davidson AD, Shoemaker KT et al (2014) Imputation of missing data in life-history trait datasets: which approach performs the best? Methods Ecol Evol 5:961–970. https://doi.org/10.1111/2041-210X.12232
Peterken GF (1974) A method for assessing woodland flora for conservation using indicator species. Biol Conserv 6:239–245. https://doi.org/10.1016/0006-3207(74)90001-9
Pla L, Casanoves F, Di Rienzo J (2011) Quantifying functional biodiversity. Springer Dordr
Poorter H, Jagodziński AM, Ruiz-Peinado R et al (2015) How does biomass distribution change with size and differ among species? An analysis for 1200 plant species from five continents. New Phytol 208:736–749. https://doi.org/10.1111/nph.13571
Purcel A (2009) Obce gatunki drzew i krzewów w Wielkopolskim Parku Narodowym - ich występowanie i rola w biocenozach parku. Morena 14:35–191
Pyšek P, Jarošík V, Pergl J et al (2014) Temperate trees and shrubs as global invaders: the relationship between invasiveness and native distribution depends on biological traits. Biol Invasions 16:577–589. https://doi.org/10.1007/s10530-013-0600-2
Pyšek P, Manceur AM, Alba C et al (2015) Naturalization of central European plants in North America: species traits, habitats, propagule pressure, residence time. Ecology 96:762–774. https://doi.org/10.1890/14-1005.1
R Core Team (2023) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Richardson DM (2008) Forestry Trees as Invasive Aliens. Conserv Biol 12:18–26. https://doi.org/10.1111/j.1523-1739.1998.96392.x
Richardson DM, Pysek P, Rejmanek M et al (2000) Naturalization and invasion of alien plants: concepts and definitions. Divers Distrib 6:93–107. https://doi.org/10.1046/j.1472-4642.2000.00083.x
Santos T (2018) PVR: phylogenetic eigenvectors regression and phylogentic signal-representation curve. https://doi.org/10.32614/CRAN.package.PVR
Sapsford SJ, Brandt AJ, Davis KT et al (2020) Towards a framework for understanding the context dependence of impacts of non-native tree species. Funct Ecol 34:944–955. https://doi.org/10.1111/1365-2435.13544
Schooler SS, McEvoy PB, Coombs EM (2006) Negative per capita effects of purple loosestrife and reed canary grass on plant diversity of wetland communities. Divers Distrib 12:351–363. https://doi.org/10.1111/j.1366-9516.2006.00227.x
Seebens H, Clarke DA, Groom Q et al (2020) A workflow for standardising and integrating alien species distribution data. NeoBiota 59:39–59. https://doi.org/10.3897/neobiota.59.53578
Starfinger U, Kowarik I, Rode M, Schepker H (2003) From Desirable Ornamental Plant to Pest to Accepted Addition to the Flora? – the Perception of an Alien Tree Species Through the Centuries. Biol Invasions 5:323–335. https://doi.org/10.1023/B:BINV.0000005573.14800.07
Stekhoven DJ, Buhlmann P (2012) MissForest–non-parametric missing value imputation for mixed-type data. Bioinformatics 28:112–118. https://doi.org/10.1093/bioinformatics/btr597
Stohlgren TJ, Jarnevich C, Chong GW, Evangelista PH (2006) Scale and plant invasions: a theory of biotic acceptance. Preslia 78:405–426
Strayer DL, Eviner VT, Jeschke JM, Pace ML (2006) Understanding the long-term effects of species invasions. Trends Ecol Evol 21:645–651. https://doi.org/10.1016/j.tree.2006.07.007
Swenson NG, Enquist BJ (2009) Opposing assembly mechanisms in a Neotropical dry forest: implications for phylogenetic and functional community ecology. Ecology 90:2161–2170. https://doi.org/10.1890/08-1025.1
Tecco PA, Díaz S, Cabido M, Urcelay C (2010) Functional traits of alien plants across contrasting climatic and land-use regimes: do aliens join the locals or try harder than them? J Ecol 98:17–27. https://doi.org/10.1111/j.1365-2745.2009.01592.x
Tsirogiannis C, Sandel B (2016) PhyloMeasures: a package for computing phylogenetic biodiversity measures and their statistical moments. Ecography 39:709–714. https://doi.org/10.1111/ecog.01814
Tucker CM, Cadotte MW, Carvalho SB et al (2017) A guide to phylogenetic metrics for conservation, community ecology and macroecology: A guide to phylogenetic metrics for ecology. Biol Rev 92:698–715. https://doi.org/10.1111/brv.12252
Urban J, Tatarinov F, Nadezhdina N et al (2009) Crown structure and leaf area of the understorey species Prunus serotina. Trees 23:391–399. https://doi.org/10.1007/s00468-008-0288-6
Vanhellemont M, Verheyen K, Keersmaeker L et al (2009) Does Prunus serotina act as an aggressive invader in areas with a low propagule pressure? Biol Invasions 11:1451–1462. https://doi.org/10.1007/s10530-008-9353-8
Verheyen K, Vanhellemont M, Stock T, Hermy M (2007) Predicting patterns of invasion by black cherry (Prunus serotina Ehrh.) in Flanders (Belgium) and its impact on the forest understorey community. Divers Distrib 13:487–497. https://doi.org/10.1111/j.1472-4642.2007.00334.x
Violle C, Navas M-L, Vile D et al (2007) Let the concept of trait be functional! Oikos 116:882–892. https://doi.org/10.1111/j.0030-1299.2007.15559.x
Wiatrowska B, Kurek P, Moroń D et al (2023) Linear scaling – negative effects of invasive Spiraea tomentosa (Rosaceae) on wetland plants and pollinator communities. NeoBiota 81:63–90. https://doi.org/10.3897/neobiota.81.95849
Wild J, Kaplan Z, Danihelka J, et al (2019) Plant distribution data for the Czech Republic integrated in the Pladias database. Preslia 91:1–24. https://doi.org/10.23855/preslia.2019.001
Wohlgemuth T, Gossner MM, Campagnaro T et al (2022) Impact of non-native tree species in Europe on soil properties and biodiversity: a review. NeoBiota 78:45–69. https://doi.org/10.3897/neobiota.78.87022
Woziwoda B, Dyderski MK, Jagodziński AM (2019) Effects of land use change and Quercus rubra introduction on Vaccinium myrtillus performance in Pinus sylvestris forests. For Ecol Manag 440:1–11. https://doi.org/10.1016/j.foreco.2019.03.010
Younginger BS, Sirová D, Cruzan MB, Ballhorn DJ (2017) Is Biomass a Reliable Estimate of Plant Fitness? Appl Plant Sci 5:1600094. https://doi.org/10.3732/apps.1600094
Acknowledgements
The study was financed by National Science Centre, Poland, under project no. 2015/19/N/NZ8/03822 entitled: ‘Ecophysiological and ecological determinants of invasiveness of trees and shrubs with the examples of Padus serotina, Quercus rubra and Robinia pseudoacacia’. The study was partially supported by the Institute of Dendrology, Polish Academy of Sciences. MKD acknowledges support from the Foundation for Polish Science (FNP) from the START scholarship. We are thankful to three anonymous Reviewers for valuable comments on the earlier draft of the study.
Author information
Authors and Affiliations
Contributions
Conceptualization, M.K.D and A.M.J.; methodology, S.B., M.K.D. and A.M.J.; investigation, M.K.D.; formal analysis S.B., M.K.D.; writing—original draft preparation, S.B., M.K.D.; writing—review and editing, M.K.D., A.M.J.; funding acquisition, M.K.D. and A.M.J.
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bury, S., Jagodziński, A.M. & Dyderski, M.K. In search of per capita effects of Prunus serotina Ehrh. invasion on temperate forest understory alpha diversity. Biologia (2024). https://doi.org/10.1007/s11756-024-01766-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11756-024-01766-7