Abstract
Introduction
Failed Back Surgery Syndrome (FBSS) presents a formidable challenge, marked by the persistence of chronic lower back pain and leg pain despite undergoing surgical interventions. Multicolumn spinal cord stimulation (m-SCS) has recently emerged as a promising therapeutic strategy for addressing the pain associated with FBSS. This meta-analysis aims to study the efficacy of m-SCS in mitigating chronic back and leg pain among patients with FBSS.
Methods
A comprehensive search of electronic databases (PubMed, Web of Science, Scopus, Cochrane Library) was conducted to identify relevant studies published up to October 25th, 2023. Inclusion criteria encompassed randomized controlled trials and cohort studies evaluating the outcomes of m-SCS in patients with FBSS. The primary outcome measured was the Visual Analog Scale (VAS) score for low back and leg pain at baseline, six months, and 12 months.
Results
A total of eight studies, including 271 patients, were analyzed. At six months, there was a statistically significant reduction in the VAS scores for low back pain (MD, 4.76; 95% CI, 3.78 to 5.74) and leg pain (MD, 4.41; 95% CI, 2.93 to 5.90) compared to baseline. Similarly, at 12 months, there was a statistically significant reduction in the VAS scores for low back pain (MD, 4.77; 95% CI, 4.34 to 5.20) and leg pain (MD, 2.78; 95% CI, 0.72 to 4.85) compared to baseline.
Conclusion
m-SCS effectively manages chronic back and leg pain in FBSS patients, providing sustained pain relief. Studies with more extended follow-up periods and qualitative analysis for the functional outcomes and overall improvement for the patients with FBSS are recommended.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Failed Back Surgery Syndrome (FBSS) is a condition characterized by persistent lumbar spinal pain, which can manifest as both axial back pain and radicular leg pain [1]. This pain can persist despite surgical intervention or appear after surgical intervention, often due to causes such as persistent nerve root compression, permanent nerve root injury, arachnoiditis, discitis, facet joint pain, sacroiliac joint pain, incorrect initial diagnoses, and altered biomechanics leading to joint leading and muscular dystrophy [1, 2]. The incidence of FBSS is increasing, with rates ranging from 5 to 50% following spinal surgery, depending on the evaluation criteria [3,4,5]. This increase is in parallel with the progressive demographic aging of the population and the consequent rise in spinal degenerative changes [6].
FBSS remains a major health problem and a significant economic burden, underscoring the need for continued research and innovation in its treatment [4, 7]. Spinal Cord Stimulation (SCS) has been established as an effective treatment for FBSS-associated back pain and leg pain [8], with multicolumn lead and high-frequency SCS techniques showing particular efficacy [9]. Kumar et al. found that combining SCS with conventional medical management yielded better results than using conventional medical management alone [10]. Moreover, North et al. [11] reported that SCS was more effective than reoperation.
Recent advancements in SCS technology have revolutionized the management of chronic pain syndromes such as FBSS. Traditional SCS systems have been challenged by limitations in targeting complex pain patterns, often leading to suboptimal outcomes [9]. The introduction of Multicolumn spinal cord stimulation (m-SCS) represents a significant leap forward, allowing for more precise electrical field shaping and targeted pain relief. This technology not only offers a higher degree of customization in pain management but also potentially reduces the occurrence of unwanted stimulation side effects [12]. By directly addressing the multidimensional nature of pain experienced by FBSS patients, m-SCS provides a more robust framework for understanding and manipulating the spinal cord's response to electrical intervention [13]. The adoption of m-SCS in clinical practice is supported by a growing body of evidence suggesting improved outcomes in both pain control and functional abilities of patients, underscoring the need for a systematic review of its efficacy and safety [12].
Developing a new generation of multicolumn leads designed to target new stimulation territories represents a promising advancement in treating FBSS. These leads, which must be placed surgically, allow transverse electric fields and classical longitudinal fields, offering many more programming options and the potential for improved pain management [12, 9]. However, despite these advancements, the BP component remains a significant limiting factor for improving the quality of life in FBSS patients [13, 14].
Therefore, this meta-analysis aims to assess the efficacy of m-SCS on low back and leg pain in failed back surgery.
Methods and Materials
Protocol and Registration
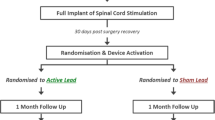
We conducted a systematic review and meta-analysis. The meta-analysis was conducted to determine the efficacy of m-SCS on back and leg pain relief in patients with FBSS. The study is performed and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement (PRISMA) [15]. The study also followed the guidelines for systematic reviews for trials of interventions for neck and back pain and related spinal disorders, as published by the Cochrane Collaboration [16]. A priori, the study protocol was registered and published in the online International Prospective Register of Systematic Reviews (PROSPERO) database (CRD42023489753).
Search Strategy
The literature search was conducted with a dedicated biomedical information specialist. The electronic databases PubMed, Web of Science, Scopus, and Cochrane Central were searched from inception through December 25th, 2023. The search strategy consisted of controlled terms and text words for m-SCS, back pain, leg pain, FBSS, and randomized controlled trials (RCTs). A broad search filter was applied to identify all the studies by using the following search strategy: (Multicolumn spinal cord stimulation OR Multi-column spinal cord stimulation OR Three-column spinal cord stimulation OR Five-column spinal cord stimulation) AND (Back pain OR Chronic back pain OR Low back pain) AND (Failed back surgery syndrome OR FBSS OR Failed spinal cord surgery OR Spine surgery).
The PRISMA diagram shows the search process and study selection (Fig. 1).
Study Selection and Eligibility Criteria
Two reviewers (HA and IS) independently screened the title and abstract according to a standardized protocol. We have decided to include only patients with m-SCS location after at least one failed back surgery to assess the back and leg pain. RCTs published in peer-reviewed international journals, with sufficient qualitative and quantitative analysis data, were included. Exclusion criteria were mono-column-SCS without FBSS, case reports and series, conference papers, posters, duplicate papers, and animal studies. We screened the full text for the back and leg pain assessment with the VAS score and the improvement at six months and 12 months after the stimulation from the baseline score. The senior author resolved disagreements.
Data Extraction and Data Items
Data were extracted independently by the same two reviewers (HA and IS) with the senior author as consensus, including author name, year of publication, study design, baseline characteristics, VAS score at baseline and 6-months, lead characteristics (number, level, and position), the definition of successful stimulation and the improvement of VAS score. The primary outcome was the VAS score at baseline and six months. Secondary outcomes included the lead characteristics (number, level, and position), VAS score at 12 months, and other methods for pain assessment.
Risk of Bias Assessment
Two authors (HA and IS) independently assessed the quality of each included study in strict accordance with the modified Newcastle–Ottawa Scale (NOS) for the cohort studies [17] and the Risk of Bias 2 (RoB 2) tool for randomized trials [18]. The senior author resolved disagreements.
Statistical Analysis
All data in this meta-analysis were calculated and pooled using (RevMan 5.4.1). A meta-analysis was conducted when two or more studies measured the same long-term pain outcome parameters. To assess the degree of correlation between m-SCS implantation and outcome parameters for continuous outcomes, mean difference (MD) and the associated 95% confidence intervals (CIs) were utilized; for dichotomous outcomes, odds ratio (OR) and 95% CI were employed. A Z-test was used to evaluate the combined MD and OR's statistical significance. As a result, a P-value of less than 0.05 was deemed statistically significant.
In addition, a significant statistical heterogeneity was measured using a Q-test. A fixed-effect model was chosen for outcome data showing low heterogeneity (I2 ≤ 30%); a random-effects model was used for other data.
Results
Study Selection
The search yielded 118 studies, with 77 remaining after duplication check. After title/abstract screening, 31 studies remained. Of the 31 studies that underwent a full-text screening, 23 were excluded because they did not meet the selection criteria (Fig. 1).
Study Characteristics
A total of 8 studies were included and summarized in (Table 1) and five studies were used for the meta-analysis, comprising results from 168 and 171 patients for 6-month and 12-month follow-ups, respectively.
The studies were conducted in 4 countries, most of them in Europe (N = 6, 75%). One study (12.5%) was conducted on multiple continents. Six studies were conducted in a single center (75%). The number of centers in the remaining studies ranged from three to 12. A controlled design was used in five studies: randomized (n = 2) and nonrandomized (n = 3). Controlled studies used a variation of SCS (n = 4) or usual care (n = 1) as a comparator. The remaining studies used an uncontrolled design (n = 3, 37.5%). A summary of the clinical characteristics of the included studies is found in Table 1.
Risk of Bias
Of the two RCTs assessed with the ROB-2 tool, one was classified as “Low risk” and the second as” some concerns” for risk of bias (Fig. 2). In the cohort studies, 4 of the six studies assessed with the NOS tool were classified as “high quality” for risk of bias, whereas the remaining two studies were classified as having “moderate quality” for risk of bias (Table 2).
Meta-Analysis
At six months, there was a statistically and clinically significant reduction in the VAS scores for low back pain (MD = 4.76; 95% CI, 3.78 to 5.74) with high heterogeneity (I2 = 88%, p < 0.001) (Fig. 3) and leg pain (MD = 4.41; 95% CI, 2.93 to 5.90) versus the baseline with high heterogeneity (I2 = 93%, p < 0.001) (Fig. 4). In both analyses, heterogeneity was not resolved, and results remain consistent during the sensitivity analysis.
Also, at 12 months, there was a statistically and clinically significant reduction in the VAS scores for low back pain (MD = 4.77; 95% CI, 4.34 to 5.20) and data was homogenous (I2 = 16%, p = 0.30) (Fig. 5) and leg pain (MD = 2.78; 95% CI, 0.72 to 4.85) versus the baseline with high heterogeneity (I2 = 91%, p < 0.001) (Fig. 6). Heterogeneity was not resolved, and results remain consistent during the sensitivity analysis.
Discussion
We found a statistically significant reduction in the VAS scores for both low back and leg pain at six months compared to the baseline. This reduction was also observed at 12 months, indicating a sustained effect.
In all studies, VAS scores were improved for low back pain after 6 and 12 months. A different observation was for the VAS scores for the leg pain. While all studies showed improvement in leg pain after 6 and 12, only one study, Rigoard [19], showed improvement without statistical significance (MD, 0.58; 95% CI, -0.84 to 2.00). Despite the lower sample of patients, it was sufficient to show a significant improvement in VAS scores for back pain. Regarding this, the authors also mentioned that they could not exclude the possibility of a potential loss of efficacy over time. This could necessitate the identification of new spatial targets of stimulation located under deliberately inactive contacts of the initially implanted SCS lead. Therefore, long-term follow-up would be necessary to determine any potential loss of efficacy over time. It is essential to highlight that most of the studies included in this research (Table 1) also reported improvements in quality-of-life measures and functional outcomes observed in the m-SCS intervention group. These findings underscore m-SCS as an effective treatment option for FBSS patients, offering them sustained pain relief and potentially enhancing their quality of life.
A previous meta-analysis on SCS in FBSS conducted by Kurt et al. [20] incorporated two studies [21, 22]. The authors observed a significant reduction in low back pain at 3, 6, and 12 months compared to the baseline, with weighted mean differences (WMD) of 3.52 (95% CI, 3.10–3.94, p < 0.01), 2.25 (95% CI, 0.40–4.11, p = 0.02), and 3.16 (95% CI, 1.58–4.75, p < 0.01), respectively. Similarly, for leg pain, there was a significant decrease at 3, 6, and 12 months compared to the baseline, with WMDs of 4.40 (95% CI, 3.97–4.82, p < 0.01), 3.25 (95% CI, 1.70–4.80, p < 0.01), and 4.01 (95% CI, 3.68–4.34, p < 0.01), respectively.
In addition, a comprehensive meta-analysis conducted by Taylor et al. [7] (n = 3025) evaluated the effectiveness of SCS and FBSS in alleviating chronic back and leg pain, considering their combined effects. The average pain relief reported across studies was 58% (95% CI: 53% to 64%, random effects) at a mean follow-up duration of 24 months. The study suggested that SCS successfully mitigated pain, regardless of the location of chronic back and leg pain. This review endorses SCS as an efficacious treatment for chronic back and leg pain, predominantly leg pain, irrespective of whether the patient has undergone back surgery previously. Notably, they discovered that the duration of pain is the only predictor of the level of pain relief following SCS [7].
A mixed method analysis conducted by Witkam et al. [23] included qualitative data from 13 patients who had SCS implants for FBSS, with a duration ranging from 3 to 20 years. Follow-up interviews conducted 25 to 35 months post-implantation revealed that 11 of these 13 patients reported reduced pain intensities, and all 11 were satisfied with the outcome of SCS. Prior to the operation, all participants anticipated a decrease in pain intensity. Quantitative analysis showed that the median baseline pain intensity score was 8.0 (6.0–9.0), and the median expected post-SCS pain intensity score was 5.0 (1.0–10.0). Despite the absence of precise pain data, patients experienced a statistically significant improvement in activity and mobility, with p-values of 0.009 for walking and 0.004 for daily activities.
The findings from this systematic review and meta-analysis have significant clinical implications for the treatment of FBSS using m-SCS. Our results demonstrate a substantial reduction in both back and leg pain, as indicated by the changes in VAS scores at six and twelve months. These outcomes suggest that m-SCS could be considered more proactively in the treatment algorithm for patients with FBSS who have not responded to conventional therapies, including single-column SCS. Furthermore, the ability of m-SCS to target specific pain territories more effectively might lead to more personalized pain management strategies, potentially reducing the need for pharmacological interventions and their associated side effects. This could encourage a shift in clinical practice guidelines towards earlier consideration of m-SCS in the treatment pathway. Additionally, the sustained pain relief observed suggests that m-SCS may also help improve the overall quality of life and functional capabilities of patients, underscoring its value as a long-term treatment option. Adoption of m-SCS based on these findings could ultimately lead to better patient management and resource utilization in healthcare systems dealing with chronic pain conditions.
Strength and Limitations
We provide up-to-date evidence of the beneficial impact of SCS on back and leg pain relief in patients with FBSS. Still, there are some limitations. First, the number of studies is small despite including studies from various designs. Second, it is essential to highlight that even though there was observed heterogeneity, previous studies on the same topic showed high heterogeneity as well as noted in Taylor et al. research substantial statistical heterogeneity (P < 0.0001) [7]. Still, we performed a sensitivity analysis, and the results were consistent.
Conclusion
Our systematic review and meta-analysis confirm that m-SCS significantly reduces chronic back and leg pain in patients with FBSS. Despite promising results, the limited number of studies and their inherent heterogeneity call for cautious interpretation and underline the necessity for further research. Future investigations should focus on long-term outcomes, optimization of stimulation parameters, and identification of predictors for treatment success to refine and bolster the application of m-SCS in managing FBSS.
Availability of Data and Materials
No datasets were generated or analysed during the current study.
References
Schofferman J, Reynolds J, Herzog R, Covington E, Dreyfuss P, O’Neill C. Failed back surgery: etiology and diagnostic evaluation. Spine J. 2003;3(5):400–3.
Hazard RG. Failed back surgery syndrome: surgical and nonsurgical approaches. Clin Orthop Relat Res. 2006;443:228–32.
Al-Kaisy A, Van Buyten J-P, Smet I, Palmisani S, Pang D, Smith T. Sustained effectiveness of 10 kHz high-frequency spinal cord stimulation for patients with chronic, low back pain: 24-month results of a prospective multicenter study. Pain Med. 2014;15(3):347–54.
Kumar K, Rizvi S. Cost-effectiveness of spinal cord stimulation therapy in management of chronic pain. Pain Med. 2013;14(11):1631–49.
Avellanal M, Diaz-Reganon G, Orts A, Soto S. One-year results of an algorithmic approach to managing failed back surgery syndrome. Pain Res Manag. 2014;19(6):313–6.
Iorio JA, Jakoi AM, Singla A. Biomechanics of degenerative spinal disorders. Asian Spine J. 2016;10(2):377–84.
Taylor RS, Desai MJ, Rigoard P, Taylor RJ. Predictors of pain relief following spinal cord stimulation in chronic back and leg pain and failed back surgery syndrome: a systematic review and meta-regression analysis. Pain Pract. 2014;14(6):489–505.
Deer TR, Falowski S, Arle JE, Vesper J, Pilitsis J, Slavin KV, et al. A systematic literature review of brain neurostimulation therapies for the treatment of pain. Pain Med. 2020;21(7):1415–20.
Remacle TY, Bonhomme VL, Renwart H-JP, Remacle JM. Effect of Multicolumn Lead Spinal Cord Stimulation on Low Back Pain in Failed Back Surgery Patients: A Three-Year Follow-Up. Neuromodulation. 2017;20(7):668–74.
Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, Molet J, et al. The effects of spinal cord stimulation in neuropathic pain are sustained: a 24-month follow-up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation. Neurosurgery. 2008;63(4):762–70; discussion 770.
North RB, Kidd DH, Farrokhi F, Piantadosi SA. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery. 2005;56(1):98–106; discussion 106.
Rigoard P, Jacques L, Delmotte A, Poon K, Munson R, Monlezun O, et al. An algorithmic programming approach for back pain symptoms in failed back surgery syndrome using spinal cord stimulation with a multicolumn surgically implanted epidural lead: a multicenter international prospective study. Pain Pract. 2015;15(3):195–207.
Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, Molet J, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain. 2007;132(1–2):179–88.
Rigoard P, Delmotte A, D’Houtaud S, Misbert L, Diallo B, Roy-Moreau A, et al. Back pain: a real target for spinal cord stimulation? Neurosurgery. 2012;70(3):574–84; discussion 584.
M Page J McKenzie P Bossuyt I Boutron T Hoffmann CD Mulrow et al The PRISMA 2020 statement: an updated guideline for reporting systematic reviews BMJ 372:n71
Stewart LA, Tierney JF, Clarke M. Reviews of Individual Patient Data, Cochrane Handbook for Systematic Reviews of Interventions . Higgins JP, Green S, editors. Chichester, UK: John Wiley & Sons, Ltd; 2011.
Ottawa Hospital Research Institute. [cited 2023 Oct 11]. Available from: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;28(366):l4898.
Rigoard P, Ounajim A, Goudman L, Bouche B, Roulaud M, Page P, et al. The Added Value of Subcutaneous Peripheral Nerve Field Stimulation Combined with SCS, as Salvage Therapy, for Refractory Low Back Pain Component in Persistent Spinal Pain Syndrome Implanted Patients: A Randomized Controlled Study (CUMPNS Study) Based on 3D-Mapping Composite Pain Assessment. J Clin Med. 2021;10(21).
Kurt E, Noordhof RK, van Dongen R, Vissers K, Henssen D, Engels Y. Spinal cord stimulation in failed back surgery syndrome: an integrative review of quantitative and qualitative studies. Neuromodulation. 2022;25(5):657–70.
Eldabe S, Kumar K, Buchser E, Taylor RS. An analysis of the components of pain, function, and health-related quality of life in patients with failed back surgery syndrome treated with spinal cord stimulation or conventional medical management. Neuromodulation. 2010;13(3):201–9.
Rigoard P, Basu S, Desai M, Taylor R, Annemans L, Tan Y, et al. Multicolumn spinal cord stimulation for predominant back pain in failed back surgery syndrome patients: a multicenter randomized controlled trial. Pain. 2019;160(6):1410–20.
Witkam RL, Kurt E, van Dongen R, Arnts I, Steegers MAH, Vissers KCP, et al. Experiences from the patient perspective on spinal cord stimulation for failed back surgery syndrome: A qualitatively driven mixed method analysis. Neuromodulation. 2021;24(1):112–25.
Choi J-G, Ha S-W, Son B-C. Comparison of Clinical Efficacy and Computed Tomographic Analysis of Lead Position Between Three-Column and Five-Column Paddle Leads Spinal Cord Stimulation for Failed Back Surgery Syndrome. World Neurosurg. 2017;97:292–303.
Remacle T, Mauviel S, Renwart H-J, Ghassempour K, Belle F, Lückers O, et al. Long-Term Multicolumn-Lead Spinal Cord Stimulation Efficacy in Patients with Failed Back Surgery Syndrome: A Six-Year Prospective Follow-up Study. World Neurosurg. 2020;142:e245–52.
Rigoard P, Billot M, Ingrand P, Durand-Zaleski I, Roulaud M, Peruzzi P, et al. How Should we Use Multicolumn Spinal Cord Stimulation to Optimize Back Pain Spatial Neural Targeting? A Prospective, Multicenter, Randomized, Double-Blind, Controlled Trial (ESTIMET Study). Neuromodulation. 2021;24(1):86–101.
Rigoard P, Ounajim A, Goudman L, Banor T, Héroux F, Roulaud M, et al. The Challenge of Converting “Failed Spinal Cord Stimulation Syndrome” Back to Clinical Success, Using SCS Reprogramming as Salvage Therapy, through Neurostimulation Adapters Combined with 3D-Computerized Pain Mapping Assessment: A Real Life Retrospective Study. J Clin Med. 2022;11(1).
Acknowledgement
Mohamed Abouzid is a participant of STER Internationalization of Doctoral Schools Program from NAWA Polish National Agency for Academic Exchange No. PPI/STE/2020/1/00014/DEC/02.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
HA, IS: conceptualization and methodology. IS and HA: investigation and data curation. HA: formal analysis. IS, HA and MAzid: Writing - Original Draft. HA, IS and MAzid: Supervision. IS: Project administration. MAzid: Writing - Review & Editing. All authors read and approved the final content.
Corresponding authors
Ethics declarations
Ethical Approval
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Atwan, H., Serag, I. & Abouzid, M. Multicolumn Spinal Cord Stimulation for Chronic Back and Leg Pain in Patients with Failed Back Surgery Syndrome: A Systematic Review and Meta-Analysis. Curr Treat Options Neurol 26, 451–462 (2024). https://doi.org/10.1007/s11940-024-00807-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11940-024-00807-5