Abstract
Background
Our study aims to determine the predictors and patterns of relapses after curative colorectal liver metastasis (CRLM) resection.
Methods
A single-centre, retrospective study of CRLM patients operated between 2010 and 2022 was performed. The site of first recurrence was either hepatic (marginal (≤ 1 cm) or extramarginal), extrahepatic, or both. Factors that predicted relapse patterns and overall survival were determined by multivariable Cox regression analysis with backward elimination of variables.
Results
The study consisted of 258 patients, with a similar proportion of synchronous (144; 56%) and metachronous(114; 43%) metastasis. At a 43-month median follow-up, 156 patients (60.4%) developed recurrences with 33 (21.1%) in the liver, 62(24.03%) extra-hepatic recurrences, and 58 (22.48%) having both. Isolated marginal liver relapses were seen in seven (9.89%) liver recurrence patients. The median overall and relapse-free survivals were 38 months (30–54) and 13 months (11–16), respectively. The 3-year liver-relapse-free survival was 54.4% (44.9–60.6). Size of liver metastases > 5 cm (HR 2.06 (1.34–3.17), involved surgical margins (HR 2.16 (1.27–3.68)), and adjuvant chemotherapy (HR 1.89 (1.07–3.35)) were predictors of hepatic recurrences. Node positivity of primary (HR 1.61 (1.02–2.56)), presence of baseline extra-hepatic metastases (HR 0.30 (0.18–0.51)), size of liver metastases > 5 cm (HR 2.02 (1.37–2.99)), poorly differentiated histology (HR 2.25 (1.28–3.49)), presence of LVI (HR 2.25 (1.28–3.94)), and adjuvant chemotherapy (HR 2.15 (1.28–3.61)) were predictors of extra-hepatic recurrences.
Conclusion
The study found majority relapses occurred at extrahepatic sites whilst isolated marginal recurrences were few. The consistent predictors of recurrence were size and inability to deliver adjuvant therapy. A tailored adjuvant therapy might improve outcomes after liver metastasectomy in colorectal cancers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The multitude of perioperative treatment options available for resectable colorectal liver metastasis (CRLM) has reduced the probability of recurrences thus providing a high chance of cure. With the increasing safety of liver resection and advances in systemic therapy, various studies now report the 5- and 10-year survival following CRLM resection as 50% and 25%, respectively [1,2,3,4]. Despite the improvement in survival, 70% of the resected CRLM recur and 20% of these occur within the first two years within the remnant liver [5, 6]. Disease-free interval after liver resection and the patterns of recurrence have been shown to correlate with long-term prognosis [7, 8]. Location and pathology of primary tumour, R1 margin status, pre-operative CEA levels, extra-hepatic metastasis and adjuvant therapy were few of the factors attributed to recurrence [9,10,11,12]. This phenomenon of disease recurrence of after surgical resection of radiological and intra-operative disease is credited to the presence of occult micrometastasis. Planning peri-operative systemic therapy based on molecular profiling has shown to provide significant clinical benefit and is commonly used in initially unresectable CRLM [13, 14]. There is now growing body of evidence for re-resection and local ablative therapies to treat hepatic recurrences where perioperative systemic therapy has limited effect [15, 16].
The knowledge of susceptibility to liver or extrahepatic recurrences occur with the current management will help to improve outcomes by allowing us to tailor post-resection surveillance and treatment strategies. In this background, the systemic therapy could be utilised only for patients at high risk of extrahepatic recurrences whilst the intention of augmenting local therapy with aggressive surgical resection with or without the addition of hepatic artery infusion would be applicable in patients who have a higher propensity for liver relapse. Thus, our study aims to determine these patterns and predictors of relapses after curative CRLM resection to guide the optimum strategy for perioperative therapy.
Methodology
Study Design and Setting
A single-centre, retrospective study of patients treated by our hepato-biliary and colorectal disease management units at Tata Memorial Hospital (Mumbai, India). Data was collected from a prospectively maintained database and electronic medical records.
Patients
Patients of colorectal cancer with synchronous or metachronous liver metastasis who were treated with a curative liver resection between 2010 and 2022 were analysed.
The inclusion criteria were as follows:
-
(1)
Patients who were operable and underwent liver resection for CRLM with or without liver-directed therapies
-
(2)
Patients with limited extra-hepatic metastasis in the lung and peritoneum at primary presentation that were considered resectable
-
(3)
Pathologically confirmed diagnosis of CRLM
Patients with other histologies and those deemed unresectable were excluded.
Management Strategy
The primary staging modality for colorectal liver metastasis was a contrast-enhanced triple-phase computerised tomography (CECT). Magnetic Resonance Imaging (MRI) and Positron Emission Tomographies (PET) were used selectively for problem-solving in doubtful lesions as per the decision of the multi-disciplinary team (MDT). The decision for perioperative chemotherapy was also an individualised decision based on the location of the primary tumour (colon vs rectum), timing of liver metastasis (synchronous vs metachronous), disease-free interval, and burden of metastatic disease and if the primary was symptomatic. Colorectal liver metastasis were considered synchronous if they presented at the time or within 6 months of primary diagnosis [17]. Similarly, the timing of resection of liver metastasis (simultaneous vs staged) was also based on MDT decisions. When staged resections were performed, a liver-first approach was preferred. No planned positive or R1vascular resections were performed. Adjuvant chemotherapy was considered for all patients who underwent liver resection. However, the receipt of adjuvant therapy depended on complications following surgery, performance status of the patient, and willingness for systemic therapy by the patients. Thus, ability to receive adjuvant chemotherapy was more closely equated to return-to-intended-oncological therapy (RIOT). Further surveillance of patients was as per the National Comprehensive Cancer Network (NCCN) guidelines, and CEA is repeated every 3 months and CECT of thorax, abdomen, and pelvis and is done every 6 months for the first two years. Then, CEA was performed every 6 months and CECT once in every year for the first 5 years [18, 19].
Variables
Demographic variables, pathological characteristics of the primary tumour, type of metastasis, size, number and histopathology of liver metastasis, clinical risk score, neo-adjuvant systemic therapy offered, type of liver resection performed, adjunct use of liver directed therapies, patterns of recurrence and duration to develop recurrence and post-recurrence survival and therapeutic options offered were all the variables collected as a part of this study.
Outcomes
Primary end-point of the study was site of first recurrence, categorised as either hepatic, extrahepatic, or both. The liver recurrences were further classified marginal (≤ 1 cm from resection bed) or extramarginal [20]. The secondary end-points were recurrence-free survival (RFS) and overall survival (OS) after curative CRLM resection. RFS was measured from the time of resection of liver metastases until recurrence or the last follow-up whilst OS was defined from the time of resection of liver metastases until death or the last follow-up. Liver relapse-free survival (LRFS) was considered till the appearance of liver recurrence.
Statistical Analysis
Categorical data were expressed as frequencies and percentages, whereas continuous data were expressed as median and interquartile range (IQR). Survival curves were plotted using the Kaplan–Meier method. Follow-up duration was calculated using the reverse Kaplan–Meier method. Multivariable Cox regression analysis with backward elimination of variables with an exit level alpha of 0.2 was used to determine the factors that predicted the various relapse patterns. This gradual elimination of variables from higher p-values resulted in the transformation of a saturated regression model to a reduced model with important variables that allowed the best fit with the least possible number of variables from the data. All statistical analyses were conducted using SPSS v25 (IBM).
Ethics
Data collection was in accordance with the Declaration of Helsinki [21]. As per institutional protocol, a formal board review was not taken for a retrospective analysis with anonymized data and absence of patient contact.
Results
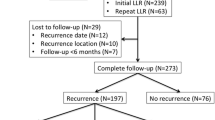
Two hundred and fifty-eight CRLM patients treated with curative liver resection were analysed as a part of our study. The study population had a similar proportion of synchronous (144; 56%) and metachronous (114; 43%) CRLM. The median number of liver metastasis was two (Interquartile range: 1–4) with a median tumour size of 3.5 cm (IQR: 2–5 cm). A total of 159 (62%) patients received at least four cycles of neoadjuvant chemotherapy. A major hepatectomy (≥ 3 segments) was performed in 109 (45.7%) patients with unplanned positive resections margins (R +) in 36 (14%) patients. A total of 216 (84%) patients received the planned adjuvant therapy. The remaining patient characteristics are elucidated in Table 1 whilst the type of resections and therapeutic adjuncts are shown in Table 2.
The recurrence patterns identified are elaborated in Table 3. One hundred and fifty-six patients (156; 60%) relapsed after curative CRLM resection of which the majority were extra-hepatic distant recurrences (120; 47%) followed by liver relapses (91; 35%). One-hundred and five patients were recurrence-free, 33 (21.1%) had relapses in the liver, 62 (24.03%) had extrahepatic recurrences, and 58 (22.48%) had both, hepatic and extrahepatic, relapses. Isolated marginal liver relapses defined as those occurring within 1 cm of the liver resection margin were seen in only 7 (9.89%) patients out of the 91 liver recurrences. Extra-hepatic recurrences were observed in the lungs (66; 55%) followed by lymph nodes (22; 18%) and peritoneum (22; 18%).
At a median follow-up of 43 months (range 30–51 months, 95% CI), the OS of patients with resected CRLM was 38 months (30–54), the RFS was 13 months (11–16), and the 3-year LRFS was 54.4% (44.9–60.6) (Figs. 1 and 2). Amongst extra-hepatic sites, peritoneal recurrences (16.65 months, HR 1.53, p = 0.137) had worse survival compared to lung (19.31 months, HR 0.88, p = 0.553) and nodal (24.57 months, HR 0.80, p = 0.381) disease. The worst prognosis was seen with brain (3.2 months, HR 4.11, p = 0.05) and bone metastases (1.2 months, HR 4.43, p = 0.002), respectively, as they were often associated with other distant sites.
Size of liver metastases > 5 cm (HR 2.06 (1.34–3.17)), involved surgical margins (HR 2.16 (1.27–3.68)), omission of adjuvant chemotherapy (HR 1.89 (1.07–3.35)) were predictors of worse liver specific relapse-free survival (Table 4). Extrahepatic relapses were predicted by node positivity of primary (HR 1.61 (1.02–2.56), p < 0.041), presence of baseline extra-hepatic metastases (HR 0.30 (0.18–0.51), p < 0.01), size of liver metastases > 5 cm (HR 2.02 (1.37–2.99), p < 0.001), poorly differentiated histology (HR 2.25 (1.28–3.49), p = 0.043), presence of lymphovascular invasion (HR 2.25 (1.28–3.94), p = 0.005), and lack of adjuvant chemotherapy (HR 2.15 (1.28–3.61), p = 0.004) (Table 4). Timing and number of metastases or the use of preoperative chemotherapy did not influence survival outcomes. Factors that significantly affected overall survival were node positivity of the colorectal primary (HR 1.75 (1.09–2.80, p = 0.020)), size of the metastasis > 5 cm (HR 1.98 (1.33–2.94, p = 0.001), poorly differentiated histology (HR 4.12 (2.47–6.86, p < 0.001), presence of extrahepatic metastasis at baseline (HR 0.41 (0.25–0.69, p = 0.001), and ability to receive adjuvant chemotherapy (HR 2.66 (1.59–4.45, p < 0.001) (Table 5).
Discussion
Our study of 258 resected colorectal liver metastasis was conducted with the main aim to identify which patients recur in liver or extrahepatic sites after curative liver resection and delineate the factors which affect this pattern of recurrence. Our study analysed data on recurrence patterns and found that majority of the relapses were within extrahepatic sites followed by the liver. However, marginal recurrences around the resection bed were minimal. Tumour size and the inability to receive adjuvant therapy were common predictors of hepatic and extra-hepatic recurrences whilst extrahepatic relapses were additionally predicted by nodal stage, extrahepatic disease at baseline, poorly differentiated histology, and lymphovascular invasion.
The prognostic implications of margins in CRLM resections have been a subject of long debate. Whilst R0 resections with at least 1 mm of clear margin should always be the goal, a microscopically positive surgical margin or a clear margin of less than 1 mm (R1) on pathology is a grey area. Though previous studies showed no significant difference in median overall survival and recurrence-free survival amongst patients with R0 or R1 resection in high risk groups, overall R1 resections were associated with higher local failure [15, 22,23,24]. Our data reveals very few had marginal recurrences (7; 2.7%) even though the margin positivity rate was 14%. Since, R0 resections were always the goal and marginal recurrences were so few in number, increasing the width of resection may not be the solution and the failure to achieve clear margins may only reflect poor tumour biology. Caution should be exercised in the interpreting the results since no patient in the present study had planned positive margins, and we do not suggest that an R1 resection is acceptable based on the present study. The practice of intentional R1 vascular margins proposed by Vigano et al. which were reported to achieve outcomes equivalent to true R0 resections is emerging and accepted though we have not applied it in our patients [17]. There is some evidence of correlation between the margin status (R0 vs R1) and OS based on tumour biology. The type of margin status in wtKRAS tumours was shown to affect both OS and liver recurrence free survival whilst it was minor in mKRAS tumours; however, more data would be required to suggest any practice modification [14].
The results from our study suggest that the ability to receive adjuvant chemotherapy after curative liver resection positively impacted survival. Thus, patients with a good performance status who recovered with negligible surgical morbidity did better. Perioperative chemotherapy is routinely recommended by current National Comprehensive Cancer Network (NCCN) guidelines based on retrospective data [25,26,27]. Buisman et al. showed in a competing risk analysis that perioperative systemic chemotherapy decreases extrahepatic recurrences in high risk patients [27]. Careful interpretation is necessary since randomised trials have not proven overall survival benefit, even though improvements in RFS have been reported [28, 29]. Current practice for adjuvant therapy in CRLM is a controversy world over in view of the conflicting results in phase III trials whose primary end point was RFS and hence were probably underpowered to detect overall survival benefit. Bearing in mind the systemic toxicity, lack of strong evidence to correlate RFS to OS, and lack of data on patient reported quality of life outcomes, we accept further evidence is necessary [14, 30]. We recommend future analysis of available data with risk stratification for recurrence based on other risk factors and research to identify better prognostic and predictive markers.
Older studies evaluating various prognostic nomograms suggest size and number of liver metastasis, lymph node status in primary tumour and pre-operative CEA levels to be the most commonly included factors [31]. Our results indicate that tumour size is of the most prognostic value amongst different components of Fong’s clinical risk score This is following multiple recent studies which showed that even with the inclusion of RAS status improved predictive power, tumour size and number are still the most significant variables [28, 32,33,34]. Thus, it is possible that different components of the risk score have differential weights, and it may be prudent to revisit the scoring system.
A frequently debated contraindication for local treatment of CRLM is the simultaneous presence of extrahepatic disease. Several retrospective studies support CRLM resection with concurrent extrahepatic disease confined to a single organ in highly selected patients [35,36,37]. Our study analysis had a higher risk of disease recurrence in the presence of baseline extra-hepatic metastasis, which is expected.
Even though the size of metastasis and inability to receive adjuvant chemotherapy are the most consistent predictors of recurrence of any recurrence, distant recurrences were additionally predicted by node-positive primary cancer, poorly differentiated histology, extrahepatic metastasis, and lymphovascular invasion. Thus, the presence of these features can guide the adjuvant therapy in patients. A tumour with liver only metastasis with a size > 5 cm without other adverse features should probably be subjected to additional liver-directed therapy in the form of hepatic artery infusional chemotherapy (HAIC) during systemic therapy. Finally, given the ambiguity surrounding the role of perioperative chemotherapy in limited, resectable CRLM, systemic therapy may be avoided in those without any of the risk factors.
Application of liver-directed therapies as adjunct in the treatment of CRLM has long been commonplace at our institute [38]. Augmentation of liver directed therapies like hepatic artery infusion chemotherapy (HAIC) or trans-arterial chemoembolization (TACE) has been applied in the management of initially unresectable and heavily treated CRLM all over the world now. The evidence for role of HAIC as adjunct to adjuvant systemic chemotherapy is limited but promises improvement in overall survival [39, 40]. This evidence is further strengthened by findings from the PACHA-01 trial where adjuvant HAIC has a role in improving liver specific recurrence free survival in high risk patients [41]. At our institute, HAIC is offered to select patients as adjuvant therapy with more than 3 liver metastasis, bilobar lesions or > 5 cm, or for unresectable disease that has failed 2 lines of chemotherapy with or without TACE [42].
Overall, elucidation of different patterns of recurrence and the factors affecting the pattern can be refined with further research to identify patients with individualised risk. This will allow formulation of more reliable precision medicine strategies to allow neoadjuvant and adjuvant therapies to be tailored for each risk group. Future trials in hepatic arterial infusion therapy can include patients with higher risk of a liver recurrence, resulting in greater efficacy. More analysis of real-world data on management of colorectal liver metastases is necessary to derive conclusions.
Our study has a few limitations as well. The relatively small sample and retrospective study design from a single institution is subject to selection bias and limited external validity. In addition, the extended period included in the study may combine management strategies from different eras and evolving systemic therapy regimens. Addition of a contrast enhanced MRI of the liver to CT has shown up to 30% change in treatment plan. Since the use of MRI was not universal in our patients, the same can be a potential limitation as well [43]. Influence of histological growth patterns (desmoplastic vs non-desmoplastic) and molecular biomarkers like RAS alterations, BRAF and TP53 mutations, microsatellite instability and subsequent mismatch repair gene deficiency and circulating tumour cells were not included in analysis and is a strong limitation to our data [32, 33, 44,45,46,47,48].
Conclusion
Amongst resected CRLM, the majority of relapses occurred at extrahepatic sites followed by that in the liver. However, isolated marginal recurrences were very few. Most consistent predictors of recurrence were size and the inability to deliver adjuvant therapy. Extrahepatic relapses were additionally predicted by nodal stage, baseline extrahepatic disease, and histologic differentiation. With further research, tailored adjuvant therapy might improve patient outcomes after liver metastasectomy in colorectal cancers.
Data Availability
No datasets were generated or analysed during the current study.
Abbreviations
- CRLM:
-
Colorectal liver metastasis
- CEA:
-
Carcinoma embryonic antigen
- MRI:
-
Magnetic Resonance Imaging
- PET:
-
Positron Emission Tomography
- MDT:
-
Multi-disciplinary team
- RIOT:
-
Return-to-intended-oncological therapy
- NCCN:
-
National Comprehensive Cancer Network
- RFS:
-
Relapse-free survival
- OS:
-
Overall survival
- LRFS:
-
Liver relapse-free survival
- HAIC:
-
Hepatic artery infusion chemotherapy
- RFA:
-
Radio-frequency ablation
- TACE:
-
Trans-arterial chemoembolization
- CD:
-
Clavien Dindo
- RAS gene:
-
Rat sarcoma gene
- BRAF gene:
-
V-Raf murine sarcoma viral oncogene homolog B1
References
Driedger MR, Cleary SP, Nagorney DM. Synchronous colorectal liver metastases: therapeutic considerations. Hepatobiliary Surg Nutr. 2021;10(5):71113–713.
Creasy JM, Sadot E, Koerkamp BG, Chou JF, Gonen M, Kemeny NE, et al. Actual 10-year survival after hepatic resection of colorectal liver metastases: what factors preclude cure? Surgery. 2018;163(6):1238–44.
Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol Off J Eur Soc Med Oncol. 2016;27(8):1386–422.
Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, Kemeny N, Brennan MF, Blumgart LH, D’Angelica M. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25(29):4575–80. https://doi.org/10.1200/JCO.2007.11.0833.
Kawaguchi Y, Lillemoe HA, Panettieri E, Chun YS, Tzeng CWD, Aloia TA, et al. Conditional recurrence-free survival after resection of colorectal liver metastases: persistent deleterious association with RAS and TP53 co-mutation. J Am Coll Surg. 2019;229(3):286-294.e1.
Kokudo N, Tada K, Seki M, Ohta H, Azekura K, Ueno M, et al. Anatomical major resection versus nonanatomical limited resection for liver metastases from colorectal carcinoma. Am J Surg. 2001;181(2):153–9.
D’Angelica M, Kornprat P, Gonen M, DeMatteo RP, Fong Y, Blumgart LH, et al. Effect on outcome of recurrence patterns after hepatectomy for colorectal metastases. Ann Surg Oncol. 2011;18(4):1096–103.
Hill CRS, Chagpar RB, Callender GG, Brown RE, Gilbert JE, Martin RCG, et al. recurrence following hepatectomy for metastatic colorectal cancer: development of a model that predicts patterns of recurrence and survival. Ann Surg Oncol. 2012;19(1):139–44.
Russolillo N, Sperti E, Langella S, Menonna F, Allieta A, Di Maio M, et al. Impact of primary tumor location on patterns of recurrence and survival of patients undergoing resection of liver metastases from colon cancer. HPB. 2020;22(1):116–23.
Dupré A, Malik HZ, Jones RP, Diaz-Nieto R, Fenwick SW, Poston GJ. Influence of the primary tumour location in patients undergoing surgery for colorectal liver metastases. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2018;44(1):80–6.
Kato T, Yasui K, Hirai T, Kanemitsu Y, Mori T, Sugihara K, et al. Therapeutic results for hepatic metastasis of colorectal cancer with special reference to effectiveness of hepatectomy: analysis of prognostic factors for 763 cases recorded at 18 institutions. Dis Colon Rectum. 2003;46(10 Suppl):S22-31.
John SKP, Robinson SM, Rehman S, Harrison B, Vallance A, French JJ, et al. Prognostic factors and survival after resection of colorectal liver metastasis in the era of preoperative chemotherapy: an 11-year single-centre study. Dig Surg. 2013;30(4–6):293–301.
Sonbol MB, Siddiqi R, Uson PLS, Pathak S, Firwana B, Botrus G, Almader-Douglas D, Ahn DH, Borad MJ, Starr J, Jones J, Stucky CC, Smoot R, Riaz IB, Bekaii-Saab T. The role of systemic therapy in resectable colorectal liver metastases: systematic review and network meta-analysis. Oncologist. 2022;27(12):1034–40. https://doi.org/10.1093/oncolo/oyac212.
Filoni E, Musci V, Di Rito A, Inchingolo R, Memeo R, Mannavola F. Multimodal management of colorectal liver metastases: state of the art. Oncol Rev [Internet]. 2024;17. Available from: https://doi.org/10.3389/or.2023.11799.
De Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250(3):440–8.
Patel RK, Rahman S, Schwantes IR, Bartlett A, Eil R, Farsad K, Fowler K, Goodyear SM, Hansen L, Kardosh A, Nabavizadeh N, Rocha FG, Tsikitis VL, Wong MH, Mayo SC. Updated management of colorectal cancer liver metastases: scientific advances driving modern therapeutic innovations. Cell Mol Gastroenterol Hepatol. 2023;16(6):881–94. https://doi.org/10.1016/j.jcmgh.2023.08.012.
Garajova I, Balsano R, Tommasi C, Dalla Valle R, Pedrazzi G, Ravaioli M, Spallanzani A, Leonardi F, Santini C, Caputo F, Riefolo M, Giuffrida M, Gelsomino F. Synchronous and metachronous colorectal liver metastases: impact of primary tumor location on patterns of recurrence and survival after hepatic resection. Acta Biomed. 2020;92(1):e2021061. https://doi.org/10.23750/abm.v92i1.11050.
National Comprehensive Cancer Network. NCCN Guidelines to Rectal cancer. https://www.nccn.org/professionals/physician_gls/pdf/rectal_blocks.pdf. Accessed 13 Apr 2024.
National Comprehensive Cancer Network. NCCN guidelines to Colon Cancer. https://www.nccn.org/professionals/physician_gls/pdf/colon_blocks.pdf. Accessed 13 Apr 2024.
Andreou A, Knitter S, Schmelzle M, Kradolfer D, Maurer MH, Auer TA, et al. Recurrence at surgical margin following hepatectomy for colorectal liver metastases is not associated with R1 resection and does not impact survival. Surgery. 2021;169(5):1061–8.
World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–4.
Symeonidis D, Tepetes K, Tzovaras G, Kissa L, Samara AA, Bompou E, et al. Colorectal cancer liver metastases: is an R1 hepatic resection accepted? Clin Pract. 2022;12(6):1102–10.
The prognostic impact of resection margin status varies according to the genetic and morphological evaluation (GAME) score for colorectal liver metastasis. J Surg Oncol. 2021;124(4):619–26. https://doi.org/10.1002/jso.26557.
Sakamoto K, Beppu T, Ogawa K, Tamura K, Honjo M, Funamizu N, et al. Prognostic impact of surgical margin width in hepatectomy for colorectal liver metastasis. J Clin Transl Hepatol. 2023;11(3):705–17.
Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371(9617):1007–16. https://doi.org/10.1016/S0140-6736(08)60455-9.
Predictive factors for the benefit of perioperative FOLFOX for resectable liver metastasis in colorectal cancer patients (EORTC Intergroup Trial 40983). Ann Surg. 2012;255(3):534–9. https://doi.org/10.1097/SLA.0b013e3182456aa2.
Buisman FE, Galjart B, van der Stok EP, Balachandran VP, Boerner T, Drebin JA, et al. Recurrence patterns after resection of colorectal liver metastasis are modified by perioperative systemic chemotherapy. World J Surg. 2020;44(3):876–86.
Kobayashi K, Ono Y, Kitano Y, Oba A, Sato T, Ito H, et al. Prognostic impact of tumor markers (CEA and CA19-9) on patients with resectable colorectal liver metastases stratified by tumor number and size: potentially valuable biologic markers for preoperative treatment. Ann Surg Oncol. 2023;30(12):7338–47.
Kanemitsu Y, Shimizu Y, Mizusawa J, Inaba Y, Hamaguchi T, Shida D, Ohue M, Komori K, Shiomi A, Shiozawa M, Watanabe J, Suto T, Kinugasa Y, Takii Y, Bando H, Kobatake T, Inomata M, Shimada Y, Katayama H, Fukuda H, JCOG Colorectal Cancer Study Group. Hepatectomy followed by mFOLFOX6 versus hepatectomy alone for liver-only metastatic colorectal cancer (JCOG0603): a phase II or III randomized controlled trial. J Clin Oncol. 2021;39(34):3789–99. https://doi.org/10.1200/JCO.21.01032.
Saude Conde R, Bregni G, Saad E, Hendlisz A, Sclafani F. JCOG0603: are we really sure this was a negative trial? J Clin Oncol. 2022;40(7):803–5. https://doi.org/10.1200/JCO.21.02299.
Matias M, Casa-Nova M, Faria M, Pires R, Tato-Costa J, Ribeiro L, et al. Prognostic factors after liver resection for colorectal liver metastasis. Acta Med Port. 2015;28(3):357–69.
Takeda Y, Mise Y, Takahashi Y, Ito H, Inoue Y, Yoshioka R, et al. Limited prognostic value of KRAS in patients undergoing hepatectomy for colorectal liver metastases. Ann Surg Oncol. 2022;29(4):2383–91.
Margonis GA, Sasaki K, Gholami S, Kim Y, Andreatos N, Rezaee N, et al. Genetic And Morphological Evaluation (GAME) score for patients with colorectal liver metastases. Br J Surg. 2018;105(9):1210–20.
Kawaguchi Y, Kopetz S, Cao HST, Panettieri E, Bellis MD, Nishioka Y, et al. Contour prognostic model for predicting survival after resection of colorectal liver metastases: development and multicentre validation study using largest diameter and number of metastases with RAS mutation status. Br J Surg. 2021;108(8):968.
Hadden WJ, de Reuver PR, Brown K, Mittal A, Samra JS, Hugh TJ. Resection of colorectal liver metastases and extra-hepatic disease: a systematic review and proportional meta-analysis of survival outcomes. HPB. 2016;18(3):209–20.
Hwang M, Jayakrishnan TT, Green DE, George B, Thomas JP, Groeschl RT, et al. Systematic review of outcomes of patients undergoing resection for colorectal liver metastases in the setting of extra hepatic disease. Eur J Cancer Oxf Engl 1990. 2014;50(10):1747–57.
Leung U, Gönen M, Allen PJ, Kingham TP, DeMatteo RP, Jarnagin WR, et al. Colorectal cancer liver metastases and concurrent extrahepatic disease treated with resection. Ann Surg. 2017;265(1):158–65.
Patkar S, Chopde A, Shetty N, Kulkarni S, Gala KB, Chandra D, et al. Multimodality liver directed treatment for colorectal liver metastasis: array of complementary options can improve outcomes - A single centre experience from India. Front Oncol. 2023;13:1073311.
Kwan J, Pua U. Review of intra-arterial therapies for colorectal cancer liver metastasis. Cancers. 2021;13(6):1371.
Buisman FE, Filipe WF, Galjart B, Grünhagen DJ, Homs MYV, Moelker A, et al. Adjuvant intra-arterial chemotherapy for patients with resected colorectal liver metastases: a systematic review and meta-analysis. HPB. 2022;24(3):299–308.
Gelli M, Ewald J, Tanguy ML, Passot G, Quenet F, Touchefeu Y, et al. Postoperative hepatic arterial chemotherapy after resection of colorectal liver metastases in patients at high risk of hepatic recurrence: a multicenter randomized phase II trial (PRODIGE 43 - PACHA-01). J Clin Oncol. 2023;41(16_suppl):3515–3515.
Sree Ganesh B, Kazi M, Goel M, et al. Feasibility of hepatic artery infusion chemotherapy for colorectal liver metastasis in an Indian setting. Indian J Surg Oncol. 2023. https://doi.org/10.1007/s13193-023-01871-0.
Görgec B, Hansen I, Kemmerich G, Syversveen T, Abu Hilal M, Belt E, Bosscha K, Burgmans M, Cappendijk V, D’Hondt M, Edwin B, van Erkel A, Gielkens H, Grunhagen D, Gobardhan P, Hartgrink H, Horsthuis K, Klompenhouwer E, Kok N, Zonderhuis B (2023) MRI in addition to CT in patients scheduled for local therapy of colorectal liver metastases (CAMINO): an international, multicentre, prospective, diagnostic accuracy trial. Lancet Oncol. 25. https://doi.org/10.1016/S1470-2045(23)00572-7.
Höppener DJ, Galjart B, Nierop PMH, Buisman FE, van der Stok EP, Coebergh van den Braak RRJ, et al. Histopathological growth patterns and survival after resection of colorectal liver metastasis: an external validation study. JNCI Cancer Spectr. 2021;5(3):pkab026.
Vermeulen PB, Colpaert C, Salgado R, Royers R, Hellemans H, Van Den Heuvel E, et al. Liver metastases from colorectal adenocarcinomas grow in three patterns with different angiogenesis and desmoplasia. J Pathol. 2001;195(3):336–42.
van Dam PJ, Daelemans S, Ross E, Waumans Y, Van Laere S, Latacz E, et al. Histopathological growth patterns as a candidate biomarker for immunomodulatory therapy. Semin Cancer Biol. 2018;52(Pt 2):86–93.
Mauri G, Monfardini L, Garnero A, Zampino MG, Orsi F, Della Vigna P, et al. Optimizing loco regional management of oligometastatic colorectal cancer: technical aspects and biomarkers, two sides of the same coin. Cancers. 2021;13(11):2617.
Kitsel Y, Cooke T, Sotirchos V, Sofocleous CT. Colorectal cancer liver metastases: genomics and biomarkers with focus on local therapies. Cancers. 2023;15(6):1679.
Acknowledgements
All the authors have gone through the final version of this manuscript and attest to the veracity of the data presented. This manuscript is an original research work.
Funding
Open access funding provided by Department of Atomic Energy.
Author information
Authors and Affiliations
Contributions
Conception of the study design was done by MK and SP. Acquisition of data, data analysis, and drafting of the manuscript were done by SNV, MK, SP, and RM. SNV and MK share joint first authorship as both have contributed equally to the manuscript. Critical revision of the manuscript was done by MK, SP, AD, AS, and MG. Final approval of the manuscript was done by MK, SP, AD, AS, and MG.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Conference Presentation
This study was accepted for presentation as On-demand Oral Presentation at 16th IHPBA World Congress, 2024
Synopsis for Table of Contents
A single-centre, retrospective study of patients who underwent curative CRLM resection showed the majority were extrahepatic relapses. Isolated marginal liver recurrences were very few irrespective of the margin positivity rate. The most consistent predictors of recurrence were size and inability to deliver adjuvant therapy.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vadisetti, S.N., Kazi, M., Patkar, S. et al. Patterns and Predictors of Recurrence After Curative Resection of Colorectal Liver Metastasis (CRLM). J Gastrointest Canc (2024). https://doi.org/10.1007/s12029-024-01105-8
Accepted:
Published:
DOI: https://doi.org/10.1007/s12029-024-01105-8