Abstract
Background
Hepatocellular carcinoma (HCC) is a highly aggressive tumor associated with significant morbidity and mortality rates. Combination therapy with immune checkpoint inhibitors (ICIs) and kinase inhibitors has emerged as a promising strategy for liver cancer treatment in recent years. However, the clinical factors predicting the outcomes of combination therapy in patients with advanced liver cancer remain uncertain. Therefore, this study investigated the relationships between clinical predictors and the efficacy of ICI plus kinase inhibitor therapy to personalize treatment plans.
Methods
We retrospectively enrolled 98 patients who received combination treatment with ICIs and kinase inhibitors for advanced HCC. Based on blood lipid levels and other clinical factors prior to treatment, we investigated potential biomarkers that could predict treatment responses in this patient population.
Results
Mean progression-free survival (PFS) and overall survival (OS) in this cohort were 10.1 and 17.2 months, respectively. Via multivariate analysis, the absence of extrahepatic metastasis, the absence of portal vein thrombosis (PVT), neutrophil-to-lymphocyte ratio (NLR) < 3.225, platelet-to-lymphocyte ratio (PLR) < 140.75, and prognostic nutritional index (PNI) ≥ 37.25 were identified as independent predictors of improved PFS. Factors associated with better OS included PLR < 140.75 and total cholesterol (TC) < 3.46 mmol/L. Univariate analysis identified significant associations of Eastern Cooperative Oncology Group performance status (ECOG PS), hepatitis B virus (HBV) DNA levels, Child–Pugh classification, alpha-fetoprotein (AFP), TC, and the receipt of regorafenib with PFS. Additionally, ECOG PS, Child–Pugh classification, AFP, PVT, NLR, PNI, and the receipt of regorafenib were significantly associated with OS.
Conclusions
PLR and TC were potential clinical predictive factors for survival outcomes in patients with advanced HCC who received ICI/kinase inhibitor combination therapy. It is important to know the clinical characteristics of patients prior to treatment initiation to optimize outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Liver cancer is the second leading cause of cancer death in China, and its mortality rate has significantly increased in the US [1]. Approximately 85–90% of patients with liver cancer have primary hepatocellular carcinoma (HCC). Various factors such as metabolic problems, infectious diseases including hepatitis, and lifestyle factors including alcoholism and smoking contribute to the development of HCC [2]. Although diagnostic techniques have advanced, approximately 70% of cases of HCC are diagnosed at middle and late stages, missing the opportunity for radical surgery. In addition, the recurrence rate of liver cancer after surgery is approximately 70%, and surgery cannot be repeated in such cases [3]. However, there has been notable improvement in the survival of patients with advanced HCC since 2017 [4].
Recently, the combination of immune checkpoint inhibitors (ICIs) and kinase inhibitors emerged as a new therapeutic strategy for advanced HCC. ICIs, consisting of programmed death-1 (PD-1) and programmed death ligand-1 (PD-L1) antibodies, are new treatment modalities with clinical benefits in patients with HCC. PD-1 inhibitors include pembrolizumab, camrelizumab, tislelizumab, and nivolumab, whereas PD-L1 inhibitors include atezolizumab, durvalumab, and envafolimab. Kinase inhibitors include apatinib, sorafenib, lenvatinib, and regorafenib. In a real-world study of patients with unresectable HCC, the combination of lenvatinib and PD-1 inhibitors prolonged progression-free survival (PFS) and overall survival (OS). Median PFS and OS were 6.9 (95% confidence interval [CI] = 6.0–7.9) and 17.8 months (95% CI = 14.0–21.6), respectively, and the objective response rate (ORR) and disease control rate (DCR) were 19.6% and 73.5%, respectively [5]. A retrospective study of elderly patients with HCC described the efficacy and safety of sorafenib or lenvatinib plus PD-1 inhibitors, with median PFS and OS of 4.6 and 17.0 months, respectively [6]. Another multicenter retrospective study of patients with advanced HCC indicated that the combination of regorafenib and PD-1 inhibitors improved ORR and DCR and extended PFS and OS [7]. Similarly, in the CARES-310 study, camrelizumab plus rivoceranib prolonged PFS (5.6 months [95% CI = 5.5–6.3]) and OS (22.1 months [95% CI = 19.1–27.2]), whereas the ORR was only 25% in unresectable HCC [8]. ICI and kinase inhibitor combination treatment has displayed promise clinically, but their efficacy remains limited [9]. In summary, although the ICI/kinase inhibitor treatment significantly improved PFS and OS in advanced HCC, the survival benefit has been limited to a small portion of the population. Therefore, there is in urgent need to identify the patients who will benefit from new therapeutic approaches.
The study aimed to identify novel predictors of clinical outcomes for patients with advanced HCC who received ICI/kinase inhibitor therapy to improve survival outcomes.
Methods and materials
Patients
In this present study, patients with advanced HCC who received combination treatment with ICIs and kinase inhibitors at Ningbo Medical Center Lihuili Hospital between January 2020 and December 2023 were enrolled. Retrospective clinical data were collected independently by two oncologists. The inclusion criteria were as follows: age > 18 years, confirmation of HCC by pathology or clinical diagnosis (imaging and alpha-fetoprotein [AFP]); diagnosis of advanced HCC based on the China liver cancer staging criteria; Eastern Cooperative Oncology Group performance status (ECOG PS) ≤ 2; synchronous treatment with one anti-PD1/anti-PD-L1 inhibitor and one kinase inhibitor; peripheral blood testing was performed within 7 working days before treatment initiation; and the presence of at least one measured lesion according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 [10]. Meanwhile, the exclusion criteria were as follows: body mass index > 30; presence of other malignant tumors; presence of immune diseases prior to systemic treatment; prior receipt of other immune biotherapies; a lack of adequate laboratory data and clinical information; and reducing blood fat treatment before body treatments.
Patients’ clinical information comprised the following variables: sex; age; ECOG PS; the extrahepatic metastasis status at baseline; the portal vein thrombosis (PVT) status; HCC treatment history; the hepatitis B virus (HBV) status; HBV DNA levels; Child–Pugh classification; the ICI and kinase inhibitor received; alpha-fetoprotein (AFP), albumin (ALB, g/L), triglyceride (TG, mmol/L), total cholesterol (TC, mmol/L), high-density lipoprotein (HDL, mmol/L), and low-density lipoprotein (LDL, mmol/L) levels; and neutrophil (×109/L), lymphocyte (×109/L), and platelet counts (×109/L) within 7 working days before treatment initiation. The prognostic nutritional index (PNI) was defined using the following formula: (10 × ALB + 0.005 × absolute lymphocyte count). The HBV DNA status was based on the guidelines for the prevention and treatment of chronic hepatitis B (version 2022). Serum HBsAg levels lower than 1000 IU/mL were indicative of non-reactivation and non-infection, whereas levels higher than 1000 IU/mL or equal to 1000 IU/mL denoted reactivation [11].
The Ethics Committee of Ningbo Medical Center Lihuili Hospital (KY2024SL072-01) approved this study, and all patients or their guardians provided informed consent before enrollment according to the Declaration of Helsinki (as revised in 2013).
Evaluation of treatment responses
Based on RECIST 1.1, the efficacy of treatment was assessed by computed tomography or magnetic resonance imaging every 8–12 weeks until disease progression, and the treatment effect was categorized as progressive disease (PD), stable disease (SD), partial response (PR), and complete response (CR). The ORR was calculated as the sum of the CR and PR rates. Meanwhile, the DCR was calculated as the sum of the CR, PR, and SD rates.
According to RECIST 1.1, PFS was defined as the duration from the initiation of anti-PD1/anti-PD-L1 or kinase inhibitor treatment to tumor progression or death. OS was defined as the time from the initiation of anti-PD1/anti-PD-L1 or kinase inhibitor therapy until the date of the last follow-up or death. Anti-PD1/anti-PD-L1 therapy was started within 7 days of the initiation of kinase inhibitor treatment. Patient follow-up was conducted via telephone by May 2024.
Evaluation of treatment toxicity
According to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 5.0) [12], the adverse events (AEs) of the combination therapy were assessed every month.
Statistical analysis
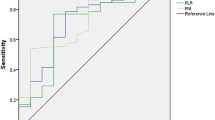
Clinical data were presented as proportions, medians, and ranges. Prognostic factors for PFS and OS were assessed by the Kaplan–Meier method. Hazard ratios (HRs) were estimated to identify independent factors via Cox regression survival. All tests were two-sided, and statistical significance was indicated by P < 0.05. Receiver operating characteristic (ROC) curves were drawn for the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), PNI, TG, TC, LDL, and HDL, and the proper cutoffs were determined using the Youden index. All statistical analyses were performed using SPSS 20.0 (IBM, Armonk, NY, USA) and R version 3.3.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patients’ characteristics
As presented in Table 1, 98 patients with advanced HCC who received combination treatment with anti-PD1/anti-PD-L1 immunotherapy and kinase inhibitors were identified. Of these patients, 87 were men, and 17 were older than 70 years. Most patients had ECOG PS 0–1 (80.6%) and Child–Pugh A (75.5%). Seventy-five patients had HBV infection, and 23 had a negative HBV status. Meanwhile, 62 patients had HBV DNA < 1000 IU/mL, and 36 had HBV DNA ≥ 1000 IU/mL. Sixty-one patients (62.2%) exhibited extrahepatic metastasis, and 41 (41.8%) had portal vein metastasis. Fifty-nine (60.2%) patients received combination therapy in the first-line setting. AFP levels exceeded 400 ng/mL in 44 patients (44.9%). In total, 95 patients (96.9%) received anti-PD-1 inhibitors, and 16 (16.3%) were treated with regorafenib.
Survival outcomes
Median PFS was 10.1 months (95% confidence interval [CI] = 8.10–12.10), and median OS was 17.2 months (95% CI = 12.29–22.11). HBV infection and the number of lines of immunotherapy were not related to PFS and OS. However, the HBV DNA status was obviously relevant for PFS (P = 0.044, Fig. 1).
Concerning clinical characteristics, univariate analysis identified ECOG PS 2 as a prognostic factor for worse PFS (P < 0.001) and OS (P = 0.003). Meanwhile, the absence of extrahepatic metastasis was linked to longer PFS (P = 0.001) and OS (P = 0.004, Fig. 2). The presence of PVT was associated with shorter PFS (P = 0.005) and OS (P = 0.016, Fig. 3). Moreover, PFS (P = 0.012) and OS (P = 0.007) were longer in patients with AFP < 400 ng/mL than in those with AFP ≥ 400 ng/mL. NLR ≥ 3.225, PLR ≥ 140.75, PNI < 37.25, and TC ≥ 3.46 mmol/L were associated with poor PFS and OS (Figs. 4, 5, 6, 7). Regarding the Child–Pugh classification, 98 patients were classified into class A or class B. Child–Pugh class A was associated with better PFS (P = 0.009) and OS (P = 0.001). Meanwhile, PFS significantly differed among the kinase inhibitors used (P = 0.014), and the receipt of regorafenib was associated with longer OS (P = 0.007, Fig. 8). However, the number of lines of prior systemic therapy, HDL, LDL, and TG were not significantly associated with PFS and OS (Tables 2, 3).
According to multivariate analysis, extrahepatic metastasis (P < 0.001; HR = 0.311; 95% CI = 0.164–0.589), PVT (P = 0.011; HR = 0.485; 95% CI = 0.278–0.846), NLR (P = 0.038; HR = 1.936; 95% CI = 1.038–3.612), PLR (P = 0.001; HR = 3.495; 95% CI = 1.693–7.213), and PNI (P = 0.021; HR = 0.439; 95% CI = 0.218–0.885) were predictive of PFS (Table 2). Conversely, PLR (P = 0.001; HR = 3.711; 95% CI = 1.726–7.978) and TC (P = 0.031; HR = 1.998, 95% CI = 1.065–3.751) were associated with OS (Table 3).
Safety
All 98 patients were included in the assessment of treatment-related AEs. The combination of ICIs and kinase inhibitors was well tolerated, and no patients died. Rash was the most common AEs, and skin toxicity occurred in 34.5% of patients. Other AEs included hypothyroidism (23.5%), gastrointestinal toxicity (25.5%), nephrotoxicity (54.1%), hepatotoxicity (26.5%), and hypertension (73.5%). Twenty-one patients experienced grade 3–4 treatment-related AEs, including hypertension, nephrotoxicity, gastrointestinal toxicity and dermatologic toxicity (Table 4).
Treatment response
The treatment response was CR, PR, SD, and PD in 0, 14 (14.3%), 70 (71.4%), and 14 patients (14.3%), respectively. The ORR and DCR were 14.3% and 85.7%, respectively.
Discussion
In this retrospective study, we focused on the clinical characteristics of patients with advanced HCC to identify meaningful predictive factors for the outcomes of ICI/kinase inhibitor combination therapy. Although combination treatment is the most common strategy for advanced HCC, only a few patients benefit from such regimens. Furthermore, there is no consensus predictor for the efficacy of combination therapy. In our study, the ORR and DCR were 14.3% and 85.7%, respectively. The absence of extrahepatic metastasis, the absence of PVT, NLR < 3.225, PLR < 140.75, and PNI ≥ 37.25 were independent prognostic factors for PFS, whereas PLR < 140.75 and TC < 3.46 mmol/L were independent prognostic factors for OS.
The absence of extrahepatic metastasis and absence of PVT were identified as independent prognostic factors for PFS in patients with advanced HCC in this study. Consistently, prior research identified the absence of extrahepatic metastasis (P = 0.002; HR = 2.244; 95%CI = 1.365–3.689) and absence of PVT (P = 0.025; HR = 1.911; 95% CI = 1.377–2.572) as predictive of better prognoses [13, 14]. Wu et al. also found in a real-world study of patients who received lenvatinib and nivolumab for advanced HCC survival was worse in patients with PVT (P = 0.01; HR = 4.3; 95% CI = 1.5–12.8) [15]. These findings could be explained by the fact that PVT can lead to portal vein blockage, thereby affecting liver function [14]. Thus, the combination of ICIs and kinase inhibitors appears beneficial in patients with HCC patients but no extrahepatic metastasis or PVT.
NLR and PLR are easily assessed serum indices. In prior research, a lower NLR was linked to better survival among patients treated with the anti-PD-1 antibody sintilimab combined with regorafenib (P = 0.002; HR = 0.518; 95% CI = 0.257–0.955) [16]. In another study, a higher PLR was linked to worse tumor responses to ICI/kinase inhibitor combination therapy (P < 0.001; HR = 0.287; 95% CI = 0.143–0.575) [17]. The present study identified NLR as an independent factor for PFS, and PLR was predictive of both PFS and OS. In the tumor microenvironment, chronic inflammation is a well-known factor associated with malignant tumors, and it influences the outcomes of cancer [20]. In a cohort of 296 patients with unresectable HCC from 14 institutions who were treated with atezolizumab plus bevacizumab, NLR < 5.0 and PLR < 300 were correlated with better PFS [18]. Furthermore, there were no significant differences in the treatment response and AE rates between low and high NLR and PLR [18]. Compared with other predictors, NLR and PLR are objective, inexpensive, and clinically available parameters.
HCC is closely related to chronic inflammation, which is associated with ICI treatment [19, 20]. A few studies described the significance of ALB levels and lymphocyte counts in patients with malignancy [21,22,23]. Our study analyzed clinical data and found that pretreatment PNI was a low-cost, reliable, and independent index, as PNI < 37.25 predicted worse PFS (P = 0.021; HR = 0.439; 95% CI = 0.218–0.885). Furthermore, PNI has been reported to be an independent predictor in gastric cancer [22, 23], non-small cell lung cancer [21, 24], nasopharyngeal cancer [25], and HCC [20].
TC metabolism plays an important role in cancers, and it influences the tumor microenvironment by reprogramming immune cell function [26, 27]. Previous studies investigated the relationship between TC and prognosis. In patients with gastric cancer who received chemotherapy and PD-1 inhibitors, Tang et al. identified TC as a clinical notable biomarker for assessing survival [28]. In patients who received anlotinib for non-small cell lung cancer, multivariate analysis revealed that higher TC levels were associated with worse OS (P = 0.003; HR = 1.773; 95% CI = 1.213–2.592) [29]. Similarly, pretreatment TC > 3.46 mmol/L was associated with worse PFS and OS in the current study. Moreover, TC was a highlighted predictor for OS individually through ICI plus kinase inhibitor.
According to univariate analysis, patients’ survival was associated with HBV DNA levels, but not the HBV infection status, in this study. Specifically, high HBV DNA levels were associated with poor PFS (8.1 months; P = 0.044) and OS (12.2 months; P = 0.136) in patients with advanced HCC, in line with previous reports [30, 31]. Lei et al. stated that among patients with HCC who received kinase inhibitors alone or in combination with PD-1 inhibitors, PFS (P < 0.001) and OS (P = 0.001) were significantly better in the non-HBV reactivation group than in the HBV reactivation group [30]. Furthermore, among patients with HBV-related HCC who received PD-1 inhibitor therapy, 24 patients with HBV DNA < 1000 copies/mL had longer recurrence-free survival (P = 0.002; HR = 7.783) and OS (P < 0.001; HR = 6.699) than 20 patients with HBV DNA ≥ 1000 copies/mL [31]. This can be explained by the fact that HBV reactivation might damage the function, proliferation, and survival of natural killer cells [32]. Accordingly, HBV reactivation might be a risk factor for patients with HCC who received ICI and kinase inhibitor combination therapy.
Regorafenib appeared to be the best kinase inhibitor for advanced HCC in this study. Regorafenib can enhance the effect of immune checkpoint inhibitor by modulating the IFN-γ/NSDHL/SREBP1/TGF-β1 axis against HCC, and this treatment potently suppresses JAK1/2-STAT and MAPK signaling to attenuate IFNγ-induced PD-L1 expression.[33.34] Moreover, regorafenib improved survival by increasing intratumoral CXCR3 + CD8 T cell infiltration and normalizing the cancer vasculature [35]. In this study, regorafenib was linked to prolonged median PFS (25.9 months; P = 0.014) and median OS (33.1 months; P = 0.007). However, multivariate analysis did not identify differences in PFS and OS among the kinase inhibitors used in this study. Thus, a randomized study of regorafenib plus ICI therapy is needed to identify the optimal combination therapy for advanced HCC.
Limitations
This study had multiple limitations. First, this study was retrospective in nature, and the sample size was small. Thus, the existence of selection or statistical bias cannot be dismissed. Second, HCC was not proven by pathology in all cases. Third, no independent verification group checked the clinical application of the cutoffs. Fourth, there was no consensus regarding the sequence of ICI and kinase inhibitor therapy. Finally, different follow-up therapies might lead to different outcomes. Thus, future studies should seek to identify the optimal ICI/kinase inhibitor combination for patients with advanced HCC.
Conclusion
This study identified extrahepatic metastasis, PVT, NLR, PLR, and PNI as independent clinical predictive factors for PFS and PLR and TC as independent predictors for OS in patients with advanced HCC who received combination therapy with ICIs and kinase inhibitors. Additionally, controlling HBV DNA levels before treatment is crucial for patients with advanced HCC and high HBV DNA levels. Although different combinations of ICIs and kinase inhibitors produced similar survival benefits, the findings suggested that regorafenib in combination with ICIs could improve survival outcomes in advanced HCC.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
Xia C, Dong X, Li H, Cao M, Sun D, He S, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J. 2022;135(5):584–90.
Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400(10360):1345–62.
Chinese Chapter of the International Hepato-Pancreato-Biliary Association; Group of liver Surgery, Surgical Society of Chinese Medical Association. Chinese multidisciplinary expert consensus on combined immunotherapy for hepatocellular carcinoma (2023 version). Zhonghua Gan Zang Bing Za Zhi. 2023; 31(1):16–34.
Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–73.
Yang X, Chen B, Wang Y, Wang Y, Long J, Zhang N, et al. Real-world efficacy and prognostic factors of lenvatinib plus PD-1 inhibitors in 378 unresectable hepatocellular carcinoma patients. Hepatol Int. 2023;17(3):709–19.
Chen B, Lei J, Zhao H, Dong J, Zeng Z, Li Y, et al. Efficacy and safety of TKI Plus PD-1 inhibitors in elderly uHCC patients: a retrospective study. J Hepatocell Carcinoma. 2022;9:1171–85.
Yan T, Huang C, Peng C, Duan X, Ji D, Duan Y, et al. A multi-center retrospective study on the efficacy and safety of regorafenib vs. regorafenib combined with PD-1 inhibitors as a second-line therapy in patients with advanced hepatocellular carcinoma. Ann Transl Med. 2023;11(2):109.
Qin S, Chan S, Gu S, Bai Y, Ren Z, Lin X, et al. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): a randomised, open-label, international phase 3 study. Lancet. 2023;402(10408):1133–46.
Dai L, Cai XC, Migaanyi J, Liu Y, Mao S, Lu C, et al. Therapeutic efectiveness and safety of sintilimab-dominated triple therapy in unresectable hepatocellular carcinoma. Sci Rep. 2021;11(1):19711.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47.
You H, Wang F, Li T, Xu X, Sun Y, Nan Y, et al. Guidelines for the prevention and treatment of chronic hepatitis B (version 2022). Zhonghua Gan Zang Bing Za Zhi. 2022;30(12):1309–31.
Freites-Martinez A, Santana N, Arias-Santiago S, Viera A. Using the common terminology criteria for adverse events (CTCAE - version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermo-Sifiliogr. 2021;112(1):90–2.
Cai M, Huang W, Huang J, Shi W, Guo Y, Liang L, et al. Transarterial chemoembolization combined with lenvatinib plus PD-1 inhibitor for advanced hepatocellular carcinoma: a retrospective cohort study. Front Immunol Front Immunol. 2022;13: 848387.
Zou X, Xu Q, You R, Yin G. Correlation and efficacy of TACE combined with lenvatinib plus PD-1 inhibitor in the treatment of hepatocellular carcinoma with portal vein tumor thrombus based on immunological features. Cancer Med. 2023;12(10):11315–33.
Wu WC, Lin TY, Chen MH, Hung YP, Liu CA, Lee RC, et al. Lenvatinib combined with nivolumab in advanced hepatocellular carcinoma-real-world experience. Invest New Drugs. 2022;40(4):789–97.
Huang J, Guo Y, Huang W, Hong X, Quan Y, Lin L, et al. Regorafenib combined with PD-1 blockade immunotherapy versus regorafenib as second-line treatment for advanced hepatocellular carcinoma: a multicenter retrospective study. J Hepatocell Carcinoma. 2022;9:157–70.
Wang ZY, Xu B, Wang LN, Zhu XD, Huang C, Shen YH, et al. Platelet-to-lymphocyte ratio predicts tumor response and survival of patients with hepatocellular carcinoma undergoing immunotherapies. Int Immunopharmacol. 2024;131: 111863.
Wu YL, Fulgenzi CAM, D’Alessio A, Cheon J, Nishida N, Saeed A, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as prognostic biomarkers in unresectable hepatocellular carcinoma treated with atezolizumab plus bevacizumab. Cancer. 2022;14(23):5834.
Keenan BP, Fong L, Kelley RK. Immunotherapy in hepatocellular carcinoma: the complex interface between inflammation, fibrosis, and the immune response. J Immunother Cancer. 2019;7(1):267.
Mei J, Sun XQ, Lin WP, Li SH, Lu LH, Zou JW, et al. Comparison of the prognostic value of inflammation-based scores in patients with hepatocellular carcinoma after anti-PD-1 therapy. J Inflamm Res. 2021;14:3879–90.
Liu N, Jiang A, Zheng X, Fu X, Zheng H, Gao H, et al. Prognostic nutritional index identifies risk of early progression and survival outcomes in advanced non-small cell lung cancer patients treated with PD-1 inhibitors. J Cancer. 2021;12(10):2960–7.
Wang X, Liu X, Dai H, Jia J. Peripheral blood nutrient indices as biomarkers for anti-PD-1 therapy efficacy and prognosis in patients with advanced gastric cancer. Oncol Lett. 2023;26(3):397.
Deng G, Zhu D, Du Z, Xue Y, Song H, Li Y. Body composition change indices combined with Prognostic Nutritional Index predicts the clinical outcomes of patients with gastric cancer treated with immune checkpoint inhibitor. Cancer Med. 2024;13(6): e7110.
Fang Q, Yu J, Li W, Luo J, Deng Q, Chen B, et al. Prognostic value of inflammatory and nutritional indexes among advanced NSCLC patients receiving PD-1 inhibitor therapy. Clin Exp Pharmacol Physiol. 2023;50(2):178–90.
Guo J, Yang Q, Jiang Q, Gu LW, Lin HX, Guo L. Integrating baseline nutritional and inflammatory parameters with post-treatment EBV DNA level to predict outcomes of patients with de novo metastatic nasopharyngeal carcinoma receiving chemotherapy combination PD-1 inhibitor. Nutrients. 2023;15(19):4262.
Zhang H, Zhao W, Li X, He Y. Cholesterol metabolism as a potential therapeutic target and a prognostic biomarker for cancer immunotherapy. Onco Targets Ther. 2021;14:3803–12.
Saad EE, Michel R and Borahay MA. Cholesterol and immune microenvironment: path towards tumorigenesis. Curr Nutr Rep. 2024; Online ahead of print.
Tang W, Li G, Lin Q, Zhu Z, Wang Z, Wang Z. Multiplex immunohistochemistry defnes two cholesterol metabolism patterns predicting immunotherapeutic outcomes in gastric cancer. J Transl Med. 2023;21(1):887.
Tang M, Song C, Zhang Y, Xu X, Wang C, Zhang Z, et al. Levels of pretreatment blood lipids are prognostic factors in advanced NSCLC patients treated with anlotinib. Lipids Health Dis. 2021;20(1):165.
Lei J, Yan T, Zhang L, Chen B, Cheng J, Gao X, et al. Comparison of hepatitis B virus reactivation in hepatocellular carcinoma patients who received tyrosine kinase inhibitor alone or together with programmed cell death protein-1 inhibitors. Hepatol Int. 2023;17(2):281–90.
Liang Y, Zhong D, Zhang Z, Su Y, Yan S, Lai C, et al. Impact of preoperative antiviral therapy on the prognosis of hepatitis B virus-related hepatocellular carcinoma. BMC Cancer. 2024;24(1):291.
Yang Y, Han Q, Hou Z, Zhang C, Tian Z, Zhang J. Exosomes mediate hepatitis B virus (HBV) transmission and NK-cell dysfunction. Cell Mol Immunol. 2017;14(5):465–75.
Xie L, Liu M, Cai M, Huang W, Guo Y, Liang L, et al. Regorafenib enhances anti-tumor efficacy of immune checkpoint inhibitor by regulating IFN-γ/NSDHL/SREBP1/TGF-β1 axis in hepatocellular carcinoma. Biomed Pharmacother. 2023;159: 114254.
Wu RY, Kong PF, Xia LP, Huang Y, Li ZL, Tang YY, et al. Regorafenib promotes antitumor immunity via inhibiting PD-L1 and IDO1 expression in melanoma. Clin Cancer Res. 2019;25(14):4530–41.
Shigeta K, Matsui A, Kikuchi H, Klein S, Mamessier E, Chen IX, et al. Regorafenib combined with PD1 blockade increases CD8 T-cell infiltration by inducing CXCL10 expression in hepatocellular carcinoma. J Immunother Cancer. 2020;8(2): e001435.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Ethical approval
This study was approved by the ethics committees of Ningbo Medical Center Lihuili Hospital.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
All the authors agree to the publication clause.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lu, Y., Lu, Y. Clinical predictive factors of the efficacy of immune checkpoint inhibitors and kinase inhibitors in advanced hepatocellular cancer. Clin Transl Oncol (2024). https://doi.org/10.1007/s12094-024-03644-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12094-024-03644-9