Abstract
Objectives
This study aims to evaluate the value of the dynamic and static quantitative metabolic parameters derived from 18F-fluorodeoxyglucose (FDG)–positron emission tomography/CT (PET/CT) in the differential diagnosis of metastatic from non-metastatic lymph nodes (LNs) in lung cancer and to validate them based on the results of a previous study.
Methods
One hundred and twenty-one patients with lung nodules or masses detected on chest CT scan underwent 18F-FDG PET/CT dynamic + static imaging with informed consent. A retrospective collection of 126 LNs in 37 patients with lung cancer was pathologically confirmed. Static image analysis parameters include LN-SUVmax and LN-SUVmax/primary tumor SUVmax (LN-SUVmax/PT-SUVmax). Dynamic metabolic parameters including the net influx rate (Ki) and the surrogate of perfusion (K1) and of each LN were obtained by applying the irreversible two-tissue compartment model using in-house Matlab software. Ki/K1 was then calculated as a separate marker. Based on the pathological findings, we divided into a metastatic group and a non-metastatic group. The χ2 test was used to evaluate the agreement of the individual and combined diagnosis of each metabolic parameter with the gold standard. The receiver-operating characteristic (ROC) analysis was performed for each parameter to determine the diagnostic efficacy in differentiating non-metastatic from metastatic LNs with high FDG-avid. P < 0.05 was considered statistically significant.
Results
Among the 126 FDG-avid LNs confirmed by pathology, 70 LNs were metastatic, and 56 LNs were non-metastatic. For ROC analysis, in separate assays, the dynamic metabolic parameter Ki [sensitivity (SEN) of 84.30%, specificity (SPE) of 94.60%, accuracy of 88.89%, and AUC of 0.895] had a better diagnostic value than the static metabolic parameter SUVmax (SEN of 82.90%, SPE of 62.50%, accuracy of 74.60%, and AUC of 0.727) in differentiating between metastatic from non-metastatic LNs groups, respectively. In the combined diagnosis group, the combined SUVmax + Ki diagnosis had a better diagnostic value in the differential diagnosis of metastatic from non-metastatic LNs, with SEN, SPE, accuracy, and AUC of 84.3%, 94.6%, 88.89%, and 0.907, respectively.
Conclusions
When the cutoff value of Ki was 0.022 ml/g/min, it had a high diagnostic value in the differential diagnosis between metastasis and non-metastasis in FDG-avid LNs of lung cancer, especially in improving the specificity. The combination of SUVmax and Ki is expected to be a reliable metabolic parameter for N-staging of lung cancer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the leading cause of cancer-related death in both men and women worldwide. In China, the mortality rate is 30%, which ranks first [1]. In recent years, morbidity and mortality have been increasing in China [1]. For patients with lung cancer, accurate N-staging is critical for developing individualized treatment plans and predicting prognosis [2]. In addition to the loss of surgical options for stage N3 patients, the 5-year survival rate is reduced to 6% [3]. Therefore, improving the accuracy of lung cancer N-staging to reduce the false-positive rate is one of the issues that has attracted clinical attention.
18F-fluorodeoxyglucose (FDG)–positron emission tomography/computed tomography (PET/CT) plays an important role in the differential diagnosis, staging, response assessment, and prognosis of lung cancer. The sensitivity and specificity of FDG PET/CT in the differential diagnosis and accurate staging of lung cancer are limited. Because FDG is not a tumor-specific imaging agent, the standard uptake value (SUV) is affected by a variety of factors [4, 5]. In China, as an endemic region for endemic infectious diseases, some benign lung diseases such as tuberculosis, infections, and inflammatory and granulomatous diseases cause FDG-avid, leading to a decrease in the specificity of FDG PET/CT in N-staging of lung cancer [6,7,8,9]. In recent years, the use of dynamic FDG PET/CT (dPET/CT) imaging in oncology has received much attention. Dynamic metabolic parameters, such as net influx rate (Ki) and tumor blood flow (K1), obtained based on the two-tissue irreversible compartment model approach, better describe the different metabolic stages of FDG and thus reflect the pathophysiological mechanisms of the disease [10, 11].
Previously, we conducted a series of studies related to the clinical application of dPET/CT in lung cancer [12,13,14,15]. In our preliminary study [12,13,14,15], we investigated the clinical value of dPET/CT in the differential diagnosis, N-staging, and epidermal growth factor receptor (EGFR) status prediction of lung cancer. The results of our preliminary study showed that the dynamic metabolic parameter Ki has a better differential diagnostic value in lung cancer differential diagnosis (cutoff value of 0.0250 ml/g/min) and EGFR status prediction (cutoff value of 0.0350 ml/g/min), especially improved specificity [13]. Of particular note, in a previous study [12], we compared the value of static metabolic parameters [SUVmax, lymph node (LN)–SUVmax/primary tumor (PT)–SUVmax] and dynamically visualized metabolic parameters (Ki and Ki/K1) in metastatic and non-metastatic FDG-avid LNs of lung cancer. We tentatively concluded that the dynamic metabolic parameters Ki and Ki/K1 with high specificity at cutoff values of 0.022 ml/g/min and 0.093, respectively (specificity of 0.918 and 0.776, and AUCs of 0.672 and 0.673, respectively) were able to better discriminate the metastatic and non-metastatic LNs from the FDG-avid LNs. For static metabolic parameters, SUVmax and LN-SUVmax/PT-SUVmax showed high sensitivity (0.826 and 0.999) at cutoff values of 4.050 and 0.236, respectively, but the specificity was suboptimal (specificity 0.388 and 0.204, and AUC 0.596 and 0.566, respectively).
To our knowledge, there are fewer previous relevant studies on the cutoff value of i. Based on our previous studies [12,13,14,15], we found that although Ki has good specificity in differential diagnosis, the sensitivity is not very reasonable. Therefore, in this study, we further investigated the clinical valueK of (SUVmax and LN-SUVmax/PT-SUVmax) and dynamic metabolic parameters (Ki and Ki/K1) in the differential diagnosis of FDG-avid LNs in lung cancer based on the cutoff value in our previous findings. The clinical value of each metabolic parameter in single or combined detection was investigated, and the results of the preliminary study were validated.
Materials and methods
Patients
The study was approved by the Ethics Committee of Cancer Hospital and Shenzhen Hospital, Chinese Academy of Medical Sciences (KYLH2022-1), and all patients signed a written informed consent before FDG PET/CT imaging.
A total of 121 patients underwent dPET/CT (chest, 65 min) + static FDG PET/CT imaging (sPET/CT, whole body, 10–20 min) from April 2022 to August 2023. All patients were found to have pulmonary nodules/masses on chest CT scans and were not receiving anti-inflammatory or anti-tumor therapy.
From the 121 patients, we retrospectively collected 126 FDG-avid LNs from 37 lung cancer patients to perform the present study. All 37 included patients enrolled had pathologically confirmed lung cancer. Postoperative pathology and/or puncture biopsy pathology confirmed 126 FDG-avid LNs as metastatic or non-metastatic LNs. The interval between the FDG PET/CT scan and the pathology results was less than 2 weeks.
Patients' FDG PET/CT scan characteristics of patients were collected, including PT-SUVmax, FDG-avid LN long diameter, short diameter, LN-SUVmax, and dynamic metabolic parameter values (including Ki and K1). Clinical information about the patients was also collected, including age, sex, type of primary lesion pathology, LN pathology results, and TNM staging [16]. LN locations were categorized according to the International Association for the Study of Lung Cancer LN map into the mediastinal region (zones 1–9) and the pulmonary hilar region (zones 10–12) [17]. The LNs enrolled in both dPET/CT and sPET/C corresponded to postoperative pathologic findings and or puncture biopsy sites and results. Finally, the results of the N-staging in dPET/CT and sPET/CT were based on the eighth edition of the TNM lung cancer classification [16].
PET/CT data acquisition, reconstruction, and analysis
Figure 1 shows the dPET/CT + sPET/CT examination process, data acquisition, image reconstruction, and metabolic parameter acquisition for the patient. All scans were performed in a Discovery MI PET/CT (GE Healthcare, Milwaukee, USA).
Dynamic parameters of Ki and K1 were then calculated and obtained based on the two-tissue irreversible compartment model. Ki/K1 was computed subsequently as a separate marker. The image-derived input function (IDIF) was extracted from the ascending aorta by drawing a 10-mm-diameter ROI on six consecutive slices in an image obtained by combining early time frames (0–60 s), where the effects of motion and partial volume are less pronounced than in the left ventricle. In addition, this study did not account for differences in blood and plasma uptake. In this model, we assumed unidirectional uptake of 18F-FDG (i.e., k4 = 0) with irreversible trapping in the tissue as 18F-FDG-6-PO4 [18]. Parametric images of each dynamic scan were generated using voxel-based analysis. Due to the large number of voxels in a whole-body image, the Lawson–Hanson non-negative least-squares algorithm was used to solve a linearized problem instead of the conventional non-linear one [19].
Pathological diagnosis
In this study, postoperative pathology and/or puncture biopsy pathology were the gold standard for follow-up.
All puncture and/or postoperative specimens were fixed in formalin, dehydrated, and embedded in paraffin. Four-micron sections of each tissue were stained with hematoxylin and eosin (H&E) and immunohistochemistry. The diagnosis was based on microscopic appearance and immunohistochemical results. The diagnosis was made independently by two experienced pathologists. In case of disagreement, the diagnosis was clarified after a full departmental discussion.
Statistical analysis
Continuous variables were reported as the median and interquartile range. Categorical variables were described as number and frequency. The χ2 test was used to evaluate the agreement of the individual and combined diagnosis of each metabolic parameter with the gold standard. The receiver-operating characteristic (ROC) analysis was performed for each parameter to determine the diagnostic efficacy in differentiating non-metastatic from metastatic LNs with high FDG-avid. The difference in the area under the curve (AUC) was determined by Delong’s test. A P value of less than 0.05 was considered statistically significant. All statistical analyses were performed with R statistical software (version 4.1.1).
Results
Patients and LN characteristics
Patient and LN characteristics are shown in Table 1. Among the 37 patients who underwent dPET/CT + sPET/CT scans, the mean age was 59.95 (59.95 ± 11.44) years, and the number of male and female patients was 24 (64.87%) and 13 (35.13%), respectively. Among the 126 LNs that were pathologically confirmed, 56 (44.44%) LNs were non-metastatic and 70 (55.56%) LNs were metastatic.
Primary tumors and FDG-avid LNs 18F-FDG PET/CT characteristics summary
Among the 37 patients, the average SUVmax of the primary tumor was 10.95 (10.95 ± 4.48). In the metastatic LNS group (N = 70), the ranges of long diameter, short diameter, SUVmax, LN-SUVmax/PT-SUVmax, Ki, and Ki/K1 were 1.95 cm [1.43;2.55], 1.30 cm [1.10;1.80], 7.75 [5.85;12.38], 0.69 [0.53;1.08], 0.023 [0.024;0.050] ml/g/min, and 0.295 [0.138;0.478], respectively. In the non-metastatic LNS group (N = 56), the ranges of long diameter and short diameter, SUVmax, LN-SUVmax/PT-SUVmax, Ki, and Ki/K1, were 1.30 cm [1.18;1.50], 1.00 cm [0.90;1.10], 3.50 [2.78;4.63], 0.45 [0.28;4.63], 0.012 [0.009;00016] ml/g/min, and 0.056 [0.0248;0.146], respectively.
Diagnostic value of individual and combined tests for each metabolic parameter
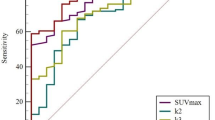
In previous studies, we have concluded that the cutoff values of 4.05, 0.236, 0.022 ml/g/min, and 0.093 for the static metabolic parameters SUVmax, LN-SUVmax/PT-SUVmax, and the dynamic metabolic parameters Ki, Ki/K1, respectively. Therefore, based on the above thresholds, we tested static metabolic parameters, and dynamic metabolic parameters individually and in combination. Table 2 and Fig. 2 show the results of ROC analysis of static and dynamic metabolic parameters when tested individually and in combination, respectively.
In separate assays, the dynamic metabolic parameter Ki [sensitivity (SEN) of 84.30%, specificity (SPE) of 94.60%, positive predictive value (PPV) of 84.29%, negative predictive value (NPV) of 94.64%, accuracy of 88.89%, and AUC of 0.895] had a better diagnostic value than the static metabolic parameter SUVmax (SEN of 82.90%, SPE of 62.50%, PPV of 82.86%, NPV of 65.50%, accuracy of 74.60%, and AUC of 0.727) in differentiating between metastatic and non-metastatic LNs groups, respectively.
In the combined diagnosis group, the combined SUVmax + Ki diagnosis had a better diagnostic value in the differential diagnosis of metastatic from non-metastatic LNs, with SEN, SPE, PPV, NPV, accuracy, and AUC of 84.3%, 94.6%, 84.29%, 94.64%, 88.89%, and 0.907, respectively.
Accuracy of metabolic parameters SUVmax and K i in N staging
Of the 126 LNs in 37 patients with lung cancer, 70 were metastatic, and 56 were non-metastatic. At the time of sPET/CT diagnosis, N-staging was consistent with pathology in 20 patients, but not in 17 patients, of which 15 patients were over-staged and 2 patients were under-staged. For the combined SUVmax + Ki diagnosis, N-staging was accurate in 30 patients, but N-staging was inconsistent with pathology findings in 7 patients, of which 3 patients were over-staged and 4 patients were under-staged. Table 3 shows the comparison of N-staging based on SUVmax and SUVmax + Ki diagnosis with pathological findings (Fig. 3).
A 66-year-old female patient. Surgical pathology confirmed squamous cell carcinoma in the upper lobe of the right lung (A, E, I, white arrow, size of 3.5 × 3.1 cm, SUVmax of 14.8). FDG PET/CT scan showed multiple FDG-avid LNs in the zone 11R, 7, and 4R. Among them, the FDG-avid LN in zone 11R (B, F, white arrow) with a size of 1.4 × 1.3 cm, SUVmax of 4.0, and Ki of 0.010 ml/g/min. The FDG-avid LN in zone 7 (C, G, white arrow) with a size of 1.0 × 0.8 cm, SUVmax of 4.4, and Ki of 0.016 ml/g/min. The FDG-avid LN in zone 4R (D, H, white arrow) with a size of 2.1 × 1.3 cm, SUVmax of 4.1, and Ki of 0.013 ml/g/min. Finally, all FDG-avid LNs in zones 11R, 7, and 4R were pathologically confirmed to be cancer-free (Fig. 4).
A 66-year-old male patient. FDG PET/CT showed a nodule in the upper lobe of the left lung (A, B), with a size of 1.0 × 0.6 cm, and SUVmax of 4.8. FDG PET/CT scan showed FDG-avid LNs in zones 4L and 4R. Among them, the FDG-avid LN in zone 4L (C, F, white arrow) was pathologically confirmed adenocarcinoma metastasis (D), with a size of 4.2 × 3.8 cm, SUVmax of 34.6, and Ki of 0.135 ml/g/min. The FDG-avid LN in zone 4R (C, F, yellow arrow) was pathologically confirmed non-metastasis (E), with a size of 1.3 × 0.9 cm, SUVmax of 4.0, and Ki of 0.015 ml/g/min.
Discussion
In lung cancer, accurate N-staging is essential for developing personalized treatment plans and determining prognosis and is one of the clinical concerns. In this study, based on the previous study, we further confirmed the high specificity of the dynamic metabolic parameter Ki in the differential diagnosis between metastatic from non-metastatic FDG-avid LNs in lung cancer. The combination of SUVmax and Ki is expected to be a reliable imaging basis for accurate N-staging of lung cancer.
Radiopharmaceutical distribution is a dynamic process that varies widely in diseases and individuals [20]. Previous studies have confirmed that dPET/CT extracts physiological and biochemical parameters that better reflect the pathophysiological mechanisms of disease compared to sPET/CT. These parameters (e.g., Ki) have been shown to discriminate between benign and malignant diseases [10,11,12,13,14,15, 19, 21,22,23]. Previous studies have confirmed the value of dPET/CT in the differential diagnosis of lung cancer and inflammatory lesions, but there are fewer studies in the differential diagnosis of LNs. Previously, in our studies related to dynamic metabolic parameters in the differential diagnosis of lung cancer LNs [12], we discussed the value of dynamic metabolic parameters (including K1, K2, Ki, and Ki/K1) in the N-staging of lung cancer. We concluded that when the cutoff values of Ki and Ki/K1 were 0.022 ml/g/min and 0.093, respectively, there was a good diagnostic value in the differential diagnosis of metastasis and non-metastasis in FDG-avid LNs of lung cancer, especially improving the specificity (AUC of 0.672 and 0.673, and specificity of 0.918 and 0.776, respectively). Therefore, to further investigate the diagnostic value and feasibility of dynamic metabolic parameters in N-staging of lung cancer based on the critical values of our previous study, we conducted this validation study.
In this study, we found that the static parameters SUVmax (cutoff value of 4.05) and LN-SUVmax/PT-SUVmax (cutoff value of 0.236) had high sensitivity (82.5% and 92.7%) and accuracy (74.60% and 61.11%), but the specificity was not satisfactory (62.50% and 17.90%). The addition of the dynamic metabolic parameter Ki (cutoff value of 0.022) resulted in a significant improvement in the differential diagnosis when SUVmax and Ki were combined (AUC of 0.907), with high sensitivity (84.30%), specificity (94.60%), and accuracy (88.89%).
Among the 37 lung cancer patients in our study, at the time of sPET/CT diagnosis, N-staging was consistent with pathology in 20 (54.05%) patients, but not in 17 (45.94%) patients, of which 15 (40.54%) patients were over-staged and 2 (5.41%) patients were down-staged. For the combined SUVmax + Ki diagnosis, N-staging was accurate in 30 (81.08%) patients, but N-staging was inconsistent with pathology findings in 7 (18.92%) patients, of which 3 (8.10%) patients were over-staged and 4 (10.81%) patients were down-staged. After adding the dynamic metabolic parameter Ki for co-diagnosis, eight patients (21.62%) diagnosed as stage N3 by SUVmax were downgraded to stage N0–2, and four (30.77%) patients underwent successful surgical treatment. Therefore, SUVmax + Ki combined diagnosis has high diagnostic value in the differential diagnosis of FDG-avid LN metastasis from non-metastasis in lung cancer, especially to improve the specificity. In particular, it improves the diagnostic accuracy of patients with clinical suspicion of stage N3, seeks surgical opportunities as much as possible, and reduces some unnecessary puncture biopsies. To assist clinicians in accurate N-staging to improve the prognosis and quality of life of lung cancer patients.
Previous studies have reported that [24,25,26], the false-positive rate of squamous cell carcinoma on sPET/CT is significantly higher than that of adenocarcinoma and small cell carcinoma. This may be because squamous cell carcinomas are mostly central bronchogenic carcinomas, often associated with obstructive pneumonia and atelectasis, which can activate macrophages and inflammatory cells and cause reactive hyperplasia of LNs, leading to false-positive sPET/CT results [27]. In our sample size of 21 LNs (21/126) from 5 patients (5/37) with squamous cell carcinoma, the PT-SUVmax ranged from 8.5 to 16.4 and LN-SUVmax ranged from 2.5 to 11.5. N-staging sPET/CT failed to accurately stage all five patients, misstating two and over-staging three patients. Three of these patients were accurately staged with the SVUmax + Ki combination. Three (3/13) of these patients had pathologically confirmed N0, but SUVmax was assessed as N3 (1/3) and N2 (2/3), and with the addition of Ki, the N3 (1/3)/N2 (1/3) stage was reduced to N0 (2/3). Among the patients in the adenocarcinoma group (N = 26), 17 patients (17/26) were accurately staged by sPET/CT, but 4 patients (4/26) were misstated and 5 patients (5/26) were over-staged. Among the over-staged patients (N = 5), PT-SUVmax ranged from 1.3 to 14.8, LN-SUVmax (N = 20) ranged from 2.6 to 17.2, and all were staged as stage N2 by sPET/CT. When SUVmax + Ki was combined with the test, the five patients who were over-staged as described above were accurately staged, with three down-staged to N0 and two down-staged to N1, all consistent with pathologic findings. Among patients with small cell carcinoma (N = 5), 3 patients (3/5) were accurately staged by sPET/CT, but 2 patients (2/5) were over-staged. Among the over-staged patients (N = 2), PT-SUVmax was 7.6 and 9.0, respectively, LN-SUVmax (N = 3) ranged from 2.6 to 4.2, and sPET/CT staging was N3 and N2, respectively. When SVUmax + Ki was detected in combination, the patients originally staged as N3 by sPET/CT were downgraded to N2, and those staged as N2 were downgraded to N0, which was consistent with the pathological results. The inclusion of the dynamic metabolic parameter Ki improved the specificity of the differential diagnosis. The combined SUVmax + Ki is expected to be a reliable metabolic parameter for lung cancer N-staging. In addition, whether the dynamic metabolic parameter Ki differs between pathology types and degree of differentiation is also of interest and one of our ongoing research topics.
There are several limitations to our study. First, we used a retrospective design due to our use of pathologic findings as the gold standard and the fact that the dPET/CT scan takes 65 min, resulting in our small sample size. In addition, prolonged scanning may lead to patient discomfort and feasibility issues. Second, motion correction was not considered in this study. It is known that motion in the chest region can affect not only the SUV but also the kinetic parameters quantification [28,29,30]. Third, SUVmax rather than SUVmean was used in this study, because we believed SUVmax to be more stable and less affected by partial volume effects.
Conclusions
When the cutoff value of the Ki was 0.022 ml/g/min, it had a high diagnostic value in the differential diagnosis between metastasis and non-metastasis in FDG-avid LNs of lung cancer, especially in improving the specificity. The combination of SUVmax and Ki is expected to be a reliable metabolic parameter for N-staging of lung cancer.
Data availability
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
References
Yang D, Liu Y, Bai C, Wang X, Powell CA. Epidemiology of lung cancer and lung cancer screening programs in China and the United States. Cancer Lett. 2020;468:82–7.
Detterbeck FC, Postmus PE, Tanoue LT. The stage classification of lung cancer: diagnosis and management of lung cancer, 3rd ed: American college of chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e191S-210S.
Woodard GA, Jones KD, Jablons DM. Lung cancer staging and prognosis. Cancer Treat Res. 2016;170:47–75.
Zhao L, He Z-Y, Zhong X-N, Cui ML. (18) FDG-PET/CT for detection of mediastinal nodal metastasis in non-small cell lung cancer: a meta-analysis. Surg Oncol. 2012;21(3):230–6.
Wu Y, Li P, Zhang H, Shi Y, Wu H, Zhang J, et al. Diagnostic value of fluorine 18 fluorodeoxyglucose positron emission tomography/computed tomography for the detection of metastases in non-small-cell lung cancer patients. Int J Cancer. 2013;132(2):E37-47.
Deppen S, Putnam JB, Andrade G, Speroff T, Nesbitt JC, Lambright ES, et al. Accuracy of FDG-PET to diagnose lung cancer in a region of endemic granulomatous disease. Ann Thorac Surg. 2011;92(2):428–32.
Ur M, Even-Sapir E. Increased (18)F-fluorodeoxyglucose uptake in benign, nonphysiologic lesions found on whole-body positron emission tomography/computed tomography (PET/CT): accumulated data from four years of experience with PET/CT. Semin Nucl Med. 2007;37(3):206–22.
Deppen SA, Blume JD, Kensinger CD, Morgan AM, Aldrich MC, Massion PP, et al. Accuracy of FDG-PET to diagnose lung cancer in areas with infectious lung disease: a meta-analysis. JAMA. 2014;312(12):1227–36.
Konishi J, Yamazaki K, Tsukamoto E, Tamaki N, Onodera Y, Otake T, et al. Mediastinal lymph node staging by FDG-PET in patients with non-small cell lung cancer: analysis of false-positive FDG-PET findings. Respiration. 2003;70(5):500–6.
Rahmim A, Lodge MA, Karakatsanis NA, Panin VY, Zhou Y, McMillan A, et al. Dynamic whole-body PET imaging: principles, potentials and applications. Eur J Nucl Med Mol Imaging. 2019;46(2):501–18.
Meijer TWH, Geus-Oei L-F, Visser EP, Oyen WJG, Looijen-Salamon MG, Visvikis D, et al. Tumor delineation and quantitative assessment of glucose metabolic rate within histologic subtypes of non-small cell lung cancer by using dynamic 18F fluorodeoxyglucose PET. Radiology. 2017;283(2):547–59.
Wumener X, Zhang Y, Wang Z, Zhang M, Zang Z, Huang B, Liu M, Huang S, Huang Y, Wang P, Liang Y, Sun T. Dynamic FDG-PET imaging for differentiating metastatic from non-metastatic lymph nodes of lung cancer. Front Oncol. 2022;10(12):1005924.
Wumener X, Zhang Y, Zang Z, Fen D, Ye X, Zhang M, et al. The value of dynamic FDG PET/CT in the differential diagnosis of lung cancer and predicting EGFR mutations. BMC Pulm Med. 2024;24:227.
Du F, Wumener X, Zhang Y, Zhang M, Zhao J, Zhou J, et al. Clinical feasibility study of early 30-minute dynamic FDG-PET scanning protocol for patients with lung lesions. EJNMMI Phys. 2024;11(1):23.
Wumener X, Ye X, Zhang Y, Jin S, Liang Y. Dynamic and static 18F-FDG PET/CT imaging in SMARCA4-deficient non-small cell lung cancer and response to therapy: a case report. Diagnostics (Basel). 2023;13(12):2048.
Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The eighth edition lung cancer stage classification. Chest. 2017;151(1):193–203.
El-Sherief AH, Lau CT, Wu CC, Drake RL, Abbott GF, Rice TW. International association for the study of lung cancer (IASLC) lymph node map: radiologic review with CT illustration. Radiogr Rev Publ Radiol Soc N Am Inc. 2014;34(6):1680–91.
Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 1983;3:1–7. https://doi.org/10.1038/jcbfm.1983.1.
Lawson CL, Hanson RJ. Solving least squares problems. Linear Least Sq Linear Inequal Constr. 1995;23:158–73.
Schmidt-Hansen M, Baldwin DR, Zamora J. FDG-PET/CT imaging for mediastinal staging in patients with potentially resectable non-small cell lung cancer. JAMA. 2015;313(14):1465–6.
Van Tinteren H, Hoekstra OS, Smit EF, van den Bergh JH, Schreurs AJ, Stallaert RA, et al. Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small-cell lung cancer: the PLUS multicentre randomised trial. Lancet. 2002;359(9315):1388–93.
Lee J, Kim YK, Seo YY, Choi EK, Lee DS, Kim YS, et al. Clinical characteristics of false-positive lymph node on chest CT or PET-CT confirmed by endobronchial ultrasound-guided transbronchial needle aspiration in lung cancer. Tuberc Respir Dis (Seoul). 2018;81(4):339–46.
Lu P, Sun Y, Sun Y, Yu L. The role of (18)F-FDG PET/CT for evaluation of metastatic mediastinal lymph nodes in patients with lung squamous-cell carcinoma or adenocarcinoma. Lung Cancer. 2014;85(1):53–8.
Sarikaya I, Yeung HWD, Erdi Y, Larson SM. Respiratory artefact causing malpositioning of liver dome lesion in right lower lung. Clin Nucl Med. 2003;28(11):943–4.
Nehmeh SA, Erdi YE, Ling CC, Rosenzweig KE, Schoder H, Larson SM, et al. Effect of respiratory gating on quantifying PET images of lung cancer. J Nucl Med Off Publ Soc Nucl Med. 2002;43(7):876–81.
Sun T, Petibon Y, Han PK, Ma C, Kim SJW, Alpert NM, et al. Body motion detection and correction in cardiac PET: phantom and human studies. Med Phys. 2019;46(11):4898–906.
Sun T, Wu T-H, Wang S-J, Yang BH, Wu NY, Mok GS. Low dose interpolated average CT for thoracic PET/CT attenuation correction using an active breathing controller. Med Phys. 2013;40(10): 102507.
Hoffman EJ, Huang SC, Phelps ME. Quantitation in positron emission computed tomography: 1. effect of object size. J Comput Assist Tomogr. 1979;3(3):299–308.
Rousset OG, Ma Y, Evans AC. Correction for partial volume effects in PET: principle and validation. J Nucl Med Off Publ Soc Nucl Med. 1998;39(5):904–11.
Chang G, Chang T, Pan T, Clark JW Jr, Mawlawi OR. Joint correction of respiratory motion artifact and partial volume effect in lung/thoracic PET/CT imaging. Med Phys. 2010;37(12):6221–32.
Acknowledgements
We would like to express our deepest gratitude to Dr. Tao Sun's team at the Paul C. Lauterbur Research Center for Biomedical Imaging, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences for generously providing us with the dynamic image and data analysis software. Their valuable contributions made this study possible. We would also like to thank our mentor, Jun Zhao, and the department head, Ying Liang, for their guidance and support during the research process. Without their expertise and guidance, this project would not have been possible.
Funding
This study was funded by the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital and Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shenzhen (E010322003)/Shenzhen Clinical Research Center for Cancer and Shenzhen High-level Hospital Construction Found.
Author information
Authors and Affiliations
Contributions
XW, JZ, and YL designed the project and wrote the manuscript. YZ analyzed data. ZZ organized data. XY provided pathologic guidance. JZ contributes to PET/CT scans. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethics statement
This study was approved by the ethics committee of Cancer Hospital and Shenzhen Hospital, Chinese Academy of Medical Sciences (KYLH2022-1). The patients/participants provided their written informed consent to participate in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wumener, X., Zhang, Y., Zang, Z. et al. The value of net influx constant based on FDG PET/CT dynamic imaging in the differential diagnosis of metastatic from non-metastatic lymph nodes in lung cancer. Ann Nucl Med (2024). https://doi.org/10.1007/s12149-024-01964-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12149-024-01964-y