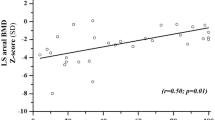
Abstract
Introduction
Bone loss is a major issue in patients affected by Duchenne muscular dystrophy (DMD), a rare musculoskeletal disorder, particularly in those treated with glucocorticoids (GCs). We aimed to assess the effectiveness of neridronate in terms of bone mineral density (BMD) changes in this population.
Methods
We retrospectively reviewed the records of patients affected by DMD receiving GCs referred to our outpatient from 2015 to 2020. All patients were treated with an intramuscular (IM) injection of neridronate (25 mg every month). Bone density was measured at the lumbar spine (LS; L1–L4 tract) using dual-energy x-ray absorptiometry (DXA) (GE Lunar), no more than 4 weeks before (T0) and after 1 year from neridronate treatment (T1).
Results
Eight boys with DMD were included with a mean age at diagnosis of 4.75 ± 2.81 years. Six of them were non-ambulant and two of them had previous low-trauma fractures (a distal femur fracture and a vertebral compression fracture, respectively). All patients were receiving deflazacort [median duration of therapy 11.5 years (interquartile range 2–25)]. At the DXA evaluation (T0), the mean L1–L4 BMD value was 0.716 ± 0.164 g/cm2. Six patients (75%) showed an L1–L4 Z-score height-adjusted of less than − 2. The mean age of neridronate initiation was 18.87 ± 6.81 years. All patients were supplemented with calcium carbonate and vitamin D at baseline. After 12 months of treatment (T1), the mean L1–L4 BMD value was 0.685 ± 0.190 g/cm2. Seven patients (87.5%) showed an L1–L4 Z-score of less than − 2. Changes in LS BMD and Z-score were not significant between T0 and T1 in our cohort (p = 0.674 and p = 0.208, respectively) as well as among non-ambulant patients with DMD without previous fragility fractures.
Conclusions
In this study, we reported for the first time that neridronate may slow bone loss in GC-treated patients with DMD at 1-year follow-up.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In Duchenne muscular dystrophy (DMD), progressive muscle weakness and chronic use of glucocorticoids (GCs) cause poor bone health and increased risk of fragility fractures. |
To date, bisphosphonates (BPs) may represent a therapeutic strategy, although limited evidence supports their use in this population. |
Neridronate, a parenteral BP, demonstrates efficacy and safety in the management of secondary osteoporosis. |
In this study, we assess the effectiveness of neridronate in the management of bone health in patients with DMD, treated with chronic GCs. |
After 1 year of treatment, no changes in bone mineral density at the lumbar spine, as well as no incident vertebral fractures, were observed in our population. |
Further well-designed trials are required to confirm the role of neridronate in the prevention of bone fragility in patients with DMD receiving chronic GCs. |
Introduction
Duchenne muscular dystrophy (DMD) is a rare X-linked neuromuscular disease (NMD), affecting about 1 on 6000 live male births [1], characterized by progressive muscle weakness, up to loss of independent ambulation by the age of 13 years old [2]. This disabling condition has several consequences on musculoskeletal health, including reduced bone density and strength and increased risk of fragility fractures. Bone loss in people with DMD depends on mechanical and biochemical mechanisms. Muscle weakness and progressive mobility loss are major culprits for poor mechanical stimulation of bone tissue causing reduced bone mineral density (BMD) [3]. Chronic muscle inflammation, observed in these patients, adversely affects signalling pathways that modulate muscle–bone cross talk [4]. In particular, several cytokines (i.e., interleukin-6, leukemia inhibitory factor), osteokines (i.e., osteopontin), and myokines (fibroblast growth factor 21, FGF21) are upregulated in patients with DMD contributing to bone loss [4]. Furthermore, destabilization of the dystrophin-associated protein complex (DAPC) and structural changes in the elastic properties of the myotendinous junctions reduced force transmission and mechanical stimuli on bone tissue [5].
Finally, although glucocorticoids (GCs) still represent the first-line intervention for delaying the decline of motor development in patients with DMD, their chronic use is associated with poor bone strength, as a consequence of impaired bone formation and mineralization of trabecular bone [6, 7]. Among GCs, deflazacort was considered less detrimental to the trabecular bone than prednisone [8], although recent studies have demonstrated that patients with DMD on daily deflazacort have a 16-fold increased risk for the first fragility fracture during 4 years of treatment [9]. Moreover, a new first-in-class steroidal anti-inflammatory drug, vamolorone, had recently shown a similar efficacy in maintaining muscle strength and physical performance with an improvement in bone turnover markers compared to the standard GC treatment in this population, along with a favorable safety profile, in terms of lack of stunting of growth [10].
To date, primary prevention of fragility fracture in this population includes supplementation of vitamin D with or without calcium, and physical activity [11]. Anti-osteoporotic drugs, such as bisphosphonates (BPs), are widely used in both primary and secondary osteoporosis, including glucocorticoid-induced osteoporosis (GIO). However, limited evidence supports their use in patients with DMD, except for those with previous vertebral or long-bone fragility fractures [12]. It is key to note that BP administration may affect the outer shape and inner structure of the growing metaphysis [13], justifying the limited use of these drugs in children.
Among available BPs, neridronate, a nitrogen-containing BP requiring parenteral administration, is effective and safe for patients with osteoporosis, including those affected by thalassemia-related bone loss. Moreover, this drug is effective in managing other conditions, such as complex regional pain syndrome type I, where localized bone loss is only one of the typical clinical findings [14,15,16,17].
This study aimed to assess the effectiveness of neridronate in terms of BMD changes in patients affected by DMD receiving GCs.
Methods
In this retrospective cohort study, we reviewed the records of patients with a genetic diagnosis of DMD receiving GCs from at least 3 months, referred to our outpatient for the management of bone fragility. Patients treated with neridronate received an intramuscular (IM) dose of 25 mg every month [18] and daily vitamin D (600 IU) and calcium supplementation (500 mg) [19]. We excluded medical records of patients aged less than 5 years old, treated previously with BPs, with severe scoliosis that may hinder instrumental acquisition and/or with any severe comorbidities (including cardiological and respiratory ones).
Our data collection included demographic, anthropometric, and anamnestic details such as age, body mass index (BMI), disease duration, and GC therapy, both as dose and duration. We included only patients that underwent dual-energy x-ray absorptiometry (DXA) measurements at our outpatient clinic. We reviewed DXA-derived (GE Lunar i-DXA) measurements of BMD at lumbar spine (LS), LS Z-score height-adjusted as recommended by the International Society for Clinical Densitometry (ISCD), as well as lateral vertebral assessment (LVA) to identify vertebral fragility fractures (VFx) performed no more than 4 weeks before (T0) and after 1 year from neridronate treatment (T1). Moreover, we provided DXA measurements performed before T0, where available.
The research was conducted in accordance with the Declaration of Helsinki and all patients signed an informed consent to provide available data for this study.
Statistical analysis was carried out using Statistical Package for the Social Sciences 25 (SPSS 25) software. Distribution of all variables was tested using Shapiro–Wilk test. Intergroup comparisons were made using Wilcoxon-Mann–Whitney test for data not normally distributed. A p value ≤ 0.05 was considered significant. Continuous variables are presented as mean ± standard deviation (SD) or median (IQR, interquartile range), and categorical variables as counts and percentage (%).
Results
A flowchart of study population selection is shown in Fig. 1. Medical records of eight boys with DMD are reported in Table 1. All patients received deflazacort at dosage of 15 mg/daily. At the DXA evaluation (T0), the mean L1–L4 BMD value was 0.716 ± 0.164 g/cm2. Six patients (75%) showed an L1–L4 Z-score of less than − 2. The mean age of neridronate initiation was 18.87 ± 6.81 years. After 12 months of treatment (T1), the mean L1–L4 BMD value was 0.685 ± 0.19 g/cm2. Seven patients (87.5%) showed an L1–L4 Z-score of less than − 2. No incident VFx at LVA and no new long-bone fractures referred by the patient were reported at T1. Moreover, no worsening of the previous VFx was observed at LVA evaluation. As shown in Table 2, changes in LS BMD and Z-score were not significant between T0 and T1 (p = 0.674 and p = 0.208, respectively). We performed a sub-analysis among the non-ambulant patients without previous fracture to identify changes in LS BMD and Z-score between baseline and T1. We observed that also in this population BMD changes were not significant (p = 0.917 and p = 0.249, respectively). Clinical and densitometric characteristics of five patients of our cohort that underwent at least three densitometric exams through our facility and relative comparisons are described in Table 3.
Discussion
In this study, we demonstrated that neridronate may slow the decline of BMD in patients with DMD receiving GCs. It has been reported that GC regimen and ambulatory status are independently associated with a significantly increased hazard ratio of first fragility fracture in this population [9]. In particular, loss of mobility with full-time wheelchair use is considered a risk factor of first fracture (greater than 75%) for every 3 months [20]. According to our data, no new fracture was reported among the four non-ambulant patients with DMD in the 12-month follow-up period.
Long-term GC therapy strongly contributes to low BMD and increased risk of VFx in patients with DMD [21]. Mayo et al. demonstrated a significant reduction in LS BMD Z-scores within the first 2 years of deflazacort therapy among boys with DMD, and this decline was consistent with subsequent years of therapy as well as with the loss of ambulation [8]. In our cohort, 1 year of neridronate administration did not significantly change LS BMD and Z-score, suggesting that this intervention may prevent bone loss in GC-treated patients with DMD. Moreover, the effectiveness of neridronate is confirmed also in non-ambulant patients, where LS BMD and Z-score did not significantly change. These results demonstrate how neridronate might mitigate BMD reduction also among patients with DMD with mobility loss.
Our findings are in line with those recently reported by Ronsley et al. in a retrospective, comparative effectiveness study [22]. They demonstrated that boys with DMD receiving intravenous (IV) administration of pamidronate or zoledronic acid had significantly lower mean decline of total-body and left hip BMD Z-scores compared to the untreated group (− 0.63 SD, p = 0.026, and − 1.04 SD, p = 0.004, respectively). It is key to note that our study provides data about BMD changes in a specific site (lumbar vertebrae) that is affected during GIO and DMD. Consistent with our findings, a recent randomized controlled trial assessing effects of five zoledronic acid infusions on BMD in patients with DMD showed higher BMD value in the treated arm versus control arm both at 12 and 24 months (both p < 0.001) [23].
Several guidelines recommend BPs use, both oral and parenteral, to treat GIO [24, 25].
In the last two decades, the benefits of BPs, in different formulations (IV or oral), in terms of BMD changes in patients with DMD have been thoroughly investigated [26]. Nevertheless, no study addressed the effectiveness of neridronate in patients affected by DMD receiving GCs.
Neridronate has a good safety profile, except for some cases of flu-like symptoms that resolved within a few days [27]. In an open-label 3-year study that assessed safety of neridronate in children and adolescents with osteogenesis imperfecta (OI), no fatal event was registered, and serious adverse events were considered not treatment-related; moreover, no cases of osteonecrosis of the jaw or atypical femur fracture were reported [28].
Considering the efficacy and safety of neridronate in children affected by idiopathic juvenile osteoporosis (IJO) or secondary osteoporosis [14, 29, 30], our data might support a role of this drug in the prevention of bone fragility in patients with DMD.
Concerning the efficacy of BPs in the secondary prevention of fragility fractures in patients with DMD treated with GCs, several studies [22, 26, 31] showed that these drugs reduce the number and severity of incident vertebral fractures, and pain after 2 years of therapy.
In this population, fragility fractures occur in about 20–40% of cases in comparison to less than 5% reported in healthy children [32]. The risk of at least one fracture, particularly of the spine and long bones, reaches 60% in patients with DMD aged 15 years [21]. The incidence of VFx is even higher in children with DMD on chronic GCs therapy [33, 34]. In our cohort of long-term GC-treated patients with DMD, no fracture was reported at 1-year follow-up (both at LVA evaluation and as referred by our population).
The main strength of our study is to have focused our investigations on the most commonly involved skeletal site in patients affected by DMD, particularly in those receiving GCs.
The main limitations of our study are the retrospective design with a low number of participants, 1-year follow-up, and the lack of a matched control population. In addition, we did not provide data about serum and urinary bone turnover markers during the 12 months of treatment. Finally, only LVA without radiographic imaging was available for the assessment of VFx. Despite these limitations, our data are noteworthy because we first described the effectiveness of the only BP (i.e., neridronate) already approved by the Italian national regulatory agency (AIFA) for the prevention and treatment of fragility fractures in the pediatric population (i.e., OI), in the management of bone loss in patients with DMD receiving GCs.
Conclusions
Our study suggests that IM administration of neridronate might slow the decline of bone loss at LS in boys with DMD receiving GCs. Moreover, after 1 year of treatment, no patient reported incident VFx. Further studies with long-term follow-up should be performed to confirm our findings.
References
Ryder S, Leadley RM, Armstrong N, et al. The burden, epidemiology, costs and treatment for Duchenne muscular dystrophy: an evidence review. Orphanet J Rare Dis. 2017;12(1):79. https://doi.org/10.1186/s13023-017-0631-3.
Duan D, Goemans N, Takeda S, Mercuri E, Aartsma-Rus A. Duchenne muscular dystrophy. Nat Rev Dis Primers. 2021;7(1):13. https://doi.org/10.1038/s41572-021-00248-3.
Iolascon G, Paoletta M, Liguori S, Curci C, Moretti A. Neuromuscular diseases and bone. Front Endocrinol (Lausanne). 2019;22(10):794. https://doi.org/10.3389/fendo.2019.00794.
Starosta A, Konieczny P. Therapeutic aspects of cell signaling and communication in Duchenne muscular dystrophy. Cell Mol Life Sci. 2021;78(11):4867–91. https://doi.org/10.1007/s00018-021-03821-x.
Welser JV, Rooney JE, Cohen NC, et al. Myotendinous junction defects and reduced force transmission in mice that lack alpha7 integrin and utrophin. Am J Pathol. 2009;175:1545–54. https://doi.org/10.2353/ajpath.2009.090052.
Birnkrant DJ, Bushby K, Bann CM, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol. 2018;17(3):251–67. https://doi.org/10.1016/S1474-4422(18)30024-3.
Bylo M, Farewell R, Coppenrath VA, Yogaratnam D. A review of deflazacort for patients with Duchenne muscular dystrophy. Ann Pharmacother. 2020;54(8):788–94. https://doi.org/10.1177/1060028019900500.
Mayo AL, Craven BC, McAdam LC, Biggar WD. Bone health in boys with Duchenne muscular dystrophy on long-term daily deflazacort therapy. Neuromuscul Disord. 2012;22(12):1040–5. https://doi.org/10.1016/j.nmd.2012.06.354.
Joseph S, Wang C, Bushby K, et al. Fractures and linear growth in a nationwide cohort of boys with Duchenne muscular dystrophy with and without glucocorticoid treatment: results from the UK NorthStar Database. JAMA Neurol. 2019;76(6):701–9. https://doi.org/10.1001/jamaneurol.2019.0242.
Mah JK, Clemens PR, Guglieri M, et al. Efficacy and safety of vamorolone in Duchenne muscular dystrophy: a 30-month nonrandomized controlled open-label extension trial. JAMA Netw Open. 2022;5(1):e2144178. https://doi.org/10.1001/jamanetworkopen.2021.44178.
Bianchi ML, Morandi L, Andreucci E, Vai S, Frasunkiewicz J, Cottafava R. Low bone density and bone metabolism alterations in Duchenne muscular dystrophy: response to calcium and vitamin D treatment. Osteoporos Int. 2011;22(2):529–39. https://doi.org/10.1007/s00198-010-1275-5.
Sbrocchi AM, Rauch F, Jacob P, et al. The use of intravenous bisphosphonate therapy to treat vertebral fractures due to osteoporosis among boys with Duchenne muscular dystrophy. Osteoporos Int. 2012;23:2703–11. https://doi.org/10.1007/s00198-012-1911-3.
Land C, Rauch F, Glorieux FH. Cyclical intravenous pamidronate treatment affects metaphyseal modeling in growing patients with osteogenesis imperfecta. J Bone Miner Res. 2006;21(3):374–9. https://doi.org/10.1359/JBMR.051207.
Forni GL, Perrotta S, Giusti A, et al. Neridronate improves bone mineral density and reduces back pain in β-thalassaemia patients with osteoporosis: results from a phase 2, randomized, parallel-arm, open-label study. Br J Haematol. 2012;158(2):274–82. https://doi.org/10.1111/j.1365-2141.2012.09152.x.
Iolascon G, Moretti A. Pharmacotherapeutic options for complex regional pain syndrome. Expert Opin Pharmacother. 2019;20(11):1377–86. https://doi.org/10.1080/14656566.2019.1612367.
Rossini M, Adami S, Bertoldo F, et al. Guidelines for the diagnosis, prevention and management of osteoporosis. Reumatismo. 2016;68(1):1–39. https://doi.org/10.4081/reumatismo.2016.870.
Adami S, Gatti D, Colapietro F, et al. Intravenous neridronate in adults with osteogenesis imperfecta. J Bone Miner Res. 2003;18(1):126–30. https://doi.org/10.1359/jbmr.2003.18.1.126.
Gatti D, Rossini M, Viapiana O, Idolazzi L, Adami S. Clinical development of neridronate: potential for new applications. Ther Clin Risk Manag. 2013;9:139–47. https://doi.org/10.2147/TCRM.S35788.
Ross AC, Taylor CL, Yaktine AL, Valle HBD, editors. Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Washington, DC: The National Academies Press; 2011.
James KA, Cunniff C, Apkon SD, et al. Risk factors for first fractures among males with Duchenne or Becker muscular dystrophy. J Pediatr Orthop. 2015;35(6):640–4. https://doi.org/10.1097/BPO.0000000000000348.
Ward LM, Hadjiyannakis S, McMillan HJ, Noritz G, Weber DR. Bone health and osteoporosis management of the patient with Duchenne muscular dystrophy. Pediatrics. 2018;142(Suppl 2):S34–42. https://doi.org/10.1542/peds.2018-0333E.
Ronsley R, Islam N, Kang M, et al. Effects of bisphosphonate therapy on bone mineral density in boys with Duchenne muscular dystrophy. Clin Med Insights Endocrinol Diabetes. 2020;6(13):1179551420972400. https://doi.org/10.1177/1179551420972400.
Zacharin M, Lim A, Gryllakis J, et al. Randomized controlled trial evaluating the use of zoledronic acid in Duchenne muscular dystrophy. J Clin Endocrinol Metab. 2021;106(8):2328–42. https://doi.org/10.1210/clinem/dgab302.
Lekamwasam S, Adachi JD, Agnusdei D, et al. An appendix to the 2012 IOF-ECTS guidelines for the management of glucocorticoid-induced osteoporosis. Arch Osteoporos. 2012;7:25–30.
Tarantino U, Iolascon G, Cianferotti L, et al. Clinical guidelines for the prevention and treatment of osteoporosis: summary statements and recommendations from the Italian Society for Orthopaedics and Traumatology. J Orthop Traumatol. 2017;18(Suppl 1):3–36. https://doi.org/10.1007/s10195-017-0474-7.
Tian C, Wong BL, Hornung L, et al. Oral bisphosphonate treatment in patients with Duchenne muscular dystrophy on long term glucocorticoid therapy. Neuromuscul Disord. 2020;30(7):599–610. https://doi.org/10.1016/j.nmd.2020.06.005.
Corrado A, Colia R, Cantatore FP. Neridronate: from experimental data to clinical use. Clin Med Insights Theraps. 2017. https://doi.org/10.1177/1179559X17732971.
Idolazzi L, Fassio A, Viapiana O, et al. Treatment with neridronate in children and adolescents with osteogenesis imperfecta: data from open-label, not controlled, three-year Italian study. Bone. 2017;103:144–9. https://doi.org/10.1016/j.bone.2017.07.004.
Maines E, Monti E, Doro F, Morandi G, Cavarzere P, Antoniazzi F. Children and adolescents treated with neridronate for osteogenesis imperfecta show no evidence of any osteonecrosis of the jaw. J Bone Miner Metab. 2012;30(4):434–8. https://doi.org/10.1007/s00774-011-0331-3.
Gatti D, Antoniazzi F, Prizzi R, et al. Intravenous neridronate in children with osteogenesis imperfecta: a randomized controlled study. J Bone Miner Res. 2005;20(5):758–63. https://doi.org/10.1359/JBMR.041232.
Nasomyont N, Tian C, Hornung L, et al. The effect of oral bisphosphonate therapy on vertebral morphometry and fractures in patients with Duchenne muscular dystrophy and glucocorticoid-induced osteoporosis. Muscle Nerve. 2021;64(6):710–6. https://doi.org/10.1002/mus.27416.
Perera N, Sampaio H, Woodhead H, Farrar M. Fracture in Duchenne muscular dystrophy: natural history and vitamin D deficiency. J Child Neurol. 2016;31(9):1181–7. https://doi.org/10.1177/0883073816650034.
Singh A, Schaeffer EK, Reilly CW. Vertebral fractures in Duchenne muscular dystrophy patients managed with deflazacort. J Pediatr Orthop. 2018;38(6):320–4. https://doi.org/10.1097/BPO.0000000000000817.
Buckner JL, Bowden SA, Mahan JD. Optimizing bone health in Duchenne muscular dystrophy. Int J Endocrinol. 2015. https://doi.org/10.1155/2015/928385.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article. No Open Access Fee was received by the journal for the publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Author Contributions
Conceptualization, Giovanni Iolascon, Antimo Moretti and Sara Liguori; methodology, Francesca Gimigliano and Marco Paoletta; data collection and analysis, Sara Liguori; writing—original draft preparation, Sara Liguori and Marco Paoletta; writing—review and editing, Antimo Moretti, Francesca Gimigliano and Giovanni Iolascon; supervision, Antimo Moretti and Giovanni Iolascon. All authors have read and agreed to the published version of the manuscript.
Disclosures
Antimo Moretti, Sara Liguori, Marco Paoletta, Francesca Gimigliano and Giovanni Iolascon have no disclosures for any personal, financial, commercial, or academic conflicts of interest separately.
Compliance with Ethics Guidelines
This cohort study respects the Declaration of Helsinki and all the criteria of a prospective study of real practice, approved by the Ethical Committee of University of Campania “Luigi Vanvitelli”. All the participants were asked to carefully read and sign an informed consent, and researchers agreed to protect the participants’ privacy.
Data Availability
The dataset of the current study is available from the corresponding author on reasonable request.
Funding
Open access funding provided by Università degli Studi della Campania Luigi Vanvitelli within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Moretti, A., Liguori, S., Paoletta, M. et al. Effectiveness of Neridronate in the Management of Bone Loss in Patients with Duchenne Muscular Dystrophy: Results from a Pilot Study. Adv Ther 39, 3308–3315 (2022). https://doi.org/10.1007/s12325-022-02179-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02179-1