Abstract
Introduction
Atopic dermatitis is a complex, chronic, inflammatory skin disease that requires long-term control of symptoms like itch and sleep loss and improvement in quality of life, in addition to reduction of clinical signs. Lebrikizumab is a selective interleukin-13 inhibitor approved in the European Union, United Kingdom, United Arab Emirates, Canada, and Japan for treatment of moderate-to-severe atopic dermatitis in adults and adolescents. Here, we assess the magnitude of changes across signs and symptoms of atopic dermatitis with lebrikizumab monotherapy over the 16-week induction period in two phase 3 studies, ADvocate1 and ADvocate2.
Methods
Eligible adults (aged ≥ 18 years) and adolescents (aged 12 to < 18 years and weighing ≥ 40 kg) with moderate-to-severe atopic dermatitis were randomized to receive either 250 mg of lebrikizumab or placebo subcutaneously every two weeks. Least squares mean percentage change from baseline through week 16 was compared between lebrikizumab and placebo using mixed model repeated measure analysis for the following endpoints: Eczema Area and Severity Index (EASI), Pruritus Numeric Rating Scale (NRS), Sleep-Loss Scale, Patient-Oriented Eczema Measure (POEM), and Dermatology Life Quality Index (DLQI).
Results
In both trials, significant (P < 0.05) improvements were observed for lebrikizumab treatment compared with placebo at each 2-week timepoint for EASI, Pruritus NRS, Sleep-Loss Scale, and POEM, and at each 4-week timepoint for DLQI, through week 16. Statistically significant (P < 0.001) improvements were observed at 16 weeks for lebrikizumab treatment versus placebo in ADvocate1/ADvocate2 for EASI (71.9%/75.0% vs. 35.6%/43.3%), Pruritus NRS (53.3%/46.3% vs. 21.4%/18.0%), Sleep-Loss Scale (57.7%/55.6% vs. 23.9%/25.5%), POEM (54.4%/45.8% vs. 18.8%/16.9%), and DLQI (64.2%/60.5% vs. 28.5%/32.2%). Patient photos show improvements in skin appearance when disease measures improve.
Conclusions
Lebrikizumab monotherapy resulted in significant and fast improvements in multiple dimensions of disease (clinical signs, symptoms, and quality of life) over 16 weeks in patients with moderate-to-severe atopic dermatitis.
Trial Registration
ClinicalTrials.gov identifiers, NCT04146363; NCT04178967.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Lebrikizumab is an interleukin-13 inhibitor approved in the European Union, United Kingdom, United Arab Emirates, Canada, and Japan and under review in other geographies for the treatment of moderate-to-severe atopic dermatitis. |
Atopic dermatitis is a multidimensional disease that requires improvement in clinical signs, symptoms, and quality of life to provide adequate disease control. |
What was learned from the study? |
Patients with moderate-to-severe atopic dermatitis showed significant improvements in clinical signs and symptoms starting at week 1, with significantly better quality of life over the 16 week-induction of lebrikizumab monotherapy every two weeks compared with placebo. |
These results expand our understanding of the impact of lebrikizumab across multiple dimensions of atopic dermatitis disease. |
Introduction
Lebrikizumab is a selective interleukin-13 inhibitor approved in the European Union, United Kingdom, United Arab Emirates, Canada, and Japan and under review in other geographies for the treatment of moderate-to-severe atopic dermatitis in adults and adolescents. Atopic dermatitis is a chronic, pruritic, inflammatory skin disease that can negatively affect sleep, daily activities, work productivity, social well-being, and overall quality of life [1]. Atopic dermatitis severity is usually measured by objective skin severity scores, overlooking other impacts of the disease. Consequently, a multidimensional approach is needed to fully understand the response provided by lebrikizumab. The objective of this analysis was to assess the magnitude of changes across all dimensions of the disease, including physical signs, key symptoms, and quality of life, during the first 16 weeks of lebrikizumab treatment in patients with moderate-to-severe atopic dermatitis enrolled in the phase 3 monotherapy trials, ADvocate1 and ADvocate2.
Methods
Study Design
The analysis included data from patients enrolled in ADvocate1 (NCT04146363) and ADvocate2 (NCT04178967), two identically designed 52-week randomized, double-blind, placebo-controlled phase 3 trials. Details of ADvocate1 and ADvocate2 have been published previously [2]. Briefly, eligible adults (≥ 18 years old) and adolescents (12 to < 18 years old and weighing ≥ 40 kg) who had moderate-to-severe atopic dermatitis with an Eczema Area and Severity Index (EASI) score of ≥ 16, Investigator’s Global Assessment (IGA) score of ≥ 3, body surface area (BSA) involvement of ≥ 10%, and chronic atopic dermatitis for 1 year or more were enrolled. Both trials had a 16-week induction period followed by a 36-week maintenance period (Fig. 1). At the baseline visit, patients were randomized 2:1 to receive either lebrikizumab 250 mg (loading dose of 500 mg given at baseline and week 2) or placebo administered by subcutaneous injection every 2 weeks. Randomization was performed with stratification according to geographic region (United States vs. European Union vs. the rest of the world), age group (adolescent vs. adult), and disease severity (IGA score of 3 vs. 4). Use of topical or systemic treatments through week 16 was not allowed except as rescue therapy.
Study design of ADvocate1 and ADvocate2 16-week induction period [2]. Lebrikizumab-treated patients received a 500-mg loading dose at weeks 0 and 2. LD loading dose, LEB lebrikizumab, PBO placebo, Q2W, every 2 weeks
Both trials were approved by the appropriate ethics review boards across approximately 100 study sites in the United States, Canada, Europe, and the Asia–Pacific area and were performed in accordance with the Helsinki Declaration of 1964, and its later amendments. All patients provided written informed consent to participate in the trials. Patients provided consent for the use of their photographs for publication.
Outcome Measures
The outcome measures are the percentage change from baseline through week 16 for five endpoints: EASI, Pruritus Numeric Rating Scale (NRS), Sleep-Loss Scale, Patient-Oriented Eczema Measure (POEM), and Dermatology Life Quality Index (DLQI). EASI is used by investigators to assess the extent and severity of disease in four body regions (head and neck, trunk, upper limbs, and lower limbs) and measures four clinical signs (erythema, induration/papulation, excoriation, and lichenification) [3]. Total EASI ranges from 0 = clear to 72 = maximum severity. Itch was measured using the Pruritus NRS, a patient-reported, single-item, 11-point scale [4]. Patients rated their worst itch severity over the past 24 h using a daily electronic diary, with 0 = no itch and 10 = worst itch imaginable. The extent of itch interference on sleep was assessed using the Sleep-Loss Scale, which was reported daily by patients on a 5-point Likert scale, where 0 = no itch interference with sleep and 4 = unable to sleep at all due to itch. Weekly scores for Pruritus NRS and Sleep-Loss Scale were calculated by averaging the scores from the previous 7 days with at least 1 non-missing day. Patient perception of atopic dermatitis severity over the previous week was assessed using POEM, a 7-item, patient-reported questionnaire that evaluates how often the patient experienced skin dryness, pruritus, flaking, cracking, sleep loss, bleeding, and weeping/oozing per week [5]. Each POEM response is scored from 0 to 4 with total scores ranging from 0 = clear to 28 = very severe disease. The effect of atopic dermatitis on the patient’s quality of life over the previous week was evaluated using DLQI [or its children’s version (CDLQI) for patients ≤ 16 years old], a 10-item, patient-reported questionnaire that assesses symptoms, feelings, daily life activities, personal relationships, and treatment [6, 7]. Each DLQI response was scored from 0 to 3 with total scores ranging from 0 = no impact on quality of life to 30 = maximum impact.
Statistical Analyses
Efficacy analyses in ADvocate1 were conducted on the intent-to-treat population (ITT; n = 424). In ADvocate2, a total of 18 patients from a single study site were excluded from the ITT population since the eligibility criteria of having moderate-to-severe atopic dermatitis could not be confirmed. Efficacy analyses in ADvocate2 were therefore conducted on a modified intent-to-treat population (mITT; n = 427).
Mixed-effects model of repeated measures (MMRM) was used to evaluate the differences in percentage change from baseline between lebrikizumab and placebo from baseline through week 16. Baseline characteristics were summarized using descriptive statistics by study and treatment arm. The MMRM model included treatment, baseline value, visit, the interaction of baseline value-by-visit, the interaction of treatment-by-visit, geographic region, age, and baseline IGA score as factors. For continuous endpoints, patients who received topical or systemic rescue medication, or discontinued treatment due to any reason had values set to missing subsequent to this time through week 16. MMRM was used to handle all missing data. All analyses were post hoc without multiplicity controls and nominal P values were reported. Significant differences were tested at a two-sided alpha level of 0.05. Analyses were performed using Statistical Analysis System, version 9.4 (SAS Institute, Cary, NC, USA).
Results
The demographics and disease characteristics of the patients were similar across ADvocate1 and ADvocate2 and across treatment groups (Table 1). In ADvocate1, patients had a mean age of 35.5 years, 13.0% were adolescents, and 50.5% were female. The patient population was 68.2% White, 16.5% Asian, and 11.6% Black or African American. In ADvocate2, patients had a mean age of 36.2 years, 11.0% were adolescents, and 49.4% were female. The patient population was 59.3% White, 28.6% Asian, and 8.2% Black or African American. The percentages of patients with severe disease as defined by IGA = 4 were 40.3% and 36.8%, and the mean BSA affected were 46.1% and 46.0% in ADvocate1 and ADvocate2, respectively. The mean EASI scores at baseline were 29.6 and 29.7; mean Pruritus NRS were 7.3 and 7.1; mean Sleep-Loss Scale were 2.3 and 2.2; and mean POEM scores were 20.8 and 20.8 in ADvocate1 and ADvocate2, respectively. The mean DLQI was 15.4 and 15.5 for patients > 16 years and 13.4 and 12.8 for patients ≤ 16 years (CDLQI) in ADvocate1 and ADvocate2, respectively. At baseline, all patients had shown inadequate response to previous topical treatments or had been advised not to use them [2].
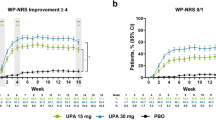
In both trials, improvements were significantly greater for lebrikizumab treatment compared with placebo at each 2-week timepoint for EASI, Pruritus NRS, Sleep-Loss Scale, and POEM (Fig. 2). DLQI, which was measured every 4 weeks after baseline assessment, showed statistically significant improvements with lebrikizumab therapy at each 4-week timepoint. In EASI, the least squares mean (± standard error) percentage change from baseline to week 16 showed reductions of 71.9% (± 2.7%) and 75.0% (± 2.3%) among patients receiving lebrikizumab compared with reductions of 35.6% (± 3.9%) and 43.3% (± 3.3%) among those receiving placebo in ADvocate1 and ADvocate2, respectively (P < 0.001 for all comparisons). As early as week 1, significant reductions in itch and sleep loss due to itch were seen in patients receiving lebrikizumab versus placebo (P < 0.05 for all comparisons, see Fig. 2b, c). At week 16, the percentage change from baseline for Pruritus NRS showed reductions of 53.3% (± 2.6%) and 46.3% (± 2.8%) versus 21.4% (± 3.9%) and 18.0% (± 4.1%), and the reductions in Sleep-Loss Scale were 57.7% (± 2.8%) and 55.6% (± 3.0%) versus 23.9% (± 4.3%) and 25.5% (± 4.4%) for patients receiving lebrikizumab versus placebo in ADvocate1 and ADvocate2, respectively (P < 0.001 for all comparisons). Lebrikizumab also significantly reduced patient-reported symptoms of atopic dermatitis and impairment on quality of life. At week 16, the percentage change from baseline for POEM showed reductions of 54.4% (± 2.3%) and 45.8% (± 2.5%) versus 18.8% (± 3.5%) and 16.9% (± 3.7%), and for DLQI showed reductions of 64.2% (± 6.0%) and 60.5% (± 6.7%) versus 28.5% (± 6.7%) and 32.2% (± 7.1%) for patients receiving lebrikizumab versus placebo in ADvocate1 and ADvocate2, respectively (P < 0.001 for all comparisons). The improvements in all five outcome measures through week 16 are illustrated in Fig. 3.
Time course of least squares mean percentage change from baseline to week 16 in ADvocate1 and ADvocate2 for a EASI, b Pruritus NRS, c Sleep-Loss Scale, d POEM, and e DLQI. DLQI analysis is based on patients with age > 16 years. *P < 0.05 vs. PBO; **P < 0.01 vs. PBO; ***P < 0.001 vs. PBO. DLQI Dermatology Life Quality Index, EASI Eczema Area and Severity Index, LEB lebrikizumab, NRS Numeric Rating Scale, POEM Patient-Oriented Eczema Measure, PBO placebo, Q2W every 2 weeks
Spider plots of least squares mean percentage change from baseline at weeks 4, 8, 12 and 16. Percentage change from baseline was calculated by (least squares mean change from baseline/overall mean at baseline) × 100% for DLQI, POEM, and Sleep-Loss Scale. ***P < 0.001 vs. PBO. DLQI Dermatology Life Quality Index, EASI Eczema Area and Severity Index, LEB lebrikizumab, NRS Numeric Rating Scale, PBO placebo, POEM Patient-Oriented Eczema Measure, Q2W every 2 weeks
The rapid improvement in skin appearance can be seen in photos taken of two patients at baseline and at 2, 8, and 16 weeks of lebrikizumab treatment (Fig. 4). At baseline, both patients had severe disease as defined by IGA = 4. For the patient shown in Fig. 4a, the improvement in EASI from baseline to 2, 8, and 16 weeks was 94.8%, 95.8%, and 100%, respectively. At week 16, Pruritus NRS, POEM, and DLQI all showed 100% improvement from baseline as reported by the patient. For the patient shown in Fig. 4b, the improvement in EASI from baseline to 2, 8, and 16 weeks was 13.3%, 90.8%, and 93.9%, respectively. At week 16, the improvement in Pruritus NRS, POEM, and DLQI from baseline was 21.0%, 39.3%, and 70.6%, respectively.
Skin improvements in two patients treated with lebrikizumab. a Antecubital fossa and b hand at baseline and at 2, 8, and 16 weeks of lebrikizumab treatment. Corresponding values and percent improvements from baseline in EASI, Pruritus NRS, POEM, and DLQI are listed below each photo. © 2024 Eli Lilly and Company. All rights reserved. Permission for any use should be sought from Eli Lilly and Company. DLQI Dermatology Life Quality Index, EASI Eczema Area and Severity Index, NO not observed, NRS Numeric Rating Scale, POEM Patient-Oriented Eczema Measure
Discussion
Due to the complexity of atopic dermatitis in both signs and symptoms, no single outcome measure fully captures the patient burden of atopic dermatitis. Beyond the visible symptoms, skin appearance can cause patients to feel self-conscious and avoid social interactions [8]. Many patients experience intense itching, which can disrupt sleep and impair quality of life [9]. The ADvocate1 and ADvocate2 trials demonstrated the efficacy and safety of lebrikizumab monotherapy for adults and adolescents with moderate-to-severe atopic dermatitis who cannot use or do not respond sufficiently to topical treatments [2]. Reductions in itch and sleep loss due to itch have been shown to occur within the first few days of lebrikizumab therapy [10]. This post hoc analysis expands upon previous reports by assessing the magnitude of changes across different measures of the disease, the clinician-evaluated measure of EASI, as well as the patient-reported measures of Pruritus NRS, Sleep-Loss Scale, POEM, and DLQI during the first 16 weeks of treatment.
Significant improvements with lebrikizumab compared to placebo were seen at each 2-week timepoint for EASI, Pruritus NRS, Sleep-Loss Scale, and POEM, and at each 4-week timepoint for DLQI. As early as week 1, patient-reported scores with respect to itch and sleep loss due to itch were significantly better among those receiving lebrikizumab compared to placebo. By week 2, significant changes were seen in both clinical (EASI) and patient (POEM) assessments of atopic dermatitis severity, and, by week 4, the first post-baseline DLQI assessment showed significant improvements in quality of life with lebrikizumab treatment. This multidimensional response can be clearly seen in the spider plots (Fig. 3). Individual examples of the multidimensional response are seen in patient photos, which show how treatment with lebrikizumab translates to improvements in skin, itch, and quality-of-life dimensions.
Our analysis represents the average multi-dimensional improvement with lebrikizumab across all patients, including nonresponders and those with different atopic dermatitis phenotypes. Atopic dermatitis patients present with varying levels of severity in different disease measures. The results of this intention-to-treat analysis cannot represent the response that lebrikizumab can have for an individual patient across all dimensions of the disease.
A limitation of this analysis is that the time period was relatively short at 16 weeks. Furthermore, we did not include data from patients using topical or systemic rescue medication; consequently, the impact of their use on these results was not fully evaluated. The ADvocate1 and ADvocate2 studies have maintenance periods to 52 weeks and additional long-term studies will examine the effectiveness of lebrikizumab treatment for long-term management of atopic dermatitis.
Conclusions
Lebrikizumab monotherapy resulted in significant improvements in five measures representative of three dimensions of disease (physical signs, key symptoms, and quality of life) over 16 weeks in patients with moderate-to-severe atopic dermatitis. Rapid reductions in itch and itch interference on sleep were seen in patients with previous failure to topical therapies. These data expand upon previous reports by demonstrating that lebrikizumab improves atopic dermatitis across multiple dimensions that reflect not only the clinician-assessed improvement in EASI but also patient-reported disease severity and quality of life, with significant differences seen as early as week 1 for Pruritus NRS and Sleep-Loss Scale, by week 2 for EASI and POEM, and by week 4 for DLQI.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available upon request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at https://vivli.org/.
References
Grant L, Seiding Larsen L, Trennery C, et al. Conceptual model to illustrate the symptom experience and humanistic burden associated with atopic dermatitis in adults and adolescents. Dermatitis. 2019;30(4):247–54.
Silverberg JI, Guttman-Yassky E, Thaci D, et al. Two phase 3 trials of lebrikizumab for moderate-to-severe atopic dermatitis. N Engl J Med. 2023;388(12):1080–91.
Hanifin JM, Thurston M, Omoto M, Cherill R, Tofte SJ, Graeber M. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group Exp Dermatol. 2001;10(1):11–8.
Yosipovitch G, Reaney M, Mastey V, et al. Peak Pruritus Numerical Rating Scale: psychometric validation and responder definition for assessing itch in moderate-to-severe atopic dermatitis. Br J Dermatol. 2019;181(4):761–9.
Charman CR, Venn AJ, Williams HC. The patient-oriented eczema measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch Dermatol. 2004;140(12):1513–9.
Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–6.
Lewis-Jones MS, Finlay AY. The Children’s Dermatology Life Quality Index (CDLQI): initial validation and practical use. Br J Dermatol. 1995;132(6):942–9.
Silverberg JI, Gelfand JM, Margolis DJ, et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann Allergy Asthma Immunol. 2018;121(3):340–7.
Silverberg JI, Garg NK, Paller AS, Fishbein AB, Zee PC. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135(1):56–66.
Yosipovitch G, Lio PA, Rosmarin D, et al. Lebrikizumab improved itch and reduced the extent of itch interference on sleep in patients with moderate-to-severe atopic dermatitis: two randomized, placebo-controlled, phase III trials. Br J Dermatol. 2024;190(2):289–91.
Acknowledgements
Almirall, S.A. has licensed the rights to develop and commercialize lebrikizumab for the treatment of dermatology indications, including atopic dermatitis, in Europe. Lilly has exclusive rights for the development and commercialization of lebrikizumab in the United States and the rest of the world outside of Europe.
We thank the patients who participated in ADvocate1 and ADvocate2 and their caregivers, without whom this work would not have been possible.
Medical Writing/Editorial Assistance
Medical writing and editing support were provided by Laura Mizoue and Antonia Baldo of Syneos Health, funded by Eli Lilly and Company.
Funding
This study was funded by Dermira, Inc., a wholly owned subsidiary of Eli Lilly and Company. The journal’s Rapid Service and Open Access Fee was funded by Eli Lilly and Company.
Author information
Authors and Affiliations
Contributions
Marta Casillas, Yuxin Ding, Fan Emily Yang, and Evangeline Pierce contributed to study conception and design, data analysis and interpretation, and manuscript drafting and revision. Eric Simpson, Pablo Fernández-Peñas, Marjolein de Bruin-Weller, Peter A. Lio, Chia-Yu Chu, Khaled Ezzedine, Helena Agell, and Thomas Bieber contributed to data interpretation and manuscript revision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Eric Simpson has received grants from or serves as Principal Investigator for: AbbVie, Amgen, Arcutis, ASLAN Pharmaceuticals, Castle Biosciences, CorEvitas, Dermavant, Dermira, Inc., Eli Lilly and Company, Incyte Corporation, Kymab, Kyowa Hakko Kirin, LEO Pharma, Pfizer, Regeneron, Sanofi, and Target RWE; and has received personal fees from: AbbVie, Amgen, Arena Pharmaceuticals, ASLAN Pharmaceuticals, Boston Consulting Group, Collective Acumen, Dermira, Inc., Eli Lilly and Company, Evidera, Excerpta Medica, ForteBio, Galderma, GlaxoSmithKline, Incyte Corporation, Janssen, Kyowa Kirin, LEO Pharma, Medscape, Merck, Pfizer, Physicians World, Regeneron, Roivant Sciences, Sanofi Genzyme, Trevi Therapeutics, Valeant Pharmaceuticals, and WebMD; Pablo Fernández-Peñas has served on advisory boards for: AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squib, Celgene, Eli Lilly and Company, Janssen, LEO Pharma, Merck, Merck Sharp & Dohme, Novartis, Roche, Sanofi, and Sun Pharma; has given educational lectures for: AbbVie, Amgen, Avène, Eli Lilly and Company, Galderma, Janssen, La Roche-Posay, LEO Pharma, Merck, Novartis, Pfizer, Roche, Sanofi, Schering-Plough, Sun Pharma, and UCB Pharma; has conducted clinical trials for: AbbVie, Amgen, Arena Pharmaceuticals, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, CSL Behring, Dermira, Eisai, Eli Lilly and Company, Galderma, GlaxoSmithKline, Janssen, Jiangsu Hengrui Pharmaceuticals, Kyowa Hakko Kirin, LEO Pharma, miRagen, Novartis, OncoSec, Pfizer, Regeneron, Roche, Sun Pharma, UCB Pharma, and XOMA Corporation; Marjolein de Bruin-Weller has served as a consultant, speaker, advisor, and/or advisory board member for: AbbVie, Almirall, Arena Pharmaceuticals, ASLAN Pharmaceuticals, Eli Lilly and Company, Galderma, Janssen, LEO Pharma, Pfizer, Regeneron, and Sanofi Genzyme; Peter A. Lio has received grants as an investigator, honoraria for lecturing, and/or consulting fees from: AbbVie, AOBiome, Arbonne, Burt’s Bees, Dermavant, Dermira, Eli Lilly and Company, Exeltis, Franklin BioScience/Altus Labs, Incyte Corporation, IntraDerm, Johnson & Johnson, Kiniksa Pharmaceuticals, La Roche-Posay/L’Oréal, LEO Pharma, Menlo Therapeutics, National Eczema Association, Pfizer, Pierre Fabre, Realm Therapeutics, Regeneron/Sanofi Genzyme, Theraplex, TopMD, UCB Pharma, Unilever, and Verrica Pharmaceuticals; Chia-Yu Chu is an investigator, consultant, speaker, and/or advisory board member for: AbbVie, Amgen, Dermira, Eli Lilly and Company, GlaxoSmithKline, Janssen, Mylan, Novartis, Oneness Biotech, Pfizer, Regeneron, Roche, Sanofi, United BioPharma, and Viatris; Khaled Ezzedine is a consultant for AbbVie, Incyte, La Roche-Posay, Pfizer, Pierre Fabre, Sanofi, Eli Lilly and Company, and Almirall; Helena Agell is an employee of: Almirall, S.A.; Marta Casillas, Yuxin Ding, Fan Emily Yang, and Evangeline Pierce are employees and shareholders of: Eli Lilly and Company; Thomas Bieber is a speaker, consultant, and/or investigator for: AbbVie, Affibody, Almirall, AnaptysBio, Arena Pharmaceuticals, Asana BioSciences, ASLAN Pharmaceuticals, Bayer Pharmaceuticals, BioVersys, Boehringer Ingelheim, Bristol-Myers Squibb, Connect Biopharma, Dermavant, Domain Therapeutics, Eli Lilly and Company, EQRx, Galderma, GlaxoSmithKline, Glenmark Pharmaceuticals, Incyte Corporation, Innovaderm Research, IQVIA, Janssen, Kymab, Kyowa Kirin, L’Oréal, LEO Pharma, LG Chem, Merck Sharp & Dohme, Novartis, Numab, OM Pharma, Pfizer, Pierre Fabre, Q32 Bio, RAPT Therapeutics, Sanofi Regeneron, and UCB Pharma; and is Founder and Chairman of the Board of the non-profit biotech: Davos Biosciences. Dr. Bieber was affiliated with University Hospital Bonn, Bonn, Germany at the time of the study and is currently affiliated with Medicine Campus, Davos, Switzerland.
Ethical Approval
Both trials were approved by the appropriate ethics review boards across approximately 100 study sites in the United States, Canada, Europe, and the Asia–Pacific area and were performed in accordance with the ethical principles of the Declaration of Helsinki and Good Clinical Practice guidelines. All patients provided written informed consent to participate in the trials. Patients provided written consent for use of their photographs for publication.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Simpson, E., Fernández-Peñas, P., de Bruin-Weller, M. et al. Improvement Across Dimensions of Disease with Lebrikizumab Use in Atopic Dermatitis: Two Phase 3, Randomized, Double-Blind, Placebo-Controlled Monotherapy Trials (ADvocate1 and ADvocate2). Adv Ther (2024). https://doi.org/10.1007/s12325-024-02974-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12325-024-02974-y