Abstract
Introduction
As of March 2024, resmetirom is the first and only therapy approved in the United States (US) for the treatment of adults with non-cirrhotic nonalcoholic steatohepatitis (NASH) with moderate-to-advanced liver fibrosis (MALF) consistent with stages F2/F3 fibrosis. Estimates of the diagnosed, treatment-eligible NASH population are poorly understood due to diagnostic variability. This study provides a contemporary estimate of the size of the US resmetirom treatment-eligible population.
Methods
A dynamic population calculator was developed combining literature, screening guidelines, resmetirom study criteria, and analyses of the NHANES 2017–March 2020 cycle. It computes NASH prevalence, proportion non-cirrhotic NASH with MALF, Year 1 diagnosis, and new diagnoses in Years 2 and 3. NASH prevalence was estimated by applying the American Association of Clinical Endocrinology screening algorithm and recommended NIT cut-offs in the NHANES dataset. The proportion of non-cirrhotic NASH with MALF was informed by analyses of the Forian US integrated medical claims database using NASH and cirrhosis-specific ICD-10-CM codes and FIB-4 scores. NASH diagnosis rates were obtained from published estimates and NHANES responses. Treatment-eligible population growth was projected using published incidence data. Estimates were compared to a NASH budget-impact-analysis (BIA) from the Institute for Clinical and Economic Review (ICER).
Results
In the base case, a NASH prevalence of 4.6% was modeled (range 1.3–14.2%). This value was multiplied by the proportion estimated to have non-cirrhotic MALF (i.e., 35%). Published analyses suggest a diagnosis rate of ~ 10% (range 3.3–14.3%) and ~ 16% year-over-year growth in the treatment-eligible population. Assuming a 1-million commercial-member population, the resmetirom treatment-eligible population was estimated as 1255–1699 in Years 1–3 following approval. Sensitivity analyses were conducted and comparison to the ICER BIA was influenced by different diagnosis rates.
Conclusion
Estimation of the treatment-eligible population for resmetirom depends importantly on NASH diagnosis rates, which are predicted to be < 15% in the 3 years after drug approval.
Plain Language Summary
Nonalcoholic steatohepatitis (NASH) is an advanced form of nonalcoholic fatty liver disease. Previously there were no treatments for NASH in the United States (US), but as of March 2024, the US Food and Drug Administration (FDA) approved resmetirom (REZDIFFRA™), a once-daily, oral therapy, in conjunction with diet and exercise, under accelerated approval for the treatment of adults (aged 18 years or older) with non-cirrhotic NASH with moderate-to-advanced liver fibrosis (MALF), consistent with stages F2–F3. It is not well understood how many diagnosed patients with NASH would be eligible for treatment with resmetirom; thus, this study aimed to estimate the size of the US resmetirom treatment-eligible population. To do so, we created a flexible population calculator that considers how many people have NASH, what proportion would be eligible for resmetirom treatment—i.e., have non-cirrhotic NASH with MALF—and of those how many people would be diagnosed. We used published literature, screening guidelines, resmetirom study criteria, and analyses of national surveys to inform our range of estimates. In the main analysis, we modeled a NASH prevalence of 4.6% (range 1.3–14.2%), which was then limited to the proportion estimated to have non-cirrhotic NASH with MALF (i.e., 35%) and diagnosed (i.e., 10%, range 3.3–14.3%). A year-over-year growth of approximately 16% in the treatment-eligible population was modeled in years following approval. Assuming a population of 1 million commercial insurance enrollees, the resmetirom treatment-eligible population was estimated to be 1255–1699 in Years 1–3 following approval. We assessed alternative scenarios and have compared our results to existing models.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
In March 2024, the Food and Drug Administration (FDA) granted resmetirom (REZDIFFRA™) accelerated approval for the treatment of adults with non-cirrhotic nonalcoholic steatohepatitis (NASH) with moderate-to-advanced liver fibrosis (MALF) in the United States (US), necessitating a better understanding of the resmetirom treatment-eligible population. |
A population-calculator model, combining analyses of the National Health and Nutrition Examination Survey (NHANES) 2017–March 2020 cycle survey data and a range of published sources, was developed to provide a contemporary estimate of the resmetirom treatment-eligible population in a commercial, Medicare, or Medicaid insurance plan. |
Over a 3-year period post-FDA approval, for a hypothetical 1-million-member commercial health plan, the model estimated that 1699 adults will be eligible for treatment with resmetirom. |
Modeling of alternative diagnosis rates yielded treatment-eligible population estimates of 852–2238 adults per 1 million over 3 years. |
The model's flexibility, drawing from various published sources, analyses of NHANES survey data, and three national claims databases which utilized data as recent as 2022, offers valuable insights that may be used by payers and the healthcare delivery system to inform future research in assessing the long-term clinical and economic benefits of novel therapies in NASH. |
Introduction
Nonalcoholic fatty liver disease (NAFLD), now recommended to be referred to as metabolic dysfunction-associated steatotic liver disease (MASLD) by liver societies such as the American Association for the Study of Liver Diseases, is a condition in which excess fat is stored in the liver (hepatic steatosis) [1,2,3,4]. NAFLD is further classified into nonalcoholic fatty liver (NAFL) and nonalcoholic steatohepatitis (NASH), now recommended to be referred to as metabolic dysfunction-associated steatohepatitis (MASH). NASH is distinguished from NAFL by the presence of inflammation and hepatic injury, and, in some instances, progressive fibrosis [1,2,3,4]. Liver-related consequences of NASH include portal hypertension, liver failure, liver transplantation, and hepatocellular carcinoma. Among the histological features of NAFLD/NASH, fibrosis stage is consistently the most important prognostic factor for predicting liver-related morbidity and mortality [5,6,7]. Liver biopsy is the reference standard for diagnosis of and determination of fibrosis stages in NASH; however, due to the invasive nature, cost, and risks associated with biopsies, non-invasive tests (NITs) are commonly used to screen for potential NAFLD/NASH, including with blood-based biomarkers and/or imaging tests, such as fibrosis-4 index (FIB-4), aspartate aminotransferase to platelet ratio index, and vibration-controlled transient elastography [8,9,10,11,12].
NASH is recognized to contribute substantially to the healthcare burden in the United States (US) [13]. An analysis of Optum Clinformatics® Drug Mart (CDM) (2015–2022) estimated the mean annual per-patient cost for non-cirrhotic NASH with moderate-to-advanced liver fibrosis (MALF), consistent with stages F2–F3, and cirrhosis at US$20,031 and $58,128, respectively [14]. Increased costs associated with NASH have been observed controlling for various demographic and clinical characteristics, including age, sex, race, region of residence, smoking status, and health status characteristics in Tapper et al. (2023), [15] and type 2 diabetes (T2D) (a commonly comorbid condition) in Fishman et al. (2023) [16].
Despite the condition’s clinical significance and the substantial costs, historically, there were no US regulatory-approved therapies for NASH [9, 17]. However, in March 2024, the US Food and Drug Administration (FDA) granted approval of resmetirom (REZDIFFRA™; Madrigal Pharmaceuticals, West Conshohocken, PA, USA), a once-daily, oral, thyroid hormone receptor-beta agonist, for treatment (in conjunction with diet and exercise) of adults (aged 18 years or older) with non-cirrhotic NASH with MALF, consistent with stages F2 to F3 fibrosis [18]. While liver societies now recommend the term MASH, this manuscript will refer to the disease space as NASH to align with the indication approved in the FDA label for Rezdiffra™ [4, 18].
With the US approval of resmetirom, other therapies currently in clinical development, [9, 17] and inherent uncertainty in NIT-based prevalence estimates, contemporary estimates for the prevalence of diagnosed non-cirrhotic NASH with MALF may be of interest to healthcare decision-makers. However, estimates of the prevalence of NASH, and treatment-eligible subgroups, are characterized by significant uncertainty and heterogeneity. For example, previous real-world NASH cost studies often relied on outdated information/data sources and focused primarily either on patients with NAFLD or with severe complications potentially associated with cirrhosis [7, 19, 20]. In other cases, generalizability of estimates was uncertain, such as in Younossi et al. (2016), a meta-analysis which estimated NASH prevalence among NAFLD for the period 1989–2015 [7]. While the global prevalence of biopsy-confirmed patients with NASH was identified, a potential selection bias was reported, in that biopsy candidates were selected based on suspicion of steatohepatitis, and therefore may not be representative of the more general population. This potential selection bias was addressed through examination of patients who underwent voluntary liver biopsy, among whom 7–30% were diagnosed as having NASH [7]. The lack of confirmatory laboratory measurements and/or a NASH-specific diagnosis code prior to October 1, 2015 also contributed to uncertainty, such as in Loomba et al. (2020), a retrospective analysis of claims data from a 20% representative sample of Medicare beneficiaries between 2007 and 2015 to identify patients with NAFLD/NASH [20]. NAFLD could not be conclusively differentiated from NASH, as, prior to 2015, the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) only provided a diagnosis code for NAFLD (a NASH-specific diagnosis code was introduced in ICD-10-CM). Given this constraint, the study offered an approximation, suggesting that ~ 20% of patients within the NAFLD cohort were likely to have NASH [20]. In other studies of NASH prevalence, uncertainty is reflected in the wide ranges of estimates reported. For example, Younossi et al. (2022) reported prevalence of NAFLD in adults from 25–48%, of which 13–65% had NASH [19]. Murag et al. (2021), using imaging data, estimated global prevalence of NAFLD at 25.2% (and NASH at 3–5%) [21]. However, analysis of imaging data in the US suggested an NAFLD prevalence of 34%, based on the National Health and Nutrition Examination Survey (NHANES) [22]. These various studies underscore the substantial uncertainty present in prior estimates of NASH prevalence and the need for contemporary estimates utilizing recent datasets that concentrate on a NASH-specific population.
Hence, this analysis seeks to estimate the size of the resmetirom treatment-eligible population over a 3-year time horizon, combining multiple sources of evidence to characterize uncertainty. The resulting population projections can be used to inform future budget impact analyses and quantification of societal costs related to the uptake of novel therapies in NASH.
Methods
Model Overview
A dynamic population-calculator model was developed to estimate the number of adults in the US with diagnosed non-cirrhotic NASH with MALF, which corresponds approximately to NASH with stages F2–F3 fibrosis. The model considers a 3-year time horizon, with the population in the first year following FDA approval reflecting the estimated prevalence of diagnosed non-cirrhotic NASH with MALF, and the population in subsequent years accounting for the projected growth of the treatment-eligible population.
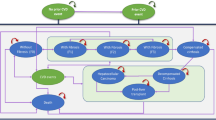
Calculations have been performed as follows. The model starts with an estimate of the prevalence of NASH in US adults (aged 18 years or older). Next, prevalence is scaled by an estimate of the proportion with MALF and without cirrhosis, thus excluding consideration of NASH fibrosis stages F0–F1 or cirrhosis (F4), to align with the FDA-approved indication for resmetirom [18]. Finally, the model adjusts the resulting population by the proportion that has been identified/diagnosed by a healthcare provider. This produces an estimate for the prevalence of the treatment-eligible population in Year 1. A year-over-year growth rate is applied to project the expected increase in size of the treatment-eligible population for Years 2 and 3. Details on each level of the calculation are provided in subsequent sections; see Fig. 1 for a model schematic. For illustrative purposes, population estimates are calculated for a hypothetical 1-million-member commercial-insurance health plan, i.e., model base case settings.
Model schematic with base case estimates displayed. Within a plan of 1,000,000, the treatment-eligible NASH population is restricted to 1255 adults diagnosed with non-cirrhotic NASH with MALF. This population is expected to grow by 205 patients in Year 2 and 238 patients in Year 3. MALF moderate-to-advanced liver fibrosis, NASH nonalcoholic steatohepatitis
The model was developed in Microsoft Excel 2016 (Microsoft, Redmond, WA, USA), and followed recommendations of the International Society for Pharmacoeconomics and Outcomes Research (Modeling Good Research Practices, 2012) [23]. The model is based on previously conducted studies and does not contain any new studies with human participants performed by any of the authors.
Identification of Target Population
Number of Adults in Plan Population
The model considers a hypothetical population enrolled in a nationally representative commercial, Medicare, or Medicaid health plan. For the three modeled health plan types, the proportion of adult enrollees (aged 18 years or older) was estimated from the 2021 US Census Bureau’s “Health insurance coverage status and type of coverage by selected characteristics for all people” in the base case (Table 1) [24]. In scenario analyses, additional sources were considered for the age distribution within commercial and Medicare health plans, including state health access data assistance (SHADA) for commercial plans, and the Centers for Medicare & Medicaid Services (CMS) for the Medicare population (Table 2) [25, 26].
Prevalence of NASH in the US Adult Population
In the default, or base case analysis, the NHANES 2017–March 2020 cycle was used to estimate NASH prevalence representative of the civilian, not-institutionalized, US population [22]. A strength of this approach, which relied on NIT-based screening for NASH as opposed to relying solely on NASH diagnoses, is its potential to capture both diagnosed and undiagnosed NASH cases [22]. In the base case, US NASH prevalence was estimated by applying the 2022 American Association of Clinical Endocrinology (AACE) screening algorithm and NASH diagnostic guidelines recommended NIT cut-offs [3, 8,9,10, 12, 22, 27, 28]. The AACE recommends screening for NASH in the presence of risk factors for NAFLD (including prediabetes, T2D, obesity, ≥ 2 cardiometabolic risk factors, steatosis on imaging, or elevated aminotransferases), followed by fibrosis risk stratification first using FIB-4 (< 1.30 = low, 1.30–2.67 = indeterminate, ≥ 2.67 = high) and then with liver-stiffness measure (LSM) measured in kilopascals (kPa) by elastography (< 8 kPa = low, 8–12 kPa = indeterminate, ≥ 12 kPa = high) if FIB-4 is the [22, 27, 29]. Application of the AACE screening guidelines in the NHANES dataset yielded an estimated NASH prevalence of 4.60% (Table 1) [22, 27].
Table 3 presents eight additional scenario analyses used to estimate NASH prevalence among US adults. Five of the scenarios offer alternative analyses of the NHANES 2017–March 2020 cycle data. The scenarios apply different NIT cut-off values, acknowledging the variation in sensitivity/specificity of various NIT-based screening approaches, as detailed in the literature [23, 30,31,32,33]. For example, Eddowes et al. (2019) reported that steatosis can be identified using a controlled attenuation parameter (CAP) score of ≥ 302 decibel/meter (dB/m), corresponding to a liver fat content ≥ 5% [area under the curve (AUC) = 0.87, sensitivity = 0.80, specificity = 0.83] [32]. Within the population with liver steatosis, the subset of patients with definite NASH—defined as a NAFLD Activity Score (NAS) ≥ 5—can be identified using the FibroScan®-AST (FAST) score, with different cut-off values producing various estimates for prevalence. Lee et al. (2022) [30] found that a FAST cut-off of 0.48 provided the highest AUC (0.752) for ruling-in definite NASH, which, in NHANES, led to an overall prevalence of 3.0% when in combination with CAP ≥ 302 dB/m. The FAST score cut-offs of 0.35 (90% sensitivity) and 0.67 (90% specificity) presented in Newsome et al. (2020) [31] were also used to provide high and low estimates of prevalence, which, when combined with CAP ≥ 302 dB/m, resulted in prevalence estimates of 1.30% and 4.80%.
Additional prevalence estimates were obtained from NHANES by applying screening steps from the MAESTRO-NASH clinical study of resmetirom [20, 22, 27, 33,34,35]. Screening criteria modeled included medical history of NAFLD, presence of ≥ 3 risk factors (age > 50 years, BMI > 30 kg/m2, AST > 20 U/L or AST/ALT ≥ 1, T2D, dyslipidemia, hypertension, metabolic syndrome), LSM ≥ 8.5 kPa, and CAP ≥ 280 dB/m [34, 35]. To identify individuals likely to have a medical history of NAFLD, two scenarios were modeled with varying access to care and evidence of other liver diseases. In the first scenario, access to care was restricted to ≥ 1 healthcare visit in the last year, and no evidence of other liver disease. This approach likely resulted in the inclusion of more patients who have received a NASH diagnosis. In the second scenario, no restriction on access to care was applied. The decision to remove this limitation on access to care was made to encompass a broader pool of potential patients with NASH, even though it may be less reflective of typical clinical practice compared to scenarios with access restrictions. This approach likely resulted in the inclusion of a greater number of patients, incorporating more undiagnosed NASH cases into the estimate. The application of the MAESTRO-NASH screening approach yielded prevalence estimates of 5.00% and 6.30% (with/without the access-to-care restriction, respectively) [22].
Finally, three additional prevalence values were also considered from the literature [7, 19, 20]. Younossi et al. (2016) reported an overall prevalence range for NASH of 1.5% and 6.45% [7]. The model applies the midpoint of this range: 3.98%. Similarly, Loomba et al. (2020) reported a prevalence of NASH in the US of 3.0–5.0%, based on which the model applies the midpoint: 4.0% [20]. Lastly, a comprehensive global assessment of NAFLD and NASH prevalence found that the prevalence of NAFLD ranged from 25 to 48%, with a midpoint of 36.50%, and that NASH prevalence among adults with NAFLD ranged from 13 to 65%, with a midpoint of 39%. Utilizing the midpoint values, the calculated NASH prevalence within the overall adult population was 14.24% [19].
Prevalence of Non-Cirrhotic NASH with MALF (Consistent with Stages F2/F3)
The base case percentage of the non-cirrhotic NASH with MALF population was informed from an observational retrospective cohort study using the Forian US integrated medical claims database [22, 36]. To identify patients with NASH, the NASH-specific ICD-10-CM code K75.81, introduced in 2015, was used within an identification period from January 1, 2015, to December 31, 2020 [36]. A subgroup of NASH-coded patients had sufficient laboratory data available to estimate fibrosis staging and were thus stratified based on their FIB-4 values (n = 18,612) [36]. Patients were classified as having F4 fibrosis if they had a FIB-4 score ≥ 1.3 and either a platelet count < 150 × 109/L, a bilirubin level > 2 × the upper limit of normal (ULN; using 1.2 mg/dL as the ULN), or diagnosis/procedure claims indicative of advanced, decompensated liver disease, including gastroesophageal varices, liver transplant, liver failure, and/or variceal bleeding [37,38,39]. Patients with FIB-4 < 1.3 were classified as not having F2 or F3 [36]. The remaining patients were classified as having either F2 (n1 = 3735) or F3 (n2 = 2725) [36]. Therefore, taking patient counts estimates using FIB-4 for F2 and F3 over the total subgroup patient count, we get (3735 + 2725)/18,612 ≈ 35% of the patients were diagnosed with F2/F3 NASH (Table 1) [36]. This value aligns both with that applied in a recent report published by the Institute for Clinical and Economic Review (ICER) assessing the budget impact of novel therapies for NASH, [40] as well as estimates using LSM kPa in NHANES [22].
Diagnosis Rates of NASH
This step accounts for patients who have been diagnosed among those with non-cirrhotic NASH with MALF. In practice, NASH is likely underdiagnosed as, first, the identification of NASH may be hindered by absent or nonspecific symptoms at noncirrhotic stages, compounded by the absence of universally established routine screening standards; second, while liver biopsy stands as the reference standard for NASH diagnosis, its use is limited by high costs, invasiveness, susceptibility to sampling errors, and the need for specialized expertise to conduct the procedure; and, third, the US lacked any approved treatments for NASH until March 2024 [9, 17, 41], which may have limited efforts to diagnose. It has been estimated that over 50% of adults with NAFLD risk factors (e.g., obesity or T2D) go unscreened [42]. In a recent survey that tested for NAFLD in previously unscreened at-risk individuals, 16% had NASH [43].
Hence, a key aspect of estimating the size of the treatment-eligible population involves determining the diagnosis rate of non-cirrhotic NASH with MALF within the population. For the base case, this rate was obtained from Fishman et al. (2022) (described in the preceding section), which reported a literature-based prevalence estimate of diagnosed NASH as approximately 10% of the overall prevalence [36]. In this model, it is assumed that the diagnosis rate of NASH is consistent across all fibrosis stages. This assumption could overstate the diagnosis rate of F2/F3 if diagnosed patients typically have more severe disease (e.g., cirrhosis/F4). To explore this assumption, alternative sources were also considered [22, 44]. First, the NHANES 2017–March 2020 cycle was used to identify patients with F2/F3 NASH who indicated that they had been diagnosed with liver disease in a clinical setting. These patients represented 14.3% of the total number of patients classified as having F2/F3 NASH [22]. Second, a recent estimate for diagnosed NASH prevalence (0.15%) [44] was divided by the base case estimate for overall US prevalence (4.6%, from the application of the AACE screening guidelines in the NHANES dataset) [22] to obtain an estimated diagnosis percentage of 3.26%. Finally, a scenario assuming the unlikely case of 100% diagnosis rate among the non-cirrhotic NASH with MALF population was also examined to provide additional context. See Tables 1 and 4 for a complete summary of diagnosis rates applied in the base case and scenario analyses, respectively.
Year-to-Year Growth in Treatable Patients
Estimates for the growth of the treatment-eligible population over the 3-year time horizon were obtained from published estimates for incidence of non-cirrhotic NASH with MALF. In the base case, the annual incidence was calculated by dividing the projected number of newly coded patients with F2/F3 NASH in 2020 (73,085) in the US population by the total adult population (257,279,447), resulting in a rate of 28.4 cases per 100,000 adults [36, 45]. This projected number of newly coded patients with F2/F3 NASH in 2020 was obtained via a longitudinal observational retrospective cohort study of coded patients with NASH following the introduction of NASH-specific ICD-10-CM codes, conducted using the Forian US integrated medical claims database for the years 2015–2020, as reported in Fishman et al. (2022) [36]. To increase the completeness of estimates in the model, other diagnosed NASH incidence rates were obtained from analyses conducted using the Optum CDM and Veradigm® EHR (linked with Komodo Health claims), which provided a range of values (20.4–64.0 per 100,000 adults) [46]. As these rates were reported independent of fibrosis stage, it was assumed that the fraction of incident cases with F2/F3 fibrosis matched the default estimate for the fraction of F2/F3 NASH within the prevalent population (35%, see Sect. 2.2.3). Finally, an estimate for overall NASH incidence in 2017, per Younossi et. al. (2019), was converted to a rate and scaled by the default percentage for F2/F3 (35%) and default percentage for diagnosis rate (10%) to obtain an incidence rate for diagnosed F2/F3 NASH [13, 36]. Estimates for the growth in treatment-eligible patients, in terms of annual incidence per 100,000 adults, are presented in Table 5. To standardize the units, the overall 3-year growth in the patient population, determined via the incidence rates described above, was used to calculate a year-over-year percentage growth in the treatment-eligible population. The percentage growth year-over-year depends on the calculated prevalence estimate for diagnosed non-cirrhotic NASH with MALF. Using the base case input parameters described above, the year-over-year growth rate was calculated as 16%.
The introduction of a novel therapy in a therapeutic area where no treatment is approved may change the size of the treatment-eligible population over time due to increased testing/screening or updated disease management guidelines. Although the analysis excludes this in the base case, this assumption is tested in scenario analysis via an additive adjustment factor sourced from Oka et al. (2023), which found that the year-over-year percentage growth in treated patients increased by 3.175% on average (in absolute terms) after the introduction of a drug with a novel mechanism of action (Table 5) [47].
It should be noted that treatment-eligible patients in Year 1 may lose eligibility for resmetirom in subsequent years due to progression to cirrhosis (F4). The fraction of patients developing cirrhosis within the modeled time frame is expected to be low, and, as a conservative assumption (i.e., possibly overstating the future eligible population), progression was not considered in these projections (Table 6).
Results
Base Case Results
The base case scenario modeled a commercially insured health plan with 1 million members, of whom 77.98% are aged 18 years or older. Consequently, the total adult population within the health plan is calculated to be 779,803. Using the base case inputs described in preceding sections, the total number of adults with NASH was estimated at 35,871 with a subset of 12,555 patients having non-cirrhotic NASH with MALF. Applying the 10% diagnosis rate from Fishman et al. (2022) yielded 1255 resmetirom treatment-eligible patients in Year 1 [36].
Annual growth was also incorporated into the model for Years 2 and 3 using the incidence estimate obtained from Fishman et al. (2022) [36]. Using this input, the treatment-eligible patient population was projected to rise to 1699 patients by Year 3. See Table 1 for a summary of population counts by year and patient category.
Scenario Analyses
Scenario analyses were conducted by varying inputs at each level of the calculations while holding other variables fixed at base case values. Table 2 illustrates scenarios for alternative health plans as well as using additional sources for the age distribution within commercial and Medicare health plans. Tables 3, 4, and 5 present the estimated treatment-eligible patient population in Years 1 and 3 within the representative 2021 US Census Data commercial plan, considering various input configurations.
Estimates for the size of the Year 1 treatment-eligible patient population varied from 355 to 3885 when considering all sources for overall NASH prevalence and holding other inputs constant (Table 3). Holding overall NASH prevalence constant and varying the diagnosis rate yielded Year 1 population estimates ranging from 409 to 1795; for context, 12,555 patients would be resmetirom treatment-eligible in Year 1 when modeling that 100% of prevalent non-cirrhotic NASH with MALF cases are diagnosed (Table 4).
The estimated Year 3 patient population ranged from 1306 to 1699 over the incidence sources considered (Table 5). Including the 3.175% annual growth rate in the treatment-eligible patients due to the introduction of a new molecule in the marketplace (as in Oka et al. [2023]) [47] raised the default Year 3 treatment-eligible patient population from 1699 to 1793 (Table 5).
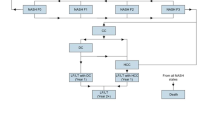
Finally, in an additional scenario, the total number of resmetirom treatment-eligible adults in the overall US population was estimated, as opposed to in a hypothetical commercial health plan with 1 million members (Fig. 2). In this scenario, the total US population was estimated at 331,501,080, with 257,279,447 (77.6%) individuals identified as aged 18 years or older for the year 2020 [45]. Of this population, 3.5% was modeled as prevalent NASH, as this percentage represents the midpoint of likely NASH prevalence (range:1.0–6.0%), and, consistent with the base case, 35.0% of adults with NASH were assumed to have non-cirrhotic NASH with MALF, [36] and 10% to have received a diagnosis [36]. This led to an estimated 315,167 patients with diagnosed non-cirrhotic NASH with MALF in Year 1. Utilizing the default scenario for incidence, a growth rate of 21.0% was estimated and we assumed no growth in the number of diagnoses. The growth in treatment-eligible patient counts for Years 2 and 3 were estimated as 66,144 and 80,026, respectively. This corresponds to a cumulative total resmetirom treatment-eligible population count of 381,311 and 461,337 in Year 2 and Year 3, respectively, post-FDA approval period.
Discussion
This population-calculator model allows estimation of the resmetirom-eligible population in a commercial, Medicare, or Medicaid insurance plan, following modeling best practices [23]. With the recent approval of resmetirom, projecting the likely patient counts of resmetirom treatment-eligible patients is of key importance in understanding financial implications for the healthcare delivery system.
Our estimates for the size of the treatment-eligible population differ from those presented in ICER’s recent budget impact analysis of resmetirom and obeticholic acid (OCA) [40]. The base case estimate for adult NASH prevalence in this study is slightly higher (4.6% vs. 4.0%). The estimates for the proportion of patients with NASH with F2/F3 fibrosis were identical across studies (35%), although the sources used to obtain this value differed [22, 48]. Larger discrepancies are present in the estimates for the baseline diagnosis rate for treatment-eligible patients, as well as estimates for the growth over time in the treatment-eligible population. ICER’s analysis did not consider current diagnosis rates; instead, it assumed that 20% of prevalent treatment-eligible patients would initiate treatment in each of the first 5 years following the introduction of resmetirom. The sources identified in our analysis suggested that approximately 3–14% of treatment-eligible patients are currently diagnosed, implying that the true size of the current treatment-eligible population is likely less than 20% of the total treatment-eligible patient population. In addition, ICER’s assumptions that all prevalent eligible patients with NASH will be diagnosed and treated within 5 years imply faster growth in the treatment-eligible patient population compared to the historical incidence estimates cited here. It should be noted, however, that a significant public health campaign to identify and treat patients with NASH at risk of developing cirrhosis could increase diagnosis rates. Conversely, historical evidence of the low treatment rates of hepatitis C following introduction of new therapies, [49] and the more recent challenges in the uptake of vaccination during the Coronavirus Disease 2019 (COVID-19) pandemic, [50] illustrate that uptake may be slow even in the presence of such public health campaigns. Overall, our model estimated that, in the base case scenario with a 1-million-member commercially-insured health plan, 1699 will be treatment-eligible for resmetirom within 3 years of its introduction, compared to 6550 when applying ICER’s input values and assumptions.
There are several primary strengths of this analysis. The model tested various recent data sources at each step of the identification of the treatment-eligible population, to characterize heterogeneity and uncertainty in the existing evidence base. Analyses using NHANES allowed for capturing both diagnosed and undiagnosed prevalent patients with NASH, potentially offering a more accurate reflection of real-world patient counts than possible with administrative claims (reflecting diagnosed cases only), considering the common underdiagnosis of the disease [22]. Across the NHANES default and scenario analyses, different NIT-based cut-off values were tested in recognition of the impact of their varying sensitivity and specificity on prevalence estimates. In addition, three literature-based scenario analyses were also considered, [7, 19, 20, 22] enhancing robustness by considering NASH diagnosed using specific ICD-10-CM codes following October 1, 2015. These scenarios drew from studies of several data sources including the Forian US integrated medical claims database, Optum's de-identified CDM Database, and Veradigm’s® EHR linked to the Komodo Health claims database. These studies, Fishman et al. (2022) [36] and Rochon et al. (2023), [46] illustrate the variability and uncertainties associated with projecting patient counts, which depend on factors such as inclusion criteria, data sources, underlying assumptions, and diagnosis rates. Nonetheless, the flexible framework of the model presented allows for the incorporation of these variables into the analysis. The advantage of the three contemporary data sources lies in overcoming the previously mentioned limitation that older published studies often relied on data predating the introduction of NASH-specific ICD-10-CM diagnosis codes, leading to uncertainty regarding the size of the diagnosed NASH population and requiring additional assumptions.
Another strength of this study lies in the use of NITs applied in the contemporary NHANES data to support the projection of non-cirrhotic NASH with MALF population counts [22]. While liver biopsy serves as the reference standard for diagnosing NASH, its invasive nature, cost, and associated risks make NITs more practical for initial identification/screening of potential NAFLD/NASH cases in clinical practice. Our model incorporates a variety of NIT techniques, aligning with NASH diagnostic guidelines that recommend different NIT cut-offs and procedures [3, 8,9,10, 12, 22, 27, 28]. Notably, the usage of these techniques can vary in actual clinical practice depending on the NIT screening testing methods or algorithms used. To address uncertainties associated with NITs, this analysis tested multiple cut-off values and screening approaches, which have variable predictive performance (i.e., sensitivity and specificity), [22] as user-modifiable input parameters.
The model has certain limitations. Inputs derived from the NHANES 2017–March 2020 cycle using NITs for NASH and fibrosis estimation are subject to uncertainty. Consequently, the various NIT criteria applied in the NHANES analysis produced a range of prevalence estimates, reflecting differences in their predictive performance. Literature-based estimates for the prevalence of NASH also vary significantly in several factors, including the modalities used for case identification and the populations in which prevalence is assessed. Prevalence estimates are expected to be generalizable to the near-term future (e.g., the 3-year horizon of this analysis); however, extrapolation further into the future may require accounting for developments in epidemiological and environmental factors, such as changes in the population’s age distribution and dietary patterns (considering associations of overweight, obesity, and T2D with NAFLD and NASH).
In addition, the model’s projections for population growth, which are based on retrospective estimates for disease incidence, do not capture changes in disease testing protocols that could follow the introduction of an approved therapy. Specifically, a greater effort to screen for NASH in at-risk individuals following the recent introduction of resmetirom could rapidly increase the size of the treatment-eligible population. To mitigate this uncertainty, we incorporate a scenario that the models anticipated growth following the introduction of an innovative therapy, as described in Oka et al. (2023), [47] who reported an average increase of 3.175% in the year-over-year growth rate of newly treated patients.
Other studies have also reported that market size may increase following the approval of a novel therapy within a disease area [51, 52]. Lakdawalla et al. (2007) estimated an average increase of 20% using a microsimulation model, although the modeled population was individuals aged 55 years or older, compared to those aged 18 years or older, as was examined in our model [51]. Another potential indicator for market growth associated with greater incentive to diagnose (e.g., with introduction of a new therapy) is “coding intensity” adjustment, [53,54,55] currently standing at 5.9%, and reflecting CMS’ expectation of higher diagnosis rates in Medicare Advantage (MA) vs. traditional fee-for-service (FFS) Medicare, as MA reimbursement financially incentivizes reporting all diagnoses while FFS does not. This estimate, while significantly lower than that reported by Lakdawalla et al., could still overstate market growth as the coding-intensity adjustment reflects increased diagnosis rates among patients who seek care, but, as reported by Stocking et al. (2023), a large proportion of patients with symptoms of chronic conditions do not seek treatment [56]. Other factors that may influence diagnosis rates, such as direct-to-consumer advertising [57] and the number of physicians diagnosing NASH, have not been considered in this analysis. However, scenario analyses varying the diagnosis rates were conducted to address these potential limitations. Together, these considerations underscore the need to account for multifaceted influences on diagnosis rates in modeling the treatment-eligible population.
Future research may build on the estimates presented in this study, by incorporating incidence of NASH, progression of fibrosis in existing patients with NASH, and changes in screening protocols. Additionally, future directions for this model may include the development of a budget impact model, evaluating net-cost impacts of treatment and the magnitude and timing of such impacts, as both dimensions may be relevant to healthcare decision-makers given the potential for novel therapies to reduce progression to long-term, costly outcomes. For example, treatment may reduce the incidence of advanced liver disease (F4 and end-stage liver events) and, accordingly, could impact national healthcare spending and patient cost burden, as reflected by ICER’s cost-effectiveness analysis, indicating that resmetirom may reduce lifetime medical costs and improve patient outcomes relative to the current standard of care [40].
Conclusion
With the emergence of resmetirom as the first FDA-approved therapy for NASH, it is important to provide healthcare decision-makers with contemporary estimates of the size of the resmetirom treatment-eligible population. For a hypothetical 1-million-member commercially-insured health plan, the conceptual framework presented in this study estimated, in the base case, that 1255 patients will be resmetirom treatment-eligible in Year 1 following its approval, increasing to 1699 in Year 3. These estimates correspond to 0.13% and 0.17% of the health plan's population being identified as treatment-eligible for Years 1 and 3, respectively. A wide range of scenario analyses were conducted, varying each major input of the conceptual framework, to characterize the impact of heterogeneity and uncertainty in the current NASH evidence, based on estimates of the treatment-eligible population. The flexibility of the model presented allows for a comprehensive and nuanced understanding of the potential prevalence and incidence of NASH to inform decision-making.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. Data informing the study are publicly available, and description of methods for replication of this analysis is included in the manuscript.
References
Sheth GS, Chopra S. Epidemiology, clinical features, and diagnosis of nonalcoholic fatty liver disease in adults. In Lindor KD and Robson KM (Ed), UpToDate. 2022.
American Liver Foundation. Nonalcoholic Fatty Liver Disease (NAFLD) 2024 [updated May 16th, 2024]. Available from: https://liverfoundation.org/for-patients/about-the-liver/diseases-of-the-liver/non-alcoholic-fatty-liver-disease/
Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023;77:5.
New MASLD Nomenclature | AASLD 2024. Available from: https://www.aasld.org/new-masld-nomenclature
Angulo P, Machado MV, Diehl AM. Fibrosis in nonalcoholic fatty liver disease: mechanisms and clinical implications. Semin Liver Dis. 2015;35(2):132–45.
Hagström H, Nasr P, Ekstedt M, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017;67(6):1265–73.
Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84.
Ajmera V, Loomba R. Imaging biomarkers of NAFLD, NASH, and fibrosis. Mol Metab. 2021;50: 101167.
Alkhouri N, Tincopa M, Loomba R, et al. What does the future hold for patients with nonalcoholic steatohepatitis: diagnostic strategies and treatment options in 2021 and beyond? Hepatol Commun. 2021;5(11):1810–23.
Patel K, Sebastiani G. Limitations of non-invasive tests for assessment of liver fibrosis. JHEP Rep. 2020;2(2): 100067.
MP C, NH A. Noninvasive assessment of hepatic fibrosis: Overview of serologic tests and imaging examinations.: UpToDate; [April 8, 2022]. Available from: https://www.uptodate.com/contents/noninvasive-assessment-of-hepatic-fibrosis-overview-of-serologic-tests-and-imaging-examinations
Yen YH, Kuo FY, Kee KM, et al. APRI and FIB-4 in the evaluation of liver fibrosis in chronic hepatitis C patients stratified by AST level. PLoS ONE. 2018;13(6): e0199760.
Younossi ZM, Tampi R, Priyadarshini M, et al. Burden of illness and economic model for patients with nonalcoholic steatohepatitis in the United States. Hepatology. 2019;69(2):564–72.
J.C. F, C. Q, Y. K, et al. Cost burden of cirrhosis and liver disease progression in metabolic dysfunction-associated steatohepatitis: A US cohort study. J Manag Care Spec Pharm. 2024:1–13.
Tapper EB, Bonafede M, Fishman J, et al. Healthcare resource utilization and costs of care in the United States for patients with non-alcoholic steatohepatitis. J Med Econ. 2023;26(1):348–56.
Fishman J, Tapper EB, Dodge S, et al. The incremental cost of non-alcoholic steatohepatitis and type 2 diabetes in the United States using real-world data. Curr Med Res Opin. 2023;39(11):1425–9.
Dufour JF, Anstee QM, Bugianesi E, et al. Current therapies and new developments in NASH. Gut. 2022;71(10):2123–34.
Madrigal Pharmaceuticals Inc. Rezdiffra (resmetirom): Highlights of prescribing information. West Conshohocken, PA: Madrigal Pharmaceuticals, Inc.; 2024.
Younossi ZM, Henry L. Fatty liver through the ages: nonalcoholic steatohepatitis. Endocr Pract. 2022;28(2):204–13.
Loomba R, Wong R, Fraysse J, et al. Nonalcoholic fatty liver disease progression rates to cirrhosis and progression of cirrhosis to decompensation and mortality: a real world analysis of Medicare data. Aliment Pharmacol Ther. 2020;51(11):1149–59.
Soumya M, Aijaz A, Donghee K. Recent epidemiology of nonalcoholic fatty liver disease. Gut Liver. 2021;15(2):206–16.
Fishman J, O’Connell T, Parrinello C, et al. Prevalence of nonalcoholic steatohepatitis and associated fibrosis stages among US adults, using imaging- vs biomarker-based non-invasive tests. JHEOR. 2024;2:89.
Caro JJ, Briggs AH, Siebert U, et al. Modeling good research practices—overview: a report of the ISPOR-SMDM modeling good research practices task force–1. Value Health. 2012;15(6):796–803.
U.S. Census Bureau. Table H-01. Health Insurance Coverage Status and Type of Coverage by Selected Characteristics for All People: 2021 2022. Available from: https://www2.census.gov/programs-surveys/cps/tables/hi/2022/h_01.xlsx
State Health Access Data Assistance Center. Health Insurance Coverage Type by Age 2023. Available from: https://statehealthcompare.shadac.org/table/4/health-insurance-coverage-type-by-age
Centers for Medicare & Medicaid Services. Medicare Beneficiaries USE of MEDICARE SERVICES - (Year reported: 2019) 2021 [August 31, 2023]. Available from: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Beneficiary-Snapshot/Downloads/Bene_Snaphot.pdf
Cusi K, Isaacs S, Barb D, et al. American association of clinical endocrinology clinical practice guideline for the diagnosis and management of nonalcoholic fatty liver disease in primary care and endocrinology clinical settings: co-sponsored by the American association for the study of liver diseases (AASLD). Endocr Pract. 2022;28(5):528–62.
Madrigal Pharmaceuticals Initiates the MAESTRO-NASH Outcomes Study Evaluating Resmetirom for the Treatment of Patients with Compensated NASH Cirrhosis | Madrigal Pharmaceuticals 2024. Available from: https://ir.madrigalpharma.com/news-releases/news-release-details/madrigal-pharmaceuticals-initiates-maestro-nash-outcomes-study
American Heart Association. What Is Metabolic Syndrome? 2021. URL: https://www.heart.org/-/media/Files/Health-Topics/Answers-by-Heart/What-Is-Metabolic-Syndrome.pdf.
Lee JS, Lee HW, Kim BK, et al. Comparison of fibroscan-aspartate aminotransferase (FAST) Score and other non-invasive surrogates in predicting high-risk non-alcoholic steatohepatitis criteria. Front Med (Lausanne). 2022;9: 869190.
Newsome PN, Sasso M, Deeks JJ, et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol. 2020;5(4):362–73.
Eddowes PJ, Sasso M, Allison M, et al. Accuracy of fibroscan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156(6):1717–30.
Loomba R, Harrison SA, Taub RA, et. Utility of FIB-4, MRE, MRI-PDFF, & FibroScan to identify patients with at-risk F2/F3 NASH based on screening data from a 2000 patient biopsy-confirmed cohort of resmetirom phase 3 clinical trial (MAESTRO-NASH). American Association for the Study of Liver Disease. The Liver Meeting, Washington DC, November 4–8 2022. URL: https://www.aasld.org/the-liver-meeting/utility-fib-4-mre-mri-pdff-and-fibroscan-identify-patients-risk-f2-f3-nash-based.
Harrison SA, Taub R, Neff GW, et al. Resmetirom for nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled phase 3 trial. Nat Med. 2023;29(11):2919–28.
Harrison SA, Ratziu V, Anstee QM, et al. Design of the phase 3 MAESTRO clinical program to evaluate resmetirom for the treatment of nonalcoholic steatohepatitis. Aliment Pharmacol Ther. 2024;59(1):51–63.
Fishman J, Hubbert A, Van der Pluijm W, et al. An epidemiological estimate of the prevalence of the coded nonalcoholic steatohepatitis (NASH) population in the us using an administrative claims dataset. ISPOR Europe. 2022.
Brown RS Jr. Current management of thrombocytopenia in chronic liver disease. Gastroenterol Hepatol (N Y). 2019;15(3):155–7.
Hagström H, Adams LA, Allen AM, et al. Administrative coding in electronic health care record-based research of NAFLD: an expert panel consensus statement. Hepatology. 2021;74(1):474–82.
Vieira Barbosa J, Lai M. Nonalcoholic fatty liver disease screening in type 2 diabetes mellitus patients in the primary care setting. Hepatol Commun. 2021;5(2):158–67.
JA T, K S, SM F, et al. Resmetirom and obeticholic acid for non-alcoholic steatohepatitis (NASH); evidence report. Institute for Clinical and Economic Review. May 25, 2023.
Yasar O, Long P, Harder B, et al. Machine learning using longitudinal prescription and medical claims for the detection of non-alcoholic steatohepatitis (NASH). BMJ Health Care Inform. 2022;29:1.
Said A, Gagovic V, Malecki K, et al. Primary care practitioners survey of non-alcoholic fatty liver disease. Ann Hepatol. 2013;12(5):758–65.
Eskridge W, Vierling JM, Gosbee W, et al. Screening for undiagnosed non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH): a population-based risk factor assessment using vibration controlled transient elastography (VCTE). PLoS ONE. 2021;16(11): e0260320.
Tapper EB, Krieger N, Przybysz R, et al. The burden of nonalcoholic steatohepatitis (NASH) in the United States. BMC Gastroenterol. 2023;23(1):109.
US Census Bureau. National Population Projections Datasets of the US Census Bureau [2023.05.23]. Available from: https://www.census.gov/
Rochon H, Qian C, Bogdanov A, et al. Characterizing the prevalence of nonalcoholic steatohepatitis in the US using two independent real-world cohorts. AMCP 2023.
Oka T, Narukawa M. Emergence of new drugs for intractable diseases is associated with an increase in the number of patients diagnosed thereafter with those intractable diseases. Ther Innov Regul Sci. 2023;57(1):70–8.
Singh S, Allen AM, Wang Z, et al. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13(4):643–54.
Thompson W. W, Symum H, Sandul A, et al. Vital signs: hepatitis C treatment among insured adults—United States, 2019–2020 2022 [cited 71]. Available from: https://www.cdc.gov/mmwr/volumes/71/wr/mm7132e1.htm#suggestedcitation
Dong E, Nixon K, Gardner LM. A population level study on the determinants of COVID-19 vaccination rates at the US county level. Sci Rep. 2024;14(1):4277.
Lakdawalla D, Goldman D, Michaud P-C, et al. Global pharmaceutical policy model (GPPM) technical appendix 2007.
Ward AS, Van Nuys K, Lakdawalla D. Impacts of first-in-class drug approvals on future in-class innovation. USC Schaeffer. 2022.
Jacobs PD, Kronick R. The effects of coding intensity in medicare advantage on plan benefits and finances. Health Serv Res. 2021;56(2):178–87.
Kronick R, Welch WP. Measuring coding intensity in the medicare advantage program. Medicare Medicaid Res Rev. 2014;4:2.
Levitt L, Biniek JF, Kronick R, et al. Unpacking the controversy over medicare advantage: kaiser family foundation; 2023. Available from: HWS-Medicare-Advantage-Transcript.pdf (kff.org)
Stocking A, Duncan I, Huynh N. Estimating underdiagnosis of patients in chronically ill populations. North Am Actuarial J. 2023;25:1–10.
Porter D. Direct-to-Consumer (DTC) Pharmaceutical marketing: impacts and policy implications. School of public, nonprofit and health administration review. 2011;7.
Acknowledgements
Medical Writing, Editorial, and Other Assistance
The authors would like to acknowledge the medical writing support provided by Hélène Parisé (Medicus Economics, LLC) funded by Madrigal Pharmaceuticals, Inc.
Funding
Design, study conduct, and financial support (including the journal’s Rapid Service Fee and Open Access Fee) for the study were provided by Madrigal Pharmaceuticals, Inc.
Author information
Authors and Affiliations
Contributions
Jesse Fishman, Yestle Kim, Zachary J. Smith, Tom O’Connell, and Eric M. Bercaw participated in the design and conduct of the research. Eric M. Bercaw and Zachary J. Smith participated in the development of the tool, with critical review by Jesse Fishman, Yestle Kim, and Tom O’Connell. All authors participated in interpretation of the findings, and in writing and review of the manuscript. All authors maintained control over the final content included in the manuscript and provided their approval of the submission.
Corresponding author
Ethics declarations
Conflicts of Interest
Jesse Fishman was previously an employee of Madrigal Pharmaceuticals, Inc. and previously owned stock options from Madrigal Pharmaceuticals, Inc. Yestle Kim is an employee of Madrigal Pharmaceuticals, Inc., and may own stocks/and or options from Madrigal Pharmaceuticals Inc. Michael R. Charlton has received advisory and consulting honoraria from Madrigal, Novo Nordisk, Cytodyn, Terns, Alnylam, AMR, Glympse, Northsea, Sagmimet, Genentech, and Merck; he has also received research grants from Pfizer and Madrigal, unrelated to this work. Eric M. Bercaw and Tom O’Connell are employees of Medicus Economics, LLC. Medicus Economics, LLC received consulting fees for research from Madrigal Pharmaceuticals, Inc. Zachary J. Smith was previously an employee of Medicus Economics, LLC at the time of development of this manuscript. Medicus Economics, LLC received consulting fees for research from Madrigal Pharmaceuticals, Inc.
Ethical Approval
This manuscript is based on previously conducted studies and does not contain any new studies with human participants performed by any of the authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Fishman, J., Kim, Y., Charlton, M.R. et al. Estimation of the Eligible Population For Resmetirom Among Adults in the United States for Treatment of Non-Cirrhotic NASH with Moderate-to-Advanced Liver Fibrosis. Adv Ther (2024). https://doi.org/10.1007/s12325-024-02989-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12325-024-02989-5