Abstract
Human Zn and Fe deficiencies can be reduced through agronomic biofortification, but information on factors influencing maize grain-Zn and -Fe levels remain scanty. This analysis: (1) Establishes the global distribution of Zn and Fe concentrations in maize grain; (2) assess the contribution of different agronomic practices to the effectiveness of Zn fertilizers for increasing grain yields, and Zn and Fe levels in maize grain; and (3) identify key biophysical factors and metrics to more effectively guide agronomic biofortification of Zn. Using 5874 data points in 138 published papers from 34 countries, we estimated a 7.5% probability of grain-Zn concentrations exceeding the benchmark target of 38 mg kg−1. Using 3187 data points from 65 studies across 27 countries we estimated a 8.5% probability of grain-Fe concentrations exceeding the target of 60 mg kg−1. Our 70-paper meta-analysis revealed that applying Zn and/or Fe in combination with inorganic NPK fertilizer can increase maize-grain-Zn and-Fe concentrations by 31% (p < 0.01) relative to the control (NPK only). In 52% and 37.5% of the studies respectively, grain-Zn and -Fe levels showed significant and concomitant increase with grain-yield increases. Soil organic matter, pH, soil-available Zn, organic input applications, and N, Zn and Fe application rates and methods were among the key factors influencing grain Zn and Fe. We conclude there is substantial room for increasing maize-grain Zn and Fe concentrations, and applying Zn, especially in combined soil and foliar applications, gives substantial increases in grain-Zn and -Fe concentrations. This global review reveals large data gaps on maize-grain nutrient levels, and we call for routine collection of such information in future research.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Globally, billions of people are at risk from micronutrient deficiencies (Kumssa et al., 2015) such as iodine, vitamin A, iron (Fe) and zinc (Zn; Bailey et al., 2015; Han et al., 2022). Zn and Fe deficiencies are reported to affect up to 3 billion people (Dwivedi et al., 2012; Akhtar et al., 2018) and cause an estimated 2–5% reduction in the gross domestic products of developing countries (Stein, 2014). Zn deficiency alone affects up to 2 billion people worldwide (Ahsan et al., 2021; Khan et al., 2022) resulting in more than 0.5 million deaths per year in infants and children below 5-years (Krebs et al., 2014; Khalid et al., 2014). The latest Global Burden of Disease Study from 195 countries revealed that Fe deficiency contributed to around 30 million disability-adjusted life-years in 2017, which is the highest amongst other nutritional deficiencies (Wang et al., 2022). Where Fe deficiencies exist, Zn deficiencies will possibly co-occur, leading to multiple micronutrient deficiencies (Chadare et al., 2022).
Insufficient dietary intake is one of the leading causes of Zn and Fe deficiencies (Aiqing et al., 2022; Han et al., 2022), where at least one-in-five humans is at risk of Zn deficiency associated with low dietary intake (Boonchuay et al., 2013; Sandstead et al., 2014). The highest age-standardized prevalence rate of dietary Fe deficiency is found in South Asia (27,404 per 100,000 population) and western sub-Saharan Africa (SSA) (21,259 per 100,000 population) in 2019 (Han et al., 2022). Compared to other regions of the world, Zn and Fe deficiencies have remained particularly high in SSA (Kumssa et al., 2015; Chadare et al., 2022). This is partly attributed to less total food intake and dietary diversity (Beal et al., 2017), poorer soil quality (Manzeke et al., 2019) and reliance on plant-based foods containing high levels of anti-nutritional factors (Gibson et al., 2010).
Zn and Fe deficiencies are high especially in populations consuming cereals as their staple food, and even more so where micronutrient soil levels and bio-availabilities are low (Boonchuay et al., 2013; Wang et al., 2020; Aiqing et al., 2022). Among the major cereals, maize, rice and wheat which are typically low in Zn and Fe contents constitute nearly two thirds of the energy intake of humans particularly in the developing countries (Saha et al., 2015). The regions in the world with Zn-deficient soils are also characterized by widespread Zn deficiency in humans (Fig. 1; Cakmak, 2008). The most significant overlaps between Zn deficiency in soils and humans occurs in SSA, South Asia and Southeast Asia (Fig. 1). Nearly 50% of the cereal-growing areas in the world have soils with low plant availability of Zn (Cakmak, 2008). In arable soils in SSA, Zn deficiency tops the list of micronutrient deficiencies in the soil (Kihara et al., 2020). Crops often depend on the native soil as a source of micronutrients such as Zn and Fe. Micronutrient deficiencies in soil are aggravated by imbalanced applications of nitrogen (N), phosphorus (P) and potassium (K) fertilizers without micronutrients (Sahrawat et al., 2010; Kihara et al., 2017; 2020). This can deplete micronutrient reserves in the soil through increased harvest in the absence of replacement applications.
Not only do Zn and Fe deficiencies in soils affect human nutrition, but also the crop productivity. Further, Zn and Fe act as co-factors for numerous enzyme functions and play vital roles in various physiological process in plants, including photosynthesis, respiration, electron transport, protein metabolism, chlorophyll synthesis and hormonal regulations (Mallikarjuna et al., 2020). Deficiencies of nutrients in humans may be addressed through a number of approaches including agronomic biofortification, genetic improvement, dietary diversification, food fortification or supplementation (Akhtar et al., 2018; Garg et al., 2018; Ramzan et al., 2020). Agronomic biofortification relies on soil or foliar application of fertilizers (mineral and organic) and/or increasing their solubilization and/or mobilization from the soil to the edible parts of plants (Garg et al., 2018; Bhatt et al., 2020).
Since nutrients in food crops mainly come from the soil, the adoption of good agronomic practices can play a key role in addressing nutrient deficiencies. Agronomic practices including optimizing fertilizer application rates, methods and timing of application, organic amendments, tillage practice and water management are recognized as important determinants for boosting various nutrients’ levels in produce (Wasaya et al., 2018; Simić et al., 2020; Manzeke-Kangara et al., 2021). In that regard, understanding the extent to which various agronomic interventions can increase Zn and Fe concentrations in cereal grains is central to sustainably enhancing food and nutrition security. Biophysical factors such as soil organic matter content, soil pH and availability of macro- and micronutrients have been reported to affect the availability of micronutrients to plants and their concentrations in the grain (Cakmak et al., 2008; Alloway, 2009; Wood and Baudron, 2018; Manzeke-Kangara et al., 2021). Although various studies and reviews exist, there is little information on how much the various biophysical factors affect Zn and Fe concentrations in maize grain. Maize is the second major cereals after wheat, as a major staple worldwide. Improving its productivity and nutritional quality continues to play a vital role in enhancing food and nutrition security. The worldwide distribution of attainable Zn and Fe in maize grain, and the probability of achieving target concentrations have not been covered by past studies and reviews. The simultaneous improvement of grain yield and nutritional quality (e.g., increasing protein and macro- and micronutrients concentration, and decreasing anti-nutrients such as phytic acid) is important for enhancing food and nutrition security. Yet, it is not clear how increases in maize grain yield influence the concentrations of Zn and Fe, or even proteins and phosphorous (P) in the grain. Therefore, the objectives of this review are to: (1) establish the global distribution of Zn and Fe concentrations in maize grain and the probability of attaining nutrient concentration targets; (2) assess the contribution of different agronomic practices to the effectiveness of Zn fertilizers for increasing grain yields, and Zn and Fe levels in maize grain; and (3) identify key biophysical factors and metrics to more effectively guide agronomic biofortification of Zn. The main hypotheses of the study are: (1) benchmark concentrations of Zn and Fe in maize grain are attainable when supported with agronomic innovations; (2) there are significant differences in grain concentrations of Zn and Fe due to different agronomic practices; and (3) levels of grain-maize Zn and Fe are influenced by soil biophysical factors.
2 Review and analyses
2.1 Study focus
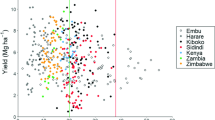
The geographic focus of this work was global, and maize (Zea mays L.) was chosen as the test crop because it is a staple food crop in many parts of the world. It is also a crop of strategic importance in food-insecure parts of the world such as the SSA (Sileshi and Gebeyehu, 2021), where Zn deficiency is widespread in both soils and human populations (Wessells and Brown, 2012; Fig. 1). Together with wheat and rice, maize accounts for an estimated 42% of the world’s food calories and 37% of protein intake (Erenstein et al., 2022). Maize is annually cultivated on ~ 206 million ha of land globally (FAOSTAT, accessed, 21 June 2023), making it the second most widely cultivated crop after wheat. Despite the lower cultivated area, the global maize crop annually produces 1.2 billion tonnes, compared with wheat’s 770 million tonnes (FAOSTAT, 2023). In SSA, the cultivated maize area annually is 3.6 times more than wheat (Sileshi & Gebeyehu, 2021). Maize is also marketed in 50% higher volumes, than both rice and wheat globally (Erenstein et al., 2022). Recent spatially-explicit analyses by Gashu et al. (2021) indicate that grain-Fe and -Zn concentrations are lower in maize than wheat and rice, and populations relying on maize-based diets are likely to have lower intakes of these nutrients. Therefore, identifying agronomic and environmental factors that increase Zn and Fe contents in maize grain was deemed an important contribution to the strategy for improving their availability in the diets of populations that depend on maize as their staple food.
Locations for data included in the study (the red dots) superimposed on map of prevalence of Zn deficiency in humans. White areas indicate no data. Prevalence map was adapted from Wessells and Brown (2012). The Zn deficiency prevalence in humans was estimated by Wessels and Brown (2012) using the National Food Supplies
2.2 Data sources, inclusion and exclusion criteria
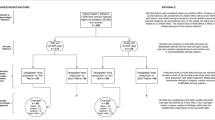
These search strategies followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Reporting Literature Searches in Systematic Reviews (PRISMA-S) (Rethlefsen et al., 2019). A systematic literature search was implemented in Web of Science using the following search string: ("soil fertili*" OR "manure" OR "fertilizer") AND ("maize grain" OR "corn grain") AND ("nutri*" or “mineral” OR "zinc" OR "iron") AND ("concentration" OR "content" OR "uptake" OR "compo*"), and Google Scholar: ("soil fertili*" OR "manure" OR "fertilizer") AND ("maize grain" OR "corn grain") AND ("nutri*" OR "zinc" OR "iron") AND ("concentration" OR "content" OR "uptake" OR "compo*") -blood -digestibility -biofuel -greenhouse -pot -enzyme. Additional searches were conducted to locate studies on genetic variability of maize in terms of grain Zn and Fe contents using the following search keywords in Google Scholar: (Geneti* OR Genotyp*) AND ("maize" OR "corn" AND "grain zinc" OR “Zn” OR "grain iron" OR “Fe” OR “mineral”) AND ("variation") until March 2024. Two Boolean operators “AND” and “OR” were used to combine key words while truncation using asterisk (*) was applied to retrieve all relevant variants of the keywords. These searches returned 1996 and 379 hits in Google Scholar and Web of Science, respectively. We restricted our search to return only studies published since the years 2000 because as older studies may not reflect current situations, based on subject expert’s knowledge. Additional studies were identified and included by examining the reference sections of the research papers found using the search strategies stated above (see PRISMA flow chart in Fig. 2).
The following inclusion criteria were used: (1) the study reported maize grain nutritional quality parameters; (2) in the case of studies analyzing genotypes and/or where specific treatments were not compared, all recommended agronomic management practices were applied consistently; (3) survey or experimental data are based on field sampling or randomized and controlled field trials; and (4) the treatments were replicated. Studies were excluded based on the following criteria: (1) the study was conducted under greenhouse condition or pot experiment; (2) the study lacked all of the keywords of the search string in the title/topic; (3) the titles/topics were outside our research objectives; (4) the study was done on a different crop, not maize or maize grown for other purposes such as silage; (5) the study reported nutrient concentrations in fresh maize grains such as baby corn; (6) the study did not report treatment means, but reported other measures such as median and the corresponding author did not share raw data after the data were requested; (7) it was a review paper; (8) not published in a peer reviewed journal article; and for experimental data, (9) the study reported results in a manner that contradicts the treatment design; and (10) the study presented results summarized across location/sites instead of specific treatments or studied varieties in a multi-location study. These exclusion criteria were chosen to make the analysis more focused and control the quality of publications selected. Quality of the selected publications was assessed by full-text reading of the selected publications to substantiate the presented evidence, and confirmed by two independent reviewers as proposed in (Koutsos et al., 2019). Additionally, the data extraction team quality-controlled each other’s datasets.
2.3 Data extraction and processing
From each study we extracted data on site characteristics including: the geographic coordinates; year of study; rainfall received in the season; soil clay, sand, and silt; soil pH; soil organic carbon (SOC), or soil organic matter (SOM); total nitrogen (N); available phosphorus (P) and potassium (K); and Zn and Fe concentrations in the soil. In addition, we extracted the World Reference Base (WRB) soil type from SoilGrid using the geographic coordinates of the study sites. We also extracted information on agronomic practices including tillage; irrigation; bacterial inoculation; and the N, P, K; Zn and Fe application rates and methods wherever available (Table 1). In addition, we extracted grain yield and grain concentrations of various nutrients, and their measures of variability. The extracted data were organized in Microsoft Excel and processed to facilitate visualization and statistical analyses.
Using the soil clay, sand and silt, we created soil texture categories following the United States Department of Agriculture classification (USDA, 1993): clay (≥ 40% clay, ≤ 45% sand and < 40% silt), clay loam (27–40% clay and 20–46% sand), loamy (7–27% clay, 28–50% silt, ≤ 52%), sandy clay (≥ 35% clay and ≥ 45% more sand), silt (≥ 80% silt and < 12% clay), silty clay (≥ 40% clay and ≥ 40% silt), silty clay loam (27–40% clay and ≤ 20% sand), silty loam (≥ 50% silt, 12–27% clay, or 50–80% silt and < 12% clay), sandy clay loam (20–35% clay, < 28% silt and > 45% sand). Finally, we grouped these texture categories into three, namely, coarse (sand, sandy loam, sandy clay, sandy clay loam), medium (loam, silt loam, silt) and fine (clay, clay loam, silty clay, silty clay loam) to facilitate meta-analysis.
We also classified the soil type as calcareous, silicic or mafic based on their parent materials (Gray et al., 2011). Calcareous parent materials include limestone, dolomite, calcareous shale and sands with > 50% CaCO3 or MgCO3 (Gray et al., 2011). Silicic (also called acidic) refers to rocks that contain significant amounts of silica (> 68% Si), while intermediate parent materials contain 52–68% Si. The term mafic (basic) is applied to rocks with relatively low amounts of silica (45–52% Si) (Gray et al., 2011).
Some authors had reported SOM while others had reported SOC. To facilitate the analyses, we converted all SOM data to SOC by dividing SOM by 2 following Pribyl (2010). After a review of empirical studies and theoretical considerations, Pribyl (2010) argued that Bemmelen factor (1.724) is too low for most soils and proposed 2 as a better correction factor. Then we grouped into three categories: low (SOC < 10 g kg−1), medium (SOC 10–20 g kg−1) and high (SOC > 20 g kg−1) in preparation for meta-analysis.
Studies had also used different soil-extraction methods and expressed soil data in different units. Soil extraction methods are based on different mechanisms and vary in their extraction efficiency (Breure et al., 2022). Comparing the results of different soil-testing methods is often not straightforward and requires pedo-transfer functions to translate the outcome of one soil test into another (Breure et al., 2022). Therefore, before combining such data for meta-analysis, we converted them into a single unit using pedo-transfer functions. For example, soil pH was given in different measurements. Therefore, we converted the reported values to pH in CaCl2 using the following pedo-transfer functions: pH_CaCl2 = 0.918pHw-0.3556; pH_CaCl2 = 0.321 + 0.987pH_KCl following Van Lierop (1981). To facilitate meta-analysis, we created soil pH categories as acidic (pH < 6.5), neutral (pH 6.5–7.5) and alkaline (pH > 7.5) following Xu et al. (2022).
Soil available P was reported based on AB-DTPA, Bray 1, DTPA, Mehlich, Olsen or resin extraction methods. We converted P extracted using the different methods to Olsen P using regression equations in McDowell et al. (2013) and Steinfurth et al. (2021). For ease of inclusion in the meta-analysis, we grouped the Olsen P values into three categories: low (< 15 mg/kg), medium (15–22 mg/kg) and high (> 22 mg/kg) based on critical values from earlier studies (e.g., Tang et al., 2009; Chen et al., 2015). According to Chen et al. (2015) the critical level of Olsen P for maize grain yield was 19.1 mg kg−1 in Luvic Xerosols in China. Using the three models, Tang et al. (2009) determined the critical value of Olsen P for maize as 15.3 mg kg−1 (ranged 12.1–17.3 mg kg−1). Below the critical level, the crop can no longer absorb sufficient P for growth and yield reductions are expected. Soil Zn was analyzed using different methods including DPTA, AB-DPTA, HCl, etc. Therefore, we converted all values to DPTA following Abreu et al. (2002) as DTPA solution was the most efficient extractant for the determination of bioavailable Zn.
Different studies also reported the control in different ways. To facilitate the meta-analysis, we calculated the response ratio with careful case-matching of the treatment with the correct control. We used a single control here, defined as the plot receiving NPK because, for a global meta-analysis, a reasonable control would be a fully fertilized treatment receiving all the three key macronutrients (i.e., N, P and K). Accordingly, in the meta-analysis we only included studies that had applied Zn in combination with NPK or any other nutrient and we assessed performance against NPK. This approach gives conservative estimates of the effect sizes for the target variables.
2.4 Data analyses
2.4.1 Empirical distributions
We used data from 168 and 65 published studies to establish the empirical distribution of grain Zn and Fe concentrations, respectively. First, we created the frequency distributions of the Zn and Fe values by setting the number of bins to optimal following the zero-stage rule (Wand, 1997). Then, we added the Gaussian kernel density (a smooth estimator of the histogram) and the normal distribution for Zn and Fe concentrations separately. Using the frequency distributions, we created ogives (cumulative distribution functions) for Zn and Fe to estimate the probability (ϕ) of exceeding the target values of Zn (38 mg kg−1) and Fe (60 mg kg−1) in maize grain set by HarvestPlus (Bouis et al., 2011). Using the Extreme Studentized Deviate test we also identified outliers in the distribution of each variable. This test provides objective procedures for detecting multiple outliers in the distribution of data via repeated iterations. This procedure starts with the most extreme values in the sample, and removes them successively until 20% of the sample has been tested and the R statistic is below its critical value (Rcrit at P < 0.05) (Rosner 1983). Once outliers were identified, we went back to the original study to verify the values, and we found the values have problems either with measurement or conversion into the unit (mg kg−1) we used.
Since one of our objectives was to establish the range of values of grain-Zn and-Fe concentrations, we estimated the means, medians, the lower and upper quantiles (Q1 and Q3) and the uncertainty around them represented by 95% confidence limits (here after CL). We calculated the CLs for these statistics using bootstrapping with 9999 replicates. We applied bias-corrected bootstrapping as it is more accurate than the simple and percentile methods albeit being complex. We used the Paleontological Statistics (PAST) package (https://past.en.lo4d.com/windows) for estimating bootstrapped CLs.
In addition, we visualized the data using box and violin plots to understand the characteristics of distributions of grain Zn and Fe. These types of visualization have been recommended because they allow examination of the underlying differences in distribution (Krzywinski & Altman, 2014; Spitzer et al., 2014) and provide a more comprehensive summary of uncertainty. A combination of box and violin plot was specifically designed to identify multimodality by exposing the waist between two or more modes of a distribution (Spitzer et al., 2014). The violin plot readily shows the shape of a dataset by using a kernel density function.
2.4.2 Meta-analysis
Meta-analysis was conducted for estimating changes in the effect size (i.e., the magnitude of change relative to the NPK fertilizer) with treatments and environmental variables for the dataset that applied Zn fertilizer. Table 2 provides detailed description of the treatments used in the narrative review and meta-analysis. For the meta-analysis, we selected a subset of the studies where zinc was applied for which the response ratio (RR) could be calculated consistently as the change of the study variable in the Zn-applied group (treatment) compared to NPK group (control). These included 56, 49 and 11 studies for Zn effects on grain yields, grain Zn, and grain Fe concentrations, respectively. We used the natural logarithm of the response ratio (lnRR) as the effect size calculated as follows (Gurevitch and Hedges, 1999):
where T and C are the values of the target variable from the treatment (see Table 2 for details) and control, respectively. The target variables were grain yield, grain-Zn and-Fe concentrations. For all target variables we defined the “control” as the treatment receiving inorganic NPK fertilizer. Once analyses were completed, we back-transformed lnRR and its 95% CLs into the arithmetic domain. In the rest of the manuscript, we presented the effect sizes estimated in percentage for better understanding. For this purpose, we re-expressed lnRR or RR into percentage change in the target variables as follows (Zhang et al., 2021):
Prior to meta-analysis, publication bias is often assessed using funnel plots of effect sizes. However, funnel plots require a measure of variance, such as the standard error of means (SE), but most studies selected for this analysis did not report SE values. Another method of visualizing publication bias is through plotting the distribution of effect sizes (Sileshi et al., 2008; Basche & DeLonge, 2017; Thapa et al., 2018; O’Brien and Hatfield, 2019). Therefore, we investigated publication bias through visualization of histograms and the normal quantile–quantile (Q–Q) plots of the log-transformed response ratios, i.e., ln(RR) for each variable (Wang & Bushman, 1998; Sileshi et al., 2008; O’Brien and Hatfield, 2019; Fig. 2). We also formally tested normality of the lnRR values using the Shapiro-Wilk (SW) and Anderson-Darling (AD) tests values of ln(RR) of grain yield, grain-Zn and-Fe concentrations. Although the distributions revealed significant (P < 0.0001) departures from normality, the Q-Q plots did not reveal publication bias. The distribution of ln(RR) of grain yield had 4 negative and 5 positive outliers, while ln(RR) of grain Zn did not have outliers. The ln(RR) of Fe had 2 negative and one positive outliers. Therefore, we performed all analyses after dropping outliers.
Density plots (top) and normal Q-Q plots (bottom) of ln(RR) of response ratios of grain yield a, grain-Zn concentrations b and grain-Fe concentrations c in studies with Zn and Fe application. The rugged and smooth blue lines in the density plots represent the kernel density and normal probability distributions of the observed values, respectively
We used a linear, mixed-effects modelling framework to estimate the variation in effect sizes of maize-grain yield, and Zn and Fe concentrations with biophysical variables and agronomic practices. These practices included inoculation, irrigation, tillage, and application of organic inputs and NPK fertilizer. We entered the chosen covariates as fixed effects and the study (each published paper) as the random effect, which represents the clustering structure in our study population. The biophysical variables were: (1) parent material (calcareous, mafic, intermediate and silicic); (2) soil texture (coarse, fine, medium); (3) soil pH (acidic, alkali, neutral); and (4) soil organic carbon levels (high, low, medium). The agronomic practices compared were: (1) inoculation using Zn-solubilizing bacteria (with vs. without); (2) irrigation (with vs. without); (3) tillage (tilled, not tilled, unknown); (4) organic matter amendment (with vs. without); (5) Zn application method (foliar, seed, soil, combined foliar and soil); and (6) Fe applications (with vs. without). As treatment, the Zn and Fe applications were combined with N, P and/or K applications. We performed these analyses to determine how effect sizes vary with each of these variables. We also explored the changes in effect sizes of grain-Zn concentrations with soil texture, pH, SOC and Olsen P due to foliar and soil application methods. We did not explore other variables (e.g., variety information, actual rainfall and temperature during the experiment) because of many missing values. Due to the sparsity of data, we also did not explore a combination of variables or their interaction effects.
Our data were highly unbalanced in terms of the number of studies and observations (sample sizes) in each categorical variable. Therefore, we used the Kenward–Roger method for approximating the degrees of freedom and correction of the standard error estimate (Spilke et al., 2005). The Kenward–Roger method incorporates a correction to reduce bias in the estimated variance–covariance matrix of fixed effects. We chose this method because comparison of a large number of unbalanced designs common in agricultural research has shown that it is preferable to other approaches in terms of the nominal type I error rate control (Spilke et al., 2005). For each analysis we calculated the intraclass correlation coefficient (ICC) or repeatability for mixed-effects models, as the ratio of the covariance estimate of the random effect (i.e., study) and the covariance estimate for the residual + random effect. The ICC estimates measure how much of the total variance in the outcome is accounted for by the random effect (Chen et al., 2018). The ICC is also a reliability metric that gauges similarity when, for example, entities are measured under similar, or even the same, well-controlled conditions (Chen et al., 2018). Reliability is defined as the agreement or consistency across two or more measurements, and the ICC was developed for this purpose (Chen et al., 2018). The ICC can also help determine whether a mixed model is even necessary. An ICC close to zero means the observations within clusters (e.g., studies in our case) are no more similar than observations from other clusters, and hence specification of a random effect might not be necessary. ICC values close to 1 indicates that the sample variance is due to the variance between clusters rather than variance within clusters. In most cases, the ICC values were close to 1 and hence inclusion of “study" as the random effect was justified. Table S2 in the Online Resource 2 provides the ICC, statistical significance of each of the fixed effects and the significance of the residual variances.
We represented the uncertainty around the estimated values using 95% confidence intervals (CI) estimated using the mixed effects models.
2.4.3 Correlation and regression analyses
We performed correlation analyses between: grain yield; grain-Zn; -Fe concentrations; their response ratios; soil nitrogen (N); available phosphorus (P); potassium (K); Zn; and grain-Zn and -Fe concentrations as well as the application rates of N, P, K and Zn to reveal the magnitude and direction of correlations. To avoid the Simpson paradox effect, we performed the correlation analyses for sites where Zn was applied. We performed all correlation and regression analyses on data transformed to the natural logarithm.
To explore the relationships between grain yield and grain nutrient concentration, we used linear regression. We performed regression analyses for each study separately, because combining studies can increase the background noise from the differences in study conditions, and often results in counter-intuitive findings (often referred to as aggregation bias). Our initial tests using the Johnson-Neyman technique (White, 2003) revealed heterogeneous residual variance and differences in intercepts. Therefore, we selected studies where two target variables were measured concurrently and where the reported number of means is sufficient (≥ 5) to run linear regression analysis. We then transformed the target variables into natural logarithms and run the analysis.
In addition, we performed boundary analysis (following Kihara et al., 2017) to establish the maximum grain-Zn and-Fe concentrations that can be expected given N and P application rates, soil total N, available P, SOC, soil Fe to Zn ratio and soil available P to Zn ratio. Wherever parametric models do not fit the data, we used weighted scatterplot smoothing (LOESS) implemented in the PAST statistical package. PAST estimates the 95% confidence bands for the LOESS curves by bootstrapping using 9,999 random replications.
2.4.4 Principal component analysis
We performed principal component analysis (PCA) to determine factors that affect the response ratio of grain-Zn concentrations (RRZn); PCA could not be performed for the response ratio of grain Fe due to the inadequate number of data points. We included soil pH, SOC, available P (Olsen P), available K, soil-Zn concentrations, N and P application rates on one hand, and RRZn on the other hand, from 20 studies that have reported all of those variables. A common issue in PCA is how to choose between raw data and standardized or transformed variables. The use of raw data is fine if all variables have the same units of measurement. However, the variables available in our dataset were expressed in different units. If the raw data is used, PCA tends to give more weight to those variables that have higher variances than to those with lower variances (Jolliffe and Cadima, 2016). Therefore, we standardized each variable (\(\:\stackrel{-}{Y}\)) of 0 and standard deviation (\(\:{Z}_{i}=\frac{{Y}_{i}-\stackrel{-}{Y}}{\sigma\:}\). We used this approach to remove scale differences between the variables (Jolliffe and Cadima, 2016).
3 Results and discussion
3.1 Studies retrieved and used for the various analyses
Based on the inclusion/exclusion criteria and quality checks, 214 studies were selected out of the 346 studies initially identified at article screening stage, after 132 publications were dropped by quality checks (Fig. 3; Online Resource 1). Survey data obtained from five studies were analysed together with experimental data to show the global distributions of maize grain Zn and Fe. The five survey studies collected samples from farmer’s fields while those that sampled grains from the market and storage facilities were excluded due to quality concerns (for instance, Dickinson et al. 2014).
3.2 Distributions of Zn and Fe concentrations in maize grain
A total of 138, 65, 27, 16 and 21 studies (Fig. 2) were used to establish the empirical distribution of Zn, Fe, protein, nitrogen (N) and phosphorus (P) concentrations in maize grain, respectively. These distributions provide baselines against which future improvements in nutrient densities can be judged (Fig. 4; Table 3; Fig. S1 in the Online Resource 1). These variables were either asymmetrical (skewed) or multimodal in their distribution (Fig. 4 ; Fig. S1 in the Online Resource 1). In asymmetric distributions, the median is preferred to the mean because it always falls closest to the mode. Therefore, in the following passages our inferences are based on the medians, their 95% confidence limits and the empirical quartiles.
For the first time, we provide the distributions and the expected values of Zn and Fe concentrations in maize grain at the global level (Fig. 4; Table 3). The grain-Zn concentrations are right-skewed, and the 95% CLs indicated significant differences between the mean and the median of the distribution (Fig. 4a). Before removing outliers, the mean and median were estimated at 24.2 and 22.3 mg Zn kg−1, respectively. After removing 50 outliers, i.e., grain-Zn concentrations greater than 61 mg kg−1 in Fig. 4a (R > Rcritical at P < 0.05), the median value of 22.3 mg kg−1 was retained and deemed to be a more realistic estimate of the expected maize-grain Zn concentrations across maize genotypes under the prevailing agronomic management practice. Similarly, grain-Fe values greater than 112 mg kg−1 were outliers. Before removing outliers, the mean and median grain-Fe concentrations were 30.1 and 22.4 mg kg−1, respectively. After removal of outliers, the median grain-Fe concentration was 22.0 mg kg−1 (Fig. 4b), and this value was deemed a realistic estimate of maize grain-Fe concentrations across genotypes and management regimes.
Across all treatments and genotypes available, the probability of grain-Zn concentrations exceeding the benchmark of 38 mg kg−1 (see Bouis et al., 2011) was only 7.5%, while the corresponding probability of grain-Fe concentrations exceeding 60 mg kg−1 was 8.5%. When treatments with and without Zn application were considered separately, the probability of exceeding 38 mg Zn kg−1 was 31.6% where Zn was applied but only 1.4% without Zn application (Fig. 4c). The probability of grain-Fe concentrations exceeding 60 mg kg−1 was 47.1% where Zn was applied, but only 4.0% in the absence of Zn application (Fig. 4d).
Histograms a and b representing the empirical distributions of Zn and Fe concentrations (in mg kg−1) in maize grain, and ogives (c and d) showing the probability of achieving the target Zn and Fe concentration with application of Zn and without Zn application. The jagged and smooth lines in (a) and (b) represent the kernel density and normal probability distributions of the observed values. Values in parentheses in (a) and (b) represent the 95% CLs of means, medians, and the first and third quantiles (Q1 and Q3) estimated after removing outliers. The value of ϕ in c and d represents the probability of exceeding the target Zn and Fe grain concentrations of 38 and 60 mg kg−1 (red lines).
Density distributions of the response ratios of grain yield, grain-Zn and -Fe concentrations in studies with Zn (labelled “With Zn”) and without Zn application (labelled “Without”) show that effect sizes of all target variables are smaller in treatments without Zn than with Zn (Fig. 5). The 95% CLs of both the medians and means of the response ratios indicate that grain yield was significantly increased with Zn application relative to the NPK control (Fig. 5a). On the other hand, where Zn was not applied, the yield increase was not significantly different from the NPK control. In 27 out of the 56 studies, treatments including Zn application significantly increased grain yields relative to the NPK control (Fig. S2 in the Online Resource 1). In the remaining 29 studies, the differences between treatments and the NPK control were not statistically significant (Fig. S2 in the Online Resource 1).
The response ratios of grain-Zn concentrations in studies where treatments involved both Zn application (“With Zn”) and no Zn application (“Without”) indicate that the difference between mean response ratios With Zn (1.36) and Without (1.05) is 0.31 with a bootstrapped 95% CL of (0.26, 0.35; Fig. 5b). The two means were significantly different (Monte Carlo permutation P < 0.0001). The median value of the response ratio to Zn application is 1.28 (95% CL: 1.26, 1.30). That means Zn application increased grain-Zn concentrations on average by 28% over and above what is achievable by NPK fertilizer alone or any other combination that does not include Zn inputs. In 40 out of 49 studies, treatments including Zn application have significantly increased grain-Zn concentrations relative to the NPK control, while for only 9 studies, the treatments and control are not significantly different (Fig. 3a in the Online Resource 1). The above findings indicate that more opportunities exist for improving maize grain-Zn concentrations than maize grain yields through Zn fertilization, over and above those achievable with NPK fertilizers alone.
The response ratios of grain-Fe concentrations were significantly increased (10% median) where Zn was applied relative to the control (Fig. 5c). In 6 out of the 10 studies, treatments including Zn application have significantly increased grain-Fe concentrations relative to the control (Fig. 3b in the Online Resource 1). Across studies, grain-Fe concentrations were also significantly increased (12% median) where Fe was applied relative to the control (Fig. 5d).
Box and violin plots of response ratios of grain yield a, grain-Zn concentration b and -Fe concentration c with and without Zn application, and Fe concentration in treatments with and without Fe application d. The category “With Zn” or “With Fe” consist of treatment combinations listed in Table 2. Distributions of the response ratios were generated after grouping treatment combinations into “With Zn or Fe” and “Without” as shown in Table 2.” Figures in parenthesis represent the 95% confidence intervals of the mean and median estimated using 9999 bootstrap replicates
3.3 Association between grain yield, grain Zn, Fe, P and protein concentrations
Regression analyses among the different variables (e.g., grain yield, grain Zn, Fe, protein and P), which had large sample sizes in the studies we found are presented in Table S3, Online Resource 2.
In the regression of grain-Zn concentration [ln(Zn)] on grain yield [ln(grain yield)], run separately for each study, 32 out of 61 studies (52.5%) had significantly positive slopes while in 27 studies, the slopes were not significantly different from zero (Fig. 6a). The slopes were significantly negative in two studies (6.7%). This indicates that in the majority of cases increases in grain yield and grain-Zn concentrations occur concurrently. Similarly, Chattha et al. (2017) found positive association between grain yield and grain-Zn concentration in wheat. On the other hand, Goredema-Matongera et al. (2021) suggested that application of Zn fertilizer may increase grain yield without increasing grain-Zn concentration in SSA. Even where Zn was not applied, the correlation between grain yield and grain-Zn concentrations can be positive. For example, Breure et al. (2023) found positive correlations between maize grain yield and grain-Zn concentrations within individual sites in field trials in Kenya, Zambia and Zimbabwe.
In the regression of grain-Fe concentrations [ln(Fe)] on ln(grain yield), 6 out of 16 studies (37.5%) had significantly positive slopes, and 10 studies (62.5%) had non-significant slopes (Fig. 6b). None of the slopes were significantly negative. Similarly, in the regression of Fe [ln(Fe)] on grain Zn [ln(Zn)], the slopes were significantly positive in 14 out of 24 studies (58.3%), while in the remaining 10 studies the slope were not significantly different from zero (Fig. 6c). Because no significantly negative association between grain-Zn and -Fe concentrations were found in any of the studies evaluated, our findings do not support assertions (e.g., of Saha et al., 2015) of possible depletion in grain-Fe concentrations with increase in grain-Zn concentrations.
Regression of grain protein [ln(protein)] on grain yield [ln(Grain yield)] showed significantly positive slopes in most of the studies, and none was negative (Fig. 7a). These findings contradict earlier studies (e.g., Ekpa et al., 2018; Cakmak and Kutman, 2018; Guo et al., 2020; Van Eynde et al., 2023; Breure et al., 2023) reporting reduced total protein or other nutrient concentrations with increase in maize grain yield. Similarly, slopes of regression of grain protein [ln(protein)] on grain-Zn [ln(Zn)] concentrations are significantly positive in 10 studies (55.6%), and significantly negative in only one study (Fig. 7b). Grain Zn also increased with grain P in 5 out of 8 studies (Fig. 7c). Thus the enrichment of Zn in grains can occur concomitantly with changes in other important nutritional or anti-nutritional properties (Hussain et al., 2012; Saha et al., 2015).
Forest plot of the slopes of regression of grain-Zn and -Fe concentration on grain yield a and b and grain-Fe concentrations on grain-Zn concentrations c in studies where both variables were reported. Horizontal bars represent 95% confidence limits of the slopes. The red vertical lines represent b = 0. When the 95% CL encompasses the redline, the slope is not significantly different from 0. Figure on the left and right sides of the horizontal bars represent the slope and the number of observations per study, respectively
Forest plot of the slopes of the regression of grain protein concentrations on grain yield a, protein concentrations on grain-Zn concentrations b, grain P concentrations on grain-Zn concentrations c in studies where both variables were reported. Horizontal bars represent 95% confidence limits of the slopes. The red vertical lines represent b = 0. When the 95% CL encompasses the redline, the slope is not significantly different from 0. Figures on the left and right sides of the horizontal bars represent the slope and the number of observations per study (N), respectively
3.4 Treatment effects on grain-Zn and -Fe concentrations
In the studies used in this meta-analysis, Zn and Fe were applied together with different rates of nitrogen (N), phosphorus (P) and potassium (K) fertilizers (Table 1) either alone or in combination with micronutrients and organic inputs. The combined application of NPK, micronutrient and organic inputs (NPK + Mn + OM) significantly increased grain yield by 39%, grain-Zn by 62% and -Fe concentrations by 57% relative to the NPK control (Fig. 8a-c). Similarly, application of NPK together with micronutrients (NPK + Mn) significantly increased grain yield (by 34%), grain-Zn (by 32%) and -Fe concentration (by 31%) relative to the NPK control. These results imply that application of micronutrients (Zn and Fe) in combination with NPK and organic input increase not only grain yields but also grain-Zn and -Fe concentrations in maize. The NP + Mn increased grain Zn (23%) but did not increase grain yield.
The relative increases in yield due to Zn application in the various countries ranged between 5% in Argentina and 50% in Zimbabwe and Libya (Online Resource 1 Fig. 4a). The corresponding values for relative increase in grain-Zn concentration were from 2% in Argentina to 76% in Mali (Online Resource 1 Fig. 4b). Grain-Fe concentrations significantly increased due to Zn application over the control in Pakistan (by 66%), Bangladesh (by 49%) and Egypt (15%) (Online Resource 1 Fig. 4a). In the remaining countries, grain-Fe concentrations were either lower or not significantly different from the control.
Forest plot of response ratios of grain yield and grain-Zn and -Fe concentration under different treatments. Horizontal bars represent 95% confidence limits of means estimated using linear mixed effects models. The red vertical lines represent RR = 1. When the 95% CL encompasses the redline, the response in the treatment is not significantly different from the response in the control. Figures on the left and right sides of the horizontal bars represent the marginal means and the number of studies (total sample sizes in parentheses), respectively. Treatments with fewer than 3 observations were excluded
While improved agronomic practices such as Zn and Fe application can increase Zn and Fe concentrations in maize grain by over 30%, future climate change related factors such as elevated atmospheric CO2 levels and high temperature can negate the gains due to the carbohydrate dilution effect (Al-Hadeethi et al., 2019; Beach et al., 2019; Loladze, 2014; Myers et al., 2014). For example, Al-Hadeethi et al. (2019), Beach et al. (2019) and Myers et al. (2014) estimated the reduction in Fe and Zn in maize gain at ~ 5% due to CO2 enrichment. Similarly, Loladze (2014) found that elevated levels of CO2 reduced overall concentration of 25 minerals including Zn and Fe by 8% across 130 different plants. On the other hand, effects of elevated temperatures and heat stress are variable with reported increases of Zn and protein concentrations (Velu et al., 2016) and yet also reductions in Zn, Fe and protein contents of 14–15% (Haddad et al., 2022). These observations show a clear direction on influence of CO2 but need for further work to ascertain the implications of heat stress on produce quality.
3.5 Agronomic factors moderating response ratios
3.5.1 Inoculation
Responses to applied Zn were significantly higher relative to the control with or without inoculation but these were not influenced by inoculation level (Fig. 9; Online Resource 2 Table S2). However, the number of studies and observations are too small to clearly demonstrate the benefits of inoculation and more work is needed to ascertain the gains. Nevertheless, inoculation with soil micro-organisms such as Bacillus, Pseudomonas, Rhizobium and Azotobacter enhance the solubilization and mobility of Zn to plant roots (Rengel et al., 1999; Kumar et al., 2016), aided by organic acids produced by these microbes (Hussain et al., 2020). As a result, inoculation increased grain yield by 11–20%, compared to no inoculation in wheat (Moreno-Lora et al. 2023) and 11 to 22% for maize based on seed priming (Goteti et al., 2013). The same inoculation in the study of Moreno-Lora et al., (2023) did not increase grain-Zn and Fe-concentrations but interestingly improved the P: Zn molar ratios (indication of increased Zn bio-availability).
3.5.2 Irrigation management
Irrigation did not significantly increase response ratio of grain-Zn and -Fe concentrations for these cases that were all applied with Zn (Online Resource 2 Table S2). In other words, although the responses were greater than the NPK control, response to Zn application was not increased by irrigation or no irrigation. On a study with wheat, (Bagci et al., 2007) observed Zn application combined with irrigation increased yield and water-use efficiency across different wheat genotypes in Central Anatolia. Sufficient soil moisture improves Zn solubility and diffusion in the soil solution, and can minimize water stress-induced Zn deficiency (Alloway, 2009; Bagci et al., 2007).
3.5.3 Tillage
The response ratios of grain-Zn concentration did not significantly vary with the tillage method (Online Resource 2 Table S2). But Fe response was significantly higher under tilled than no till. Regardless of tillage method, application of Zn significantly increased grain-Zn and -Fe concentration relative to the control. Wright et al. (2007) showed vertical distributions of Fe and Zn in soil profile; where Fe is concentrated in the subsoil (15–30 cm) while Zn is mostly within the topsoil. Deep tillage operations can redistribute Fe and influence grain quality. However, most studies show no response of grain Zn and Fe to tillage practices e.g. for ryegrass (García-Marco et al., 2014), pea and lupin (Stanislawska-Glubiak & Korzeniowska, 2009). However, under moisture stress, no-till practices showed significantly higher pea and lupin Fe- and Zn-concentrations than for conventional tillage (Stanislawska-Glubiak & Korzeniowska, 2009).
3.5.4 Organic input application
The relative increase in grain-Zn concentrations was significantly (P < 0.0001) higher with application of organic inputs (64%) compared to omission of organic inputs (28%). Although not statistically significant (P = 0.0942), grain-Fe concentrations also increased by 45% with organic inputs relative to the treatment where organic inputs were not applied (26%). Organic inputs such as manure application supply additional nutrients to the crops, improve soil physical structure, and improve soil microbial structures responsible for nutrient solubilizations. Significant increases in grain Zn due to application of FYM have been observed for maize in India (Goteti et al., 2013) and in Zimbabwe (Manzeke et al., 2014). Further discussions related to SOC/SOM are provided earlier.
3.5.5 Zn and Fe application
The response ratios of grain yield and Zn concentrations were significantly higher (p < 0.0001) with Zn application relative to the NPK control (Fig. 9; Online Resource 2 Table S2). Compared to either soil or foliar, a combination of soil + foliar application of Zn (i.e., Combined) resulted in the highest increases in grain yield (34%), and grain-Zn (55%) and grain-Fe (60%) concentrations in maize grain relative to the control. Foliar application is particularly effective on alkaline soils, increasing grain Zn by 59% (Fig. 10). Responses to both foliar and soil applications were also slightly better on soils with low to medium soil-available P (Olsen). Our results agree with Joy et al., (2015a) that foliar application of Zn is more effective than soil application at increasing grain-Zn concentration. However, the foliar method would be difficult to deploy by smallholder farmers especially over larger areas, due to the lack of access to machinery for spraying (Joy et al. 2015a). Soil application is associated with residual effects, but these have not been well studied and accounted for. On the other hand, foliar application of Zn has shown benefit of reducing grain-P and phytate concentrations (Cakmak et al., 2008). Zn deficiency and application of P without Zn in a Zn-deficient soil can lead to excessive P uptake and high phytate concentrations in grain (Cakmak et al., 2008; Imran et al., 2016). High phytate to Zn ratio can significantly reduce the bio-availability of Zn to humans (Imran et al., 2016). Overall, these results show that external Zn inputs need to be applied to increase Zn concentrations in maize grain. The application of Fe did not increase response ratios for grain yield or grain Zn, i.e., no difference relative to no application. However, application of Fe increased grain-Fe concentrations.
Forest plots of the response ratios of grain yield a, grain-Zn concentrations b and grain-Fe concentrations c with inoculation, irrigation, tillage, organic matter (OM), Zn application method and Fe application. Horizontal bars represent 95% confidence limits estimated using linear mixed effects models. The red vertical lines represent RR = 1. When the 95% CL encompasses the redline the treatment is not significantly different from the control. Figures on the left and right sides of the horizontal bars represent the marginal means and the number of studies and the total sample sizes (N) in parentheses, respectively
Forest plots of the response ratios of grain-Zn concentration as affected by Zn application mode: a foliar application and b soil application. Horizontal bars represent 95% confidence limits estimated using linear mixed effects models. The red vertical lines represent RR = 1. When the 95% CL encompasses the redline, the treatment is not significantly different from the control. Figures on the left and right sides of the horizontal bars represent the marginal means and the number of studies the total sample sizes (N) in parentheses, respectively. Combined application (foliar + soil) was reported in only 9 studies, and therefore the sample sizes were inadequate to analyse variations with soil factors
3.5.6 N, P and K application rates
Grain-Zn concentration correlated positively (p < 0.001) with P application rates (Table 4). Apart from the concentration, the response ratio of grain Zn correlates positively with N-application rates and negatively with K application rates. On the other hand, application rates of the three macronutrients N, P and K correlate positively with the Fe response ratio. Increases in grain-Zn and-Fe concentrations with increased N application rates in maize have been reported previously (Losak et al., 2011; Xue et al., 2014; Pasley et al., 2019). Root uptake and transport of Zn via chelation with nitrogenous compounds has also been shown to improve proportionately with N-levels and improves deposition of Zn to the seed (Cakmak et al., 2010). However, applying high rates of N is claimed to dilute grain-Zn concentration (Breure et al., 2023) due to increased growth with inadequate supply of Zn, changes in soil solution pH (Losak et al., 2011).
Variable results to P application on grain-Zn concentration have been observed in previous studies. For example, reduced grain-Zn concentration in 20% of cases from 51 studies within a global meta-analysis are observed (Zhang et al., 2021) with possible contributions of the soil P: Zn balance. There is not much data on effects of ratios of Zn with other soil parameters on agronomic gain. On wheat, P application on soils with Olsen P/ZnDTPA >25 had a negative effect on wheat yield (Sacristán et al., 2019), while at soil P levels below 11.6 mg kg−1, P application increased Zn remobilization from vegetative parts to grains and the Zn harvest index (Hui et al., 2019). On the other hand, application of high P levels on Zn-poor soils can induce Zn deficiency (Sacristán et al., 2019; 2020; Zhang et al., 2021). Thus, P application increases grain-Zn concentration and, management resulting in a high P: Zn ratio is undesirable (significant negative correlation). These results emphasize the need for optimizing nutrient management for improving Zn and Fe concentrations in maize grain.
Increased Zn and Fe application rates increase response ratios of grain Zn. However, a high rate of Zn reduces overall grain-Fe content.
Our analyses above are on agronomic factors moderating response ratios based on Zn applications but evaluations independent of Zn application are available. For example, the review of Manzeke-Kangara et al. (2023) show increased maize grain Zn and Fe concentrations of at least 33% under intercropping relative to monocultures, and > 100% due to organic inputs, and wheat grain Zn increase under reduced tillage of 33% compared to conventional tillage, yet no evidence of tillage effects on maize grain Zn. Variable results due to other agronomic management practices have been observed e.g., in a global analysis comparing N application effects on crop nutritional quality by Zhao et al. (2022), effects of cultivars (Guo et al. 2020), or microbial inoculations (Goteti et al. 2013).
3.6 Biophysical factors moderating response ratio
Grain-Zn and -Fe concentrations were positively (p < 0.001) correlated with the grain nitrogen and total protein, while also grain Zn was correlated with grain P concentration (Table 4). This indicates factors that increase grain Zn and Fe can also result in increases in other quality parameters. In the following passages, we will discuss the factors identified by the different analyses (correlations, mixed-effect models) and the mechanisms by which they affect responses in terms of grain yield, grain-Zn and -Fe concentrations.
Soil factors such as soil pH, and calcium carbonate (CaCO3) and organic matter levels influence Zn and Fe availability for plant uptake (Manzeke-Kangara et al., 2021; Zhao et al., 2014). Antagonistic interactions of soil Zn to P and Fe restrict Zn uptake even when the soil is not deficient (Losak et al., 2011; Rietra et al., 2017). From our study, the key soil parameters correlating with grain-Zn concentrations and response ratios are clay content, SOC, soil Zn and available P (Table 4 and Online Resource 2 Table S2). Benefits of Zn application are higher (P < 0.01) on medium and coarse textured soils but not under fine textured soil relative to the NPK control (Fig. 10). However, the differences in response ratios among soil texture classes were not statistically significant (P < 0.05).
Positive effects of SOC in increasing grain Zn is consistent with earlier analyses (e.g., Wood & Baudron, 2018; Gashu et al., 2021; Breure et al., 2023). Wood et al. (2018) showed increasing SOC by 1% was associated with an increase in Zn equivalent to the needs of 0.2 additional people per hectare (Wood et al., 2018). Zn and Fe are often less available in soils with low organic matter content (Mengel and Kirkby, 2001) and practices that increase soil organic matter (e.g., application of manure, crop residues) may be essential to availability of these nutrients and increase Zn concentrations in grain.
The relationships between grain-Fe concentrations and soil-P concentrations do not follow conventional parametric models (Online Resource 1 Fig. S5). Trends using LOESS smoothing show that grain-Zn concentrations remained flat below 25 mg kg−1 when Olsen P or the P: Zn ratios exceed 20 (Online Resource 1 Fig. S3). Grain-Zn concentrations increased with soil-available P levels of up to 10 mg kg−1, then decline rapidly and remained flat after 15 mg P kg−1 (Online Resouce 1 Fig. S5). Zn deficiency in crops has been associated with high levels of available P in soils (Hui et al., 2019; Sacristán et al., 2019; 2020). In winter wheat, grain-Zn concentration decreased with increasing available soil P in a linear-plus-plateau manner (Hui et al., 2019); the grain-Zn plateau reaching at 22.4 mg kg−1 at an available soil P of 14.2 mg kg−1 (Hui et al., 2019). The P–Zn antagonism has often been explained by the suppression of Zn uptake by roots (Zhang et al. 2021).
Grain yield, Zn and Fe concentrations of the grain as well as the response ratio of Zn were significantly positively correlated with soil-available Zn. Similarly, Botoman et al., (2022) found correlations between grain-Zn concentrations and the Zn concentration in the soil in Malawi. Grain-Fe concentrations increase with soil Zn content of up to 3 mg kg−1 and then decline rapidly afterwards (Online Resource 1 Fig. S5). Soil Zn determines the Zn concentration in grains (Cakmak & Kutman, 2018) and cultivating cereal in Zn-deficient soils can decrease grain Zn many-fold (Bhatt et al., 2020).
Forest plots of the response ratios of grain yield a, grain-Zn concentration b and grain-Fe concentration c with soil parent material, texture and pH. Horizontal bars represent 95% confidence limits estimated using linear mixed effects models. The red vertical lines represent RR = 1. When the 95% CL encompasses the redline, the treatment is not significantly different from the control. Figures on the left and right sides of the horizontal bars represent the marginal means and the number of studies (total sample sizes in parentheses), respectively. Grain-Fe data were not available on acidic soils
Grain-Fe concentration was significantly positively-correlated with soil pH (Table 4), although the pH range available for this analysis was small. The acidic and alkaline pH classes did not have adequate number of studies and sample sizes (Fig. 11). For maize grain yields, there was significantly higher response to Zn application on acidic and neutral than on alkaline soils (relative to NPK control). In the mixed-model analysis, response ratios of grain-Zn concentration (unlike grain yield) were significantly higher on alkaline soils (70%), and similarly, displayed a high grain-Fe response (92%) (Fig. 11b, c). This implies that application of Zn is required on soils of all pH classes. Zn and Fe are often reported to be less available in soils with high pH and high levels of calcium carbonates (CaCO3) (Mengel and Kirkby, 2001; Yamabi & Imai, 2002; Alloway, 2009). As a result, high-pH soils are associated with Zn deficiencies.
As with P: Zn ratio, grain-Zn concentration increases with soil Fe: Zn ratios of up to 20 and then declines rapidly afterwards (Online Resource 1 Fig. S5). Under Zn deficiency, an increased Fe to Zn ratio results in increased Fe uptake, because Fe predominantly occupies the metal transporters involved in both Zn2 + and Fe2 + uptake (Shinozaki & Yoshimoto, 2021). Fe concentration in maize grain was positively correlated with Fe concentration in the soil, as expected (Table 4) and as reported elsewhere (Mielki et al., 2016; Bevis & Hestrin., 2021).
Positive correlations of both grain-Zn and-Fe response ratios to soil-extractable K indicates the need for K especially for increasing Fe response. However, as shown earlier, high K application rate can decrease grain-Zn concentrations (negative effect). In maize, availability of K can counteract P-induced Zn deficiency (Stukenholtz et al., 1966) as it facilitates phytosidephore production, which increases Zn uptake (Hughes et al., 1992).
The differences for grain yield between soil parent materials were not statistically significant, but the response ratio of grain-Zn and -Fe concentrations were significantly higher on calcareous and intermediate parent material (Fig. 10). For example, relative to the NPK control, Zn application on calcareous soil increased Zn- and Fe concentrations by 44 and 43%, respectively. This is probably because Zn and Fe deficiencies are likely to occur in calcareous soils with high pH (Mengel & Kirkby, 2001; Alloway, 2009; Cakmak et al., 2010). Also, calcareous soils are less sensitive to P-induced Zn deficiency (Sacristán et al., 2019).
3.7 Key soil and management factors moderating maize zinc concentrations
In the principal component analysis, the first three principal components (PC1) explained 40.1% of the total variance, while PC2 and PC3 together explained 30.3% of the variance in the response ratio of grain-Zn concentrations (Fig. 12). In PC1, soil pH, SOC, P rate, N rate, soil available K and soil Zn were the most important variables influencing grain-Zn concentrations. SOC and soil Zn had negative loading, while all of the others had positive loadings. In PC2, soil available K and soil Zn (both with positive loading) were the key variables. In PC3, Olsen P had the highest positive loading followed by soil Zn with negative loading. Bartlett’s sphericity test on the var-covariance matrix indicated significant departure from spherical distribution (χ2 = 336.9; df = 35; P < 0.0001), and therefore the PCA applied here was deemed to provide a useful reduction in dimensionality.
Principal component biplots of standardized values of soil pH, SOC, Olsen P, available K, Zn, N rate and P rate and response ratios of grain-Zn concentrations classified as below average and above average based on their empirical distributions. The ellipses represent the 95% confidence bounds of the principal components
4 Data gaps, limitations and priorities for research and policy
Significant data gaps exist in relation to micronutrient content and antinutritional factors in maize grain, which limited our analysis to only Zn and Fe contents. For example, data are virtually lacking on concentrations of other micronutrients such as Se, and antinutritional factors such as phytate, which reduces Zn bioavailability to humans (Ekpa et al., 2018). Availability of such data could increase our understanding of how maize grain phytate content can be reduced through agronomic interventions. Combined application of P and Zn has been reported to increase Zn bioavailability to humans through a reduced grain phytate: Zn ratio (Imran et al., 2016), while Zn foliar application has shown benefit in reducing P and phytate levels (Cakmak et al., 2008). These only imply the potential of agronomic practices to improve Zn bio-availability, but more research is required.
The optimum Zn concentration in soil required for increasing grain-Zn concentration may differ from the soil concentrations required for high yields. Liu et al. (2017) showed that the optimal soil DTPA-Zn concentration was 4.7 mg kg−1 to attain high yield, but 7.6 mg kg−1 required to attain high Zn concentration in grains. Our study has focused on increasing grain Zn and Fe relative to that observed with NPK control and only few cases pushed the concentrations beyond the recommended targets.
Antagonistic interactions among soil nutrients are known to restrict uptake by crops and some insights have been provided. Beyond soils, an important priority area for research is Fe and Zn bioavailability in maize products, and the interactions between Fe and Zn with Cu, Ca and phytate and their influence on absorption. Such studies are required to better understand the underlying mechanisms.
There is an urgent need to determine environment and genotypic factors that govern variations in grain-Fe concentration and its bioavailability. The bioavailability of Fe in maize is known to be very low, largely offsetting any gains that might be made through breeding (Pixley et al., 2011). Therefore, in the short-term agronomic biofortification may provide an avenue for increasing Fe bioavailability. However, there is a dearth of information in this area.
The evidence from the present study and the literature suggests that application of Zn results in increased grain-Zn concentration (Joy et al., 2015a). The same authors reviewed the potential of Zn-enriched fertilizers to alleviate human dietary Zn deficiency and reported that 18% increase in maize-grain Zn concentration can be achieved by soil application of Zn-enriched fertilizers. Such information has great implication for national fertilizer recommendations, and also policies and price support mechanisms for the Zn fertilizer.
One of the key limitations of this review was our inability to perform analysis of genetic gain. For example, we could not quantify genetic gains because many of the studies did not compare diverse varieties and management. We only found 21 studies, and even where comparisons of genotypes exist, information on the varietal characters was not reported in full and we were unable to conduct combined analyses. Our analysis of the association of factors was also limited by incomplete reporting of important background information in the primary studies. For example, out of the studies that qualified for inclusion in this meta-analysis 76, 69, 66, 45, 41, 35 and 22% did not report the N, Fe, clay, potassium, P, SOC and Zn concentrations and pH of the soil, respectively. As a result, the effect of these variables on the response ratios could only be explored using a smaller sample size. This is a missed opportunity. Out of the studies that reported soil pH and soil available P, 31% and 17% did not report the method of pH measurement and P extraction. All this indicates that it is important to standardize the research protocols for such type of studies. In addition, most data came from short-term studies lasting 1–3 years; long-term studies (> 5 years) were uncommon. Consequently, the long-term changes in response could not be analyzed. Therefore, we recommend monitoring of changes in Zn and Fe concentrations in the grain and soil where long-term trials are underway.
A growing body of work (e.g., Bevis & Hestrin, 2021; Gashu et al., 2021; Watts et al., 2019; Joy et al., 2015b) suggests correlations between grain nutrient concentrations and soil properties such as pH and SOC as well as temperature and topographic index. But these studies also show existence of wide spatial variations e.g. for grain Zn concentrations in Ethiopia and Malawi (Gashu et al. 2021), for different grain mineral concentrations in Uganda (Bevis & Hestrin 2021) and for Zn and Fe in Tanzania and Kenya (Watts et al. 2019) The large variations imply that any attempt to combine treatment effects from multiple, geographically disparate studies, e.g., our meta-analysis, can create confounding effects, and the results should be interpreted with caution.
Significant data gaps also exist in terms of grain nutrient concentrations for most regions of the world, agroecological zones and soil types where maize is cultivated. The review also revealed regional imbalances in the representativeness of studies, with India and Pakistan constituting the majority of studies. The African region was only represented by a limited number of countries although maize is the staple crop in most African countries. While grain yield is routinely measured in almost all studies, the quality parameters including micronutrient content of grain are rarely measured. We strongly recommend routine measurement and reporting of grain-Zn and-Fe contents.
In terms of study design and measurement, uniform and consistent approaches have not been followed. For example, very few studies have measured both Zn and Fe contents of grains with and without Zn or Fe application. This makes meta-analysis of primary studies challenging. Therefore, we recommend development of a standardizing sampling and reporting framework for capturing produce quality data as part of the experimental design in agronomy and breeding programs.
5 Conclusions
This study had provided novel insights into the distribution of attainable grain-Zn and-Fe concentration relative to benchmark nutrient concentration targets, and opportunities for achieving those targets. The empirical distributions and attainable values reported here may be used as baselines for future improvements through either breeding program or agronomic biofortification. It is concluded that through agronomic biofortification it is possible to increase the Zn and Fe concentrations of maize grain by 32% and 31%, respectively, relative to NPK fertilizer alone. It is also concluded that combinations of soil and foliar Zn applications gives substantial increases in grain-Zn concentrations than either soil or foliar application. The results also suggest that the Fe concentration of maize grain can be increased through Zn application. It is further concluded that the Zn concentrations of maize grain concomitantly increase with the increase in grain yield and Fe, protein and P concentrations in the grain. The correlation and regression analyses identified some factors associated with grain-Zn and-Fe concentrations. However, in many cases the relationships were non-linear and hence some predictors turned up non-significant in the correlation analysis. Therefore, agronomists should be cautious about the possible nonlinear effects of variables and interaction among predictor variables when analyzing predictors of grain micronutrient concentrations.
Our meta-analysis highlights the following recommendations:
-
1.
There is need for optimizing nutrient management for improving Zn and Fe concentrations in maize grain
-
2.
Increasing soil organic matter (e.g., application of manure, crop residues) may be essential to increase Zn and Fe concentrations in grain.
-
3.
Zn application is required on soils of all pH classes.
-
4.
Significant data gaps exist in relation to micronutrient content of maize including effects on Zn bioavailability to humans, antagonistic interactions among soil nutrients and the underlying mechanisms.
-
5.
Research protocols must be standardized for more comprehensive and meaningful comparisons, including standardizing sampling frameworks.
-
6.
We recommend monitoring changes in Zn and Fe concentrations in the grain and soil where long-term trials are underway.
Data availability statement
The datasets used for the current study are available online (https://doi.org/10.7910/DVN/SOAWL6.v) and accessible on request.
Code is not applicable.
References
USDA. (1993). Soil Survey Manual Handbook 18. United 606 States Department of Agriculture. Soil Survey Division Staff. Soil Conservation Service.
FAOSTAT. (2023). FAOSTAT database. Food and Agriculture. Organization of the. United Nations.
Abreu, C. A., van Raij, B., Gabe, Ú., Abreu, M. F., & Paz-González, A. (2002). Efficiency of multinutrient extractants for the determining of available zinc in soils. Communications in Soil Science and Plant Analysis,33(15–18), 3313–3324. https://doi.org/10.1081/CSS-120014525
Ahsan, A. K., Tebha, S. S., Sangi, R., Kamran, A., Zaidi, Z. A., Haque, T., & Ali Hamza, M. S. (2021). Zinc micronutrient deficiency and its prevalence in malnourished pediatric children as compared to well-nourished children: a nutritional emergency. Global Pediatric Health,8, 2333794X211050316. https://doi.org/10.1177/2333794X211050316
Aiqing, Z., Zhang, L., Ning, P., Chen, Q., Wang, B., Zhang, F., Yang, X., & Zhang, Y. (2022). Zinc in cereal grains: Concentration, distribution, speciation, bioavailability, and barriers to transport from roots to grains in wheat. Critical Reviews in Food Science and Nutrition,62(28), 7917–7928. https://doi.org/10.1080/10408398.2021.1920883
Akhtar, S., Osthoff, G., Mashingaidze, K., & Labuschagne, M. (2018). Iron and zinc in maize in the developing world: deficiency, availability, and breeding. Crop Science,58(6), 2200–2213. https://doi.org/10.2135/cropsci2018.02.0133
Al-Hadeethi, I., Li, Y., Odhafa, A. K. H., Al-Hadeethi, H., Seneweera, S., & Lam, S. K. (2019). Assessment of grain quality in terms of functional group response to elevated [CO2], water, and nitrogen using a meta-analysis: Grain protein, zinc, and iron under future climate. Ecology and Evolution,9(13), 7425–7437. https://doi.org/10.1002/ece3.5210
Alloway, B. J. (2009). Soil factors associated with zinc deficiency in crops and humans. Environmental Geochemistry and Health,31(5), 537–548. https://doi.org/10.1007/s10653-009-9255-4
Bagci, S. A., Ekiz, H., Yilmaz, A., & Cakmak, I. (2007). Effects of zinc deficiency and drought on grain yield of field-grown wheat cultivars in Central Anatolia. Journal of Agronomy and Crop Science,193(3), 198–206. https://doi.org/10.1111/j.1439-037X.2007.00256.x
Bailey, R. L., West, K. P., Jr., & Black, R. E. (2015). The epidemiology of global micronutrient deficiencies. Annals of Nutrition and Metabolism,66, 22–33. https://doi.org/10.1159/000371618
Basche, A., & DeLonge, M. (2017). The impact of continuous living cover on soil hydrologic properties: A meta-analysis. Soil Science Society of America Journal,81(5), 1179–1190. https://doi.org/10.2136/sssaj2017.03.0077
Beach, R. H., Sulser, T. B., Crimmins, A., Cenacchi, N., Cole, J., Fukagawa, N. K., Mason-D’Croz, D., Myers, S., Sarofim, M. C., Smith, W., & Ziska, H. Z. (2019). Combining the effects of increased atmospheric carbon dioxide on protein, iron, and zinc availability and projected climate change on global diets: a modelling study. Lancet Planet Health,3, e307–e317. https://doi.org/10.1016/S2542-5196(19)30094-4
Beal, T., Massiot, E., Arsenault, J. E., Smith, M. R., & Hijmans, R. J. (2017). Global trends in dietary micronutrient supplies and estimated prevalence of inadequate intakes. PloS one,12(4), e0175554. https://doi.org/10.1371/journal.pone.0175554
Bevis, L. E., & Hestrin, R. (2021). Widespread heterogeneity in staple crop mineral concentration in Uganda partially driven by soil characteristics. Environmental Geochemistry and Health,43, 1867–1889. https://doi.org/10.1007/s10653-020-00698-w
Bhatt, R., Hossain, A., & Sharma, P. (2020). Zinc biofortification as an innovative technology to alleviate the zinc deficiency in human health: A review. Open Agriculture,5(1), 176–187. https://doi.org/10.1515/opag-2020-0018
Boonchuay, P., Cakmak, I., Rerkasem, B., & Prom-U-Thai, C. (2013). Effect of different foliar zinc application at different growth stages on seed zinc concentration and its impact on seedling vigor in rice. Soil science and plant nutrition,59(2), 180–188. https://doi.org/10.1080/00380768.2013.763382
Botoman, L., Chagumaira, C., Mossa, A. W., Amede, T., Ander, E. L., Bailey, E. H., & Nalivata, P. C. (2022). Soil and landscape factors influence geospatial variation in maize grain zinc concentration in Malawi. Scientific reportsReports,12(1), 7986. https://doi.org/10.1038/s41598-022-12014-w
Bouis, H. E., Hotz, C., McClafferty, B., Meenakshi, J. V., & Pfeiffer, W. H. (2011). Biofortification: A new tool to reduce micronutrient malnutrition. Food and Nutrition Bulletin,32(1_suppl1), S31–S40. https://doi.org/10.1177/15648265110321S105
Breure, M. S., Njoroge, S., Pasley, H. R., & Hoffland, E. (2023). Exploring options for increasing maize yields and grain Zn concentrations in sub-Saharan Africa. Plant and Soil,488, 625–6361. https://doi.org/10.1007/s11104-023-05998-5
Breure, M. S., Van Eynde, E., Kempen, B., Comans, R. N., & Hoffland, E. (2022). Transfer functions for phosphorus and potassium soil tests and implications for the QUEFTS model. Geoderma,406, 115458. https://doi.org/10.1016/j.geoderma.2021.115458
Cakmak, I. (2008). Enrichment of cereal grains with zinc: agronomic or genetic biofortification? Plant and soil,302, 1–17. https://doi.org/10.1007/s11104-008-9584-6
Cakmak, I., Kalayci, M., Kaya, Y., Torun, A. A., Aydin, N., Wang, Y., Arisoy, Z., Erdem, H., Yazici, A., Gokmen, O., Ozturk, L., & Horst, W. J. (2010). Biofortification and Localization of Zinc in Wheat Grain. Journal of Agricultural and Food Chemistry,58(16), 9092–9102. https://doi.org/10.1021/jf101197h
Cakmak, I., & Kutman, U. B. (2018). Agronomic biofortification of cereals with zinc: A review. European Journal of Soil Science,69(1), 172–180. https://doi.org/10.1111/ejss.12437
Chadare, F. J., Affonfere, M., Aidé, E. S., Fassinou, F. K., Salako, K. V., Pereko, K., Deme, B., Failler, P., Kakaï, R. L. G., & Assogbadjo, A. E. (2022). Current state of nutrition in West Africa and projections to 2030. Global Food Security,32, 100602. https://doi.org/10.1016/j.gfs.2021.100602
Chattha, M. U., Ali, H., Chattha, M. U., Hassan, M. U., Chattha, M. B., Nawaz, M., & Hussain, S. (2017). Biofortification of wheat cultivars to combat zinc deficiency. Frontiers in Plant Sciences,8, 281. https://doi.org/10.3389/fpls.2017.00281
Chen, G., Taylor, P. A., Haller, S. P., Kircanski, K., Stoddard, J., Pine, D. S., Leibenluft, E., Brotman, M. A., & Cox, R. W. (2018). Intraclass correlation: Improved modeling approaches and applications for neuroimaging. Human Brain Mapping,39(3), 1187–1206. https://doi.org/10.1002/hbm.23909
Chen, Y., Zhou, T., Zhang, C., Wang, K., Liu, J., Lu, J., & Xu, K. (2015). Rational phosphorus application facilitates the sustainability of the wheat/maize/soybean relay strip intercropping system. PLoS One,10(11), e0141725. https://doi.org/10.1371/journal.pone.0141725
Dickinson, N., Rankin, J., Pollard, M., Maleta, K., Robertson, C., & Hursthouse, A. (2014). Evaluating environmental and social influences on iron and zinc status of pregnant subsistence farmers in two geographically contrasting regions of Southern Malawi. Science of The Total Environment. https://doi.org/10.1016/j.scitotenv.2014.08.087
Dwivedi, S. L., Sahrawat, K. L., Rai, K. N., Blair, M. W., Andersson, M. S., and Pfeiffer, W. (2012). Nutritionally Enhanced Staple Food Crops. In J. Janick (Ed.), Plant Breeding Reviews (pp. 169–291). John Wiley and Sons, Inc. https://doi.org/10.1002/9781118358566.ch3
Ekpa, O., Palacios-Rojas, N., Kruseman, G., Fogliano, V., & Linnemann, A. R. (2018). Sub-Saharan African maize-based foods: technological perspectives to increase the food and nutrition security impacts of maize breeding programmes. Global food security,17, 48–56. https://doi.org/10.1016/j.gfs.2018.03.007
Erenstein, O., Jaleta, M., Sonder, K., Mottaleb, K., & Prasanna, B. M. (2022). Global maize production, consumption and trade: Trends and R&D implications. Food Security,14(5), 1295–1319. https://doi.org/10.1007/s12571-022-01288-7
García-Marco, S., Gómez-Rey, M. X., & González-Prieto, S. J. (2014). Availability and uptake of trace elements in a forage rotation under conservation and plough tillage. Soil and Tillage Research,137, 33–42. https://doi.org/10.1016/j.still.2013.11.001
Garg, M., Sharma, N., Sharma, S., Kapoor, P., Kumar, A., Chunduri, V., & Arora, P. (2018). Biofortified crops generated by breeding, agronomy, and transgenic approaches are improving lives of millions of people around the world. Frontiers in Nutrition. https://doi.org/10.3389/fnut.2018.00012
Gashu, D., Nalivata, P. C., Amede, T., Ander, E. L., Bailey, E. H., Botoman, L., Chagumaira, C., Gameda, S., et al. (2021). The nutritional quality of cereals varies geospatially in Ethiopia and Malawi. Nature,594, 71–76. https://doi.org/10.1038/s41586-021-03559-2
Gibson, R. S., Bailey, K. B., Gibbs, M., & Ferguson, E. L. (2010). A review of phytate, iron, zinc, and calcium concentrations in plant-based complementary foods used in low-income countries and implications for bioavailability. Food and nutrition bulletin,31(2_suppl2), S134–S146. https://doi.org/10.1177/15648265100312S206
Goredema-Matongera, N., Ndhlela, T., Magorokosho, C., Kamutando, C. N., van Biljon, A., & Labuschagne, M. (2021). Multinutrient biofortification of maize (Zea mays L.) in Africa: current status, opportunities and limitations. Nutrients,13(3), 1039. https://doi.org/10.3390/NU13031039
Goteti, P. K., Emmanuel, L. D. A., Desai, S., & Shaik, M. H. A. (2013). Prospective Zinc Solubilising Bacteria for Enhanced Nutrient Uptake and Growth Promotion in Maize (Zea mays L.). International Journal of Microbiology,2013, 1–7. https://doi.org/10.1155/2013/869697
Gray, J. M., Humphreys, G. S., & Deckers, J. A. (2011). Distribution patterns of World Reference Base soil groups relative to soil forming factors. Geoderma,160(3–4), 373–383. https://doi.org/10.1016/j.geoderma.2010.10.006
Guo, S., Chen, Y., Chen, X., Chen, Y., Yang, L., Wang, L., Qin, Y., Li, M., Chen, F., Mi, G., Gu, R., & Yuan, L. (2020). Grain Mineral Accumulation Changes in Chinese Maize Cultivars Released in Different Decades and the Responses to Nitrogen Fertilizer. Frontiers in Plant Science,10, 1662. https://doi.org/10.3389/fpls.2019.01662
Gurevitch, J., & Hedges, L. V. (1999). Statistical issues in ecological meta-analyses. Ecology,80, 1142–1149. https://doi.org/10.2307/177061
Haddad, N., El, Choukri, H., Ghanem, M. E., Smouni, A., Mentag, R., Rajendran, K., Hejjaoui, K., Maalouf, F., & Kumar, S. (2022). High-temperature and drought stress effects on growth, yield and nutritional quality with transpiration response to vapor pressure deficit in lentil. Plants,11(1), 95. https://doi.org/10.3390/plants11010095
Han, X., Ding, S., Lu, J., & Li, Y. (2022). Global, regional, and national burdens of common micronutrient deficiencies from 1990 to 2019: A secondary trend analysis based on the Global Burden of Disease 2019 study. EClinicalMedicine. https://doi.org/10.1016/j.eclinm.2022.101299
Hughes, D. F., Jolley, V. D., & Brown, J. C. (1992). Roles for potassium in the iron-stress response mechanisms of strategy I and strategy II plants. Journal of Plant Nutrition,15(10), 1821–1839. https://doi.org/10.1080/01904169209364442
Hui, X., Luo, L., Wang, S., Cao, H., Huang, M., Shi, M., Malhi, S. S., & Wang, Z. (2019). Critical concentration of available soil phosphorus for grain yield and zinc nutrition of winter wheat in a zinc-deficient calcareous soil. Plant and Soil,444(1), 315–330. https://doi.org/10.1007/s11104-019-04273-w
Hussain, S., Maqsood, M. A., Rengel, Z., Aziz, T. (2012). Biofortification and estimated human bioavailability of zinc in wheat grains as influenced by methods of zinc application. Plant and Soil, 361(1–2), 279–290. https://doi.org/10.1007/s11104-012-1217-4
Hussain, A., Zahir, Z. A., Asghar, N. H., Imran, M., Ahmad, M., & Hussain, S. (2020). Integrating the potential of Bacillus sp. Az6 and organic waste for zinc oxide bio-activation to improve growth, yield and zinc content of maize grains. Pakistan Journal of Agricultural Sciences. https://doi.org/10.21162/PAKJAS/20.8015
Imran, M., Rehim, A., Sarwar, N., & Hussain, S. (2016). Zinc bioavailability in maize grains in response of phosphorous–zinc interaction. Journal of Plant Nutrition and Soil Science,179(1), 60–66. https://doi.org/10.1002/jpln.201500441
Jolliffe, I. T., & Cadima, J. (2016). Principal component analysis: A review and recent developments. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences,374(2065), 20150202. https://doi.org/10.1098/rsta.2015.0202
Joy, E. J., Stein, A. J., Young, S. D., Ander, E. L., Watts, M. J., & Broadley, M. R. (2015a). Zinc-enriched fertilisers as a potential public health intervention in Africa. Plant and Soil, 389, 1–24. https://doi.org/10.1007/s11104-015-2430-8
Joy, E. J. M., Broadley, M. R., Young, S. D., Black, C. R., Chilimba, A. D. C., Ander, E. L., Barlow, T. S., & Watts, M. J. (2015b). Soil type influences crop mineral composition in Malawi. Science of The Total Environment, 505, 587–595. https://doi.org/10.1016/j.scitotenv.2014.10.038
Khalid, N., Ahmed, A., Bhatti, M. S., Randhawa, M. A., Ahmad, A., & Rafaqat, R. (2014). A Question Mark on Zinc Deficiency in 185 Million People in Pakistan—Possible Way Out. Critical Reviews in Food Science and Nutrition,54(9), 1222–1240. https://doi.org/10.1080/10408398.2011.630541
Khan, S. T., Malik, A., Alwarthan, A., & Shaik, M. R. (2022). The enormity of the zinc deficiency problem and available solutions; an overview. Arabian Journal of Chemistry, 15(3), 103668. https://doi.org/10.1016/j.arabjc.2021.103668.
Kihara, J., Bolo, P., Kinyua, M., Rurinda, J., & Piikki, K. (2020). Micronutrient deficiencies in African soils and the human nutritional nexus: Opportunities with staple crops. Environmental Geochemistry and Health,42(9), 3015–3033. https://doi.org/10.1007/s10653-019-00499-w
Kihara, J., Sileshi, G. W., Nziguheba, G., Kinyua, M., Zingore, S., & Sommer, R. (2017). Application of secondary nutrients and micronutrients increases crop yields in sub-Saharan Africa. Agronomy for Sustainable Development,37, 1–14. https://doi.org/10.1007/s13593-017-0431-0
Koutsos, T. M., Menexes, G. C., & Dordas, C. A. (2019). An efficient framework for conducting systematic literature reviews in agricultural sciences. Science of The Total Environment,682, 106–117. https://doi.org/10.1016/j.scitotenv.2019.04.354
Krebs, N. F., Miller, L. V., & Hambidge, M. (2014). Zinc deficiency in infants and children: a review of its complex and synergistic interactions. Paediatrics and international child health,34(4), 279–288. https://doi.org/10.1179/2046905514Y.0000000151
Krzywinski, M., & Altman, N. (2014). Visualizing samples with box plots. Nature methods,11(2), 119–120. https://doi.org/10.1038/nmeth.2813
Kumar, A., Choudhary, A. K., Pooniya, V., Suri, V. K., & Singh, U. (2016). Soil factors associated with micronutrient acquisition in crops- biofortification perspective. In U. Singh, C. S. Praharaj, S. S. Singh, & N. P. Singh (Eds.), Biofortification of Food Crops (pp. 159–176). Springer India. https://doi.org/10.1007/978-81-322-2716-8_13
Kumssa, D. B., Joy, E. J., Ander, E. L., Watts, M. J., Young, S. D., Walker, S., & Broadley, M. R. (2015). Dietary calcium and zinc deficiency risks are decreasing but remain prevalent. Scientific reports,5(1), 10974. https://doi.org/10.1038/srep10974
Lierop, W. V. (1981). Conversion of organic soil pH values measured in water, 0.01 M CaCl2 or 1 N KCl. Canadian Journal of Soil Science,61(4), 577–579. https://doi.org/10.4141/cjss81-067
Liu, D. Y., Zhang, W., Yan, P., Chen, X. P., Zhang, F. S., & Zou, C. Q. (2017). Soil application of zinc fertilizer could achieve high yield and high grain zinc concentration in maize. Plant and soil,411, 47–55. https://doi.org/10.1007/s11104-016-3105-9
Loladze, I. (2014). Hidden shift of the ionome of plants exposed to elevated CO2 depletes minerals at the base of human nutrition. Elife,3, e02245. https://doi.org/10.7554/eLife.02245
Losak, T., Hlusek, J., Martinec, J., Jandak, J., Szostkova, M., Filipcik, R., Manasek, J., Prokes, K., Peterka, J., Varga, L., Ducsay, L., Orosz, F., & Martensson, A. (2011). Nitrogen fertilization does not affect micronutrient uptake in grain maize (Zea mays L). Acta Agriculturae Scandinavica Section B - Soil & Plant Science,61(6), 543–550. https://doi.org/10.1080/09064710.2010.520729
Mallikarjuna, M. G., Thirunavukkarasu, N., Sharma, R., Shiriga, K., Hossain, F., Bhat, J. S., & Gupta, H. S. (2020). Comparative transcriptome analysis of iron and zinc deficiency in maize (Zea mays L). Plants,9(12), 1812. https://doi.org/10.3390/plants9121812
Manzeke, G. M., Mtambanengwe, F., Nezomba, H., & Mapfumo, P. (2014). Zinc fertilization influence on maize productivity and grain nutritional quality under integrated soil fertility management in Zimbabwe. Field Crops Research,166, 128–136. https://doi.org/10.1016/j.fcr.2014.05.019
Manzeke, M. G., Mtambanengwe, F., Watts, M. J., Hamilton, E. M., Lark, R. M., Broadley, M. R., & Mapfumo, P. (2019). Fertilizer management and soil type influence grain zinc and iron concentration under contrasting smallholder cropping systems in Zimbabwe. Scientific Reports,9(1), 6445. https://doi.org/10.1038/s41598-019-42828-0
Manzeke-Kangara, M. G., Joy, E. J. M., Lark, R. M., Redfern, S., Eilander, A., & Broadley, M. R. (2023). Do agronomic approaches aligned to regenerative agriculture improve the micronutrient concentrations of edible portions of crops? A scoping review of evidence. Frontiers in Nutrition,10, 1078667. https://doi.org/10.3389/fnut.2023.1078667
Manzeke-Kangara, M. G., Mtambanengwe, F., Watts, M. J., Broadley, M. R., Lark, R. M., & Mapfumo, P. (2021). Can nitrogen fertilizer management improve grain iron concentration of agro-biofortified crops in Zimbabwe? Agronomy,11(1), 124. https://doi.org/10.3390/agronomy11010124
McDowell, R. W., Noble, A., Pletnyakov, P., & Haygarth, P. M. (2013). A global database of soil plant available phosphorus. Scientific Data, 10(1), 125. https://doi.org/10.1038/s41597-023-02022-4
Mengel, K., & Kirkby, E. A. (2001). Principles of Plant Nutrition (5th ed.). Dordrecht: Kluwer Academic.
Mielki, G. F., Novais, R. F., Ker, J. C., Vergütz, L., de Castro, G. F. (2016). Iron availability in tropical soils and iron uptake by plants. Revista Brasileira de Ciência do Solo, 40(0). https://doi.org/10.1590/18069657rbcs20150174
Moreno-Lora, A., Sousa-Ortega, C., Recena, R., Perea-Torres, F., & Delgado, A. (2023). Microbial inoculants improve nutrients uptake and yield of durum wheat in calcareous soils under drought stress in the Mediterranean region. Archives of Agronomy and Soil Science,69(11), 2233–2247. https://doi.org/10.1080/03650340.2022.2146099
Myers, S. S., Zanobetti, A., Kloog, I., Huybers, P., Leakey, A. D. B., Bloom, A., et al. (2014). Increasing CO2 threatens human nutrition. Nature,510, 139–142. https://doi.org/10.1038/nature13179
O’Brien, P. L., & Hatfield, J. L. (2019). Dairy Manure and Synthetic Fertilizer: A Meta-Analysis of Crop Production and Environmental Quality. Agroecosystems Geosciences & Environment,2(1), 1–12. https://doi.org/10.2134/age2019.04.0027
Pasley, H. R., Cairns, J. E., Camberato, J. J., & Vyn, T. J. (2019). Nitrogen fertilizer rate increases plant uptake and soil availability of essential nutrients in continuous maize production in Kenya and Zimbabwe. Nutrient cycling in agroecosystems,115, 373–389. https://doi.org/10.1007/s10705-019-10016-1
Pixley, K. V., Palacios-Rojas, N., & Glahn, R. P. (2011). The usefulness of iron bioavailability as a target trait for breeding maize (Zea mays L.) with enhanced nutritional value. Field Crops Research,123(2), 153–160. https://doi.org/10.1016/j.fcr.2011.05.011
Pribyl, D. W. (2010). A critical review of the conventional SOC to SOM conversion factor. Geoderma,156(3–4), 75–83. https://doi.org/10.1016/j.geoderma.2010.02.003
Ramzan, Y., Hafeez, M. B., Khan, S., Nadeem, M., Batool, S., & Ahmad, J. (2020). Biofortification with zinc and iron improves the grain quality and yield of wheat crop. International Journal of Plant Production,14, 501–510. https://doi.org/10.1007/s42106-020-00100-w
Rengel, Z., Batten, G. D., & Crowley, D. D. (1999). Agronomic approaches for improving the micronutrient density in edible portions of field crops. Field crops research,60(1–2), 27–40. https://doi.org/10.1016/S0378-4290(98)00131-2
Rethlefsen, M., Ayala, A. P., Kirtley, S., Koffel, J., & Waffenschmidt, S. (2019). PRISMA-S: PRISMA Search Reporting Extension. https://doi.org/10.3390/agriculture11040310
Rietra, R. P., Heinen, M., Dimkpa, C. O., & Bindraban, P. S. (2017). Effects of nutrient antagonism and synergism on yield and fertilizer use efficiency. Communications in soil science and plant analysis,48(16), 1895–1920. https://doi.org/10.1080/00103624.2017.1407429
Rosner, B. (1983). Percentage points for a generalized ESD many-outlier procedure. Technometrics,25, 165–172.
Sacristán, D., González-Guzmán, A., Torrent, J., & Campillo, M. C. (2020). Optimum Olsen Phosphorus/Zinc DTPA ratio for the initial growth of maize in agricultural soils of the Mediterranean region. Journal of the Science of Food and Agriculture, 101(7), 3056–3064. https://doi.org/10.1002/jsfa.10940
Sacristán, D., González–Guzmán, A., Barrón, V., Torrent, J., & Del Campillo, M. C. (2019). Phosphorus-induced zinc deficiency in wheat pot-grown on noncalcareous and calcareous soils of different properties. Archives of Agronomy and Soil Science,65(2), 208–223. https://doi.org/10.1080/03650340.2018.1492714
Saha, S., Mandal, B., Hazra, G. C., Dey, A., Chakraborty, M., Adhikari, B., & Sadhukhan, R. (2015). Can agronomic biofortification of zinc be benign for iron in cereals? Journal of Cereal Science,65, 186–191. https://doi.org/10.1016/j.jcs.2015.06.007
Sahrawat, K. L., Wani, S. P., Pardhasaradhi, G., & Murthy, K. V. S. (2010). Diagnosis of secondary and micronutrient deficiencies and their management in rainfed agroecosystems: case study from Indian semi-arid tropics. Communications in Soil Science and Plant Analysis,41(3), 346–360. https://doi.org/10.1080/00103620903462340
Sandstead, H. H., & Freeland-Graves, J. H. (2014). Dietary phytate, zinc and hidden zinc deficiency. Journal of Trace Elements in Medicine and Biology,28(4), 414–417. https://doi.org/10.1016/j.jtemb.2014.08.011
Shinozaki, D., & Yoshimoto, K. (2021). Autophagy balances the zinc–iron seesaw caused by Zn-stress. Trends in Plant Science,26(9), 882–884. https://doi.org/10.1016/j.tplants.2021.06.014
Sileshi, G., Akinnifesi, F. K., Ajayi, O. C., & Place, F. (2008). Meta-analysis of maize yield response to woody and herbaceous legumes in sub-Saharan Africa. Plant and Soil,307(1–2), 1–19. https://doi.org/10.1007/s11104-008-9547-y
Sileshi, G. W., & Gebeyehu, S. (2021). Emerging infectious diseases threatening food security and economies in Africa. Global Food Security,28, 100479. https://doi.org/10.1016/j.gfs.2020.100479
Simić, M., Dragičević, V., Mladenović Drinić, S., Vukadinović, J., Kresović, B., Tabaković, M., & Brankov, M. (2020). The contribution of soil tillage and nitrogen rate to the quality of maize grain. Agronomy,10(7), 976. https://doi.org/10.3390/agronomy10070976
Spilke, J., Piepho, H. P., & Hu, X. (2005). Analysis of unbalanced data by mixed linear models using the MIXED procedure of the SAS system. Journal of Agronomy and crop science,191(1), 47–54. https://doi.org/10.1111/j.1439-037X.2004.00120.x
Spitzer, M., Wildenhain, J., Rappsilber, J., & Tyers, M. (2014). BoxPlotR: a web tool for generation of box plots. Nature methods,11(2), 121–122. https://doi.org/10.1038/nmeth.2811
Stanislawska-Glubiak, E., & Korzeniowska, J. (2009). Concentration of micronutrients in pea and lupin plants depending on the soil tillage system. Journal of Elementology,14(2), 357–364.
Stein, A. J. (2014). Rethinking the measurement of undernutrition in a broader health context: Should we look at possible causes or actual effects? Global Food Security,3(3–4), 193–199. https://doi.org/10.1016/j.gfs.2014.09.003
Steinfurth, K., Hirte, J., Morel, C., & Buczko, U. (2021). Conversion equations between Olsen-P and other methods used to assess plant available soil phosphorus in Europe–A review. Geoderma,401, 115339. https://doi.org/10.1016/j.geoderma.2021.115339
Stukenholtz, D. D., Olsen, R. J., Gogan, G., & Olson, R. A. (1966). On the mechanism of phosphorus-zinc interaction in corn nutrition. Soil Science Society of America Journal,30(6), 759–763. https://doi.org/10.2136/sssaj1966.03615995003000060029x
Tang, X., Ma, Y., Hao, X., Li, X., Li, J., Huang, S., & Yang, X. (2009). Determining critical values of soil Olsen-P for maize and winter wheat from long-term experiments in China. Plant and Soil,323, 143–151. https://doi.org/10.1007/s11104-009-9919-y
Thapa, R., Mirsky, S. B., & Tully, K. L. (2018). Cover crops reduce nitrate leaching in agroecosystems: A global meta-analysis. Journal of Environmental Quality,47(6), 1400–1411. https://doi.org/10.2134/jeq2018.03.0107
Van Eynde, E., Breure, M. S., Chikowo, R., Njoroge, S., Comans, R. N., & Hoffland, E. (2023). Soil zinc fertilisation does not increase maize yields in 17 out of 19 sites in sub-Saharan Africa but improves nutritional maize quality in most sites. Plant and Soil. https://doi.org/10.1007/s11104-023-06050-2
Velu, G., Guzman, C., Mondal, S., Autrique, J. E., Huerta, J., & Singh, R. P. (2016). Effect of drought and elevated temperature on grain zinc and iron concentrations in CIMMYT spring wheat. Journal of Cereal Science,69, 182–186. https://doi.org/10.1016/j.jcs.2016.03.006
Wand, M. P. (1997). Data-based choice of histogram bin width. The American Statistician,51(1), 59–64. https://doi.org/10.1080/00031305.1997.10473591
Wang, M. C., & Bushman, B. J. (1998). Using the normal quantile plot to explore meta-analytic data sets. Psychological Methods,3(1), 46.
Wang, M., Gao, H., Wang, J., Cao, C., Ying, X., Wei, Y., Yu, Z., Shao, J., Dong, H., & Yang, M. (2022). Global burden and inequality of iron deficiency: Findings from the Global Burden of Disease datasets 1990–2017. Nutrition Journal,21(1), 16. https://doi.org/10.1186/s12937-022-00771-3
Wang, M., Kong, F., Liu, R., Fan, Q., & Zhang, X. (2020). Zinc in wheat grain, processing, and food. Frontiers in nutrition,7, 124. https://doi.org/10.3389/fnut.2020.00124
Wasaya, A., Tahir, M., Yasir, T. A., Akram, M., Farooq, O., & Sarwar, N. (2018). Soil physical properties, nitrogen uptake and grain quality of maize (Zea mays L.) as affected by tillage systems and nitrogen application. Italian Journal of Agronomy,13(4), 324–331. https://doi.org/10.4081/ija.2018.1197
Watts, M. J., Middleton, D. R., Marriott, A. L., Humphrey, O. S., Hamilton, E. M., Gardner, A., Smith, M., Mccormack, A. V., Menya, D., Munishi, O. M., Mmbaga, T. B., & Osano, O. (2019). Source apportionment of micronutrients in the diets of Kilimanjaro, Tanzania and Counties of Western Kenya. Scientific reports,9(1), 14447. https://doi.org/10.1038/s41598-019-51075-2
Wessells, K. R., & Brown, K. H. (2012). Estimating the global prevalence of zinc deficiency: results based on zinc availability in national food supplies and the prevalence of stunting. PloS one,7(11), e50568. https://doi.org/10.1371/journal.pone.0050568
White, C. R. (2003). Allometric analysis beyond heterogeneous regression slopes: use of the Johnson-Neyman technique in comparative biology. Physiological and Biochemical Zoology,76(1), 135–140. https://doi.org/10.1086/367939
Wood, S. A., Tirfessa, D., & Baudron, F. (2018). Soil organic matter underlies crop nutritional quality and productivity in smallholder agriculture. Agriculture Ecosystems & Environment,266, 100–108. https://doi.org/10.1016/j.agee.2018.07.025
Wright, A. L., Hons, F. M., Lemon, R. G., McFarland, M. L., & Nichols, R. L. (2007). Stratification of nutrients in soil for different tillage regimes and cotton rotations. Soil and Tillage Research,96(1–2), 19–27. https://doi.org/10.1016/j.still.2007.02.005
Xu, D., Shen, Z., Dou, C., Dou, Z., Li, Y., Gao, Y., & Sun, Q. (2022). Effects of soil properties on heavy metal bioavailability and accumulation in crop grains under different farmland use patterns. Scientific Reports,12(1), 9211. https://doi.org/10.1038/s41598-022-13140-1
Xue, Y., Yue, S., Zhang, W., Liu, D., Cui, Z., Chen, X., Ye, Y., & Zou, C. (2014). Zinc, Iron, Manganese and Copper Uptake Requirement in Response to Nitrogen Supply and the Increased Grain Yield of Summer Maize. Plos One,9(4), e93895. https://doi.org/10.1371/journal.pone.0093895
Yamabi, S., & Imai, H. (2002). Growth conditions for wurtzite zinc oxide films in aqueous solutions. Journal of materials chemistry,12(12), 3773–3778. https://doi.org/10.1039/b205384e
Zhang, W., Zhang, W., Wang, X., Liu, D., Zou, C., & Chen, X. (2021). Quantitative evaluation of the grain zinc in cereal crops caused by phosphorus fertilization. A meta-analysis. Agronomy for Sustainable Development,41, 1–12. https://doi.org/10.1007/s13593-020-00661-0
Zhao, A. Q., Tian, X. H., Cao, Y. X., Lu, X. C., & Liu, T. (2014). Comparison of soil and foliar zinc application for enhancing grain zinc content of wheat when grown on potentially zinc-deficient calcareous soils. Journal of the Science of Food and Agriculture,94(10), 2016–2022. https://doi.org/10.1002/jsfa.6518
Zhao, Q., Cao, W., Chen, X., Stomph, T. J., & Zou, C. (2022). Global analysis of nitrogen fertilization effects on grain zinc and iron of major cereal crops. Global Food Security,33, 100631. https://doi.org/10.1016/j.gfs.2022.100631
Acknowledgement
This work is part of the CGIAR Research Initiative on Excellence in Agronomy. We would like to acknowledge all funders who supported this research through their contributions to the CGIAR Trust Fund. The authors also acknowledge Vincent Johnson, consultant editor for the Alliance science writing service, for his editorial review.
Funding
This study was supported by the Bill and Melinda Gates Foundation through the Excellence in Agronomy (EiA) initiative of the One CGIAR.
Author information
Authors and Affiliations
Contributions
Conceptualization and design: Job Kihara for conceptualization; Sileshi Gudeta for design
Data collection, synthesis and curation: Peter Bolo; Dominic Mutambu, Andrew Sila, Job Kihara, Sileshi Gudeta
Methodology, technical review and writing: Sileshi Gudeta; Job Kihara; Senthilkumar Kalimuthu, Kazuki Saito, Mina Devkota
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflicts of interest/Competing interests
The authors have no conflicts of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kihara, J., Sileshi, G.W., Bolo, P. et al. Maize-grain zinc and iron concentrations as influenced by agronomic management and biophysical factors: a meta-analysis. Food Sec. (2024). https://doi.org/10.1007/s12571-024-01478-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12571-024-01478-5