Abstract
Background
To retrospectively analyze the risk factors of liver metastases in patients with gastric cancer in a single center, and to establish a Nomogram prediction model to predict the occurrence of liver metastases.
Methods
A total of 96 patients with gastric cancer who were also diagnosed with liver metastasis (GCLM) and treated in our center from January 1, 2010 to December 31, 2020 were included. The clinical data of 1095 patients with gastric cancer who were diagnosed without liver metastases (GC) in our hospital from January 1, 2014 to December 31, 2017 were retrospectively compared by univariate and multivariate logistic regression. 309 patients diagnosed with gastric cancer in another medical center from January 1, 2014 to December 31, 2018 were introduced as external validation cohorts.
Results
Based on the training cohort, multivariate analysis revealed that tumor site (OR = 0.55, P = 0.046), N stage (OR = 4.95, P = 0.004), gender (OR = 0.04, P = 0.001), OPNI (OR = 0.95, P = 0.041), CEA (OR = 1.01, P = 0.018), CA724 (OR = 1.01, P = 0.006), CA242 (OR = 1.01, P = 0.006), WBC (OR = 1.13, P = 0.024), Hb (OR = 0.98, P < 0.001) were independent risk factors for liver metastasis in patients with gastric cancer, and Nomogram was established based on this analysis (C-statistics = 0.911, 95%CI 0.880–0.958), and the C-statistics of the external validation cohorts achieved 0.926. ROC analysis and decision curve analysis (DCA) revealed that the nomogram provided superior diagnostic value than single variety.
Conclusions
By innovatively introducing a new tumor location classification method, systemic inflammatory response indicators such as NLR and PLR, and nutritional index OPNI, the risk factors of gastric cancer liver metastasis were determined and a predictive Nomogram model was established, which can provide clinical prediction for patients with gastric cancer liver metastasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
With the deepening of the research on the occurrence and development of gastric cancer, the diagnosis and treatment of gastric cancer has been significantly improved. The treatment of gastric cancer has changed from local surgery to a comprehensive treatment based on surgery, combined with chemotherapy, radiotherapy and targeted therapy, as well as recently emerged immunotherapy. However, even so, the overall prognosis of patients with gastric cancer is still unsatisfactory in the world, and its incidence is increasing year by year [1]. The mortality rate of gastric cancer patients ranks third among all tumors, and the 5-year survival rate is only 40% or so [2]. The main reason is that the disease of most patients is already in the advanced stage or even late stage at the time of diagnosis, especially for patients with distant metastasis, who missed the best time of treatment. The liver is one of the common target organs for distant metastasis of gastric cancer [3]. In China, more than 80% of new cases have reached the advanced stage once they are diagnosed [4]. Compared with other organs, the liver is more likely to develop metastasis in gastric cancer patients, which accounts for 45% of distant organ metastases [5]. According to some studies, the incidence of liver metastases from gastric cancer ranged from 4.0% to 17.0%, and most patients lost their opportunity for radical surgical resection once being diagnosed with liver metastases [6]. The prognosis for patients with gastric cancer liver metastases is extremely poor, and the 5-year survival rate is less than 10.0% [7], which is as poor as lung, bone, brain and peritoneal metastasis. Considering the frequency of liver metastasis, which is much higher than other organs, we put emphases on it [8].
Current results have shown that there were limited treatment efficacy once patients were progressed to liver metastases, since their disease is incurable and their life expectancy is dreadfully short. In order to improve the survival of these patients, however, there are more liver-directed treatment strategies are applied to clinical application, such as surgical resection, radiofrequency, chemotherapy, and hepatic artery infusion [9,10,11,12,13]. Compared with radiofrequency ablation or chemotherapy, surgical resection of metastases was identified to be the most effective treatment [14]. An analysis of the resection of liver metastases from gastric cancer in the United Kingdom showed that for patients with liver metastases from gastric cancer, gastric cancer resection and gastric cancer resection combined with liver cancer were performed. The 1-year survival rates for patients who underwent tumor resection were 50% and 64.1%, respectively, which was a significant benefit compared to the 15.4%1-year survival rate for patients without surgery [7].
Following recent advances in treatment options for malignant diseases, prognoses after treatment of metastatic gastric malignancies have been improved. However, GCLM remains incurable and 3-year survival is not optimistic, due to the lag in the detection of liver metastases. Many GCLM patients were too late to choose beneficial treatments, and there are few articles illustrate the possibility of early prediction. This inspired us to explore the concept of early detection and early prediction of gastric cancer liver metastasis. This paper aims to analyze the independent risk factors of gastric cancer liver metastasis according to the differences of clinical indicators between patients with gastric cancer liver metastasis and patients with simple gastric cancer, and establish a prediction model based on the risk factors for gastric cancer liver metastasis. We also introduced external validation cohorts to verify the accuracy and applicability of the prediction model.
2 Materials and methods
2.1 Study population
According to the Helsinki Declaration, this study was registered at.
ResearchRegistry.com (the research registration unique identifying number was Reviewregistry8500, https://www.researchregistry.com/browse-the-registry#home/registrationdetails/637bba06d2fef700217a62d8/. This is a retrospective cohort study.
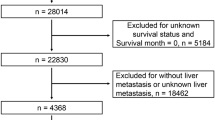
96 patients with gastric cancer with liver metastasis (GCLM) diagnosed and treated in our center from January 1, 2010 to December 31, 2020. And patients without liver metastases which were first diagnosed in our hospital from January 1, 2014 to December 31st, 2017 were also incorporated into this research. There were 1095 cases with no new distant metastasis in 5 years after surgery (follow-up until 2022). According to the normal value of digestive tract tumor indicators in the laboratory of our hospital, CEA was greater than 10 ng/ml, AFP > 10 ng/ml, CA125 > 30.2U/ml, and CA242 > 10U/m, CA199 > 27U/ml, CA724 > 6.9U/ml are regarded as elevated indicators. The basic data of the patients are shown in Table 1. And 309 patients diagnosed with gastric cancer in another medical center from January 1, 2014 to December 31, 2018 were introduced as external validation cohorts. The study was approved by the Ethics Committee of all centers. All procedures performed.
89 in our study were in line with the STROCSS criteria [15].
2.2 Inclusion and exclusion criteria.
Inclusion criteria: Patients’ age when first diagnosed were form 18–85 years-old; meet the diagnostic criteria in ‘National Comprehensive Cancer Network. Gastric cancer, version 2.2013: featured updates to the NCCN Guidelines’ [16], and were diagnosed with gastric cancer by gastroscopic biopsy and pathological examination; patients without liver metastases underwent R0 in our hospital After radical gastrectomy, according to postoperative pathology and imaging examinations, there is no distant space-occupying lesions, and there is no invasion of para-aortic lymph nodes; patients with liver metastases from gastric cancer need to be confirmed by imaging examinations to determine liver space-occupying lesions and undergo puncture or biopsy. Surgical pathological biopsy confirmed liver metastases from gastric cancer. All patients signed informed consent voluntarily. Exclusion criteria: patients without liver metastases at the first diagnosis with distant metastases during the 5-year follow-up period; patients with gastric cancer without liver metastases at the first diagnosis without surgical treatment; patients with neoadjuvant therapy in other hospitals before diagnosis and treatment in our hospital.
2.3 Follow-up and observation indicators
From January 1, 2015 to December 31, 2015, 1191 first-diagnosed patients with gastric cancer in our center and 309 patients diagnosed with gastric cancer in the other center from January 1, 2014 to December 31, 2018 were followed up in 2020 by follow-up staff. Complete follow-up was performed through outpatient reexamination and other means, and there was no new distant metastasis within 5 years. The location of gastric cancer is determined by preoperative imaging localization, intraoperative findings, description of surgical records, and postoperative pathology. According to the local anatomy of the stomach and the distribution of peripheral nerves and lymph nodes, the primary tumor is innovatively divided into: fundus Cardia and lesser curvature of gastric body; greater curvature of gastric body; gastric antrum. Clinicopathological data were evaluated by experienced pathologists, and the T stage, N stage, and differentiation were diagnosed according to the ‘National Comprehensive Cancer Network. Gastric cancer, version 2.2013: featured updates to the NCCN Guidelines’ [16]. CEA, AFP, CA125, CA242, CA199, CA724, routine blood white blood cell count, lymphocyte count, neutrophil count, platelet count, total blood biochemical protein, and serum albumin were all detected before the first diagnosis and treatment. Onodera prognostic nutrition index (OPNI = albumin value g/L + 5 × total number of lymphocytes 10^9L), neutrophil/lymphocyte ratio (NLR) and platelet count/lymphocyte ratio (PLR were all determined by the blood routine and blood biochemical results before admission for the first treatment.
2.4 Statistical analysis
Statistical software SPSS 25.0 (IBM) and R 4.2.1 were used for data analysis. Categorical variables and continuous variables were tested for differences using t test and chi-square test. Continuous variables are presented with Interquartile Range (IQR). The test power and optimal cut-off value of continuous variables such as NLR, OPNI, PLR, CEA, and AFP were determined by ROC curve. R language was used to perform univariate Logistic regression analysis to analyze the risk factors of liver metastases from gastric cancer, and the statistically significant parameters were incorporated into the multivariate Logistic regression model to find independent risk factors for liver metastases from gastric cancer, and the hazard ratio of each risk factor (OR) and 95% confidence intervals (Confidence Intervals, CI) were evaluated. The results of multivariate analysis were visualized by drawing a Nomogram. P < 0.05 was considered a statistically significant difference.
3 Results
3.1 Clinical characteristics of patients
1191 cases of gastric cancer patients in training cohort were first diagnosed with laboratory test indicators such as platelet count, white blood cell count, lymphocyte count, CEA, AFP, CA125 and other tumor indicators. During the 5 years follow-up, there were 1095 cases of GC and 96 cases of GCLM. Among the 309 cases in external validation cohort there were 281 cases of GC and 28 cases of GCLM (see Table 1).
3.2 Identification of independent prognostic factors for GCLM
Logistic univariate regression was performed on clinicopathological indicators, and the statistics found that tumor site (OR = 0.50, P = 0.010), CEA (OR = 1.00, P < 0.001), CA125 (OR = 1.02, P < 0.001), CA242 (OR = 1.02, P < 0.001), CA199 (OR = 1.00, P < 0.001), CA724 (OR = 1.02, P < 0.001), OPNI (OR = 0.90, P < 0.001), N (OR = 5.32, P < 0.001), Age (OR = 1.03, P = 0.012), gender (OR = 0.48, P < 0.001), total protein (OR = 0.97, P = 0.033), WBC (OR = 1.09, P = 0.042), Hb (OR = 0.98, P < 0.001) were all significantly correlated with liver metastasis of gastric cancer. Tumor T stage, AFP, PLR, NLR, RBC and PLT were not associated with liver metastasis of gastric cancer (Table 2). Logistic multivariate regression analysis was performed on the 13 indexes significantly correlated with the occurrence of liver metastasis of gastric cancer in the univariate analysis results. The results showed tumor site (OR = 0.55, P = 0.046), CEA (OR = 1.00, P = 0.018), CA242 (OR = 1.01, P = 0.006), CA724 (OR = 1.01, P = 0.016), OPNI (OR = 0.95, P = 0.041), N stage (OR = 4.95, P = 0.004), gender (OR = 0.40, P = 0.001), WBC (OR = 1.13, P = 0.024), Hb (OR = 0.98, P < 0.001) were independent risk factors for liver metastasis of gastric cancer (see Table 2).
3.3 Development of a prognostic nomogram in the training cohort
According to the univariate and multivariate Logistic regression results, 9 independent risk factors of gastric cancer liver metastasis were shown, and a risk prediction model of gastric cancer liver metastasis based on hematological indicators, demographic information and the primary site of gastric cancer was established. By drawing Nomogram, the risk factors of gastric cancer liver metastasis can be more intuitively displayed. By incorporating the above 9 indicators into R for analysis, a risk diagnosis and prediction model of gastric cancer liver metastasis as shown in Fig. 1 is established. This model lists the scores corresponding to different groups of each independent risk factor. By summing the scores of the factors shown in the figure of gastric cancer patients, the probability of liver metastasis of the gastric cancer patient can be obtained. The higher the total score, the higher the probability of liver metastasis. This gastric cancer patient has a higher risk of developing liver metastases. The C statistic is generally used to evaluate the prediction accuracy of the model. The closer the C index is to 1, the better the prediction effect of the model is. The C statistic of this model is 0.887 (95% CI 0.857–0.916). The Bootstrap resampling method was used for internal verification, and 1,000 samples (with repetitions) of this study were selected and included in the verification cohort. The calibration chart shown in Fig. 1 can be obtained. The closer the prediction curve is to the standard type, the better the prediction ability of the model. The calibration curves in the external validation cohorts were executed based on the nomogram model established above. And achieved a C statistic of 0.914 (95% CI 0.864–0.963).
Nomogram for predicting occurrence of liver metastasis in patients with gastric cancer (GCLM) and calibration curves in the two cohorts. [A, nomogram established by the training cohort, (C = 0.911 95% CI 0.880–0.958); B–C, calibration curves in the training and external validation cohorts, respectively.]
3.4 The performance of the ROC analysis and decision curve analysis (DCA) for predicting the probability of GCLM in the training and external validation cohorts
ROC analysis was built to compare the combined model of nomogram to single risk factors (see Fig. 2). We found that the model which achieved the AUC of 0.887 (CI 0.857–0.916) is more competitive than single variant including OPNI (AUC = 0.648), PLR (AUC = 0.610), NLR (AUC = 0.676), CEA (AUC = 0.685), AFP (AUC = 0.699), CA125 (AUC = 0.695), CA242 (AUC = 0.651), CA199 (AUC = 0.643) and CA724 (AUC = 0.619). The decision curve analysis (DCA) also revealed that the nomogram provided superior diagnostic value than single variety (see Fig. 3).
The receiver operating characteristic (ROC) curve analyses of the nomogram and single risk factors in predicting the occurrence of liver metastasis in patients with gastric cancer (GCLM). [A, B, ROC curve analyses of single risk factors including OPNI, PLR, NLR, CEA, AFP, CA125, CA242, CA199 and CA724; C, D, ROC curve analyses in the training and external validation cohorts.]
Comparison of the accuracy and clinical net benefits among all independent risk factors for prediction of occurrence of liver metastasis in patients with gastric cancer (GCLM). [A, B, decision curve analyses showed the net benefits of nomogram and other factors in predicting GCLM in the training and external validation cohorts.]
4 Discussion
Gastric cancer is a global malignant disease, with more than 1 million new cases every year, and it is the fifth most diagnosed malignant tumor in the world. Because gastric cancer is often asymptomatic in its early stages, the first diagnosis is often at an advanced stage, making it the third most common cause of cancer-related death, accounting for 784,000 deaths worldwide in 2018 [17]. Liver metastasis of gastric cancer refers to the continuous growth of gastric cancer cells, invading surrounding tissues, and returning to the portal vein system through blood vessels to reach the liver, where one or more metastases grow secondary, and these metastases are closely related to the primary tumor. Our work has several new findings, both expected and unexpected, that are noteworthy and discussed in light of existing literature and comparisons. The first is about the primary location of the tumor, which was listed as a suspected risk factor in many retrospective studies, with no statistical significance reached [18]. Metastasis studies have found that in addition to tumor invasion and hematogenous metastasis, there are still lymph node and nerve-promoting metastasis [19,20,21,22,23] pathways. Primary gastric tumors can transfer to the liver by skipping through lymph nodes in groups 7, 8, 9, and 12, or invasion of the vagus nerve. We innovatively divided the primary sites of gastric cancer into three parts: fundus and cardia and lesser curvature of gastric body, greater curvature of gastric body, and gastric antrum. Univariate and multivariate Logistic regression confirmed that they were significantly associated with liver metastasis of gastric cancer. The tumor location can be determined by preoperative CT, MRI, PET-CT or abdominal color Doppler ultrasonography with high accuracy [24, 25], which provides an effective and easy-to-obtain index for the risk assessment of liver metastases from gastric cancer.
Combined with blood test-related indicators, we narrowed the scope of risk factors for gastric cancer liver metastasis to AFP, CEA, CA125, CA242, CA199, CA724 in digestive tract tumor indicators, systemic inflammatory response indicators NLR, PLR, and nutritional indicators OPNI included in the risk factors. The results showed that tumor site, CEA, CA242, CA724, OPNI, N stage, gender, WBC and Hb were independent risk factors for liver metastasis of gastric cancer. This may show that nutrition related factors played a key role in predicting GCLM.
Systemic inflammatory response markers such as NLR and PLR are considered to be effective predictors of survival in patients diagnosed with liver disease and different cancers (eg, cholangiocarcinoma, esophageal cancer, pancreatic cancer, gastric cancer, and colorectal cancer) [26, 27]. Preoperative prediction of lymph node metastasis is of great significance. Nutritional indicators OPNI, also showed an important predictive value for the risk of liver metastases from gastric cancer in this analysis. Hematological oncology indicators CEA, CA242, and CA724 also showed high predictive significance in this prediction model.
There are few predictive models to early forecast the liver metastasis of gastric cancer, thus the construction of such a model is necessary and urgent. Millions of GC patients will benefit from it, and receive instant treatment to guarantee the best clinical profit. Our model introduced relatively comprehensive clinical features and applied external validation queue to verify the accuracy. However, we failed to obtain more detailed pathological information such as tumor histological typing, number of lymph node metastases and immunohistochemical gene targets. Therefore, we are planning a follow-up study to improve it.
5 Conclusion
In conclusion, this multi-center study demonstrated that the nomogram calculators shown good performance in predicting the occurrence of gastric cancer with liver metastasis, which may provide references for personal evaluation strategies in the future.
Data availability
No datasets were generated or analysed during the current study.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30.
Thun MJ, DeLancey JO, Center MM, et al. The global burden of cancer: priorities for prevention. Carcinogenesis. 2010;31(1):100–10.
Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–32.
Hwang JE, Kim SH, Jin J, et al. Combination of percutaneous radiofrequency ablation and systemic chemotherapy are effective treatment modalities for metachronous liver metastases from gastric cancer. Clin Exp Metastasis. 2014;31(1):25–32.
Zhang Y, Lin Y, Duan J, Xu K, Mao M, Wang X. A population-based analysis of distant metastasis in stage IV gastric cancer. Med Sci Monit. 2020;15(26): e923867.
Markar SR, Mackenzie H, Mikhail S, Mughal M, Preston SR, Maynard ND, Faiz O, Hanna GB. Surgical resection of hepatic metastases from gastric cancer: outcomes from national series in England. Gastric Cancer. 2017;20(2):379–86.
Garancini M, Uggeri F, Degrate L, et al. Surgical treatment of liver metastases of gastric cancer: is local treatment in a systemic disease worthwhile? HPB (Oxford). 2012;14(3):209–15.
Qiu MZ, Shi SM, Chen ZH, Yu HE, Sheng H, Jin Y, Wang DS, Wang FH, Li YH, Xie D, Zhou ZW, Yang DJ, Xu RH. Frequency and clinicopathological features of metastasis to liver, lung, bone, and brain from gastric cancer: a SEER-based study. Cancer Med. 2018;7(8):3662–72.
Kakeji Y, Morita M, Maehara Y. Strategies for treating liver metastasis from gastric cancer. Surg Today. 2010;40:287–94.
Kodera Y, Fujitani K, Fukushima N, et al. Surgical resection of hepatic metastasis from gastric cancer: a review and new recommendation in the Japanese gastric cancer treatment guidelines. Gastric Cancer. 2014;17:206–12.
Ojima H, Ootake S, Yokobori T, et al. Treatment of multiple liver metastasis from gastric carcinoma. World J Surg Oncol. 2007;5:70.
Garancini M, Uggeri F, Degrate L, et al. Surgical treatment of liver metastases of gastric cancer: is local treatment in a systemic disease worthwhile? HPB (Oxford). 2012;14:209–15.
Yamakado K, Nakatsuka A, Takaki H, et al. Prospective study of arterial infusion chemotherapy followed by radiofrequency ablation for the treatment of liver metastasis of gastric cancer. J Vasc Interv Radiol. 2005;16:1747–51.
Kerkar SP, Kemp CD, Avital I. Liver resections in metastatic gastric cancer. HPB (Oxford). 2010;12:589–96.
Agha R, Abdall-Razak A, Crossley E, Dowlut N, Iosifidis C, Mathew G. STROCSS Group. STROCSS 2019 guideline: strengthening the reporting of cohort studies in surgery. Int J Surg. 2019;72:156–65.
Ajani JA, Bentrem DJ, Besh S, D’Amico TA, Das P, Denlinger C, Fakih MG, Fuchs CS, Gerdes H, Glasgow RE, Hayman JA, Hofstetter WL, Ilson DH, Keswani RN, Kleinberg LR, Korn WM, Lockhart AC, Meredith K, Mulcahy MF, Orringer MB, Posey JA, Sasson AR, Scott WJ, Strong VE, Varghese TK Jr, Warren G, Washington MK, Willett C, Wright CD, McMillian NR, Sundar H. Gastric cancer, version 22013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2013;11(5):531–46.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Song JC, Ding XL, Zhang Y, Zhang X, Sun XH. Prospective and prognostic factors for hepatic metastasis of gastric carcinoma: a retrospective analysis. J Cancer Res Ther. 2019;15(2):298–304.
Nishiwaki N, Irino T, Fujiya K, Kamiya S, Hikage M, Tanizawa Y, Bando E, Kusafuka K, Terashima M. Extra-nodal metastasis should be classified separately from lymph node metastasis in gastric cancer. Eur J Surg Oncol. 2021;47(5):1055–61.
Yang HJ, Jang JY, Kim SG, et al. Risk factors of lymph node metastasis after non-curative endoscopic resection of undifferentiated-type early gastric cancer. Gastric Cancer. 2020. https://doi.org/10.1007/s10120-020-01103-2.
Kumagai K, Tanaka T, Yamagata K, et al. Liver metastasis in gastric cancer with particular reference to lymphatic advancement. Gastric Cancer. 2001;4(3):150–5.
Aurello P, et al. Influence of perineural invasion in predicting overall survival and disease-free survival in patients with locally advanced gastric cancer. Am J Surg. 2017;213(4):748–53.
Ma C, et al. Kallistatin inhibits lymphangiogenesis and lymphatic metastasis of gastric cancer by downregulating VEGF-C expression and secretion. Gastric Cancer. 2018;21(4):617–31.
Liu J, Qiu J, Wang K, Liu J, Sun X, Zhang J, Wang X, Wei J, Wu B, Wang X, Qin N. An investigation on gastric cancer staging using CT structured report. Eur J Radiol. 2021;136: 109550.
Zhang Y, Yu J. The role of MRI in the diagnosis and treatment of gastric cancer. Diagn Interv Radiol. 2020;26(3):176–82.
Fang T, Wang Y, Yin X, Zhai Z, Zhang Y, Yang Y, You Q, Li Z, Ma Y, Li C, Song H, Shi H, Zhang Y, Yu X, Gao H, Sun Y, Xie R, Xue Y. Diagnostic sensitivity of NLR and PLR in early diagnosis of gastric cancer. J Immunol Res. 2020;7(2020):9146042.
Oyama K, Oba M, Oshima Y, Shimada H. Predicting short-term life expectancy of patients with end-stage gastric cancer using Onodera’s prognostic nutritional index. Int J Clin Oncol. 2021;26(2):364–9.
Acknowledgements
We would like to thank the patients and their families for consenting to tumor acquisition in our study.
Research registration unique identifying number (UIN)
This study was registered at ResearchRegistry.com (the research registration unique identifying number was Reviewregistry8500, https://www.researchregistry.com/browse-the-registry#home/registrationdetails/637bba06d2fef700217a62d8/
Funding
This work was supported by National Natural Science Foundation of China (81970500 XF Shen, 82172645 WX Guan),Natural Science Foundation for Young Scholars of Jiangsu Province (BK20210022) and Nanjing Medical Science and Technology Development Project (YKK21078), Start-up funding for the introduction of talents in Nanjing Drum Tower Hospital (RC2022-015).
Author information
Authors and Affiliations
Contributions
H Y, H J, XF Lu, JF Du, XF Shen and WX Guan conceived the original idea of the study. H Y, H J, XF Lu, CH Bai and P S contributed to sample preparation and carried out the experiment and writing the original draft. H Y, H J, F S and SC Ai conducted all statistical analyses. QY Hu, S L and Y Y contributed to the implementation of the research and interpretation of data. X C,JF Du, XF Shen and WX Guan: Conceptualization, Writing—review & editing, Funding acquisition. All authors reviewed the manuscript,discussed the results, prepared, shaped the research and gave final approval of the manuscript to be published.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the institutional research ethics committee of the affiliated Drum Tower Hospital of Nanjing University Medical School (2017-216-01) and Chinese PLA General Hospital (S2017-147-01). In accordance with the ethics committee’s regulations, informed consent was obtained from patients included in this study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yu, H., Jiang, H., Lu, X. et al. Analysis of risk factors for liver metastasis in patients with gastric cancer and construction of prediction model: A multicenter study. Discov Onc 15, 363 (2024). https://doi.org/10.1007/s12672-024-01246-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-024-01246-z