Abstract
Positron emission tomography/computed tomography (PET/CT) has dramatically altered the landscape of noninvasive glioma evaluation, offering complementary insights to those gained through magnetic resonance imaging (MRI). PET/CT scans enable a multifaceted analysis of glioma biology, supporting clinical applications from grading and differential diagnosis to mapping the full extent of tumors and planning subsequent treatments and evaluations. With a broad array of specialized radiotracers, researchers and clinicians can now probe various biological characteristics of gliomas, such as glucose utilization, cellular proliferation, oxygen deficiency, amino acid trafficking, and reactive astrogliosis. This review aims to provide a recent update on the application of versatile PET/CT radiotracers in glioma research and clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerebral gliomas are the most common form of primary malignant brain tumors, accounting for approximately 80% of such diagnoses [1]. Effective treatment strategies and prediction of patient outcomes are strongly linked to the precise categorization of these tumors, both in terms of histological characteristics and genomic markers. The WHO 2021 update from WHO 2016 incorporated crucial genomic indicators, such as isocitrate dehydrogenase (IDH) mutations, thereby enhancing the classification and prognostic accuracy of these tumors [2, 3]. However, traditional approaches for examining tissue and molecular characteristics typically involve invasive techniques. On the other hand, imaging techniques offer a non-invasive and semi-quantitative alternative, yielding clinically significant data in preoperative settings, which may have important therapeutic implications. [4].
Magnetic resonance imaging (MRI) is currently the gold standard for brain imaging but has its limitations. MRI often faces challenges in clearly distinguishing between low- and high-grade tumors as well as between treatment-induced changes such as radiation necrosis and actual tumor progression [5, 6]. Although it provides limited information about tumor metabolism and molecular features, advanced MRI methods, such as perfusion-weighted imaging, diffusion-weighted imaging, and MR spectroscopy, have been actively investigated in research and clinical settings.
Positron emission tomography/computed tomography (PET/CT) has dramatically changed the landscape of non-invasive evaluation of gliomas. Various PET/CT radiotracers offer insights into a range of biological functions including glucose metabolism, cellular proliferation, amino acid transport, reactive astrogliosis, and hypoxia. PET/CT is invaluable for non-invasive tumor grading, differential diagnosis, prognosis prediction, recurrence evaluation, and monitoring after treatment [5,6,7,8,9,10,11]. To further enhance its transformative role and provide guidance for accurately assessing brain gliomas, the field has introduced standardized imaging protocols of various radiotracers [12,13,14]. This review aimed to provide a comprehensive overview of the PET/CT radiotracers currently used in glioma research and clinical settings. Special focus is placed on radiotracers of clinical and research importance including 18F-fluorodeoxyglucose (18F-FDG), amino acid-based radiotracers, 11C-acetate, 18F-fluorothymidine (18F-FLT), and 18F-fluoromisonidazole (18F-FMISO).
18F-Fluorodeoxyglucose (18F-FDG)
The radiotracer had a relatively long half-life of 110 min, allowing it to be transported from the central cyclotron to nearby locations. Radiosynthesis is a relatively straightforward process. Currently, 18F-FDG is the most commonly utilized radiotracer for PET/CT imaging in clinical oncology and was first used in the early 1980s for brain tumor imaging [15]. Radiotracers are highly effective in identifying rapidly proliferating cells, as these cells show an increased uptake of 18F-FDG. This is largely due to elevated levels of glucose transporters and the enzyme hexokinase, which converts both glucose and 18F-FDG into their phosphorylated form [16]. This makes 18F-FDG particularly useful for distinguishing high-grade gliomas from other gliomas [7]. Typically, more aggressive tumors demonstrate higher levels of 18F-FDG uptake, which has been proven to be a reliable prognostic marker. For example, if a previously identified low-grade tumor starts to show high uptake, it is generally an indicator of the tumor becoming anaplastic [8].
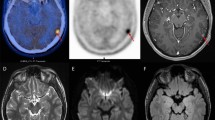
However, recent studies have highlighted the diagnostic limitations of 18F-FDG PET/CT. Non-neoplastic neurological diseases, such as bacterial abscesses, tuberculosis, fungal infections, and sarcoidosis, can mimic the appearance of brain tumors on 18F-FDG PET/CT scans [17]. Due to the naturally elevated levels of glucose metabolism in normal brain tissue, detecting tumors with only moderate increases in glucose metabolism is challenging [18]. This is particularly problematic for low-grade tumors and, in some cases, for recurrent high-grade tumors. The 18F-FDG uptake in low-grade tumors is often similar to that observed in normal white matter, and for high-grade tumors, it might be similar to or even less than the uptake in the normal gray matter. This results in a reduced sensitivity for the detection of tumor lesions. To overcome these limitations, Kim et al. explored the effects of elevated blood glucose levels by administering intravenous glucose before performing 18F-FDG PET/CT [19]. Elevated blood glucose levels led to reduced 18F-FDG uptake in normal brain tissue, thereby enhancing the ability of the scan to detect gliomas with greater sensitivity (Fig. 1). In a different approach, Johnson et al. focused on the timing of 18F-FDG PET scans, demonstrating that a delayed scanning protocol provides superior visibility of glioblastomas compared to conventional timing [20].
a Transaxial 18F-FDG PET, b glucose-loaded 18F-FDG PET, and c contrast-enhanced T1-weighted MRI images in a patient with glioblastoma. Due to the high 18F-FDG uptake in the normal cerebral cortex, the boundaries of the tumor in the para-sagittal area are not clearly distinguishable in the standard 18F-FDG PET scan. However, in the glucose-loaded 18F-FDG PET scan, the 18F-FDG uptake in the normal cerebral cortex is reduced, allowing for a clearer observation of the tumor’s 18F-FDG uptake boundaries
A major change in the conventional histology-driven classification of gliomas is the incorporation of genetic alterations. The molecular parameters outlined in the 2016 Central Nervous System (CNS) WHO classification include mutations in the isocitrate dehydrogenase enzyme isoforms 1 (IDH1) and 2 (IDH2), 1p/19q co-deletion, and H3 K27M mutations. Of these, driver mutations in IDH1 and IDH2 genes are involved in the pathogenesis and progression of gliomas, which are genetically classified into IDH mutant and IDH wild-type forms. Cytosolic IDH1 and mitochondrial IDH2 contribute to the production of nicotinamide adenine dinucleotide phosphate (NADPH) from NADP+ via oxidative decarboxylation of isocitrate to α-ketoglutarate (α-KG) [21, 22]. IDH mutation-induced NADPH reduction affects cellular defense mechanisms against oxidative stress. In addition, the mutations produce an abnormal metabolite known as 2-hydroxyglutarate instead of α-KG which competitively inhibits α-KG–dependent dioxygenases. The resultant genome-wide epigenetic changes predispose cells to malignant transformations. IDH mutations are found in more than 70% of WHO grade 2 and 3 gliomas and fewer than 10% of glioblastomas. Patients with IDH mutations exhibit longer overall survival (OS) than those with wild-type IDH [23, 24]. 18F-FDG PET/CT has been used for in vivo image-guided identification of gliomas with IDH mutations. 18F-FDG uptake in IDH1-mutant gliomas is significantly lower than that in IDH1 wild-type gliomas [8]. While IDH1 mutation was the most important factor in identifying patients with the best prognosis, increased 18F-FDG uptake provided additional prognostic information for predicting poor OS in patients with IDH1 wild-type gliomas.
Amino Acid Radiotracers
Amino acids are taken up by the cells through carrier-mediated processes [25]. This forms the basis for amino acid imaging, as multiple studies have documented an increase in amino acid transport during malignant transformation [26, 27]. In experimental animal models, it has been found that increased amino acid transport into tumor cells is facilitated by the upregulation of amino acid transporters in the blood vessels supporting the brain tumor tissue [28]. Amino acid-based radiotracers are particularly promising for brain tumor imaging. They are taken up more readily by tumors and show minimal uptake in the normal brain, thereby offering a high contrast ratio between the tumor and surrounding normal tissue for tumor delineation [29]. Amino acid radiotracers consistently outperform 18F-FDG in the diagnosis of brain tumors, particularly low-grade tumors [30,31,32].
Among these PET/CT tracers, 11C-methionine is one of the most important and highly useful for imaging L-type amino acid transporter 1 (LAT-1) [33]. It has been used globally in multiple institutions since the 1980s [33, 34]. Tracers are extensively employed in clinical settings to define the boundaries of brain tumors, staging, prognosis prediction, treatment evaluation, and recurrence identification [4, 33, 35,36,37,38]. However, due to 11C’s short half-life of 20 min, 18F-labeled aromatic amino acid analogs have been developed for tumor imaging. Developed in the late 1990s, 18-fluoride-fluoro-ethyl-tyrosine (18F-FET) is an 18F-labeled amino acid PET tracer with a longer half-life of 110 min, making it suitable for routine clinical use [39, 40]. Transport inhibition tests with specific competitive inhibitors have shown that more than 80% of 18F-FET uptake into cancer cells occurs via an L-type transport system [39]. Unlike other radiotracers, 18F-FET is neither incorporated into proteins nor metabolized once it enters the cell and essentially serves as a measure of the amino acid transport rate. Compared to 18F-FDG and 11C-methionine, 18F-FET shows lower uptake in cells related to inflammation, thereby offering greater specificity in distinguishing tumor tissue from inflammation [41,42,43]. Complementing this specificity, Vidmar et al. have demonstrated the effectiveness of 18F-FET PET in distinguishing between treatment-related changes and true progression in glioma patients [44]. In addition to 11C-methionine and 18F-FET, 3,4-dihydroxy-6-[18F]fluoro-l-phenylalanine (18F-FDOPA) is another 18F-labeled compound initially developed for measuring dopamine synthesis and is primarily used for imaging the basal ganglia [45, 46]. It is mainly transported by LAT1 in tumors and can detect both enhancing and non-enhancing tumors [47]. Additionally, its significance in clinical settings is highlighted by the effective detection of glioma recurrence or progression through the use of 18F-FDOPA PET [48].
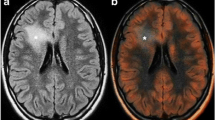
The 2016 World Health Organization (WHO) classification of cerebral gliomas has led to an improved diagnosis of oligoastrocytomas as either astrocytomas or oligodendrogliomas (OD). Gliomas with IDH1 mutations can be sub-classified into two types: those with 1p/19q co-deletion, known as ODs, and those with intact 1p/19q, identified as astrocytomas, leading to better OS in patients with OD [2]. Based on this improved classification, studies have evaluated amino acid radiotracers for the characterization of amino acid uptake in relation to IDH1 mutations and 1p/19q co-deletion status. Overall, IDH1-mutant gliomas show lower amino acid radiotracer uptake than IDH1-wildtype glioma [49,50,51]. However, amino acid radiotracer uptake in ODs is as high as that in glioblastomas, which constitutes a limitation of radiolabeled amino acids in glioma classification (Fig. 2). Therefore, amino acid radiotracer uptake for glioma grading may be more consistent in IDH1-wildtype than in IDH1-mutant tumors.
Transaxial 11C-methionine PET, contrast-enhanced T1-weighted MRI, and contrast-enhanced T2 FLAIR MRI images. a A patient with grade 2 astrocytoma, IDH-mutant. b A patient with a grade 3 oligodendroglioma. c A patient with glioblastoma. 11C-methionine has good sensitivity for detecting low-grade gliomas. However, its uptake in oligodendrogliomas is as high as in glioblastomas, which poses a significant limitation for glioma classification
11C-Acetate
11C-Acetate has long been employed as a radiotracer for cardiac oxidative metabolism by measuring clearance rates through the tricarboxylic acid cycle [52]. In the normal brain, acetate functions as an astrocyte-specific substrate and serves as an alternative energy source to glucose [53,54,55,56]. The primary mechanism of astrocyte transport and utilization of acetate involves monocarboxylate transporter 1 (MCT1) [54, 57, 58]. Astrocytes are highly sensitive to environmental conditions and undergo dynamic shifts in their molecular, functional, and morphological characteristics in response to various physical and chemical stimuli of the CNS. These astrocytes are known as reactive astrocytes, which can be seen in conditions such as Alzheimer’s disease (AD) and Parkinson’s disease (PD), as well as in patients with stroke and glioblastoma patients [59,60,61,62,63]. Recently, Nam et al. reported increased 11C-acetate uptake in reactive astrocytes in animal models of neuroinflammation, as well as in patients with AD [64]. Additionally, there have been two reports on the use of 11C-acetate PET/CT for detecting reactive astrocytes in patients with multiple sclerosis [65, 66].
In tumor imaging, 11C-acetate serves as a valuable radiotracer for identifying acetate-dependent tumors that cannot be detected using 18F-FDG PET/CT. Tumors such as renal cell carcinoma, hepatocellular carcinoma, and well-differentiated prostate cancers exhibit significant 11C-acetate uptake, which is ascribed to enhanced lipid synthesis within these tumors [67,68,69]. In contrast, there are studies implicating acetate as a substrate for lipid metabolism in high-grade gliomas [70, 71]. Using 11C-acetate, studies have suggested increased 11C-acetate uptake in patients with high-grade tumors [72,73,74,75,76]. 11C-Acetate uptake on PET/CT differed significantly between low- and high-grade gliomas and exhibited the capability to further distinguish between grade 3 and grade 4 tumors. 11C-Acetate uptake and metabolic tumor volume on PET/CT are independent prognostic factors and predict survival better than the WHO grade [75]. The high 11C-acetate uptake associated with higher-grade gliomas is inconsistent with the known finding that 11C-acetate is taken up by well-differentiated tumors in the body. This raises the question regarding the cellular origin of 11C-acetate in gliomas. In a recent study, conditioned media collected from the IDH1-wt (but not IDH1-mt) human glioblastoma cell line led to the reactivity of mouse primary astrocytes and high 11C-acetate uptake [77]. In fact, 11C-acetate uptake on PET/CT was discovered to represent reactive astrocytes in the tumor microenvironment (TME) (Fig. 3).
As an important tool to visualize reactive astrogliosis, 11C-acetate PET/CT has shown the potential for glioma grading as an important tool for visualizing reactive astrogliosis [78]. As mentioned, radiolabeled amino acids reported unexpectedly high amino acid radiotracer uptake in ODs, similar to the levels observed in high-grade IDH1-wt tumors. Unlike amino acid radiotracers, high 11C-acetate uptake was associated with high-grade IDH1-wt tumors, thus facilitating differentiation from high-grade IDH1-mt and low-grade gliomas. In particular, the low 11C-acetate uptake in ODs is advantageous for overcoming the limitations of radiolabeled amino acid tracers. In addition, 11C-acetate PET/CT appears to have other potential values in evaluating gliomas. First, reactive astrogliosis harbors cancer stem cells and defines the boundaries of advanced tumors in high-grade gliomas. Therefore, 11C-acetate PET/CT can be used to determine the surgical margins of tumors. Second, studies have reported conflicting roles of reactive astrogliosis in tumor growth, invasion, and treatment resistance in glioblastoma [79]. 11C-Acetate PET/CT can be used as a useful tool to visualize reactive astrogliosis and its effect on tumor growth in vivo following various treatment modifications.
18F-Fluorothymidine (18F-FLT)
18F-FLT was initially identified as a selective inhibitor of DNA synthesis [80]. Because thymidine is found only in DNA, the radiolabeled version is expected to indicate the rate of tissue proliferation [81]. 18F-FLT is transported into cells through either active nucleoside transporters or simple diffusion [82]. Although it does not integrate into DNA strands, it is trapped in the cell after being phosphorylated by thymidine kinase-1 (TK-1). The activity of TK-1 elevates during the cell cycle’s S-phase and is correlated with tumor growth [81, 83]. Even without becoming a part of the DNA, imaging with 18F-FLT demonstrates that cellular uptake occurs, which is related to the levels of Ki-67 expression observed in the resected tumor tissue [84].
For low-grade gliomas, 18F-FLT imaging is generally not considered useful because of the minimal uptake of the radiotracer [85]. Tumors with little or no contrast enhancement on MRI also show minimal 18F-FLT concentrations, consistent with the established correlation between 18F-FLT uptake and contrast enhancement [86, 87]. Generally, high-grade gliomas exhibit high contrast enhancement and 18F-FLT uptake, whereas low-grade gliomas do not. The efficacy of 18F-FLT in distinguishing high- from low-grade tumors has a sensitivity and accuracy of approximately 92% [88].
18F-Fluoromisonidazole (18F-FMISO)
Hypoxia is characterized by insufficient levels of oxygen, which hamper normal biological processes [89]. Hypoxia is a significant adverse factor affecting patient outcomes, particularly in high-grade gliomas [90]. The primary causes of tumor hypoxia include disrupted blood circulation due to structural and/or functional anomalies, along with rapid tumor expansion, which results in elevated oxygen needs not being met by an adequate supply [91]. Evaluating the degree of hypoxia in tumors is critical, both biologically and clinically, as tumors under hypoxic conditions have shown increased resilience to radiation treatment, heightened chemoresistance, and poor postsurgical prognoses [92].
The first radiotracer developed to detect hypoxia was 14C-misonidazole, a beta-emitting agent, introduced in 1981 [93]. This was succeeded by 18F-fluoromisonidazole (18F-FMISO) [94]. 18F-FMISO is a fat-soluble compound belonging to the 2-nitroimidazole class. These compounds enter cells through passive diffusion, and their rates of entry vary based on their fat solubility. When cells are well oxygenated, 18F-FMISO can easily exit the extracellular space. However, the reduction process continues under hypoxic conditions, resulting in its accumulation within cells [95]. Given that the level of hypoxia often correlates with the severity and aggressiveness of a tumor, 18F-FMISO is considered a valuable tool for identifying high-grade gliomas. Research has shown that 18F-FMISO provides a more accurate assessment of the extent of glioblastomas than contrast-enhanced MRI, suggesting its utility in treatment planning [96]. Additionally, hypoxia in the TME triggers the release of factors such as VEGF, which stimulates angiogenesis [97]. Barajas et al. showed that in patients with recurring high-grade gliomas undergoing bevacizumab treatment, there was a noticeable reduction in 18F-FMISO uptake [98]. This finding highlights the potential usefulness of 18F-FMISO for tracking tumor changes during anti-angiogenic therapies.
Conclusion
In summary, the evolving landscape of radiotracers in PET/CT is expanding their diagnostic and prognostic capabilities, particularly in oncology. While 18F-FDG remains a cornerstone, its limitations in cerebral gliomas have paved the way for specialized radiotracers, such as amino acids, 11C-acetate, 18F-FMISO, and 18F-FLT. Amino acid radiotracers are considered the best because of their high tumor-to-cortical background uptake and their ability to show non-enhancing tumors on MRI. The high amino acid uptake in ODs can be a limitation of radiolabeled amino acids in glioma grading. In contrast, 11C-acetate, an astrocyte-specific energy substrate, has significant clinical value in patients with glioma because it allows the visualization of reactive astrocytes in the TME. These advances not only improve diagnostic accuracy but also hold promise for personalized treatment strategies, particularly in patients with glioblastoma. The role of these radiotracers has become even more critical as we move towards more proactive approaches in medicine. Continued research is essential to unlock their full potential in treatment planning and monitoring.
Data Availability
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.
Change history
27 August 2024
A Correction to this paper has been published: https://doi.org/10.1007/s13139-024-00879-w
References
Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2015;17 Suppl 4:iv1-iv62.
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–20.
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23:1231–51.
Singhal T, Narayanan TK, Jacobs MP, Bal C, Mantil JC. 11C-Methionine PET for grading and prognostication in gliomas: a comparison study with 18F-FDG PET and contrast enhancement on MRI. J Nucl Med. 2012;53:1709–15.
Heiss WD. PET in gliomas. Overview of current studies. Nuklearmedizin. 2014;53:163–71; quiz N32.
Dhermain F. Radiotherapy of high-grade gliomas: current standards and new concepts, innovations in imaging and radiotherapy, and new therapeutic approaches. Chin J Cancer. 2014;33:16–24.
Kosaka N, Tsuchida T, Uematsu H, Kimura H, Okazawa H, Itoh H. 18F-FDG PET of common enhancing malignant brain tumors. AJR Am J Roentgenol. 2008;190:W365–9.
Kim D, Kim S, Kim SH, Chang JH, Yun M. Prediction of overall survival based on isocitrate dehydrogenase 1 mutation and 18F-FDG uptake on PET/CT in patients with cerebral gliomas. Clin Nucl Med. 2018;43:311–6.
Cui M, Zorrilla-Veloz RI, Hu J, Guan B, Ma X. Diagnostic accuracy of PET for differentiating true glioma progression from post treatment-related changes: a systematic review and meta-analysis. Front Neurol. 2021;12: 671867.
Treglia G, Muoio B, Trevisi G, Mattoli MV, Albano D, Bertagna F, et al. Diagnostic performance and prognostic value of PET/CT with different tracers for brain tumors: a systematic review of published meta-analyses. Int J Mol Sci. 2019;20:4669.
Ouyang ZQ, Zheng GR, Duan XR, Zhang XR, Ke TF, Bao SS, et al. Diagnostic accuracy of glioma pseudoprogression identification with positron emission tomography imaging: a systematic review and meta-analysis. Quant Imaging Med Surg. 2023;13:4943–59.
Law I, Albert NL, Arbizu J, Boellaard R, Drzezga A, Galldiks N, et al. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [(18)F]FDG: version 1.0. Eur J Nucl Med Mol Imaging. 2019;46:540–57.
Piccardo A, Albert NL, Borgwardt L, Fahey FH, Hargrave D, Galldiks N, et al. Joint EANM/SIOPE/RAPNO practice guidelines/SNMMI procedure standards for imaging of paediatric gliomas using PET with radiolabelled amino acids and [(18)F]FDG: version 1.0. Eur J Nucl Med Mol Imaging. 2022;49:3852–69.
Guedj E, Varrone A, Boellaard R, Albert NL, Barthel H, van Berckel B, et al. EANM procedure guidelines for brain PET imaging using [(18)F]FDG, version 3. Eur J Nucl Med Mol Imaging. 2022;49:632–51.
Di Chiro G, DeLaPaz RL, Brooks RA, Sokoloff L, Kornblith PL, Smith BH, et al. Glucose utilization of cerebral gliomas measured by [18F] fluorodeoxyglucose and positron emission tomography. Neurology. 1982;32:1323–9.
Smith TA. The rate-limiting step for tumor [18F]fluoro-2-deoxy-D-glucose (FDG) incorporation. Nucl Med Biol. 2001;28:1–4.
Omuro AM, Leite CC, Mokhtari K, Delattre JY. Pitfalls in the diagnosis of brain tumours. Lancet Neurol. 2006;5:937–48.
Demetriades AK, Almeida AC, Bhangoo RS, Barrington SF. Applications of positron emission tomography in neuro-oncology: a clinical approach. Surgeon. 2014;12:148–57.
Kim D, Ko HY, Lee S, Lee YH, Ryu S, Kim SY, et al. Glucose loading enhances the value of (18)F-FDG PET/CT for the characterization and delineation of cerebral gliomas. Cancers (Basel). 2020;12:1977.
Johnson JM, Chen MM, Rohren EM, Prabhu S, Chasen B, Mawlawi O, et al. Delayed FDG PET provides superior glioblastoma conspicuity compared to conventional image timing. Front Neurol. 2021;12: 740280.
Lee SM, Koh HJ, Park DC, Song BJ, Huh TL, Park JW. Cytosolic NADP(+)-dependent isocitrate dehydrogenase status modulates oxidative damage to cells. Free Radic Biol Med. 2002;32:1185–96.
Pollard PJ, Ratcliffe PJ. Cancer. Puzzling patterns of predisposition. Science. 2009;324:192–4.
Balss J, Meyer J, Mueller W, Korshunov A, Hartmann C, von Deimling A. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol. 2008;116:597–602.
Chen JR, Yao Y, Xu HZ, Qin ZY. Isocitrate dehydrogenase (IDH)1/2 mutations as prognostic markers in patients with glioblastomas. Medicine (Baltimore). 2016;95: e2583.
Jager PL, Vaalburg W, Pruim J, de Vries EG, Langen KJ, Piers DA. Radiolabeled amino acids: basic aspects and clinical applications in oncology. J Nucl Med. 2001;42:432–45.
Isselbacher KJ. Sugar and amino acid transport by cells in culture–differences between normal and malignant cells. N Engl J Med. 1972;286:929–33.
Busch H, Davis JR, Honig GR, Anderson DC, Nair PV, Nyhan WL. The uptake of a variety of amino acids into nuclear proteins of tumors and other tissues. Cancer Res. 1959;19:1030–9.
Miyagawa T, Oku T, Uehara H, Desai R, Beattie B, Tjuvajev J, et al. “Facilitated” amino acid transport is upregulated in brain tumors. J Cereb Blood Flow Metab. 1998;18:500–9.
Kinoshita M, Arita H, Goto T, Okita Y, Isohashi K, Watabe T, et al. A novel PET index, 18F-FDG-11C-methionine uptake decoupling score, reflects glioma cell infiltration. J Nucl Med. 2012;53:1701–8.
Chen W, Silverman DH, Delaloye S, Czernin J, Kamdar N, Pope W, et al. 18F-FDOPA PET imaging of brain tumors: comparison study with 18F-FDG PET and evaluation of diagnostic accuracy. J Nucl Med. 2006;47:904–11.
Lau EW, Drummond KJ, Ware RE, Drummond E, Hogg A, Ryan G, et al. Comparative PET study using F-18 FET and F-18 FDG for the evaluation of patients with suspected brain tumour. J Clin Neurosci. 2010;17:43–9.
Chung JK, Kim YK, Kim SK, Lee YJ, Paek S, Yeo JS, et al. Usefulness of 11C-methionine PET in the evaluation of brain lesions that are hypo- or isometabolic on 18F-FDG PET. Eur J Nucl Med Mol Imaging. 2002;29:176–82.
Glaudemans AW, Enting RH, Heesters MA, Dierckx RA, van Rheenen RW, Walenkamp AM, et al. Value of 11C-methionine PET in imaging brain tumours and metastases. Eur J Nucl Med Mol Imaging. 2013;40:615–35.
Galldiks N, Kracht LW, Dunkl V, Ullrich RT, Vollmar S, Jacobs AH, et al. Imaging of non- or very subtle contrast-enhancing malignant gliomas with [(1)(1)C]-methionine positron emission tomography. Mol Imaging. 2011;10:453–9.
Falk Delgado A, Falk DA. Discrimination between primary low-grade and high-grade glioma with (11)C-methionine PET: a bivariate diagnostic test accuracy meta-analysis. Br J Radiol. 2018;91:20170426.
Mattoli MV, Trevisi G, Scolozzi V, Capotosti A, Cocciolillo F, Marini I, et al. Dynamic (11)C-methionine PET-CT: prognostic factors for disease progression and survival in patients with suspected glioma recurrence. Cancers (Basel). 2021;13:4777.
Bag AK, Wing MN, Sabin ND, Hwang SN, Armstrong GT, Han Y, et al. (11)C-Methionine PET for identification of pediatric high-grade glioma recurrence. J Nucl Med. 2022;63:664–71.
Ninatti G, Sollini M, Bono B, Gozzi N, Fedorov D, Antunovic L, et al. Preoperative [11C]methionine PET to personalize treatment decisions in patients with lower-grade gliomas. Neuro Oncol. 2022;24:1546–56.
Heiss P, Mayer S, Herz M, Wester HJ, Schwaiger M, Senekowitsch-Schmidtke R. Investigation of transport mechanism and uptake kinetics of O-(2-[18F]fluoroethyl)-L-tyrosine in vitro and in vivo. J Nucl Med. 1999;40:1367–73.
Wester HJ, Herz M, Weber W, Heiss P, Senekowitsch-Schmidtke R, Schwaiger M, et al. Synthesis and radiopharmacology of O-(2-[18F]fluoroethyl)-L-tyrosine for tumor imaging. J Nucl Med. 1999;40:205–12.
Salber D, Stoffels G, Pauleit D, Oros-Peusquens AM, Shah NJ, Klauth P, et al. Differential uptake of O-(2–18F-fluoroethyl)-L-tyrosine, L-3H-methionine, and 3H-deoxyglucose in brain abscesses. J Nucl Med. 2007;48:2056–62.
Stober B, Tanase U, Herz M, Seidl C, Schwaiger M, Senekowitsch-Schmidtke R. Differentiation of tumour and inflammation: characterisation of [methyl-3H]methionine (MET) and O-(2-[18F]fluoroethyl)-L-tyrosine (FET) uptake in human tumour and inflammatory cells. Eur J Nucl Med Mol Imaging. 2006;33:932–9.
Dunet V, Pomoni A, Hottinger A, Nicod-Lalonde M, Prior JO. Performance of 18F-FET versus 18F-FDG-PET for the diagnosis and grading of brain tumors: systematic review and meta-analysis. Neuro Oncol. 2016;18:426–34.
Skoblar Vidmar M, Doma A, Smrdel U, Zevnik K, Studen A. The value of FET PET/CT in recurrent glioma with a different IDH mutation status: the relationship between imaging and molecular biomarkers. Int J Mol Sci. 2022;23:6787.
Matsubara K, Watabe H, Kumakura Y, Hayashi T, Endres CJ, Minato K, et al. Sensitivity of kinetic macro parameters to changes in dopamine synthesis, storage, and metabolism: a simulation study for [(1)(8)F]FDOPA PET by a model with detailed dopamine pathway. Synapse. 2011;65:751–62.
Eidelberg D, Takikawa S, Dhawan V, Chaly T, Robeson W, Dahl R, et al. Striatal 18F-dopa uptake: absence of an aging effect. J Cereb Blood Flow Metab. 1993;13:881–8.
Youland RS, Kitange GJ, Peterson TE, Pafundi DH, Ramiscal JA, Pokorny JL, et al. The role of LAT1 in (18)F-DOPA uptake in malignant gliomas. J Neurooncol. 2013;111:11–8.
Zaragori T, Ginet M, Marie PY, Roch V, Grignon R, Gauchotte G, et al. Use of static and dynamic [(18)F]-F-DOPA PET parameters for detecting patients with glioma recurrence or progression. EJNMMI Res. 2020;10:56.
Jansen NL, Schwartz C, Graute V, Eigenbrod S, Lutz J, Egensperger R, et al. Prediction of oligodendroglial histology and LOH 1p/19q using dynamic [(18)F]FET-PET imaging in intracranial WHO grade II and III gliomas. Neuro Oncol. 2012;14:1473–80.
Kim D, Chun JH, Kim SH, Moon JH, Kang SG, Chang JH, et al. Re-evaluation of the diagnostic performance of (11)C-methionine PET/CT according to the 2016 WHO classification of cerebral gliomas. Eur J Nucl Med Mol Imaging. 2019;46:1678–84.
Ponisio MR, McConathy JE, Dahiya SM, Miller-Thomas MM, Rich KM, Salter A, et al. Dynamic (18)F-FDOPA-PET/MRI for the preoperative evaluation of gliomas: correlation with stereotactic histopathology. Neurooncol Pract. 2020;7:656–67.
Pike VW, Eakins MN, Allan RM, Selwyn AP. Preparation of [1-11C]acetate–an agent for the study of myocardial metabolism by positron emission tomography. Int J Appl Radiat Isot. 1982;33:505–12.
Nicklas WJ, Clarke DD. Decarboxylation studies of glutamate, glutamine, and aspartate from brain labelled with [1-14C]acetate, L-[U-14C]-aspartate, and L-[U-14C]glutamate. J Neurochem. 1969;16:549–58.
Waniewski RA, Martin DL. Preferential utilization of acetate by astrocytes is attributable to transport. J Neurosci. 1998;18:5225–33.
Cruz NF, Lasater A, Zielke HR, Dienel GA. Activation of astrocytes in brain of conscious rats during acoustic stimulation: acetate utilization in working brain. J Neurochem. 2005;92:934–47.
Wyss MT, Magistretti PJ, Buck A, Weber B. Labeled acetate as a marker of astrocytic metabolism. J Cereb Blood Flow Metab. 2011;31:1668–74.
Cerdan S, Kunnecke B, Seelig J. Cerebral metabolism of [1,2–13C2]acetate as detected by in vivo and in vitro 13C NMR. J Biol Chem. 1990;265:12916–26.
Hassel B, Sonnewald U, Fonnum F. Glial-neuronal interactions as studied by cerebral metabolism of [2-13C]acetate and [1-13C]glucose: an ex vivo 13C NMR spectroscopic study. J Neurochem. 1995;64:2773–82.
Jo S, Yarishkin O, Hwang YJ, Chun YE, Park M, Woo DH, et al. GABA from reactive astrocytes impairs memory in mouse models of Alzheimer’s disease. Nat Med. 2014;20:886–96.
Chun H, Im H, Kang YJ, Kim Y, Shin JH, Won W, et al. Severe reactive astrocytes precipitate pathological hallmarks of Alzheimer’s disease via H(2)O(2)(-) production. Nat Neurosci. 2020;23:1555–66.
Heo JY, Nam MH, Yoon HH, Kim J, Hwang YJ, Won W, et al. Aberrant tonic inhibition of dopaminergic neuronal activity causes motor symptoms in animal models of Parkinson’s disease. Curr Biol. 2020;30(276–91): e9.
Nam MH, Cho J, Kwon DH, Park JY, Woo J, Lee JM, et al. Excessive astrocytic GABA causes cortical hypometabolism and impedes functional recovery after subcortical stroke. Cell Rep. 2020;32: 107975.
Nagashima G, Suzuki R, Asai J, Fujimoto T. Immunohistochemical analysis of reactive astrocytes around glioblastoma: an immunohistochemical study of postmortem glioblastoma cases. Clin Neurol Neurosurg. 2002;104:125–31.
Nam MH, Ko HY, Kim D, Lee S, Park YM, Hyeon SJ, et al. Visualizing reactive astrocyte-neuron interaction in Alzheimer’s disease using 11C-acetate and 18F-FDG. Brain. 2023;146:2957–74.
Takata K, Kato H, Shimosegawa E, Okuno T, Koda T, Sugimoto T, et al. 11C-Acetate PET imaging in patients with multiple sclerosis. PLoS ONE. 2014;9: e111598.
Kato H, Okuno T, Isohashi K, Koda T, Shimizu M, Mochizuki H, et al. Astrocyte metabolism in multiple sclerosis investigated by 1-C-11 acetate PET. J Cereb Blood Flow Metab. 2021;41:369–79.
Oyama N, Akino H, Kanamaru H, Suzuki Y, Muramoto S, Yonekura Y, et al. 11C-Acetate PET imaging of prostate cancer. J Nucl Med. 2002;43:181–6.
Oyama N, Okazawa H, Kusukawa N, Kaneda T, Miwa Y, Akino H, et al. 11C-Acetate PET imaging for renal cell carcinoma. Eur J Nucl Med Mol Imaging. 2009;36:422–7.
Ho CL, Yu SC, Yeung DW. 11C-Acetate PET imaging in hepatocellular carcinoma and other liver masses. J Nucl Med. 2003;44:213–21.
Mashimo T, Pichumani K, Vemireddy V, Hatanpaa KJ, Singh DK, Sirasanagandla S, et al. Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell. 2014;159:1603–14.
Masui K, Cavenee WK, Mischel PS. mTORC2 and metabolic reprogramming in GBM: at the interface of genetics and environment. Brain Pathol. 2015;25:755–9.
Liu RS, Chang CP, Chu LS, Chu YK, Hsieh HJ, Chang CW, et al. PET imaging of brain astrocytoma with 1–11C-acetate. Eur J Nucl Med Mol Imaging. 2006;33:420–7.
Yamamoto Y, Nishiyama Y, Kimura N, Kameyama R, Kawai N, Hatakeyama T, et al. 11C-Acetate PET in the evaluation of brain glioma: comparison with 11C-methionine and 18F-FDG-PET. Mol Imaging Biol. 2008;10:281–7.
Tsuchida T, Takeuchi H, Okazawa H, Tsujikawa T, Fujibayashi Y. Grading of brain glioma with 1–11C-acetate PET: comparison with 18F-FDG PET. Nucl Med Biol. 2008;35:171–6.
Kim S, Kim D, Kim SH, Park MA, Chang JH, Yun M. The roles of (11)C-acetate PET/CT in predicting tumor differentiation and survival in patients with cerebral glioma. Eur J Nucl Med Mol Imaging. 2018;45:1012–20.
Kim D, Cho A, Hwang SH, Jo K, Chang JH, Yun M. Choroid plexus as the best reference region for standardized uptake value analysis on C11-acetate PET/CT for grading and predicting prognosis in patients with cerebral gliomas. Nucl Med Mol Imaging. 2020;54:274–80.
Kim D, Ko HY, Chung JI, Park YM, Lee S, Kim SY, et al. Visualizing cancer-originating acetate uptake through MCT1 in reactive astrocytes in the glioblastoma tumor microenvironment. Neuro Oncol. 2023. https://doi.org/10.1093/neuonc/noad243.
Kim D, Chun JH, Yi JH, Ko HY, Chung JI, Lee M, et al. 11 C-Acetate PET/CT detects reactive astrogliosis helping glioma classification. Clin Nucl Med. 2022;47:863–8.
Diep YN, Park HJ, Kwon JH, Tran M, Ko HY, Jo H, et al. Astrocytic scar restricting glioblastoma via glutamate-MAO-B activity in glioblastoma-microglia assembloid. Biomater Res. 2023;27:71.
Barthel H, Cleij MC, Collingridge DR, Hutchinson OC, Osman S, He Q, et al. 3′-Deoxy-3′-[18F]fluorothymidine as a new marker for monitoring tumor response to antiproliferative therapy in vivo with positron emission tomography. Cancer Res. 2003;63:3791–8.
Salskov A, Tammisetti VS, Grierson J, Vesselle H. FLT: measuring tumor cell proliferation in vivo with positron emission tomography and 3′-deoxy-3′-[18F]fluorothymidine. Semin Nucl Med. 2007;37:429–39.
Idema AJ, Hoffmann AL, Boogaarts HD, Troost EG, Wesseling P, Heerschap A, et al. 3′-Deoxy-3′-18F-fluorothymidine PET-derived proliferative volume predicts overall survival in high-grade glioma patients. J Nucl Med. 2012;53:1904–10.
Tehrani OS, Shields AF. PET imaging of proliferation with pyrimidines. J Nucl Med. 2013;54:903–12.
Vesselle H, Grierson J, Muzi M, Pugsley JM, Schmidt RA, Rabinowitz P, et al. In vivo validation of 3′deoxy-3′-[(18)F]fluorothymidine ([(18)F]FLT) as a proliferation imaging tracer in humans: correlation of [(18)F]FLT uptake by positron emission tomography with Ki-67 immunohistochemistry and flow cytometry in human lung tumors. Clin Cancer Res. 2002;8:3315–23.
Tripathi M, Sharma R, D’Souza M, Jaimini A, Panwar P, Varshney R, et al. Comparative evaluation of F-18 FDOPA, F-18 FDG, and F-18 FLT-PET/CT for metabolic imaging of low grade gliomas. Clin Nucl Med. 2009;34:878–83.
Shinomiya A, Kawai N, Okada M, Miyake K, Nakamura T, Kushida Y, et al. Evaluation of 3′-deoxy-3′-[18F]-fluorothymidine (18F-FLT) kinetics correlated with thymidine kinase-1 expression and cell proliferation in newly diagnosed gliomas. Eur J Nucl Med Mol Imaging. 2013;40:175–85.
Nowosielski M, DiFranco MD, Putzer D, Seiz M, Recheis W, Jacobs AH, et al. An intra-individual comparison of MRI, [18F]-FET and [18F]-FLT PET in patients with high-grade gliomas. PLoS ONE. 2014;9: e95830.
Ferdova E, Ferda J, Baxa J, Tupy R, Mracek J, Topolcan O, et al. Assessment of grading in newly-diagnosed glioma using 18F-fluorothymidine PET/CT. Anticancer Res. 2015;35:955–9.
Walsh JC, Lebedev A, Aten E, Madsen K, Marciano L, Kolb HC. The clinical importance of assessing tumor hypoxia: relationship of tumor hypoxia to prognosis and therapeutic opportunities. Antioxid Redox Signal. 2014;21:1516–54.
Brown JM. Therapeutic targets in radiotherapy. Int J Radiat Oncol Biol Phys. 2001;49:319–26.
Hirata K, Yamaguchi S, Shiga T, Kuge Y, Tamaki N. The roles of hypoxia imaging using (18)F-fluoromisonidazole positron emission tomography in glioma treatment. J Clin Med. 2019;8:1088.
Hammond EM, Asselin MC, Forster D, O’Connor JP, Senra JM, Williams KJ. The meaning, measurement and modification of hypoxia in the laboratory and the clinic. Clin Oncol (R Coll Radiol). 2014;26:277–88.
Chapman JD, Franko AJ, Sharplin J. A marker for hypoxic cells in tumours with potential clinical applicability. Br J Cancer. 1981;43:546–50.
Jerabek PA, Patrick TB, Kilbourn MR, Dischino DD, Welch MJ. Synthesis and biodistribution of 18F-labeled fluoronitroimidazoles: potential in vivo markers of hypoxic tissue. Int J Rad Appl Instrum A. 1986;37:599–605.
Gronroos T, Bentzen L, Marjamaki P, Murata R, Horsman MR, Keiding S, et al. Comparison of the biodistribution of two hypoxia markers [18F]FETNIM and [18F]FMISO in an experimental mammary carcinoma. Eur J Nucl Med Mol Imaging. 2004;31:513–20.
Collet S, Guillamo JS, Berro DH, Chakhoyan A, Constans JM, Lechapt-Zalcman E, et al. Simultaneous mapping of vasculature, hypoxia, and proliferation using dynamic susceptibility contrast MRI, (18)F-FMISO PET, and (18)F-FLT PET in relation to contrast enhancement in newly diagnosed glioblastoma. J Nucl Med. 2021;62:1349–56.
Reuss AM, Groos D, Buchfelder M, Savaskan N. The acidic brain-glycolytic switch in the microenvironment of malignant glioma. Int J Mol Sci. 2021;22:5518.
Barajas RF Jr, Pampaloni MH, Clarke JL, Seo Y, Savic D, Hawkins RA, et al. Assessing biological response to bevacizumab using 18F-fluoromisonidazole PET/MR imaging in a patient with recurrent anaplastic astrocytoma. Case Rep Radiol. 2015;2015: 731361.
Funding
This study was supported by NRF-2018M3C7A1056898, NRF-2020R1A2B5B01098109, and RS-2022-00144475 from the National Research Foundation (NRF) of Korea to M.Y.
Author information
Authors and Affiliations
Contributions
Dongwoo Kim and Mijin Yun were responsible for the study design and writing the manuscript. The first draft of the manuscript was written by Dongwoo Kim and Mijin Yun, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Dongwoo Kim, Suk-Hyun Lee, Hee Sung Hwang, Sun Jung Kim, and Mijin Yun declare no competing interests..
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to a retrospective Open Access order.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, D., Lee, SH., Hwang, H.S. et al. Recent Update on PET/CT Radiotracers for Imaging Cerebral Glioma. Nucl Med Mol Imaging 58, 237–245 (2024). https://doi.org/10.1007/s13139-024-00847-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13139-024-00847-4