Abstract
Introduction
Clinical Alzheimer’s disease (AD) begins with mild cognitive impairment (MCI) and progresses to mild, moderate, or severe dementia, constituting a disease continuum that eventually leads to death. This study aimed to estimate the probabilities of transitions across those disease states.
Methods
We developed a mixed-effects multi-state Markov model to estimate the transition probabilities, adjusted for 5 baseline covariates, using the Health and Retirement Study (HRS) database. HRS surveys older adults in the United States bi-annually. Alzheimer states were defined using the modified Telephone Interview of Cognitive Status (TICS-m).
Results
A total of 11,292 AD patients were analyzed. Patients were 70.8 ± 9.0 years old, 54.9% female, and with 12.0 ± 3.3 years of education. Within 1 year from the initial state, the model estimated a higher probability of transition to the next AD state in earlier disease: 12.8% from MCI to mild AD and 5.0% from mild to moderate AD, but < 1% from moderate to severe AD. After 10 years, the probability of transition to the next state was markedly higher for all states, but still higher in earlier disease: 29.8% from MCI to mild AD, 23.5% from mild to moderate AD, and 5.7% from moderate to severe AD. Across all AD states, the probability of transition to death was < 5% after 1 year and > 15% after 10 years. Older age, fewer years of education, unemployment, and nursing home stay were associated with a higher risk of disease progression (p < 0.01).
Conclusions
This analysis shows that the risk of progression is greater in earlier AD states, increases over time, and is higher in patients who are older, with fewer years of education, unemployed, or in a nursing home at baseline. The estimated transition probabilities can provide guidance for future disease management and clinical trial design optimization, and can be used to refine existing cost-effectiveness frameworks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Transition probabilities from mild cognitive impairment (MCI) through Alzheimer’s disease (AD) severity states until death are currently not well characterized for the United States (US). |
We analyzed patients from the Health and Retirement Study (HRS), a nationally representative sample of an older US population, to understand their AD disease progression by estimating the transition probabilities between MCI and AD severity states. |
What was learned from the study? |
Within 1 year from the initial state, the estimated probability of transition to the next AD state was higher in earlier disease: 12.8% from MCI to mild AD and 5.0% from mild to moderate AD, but < 1% from moderate to severe AD. After 10 years, the probability of transition to the next state was markedly higher for all states, but still higher in earlier disease: 29.8% from MCI to mild AD, 23.5% from mild to moderate AD, and 5.7% from moderate to severe AD. |
Across all AD disease states, the probability of transition to death was < 5% after 1 year and > 15% after 10 years. |
Older age, fewer years of education, unemployment, and nursing home stay were associated with a higher risk of disease progression. |
Introduction
Alzheimer’s disease (AD) is a chronic, progressive disease that affects more than 6.5 million people in the United States (US) [1]. The prevalence of AD has been increasing, putting considerable burdens on patients, caregiver, and society 1,2,3,4]. For example, in the US, the lifetime cost of care for a patient with dementia was more than US$180,000 more than someone without dementia (2015 USD) [3], and an estimated 16 billion hours were spent by unpaid caregivers of dementia patients in 2021 [4]. The costs generally increase with disease severity [5].
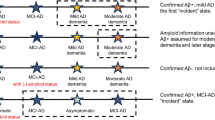
AD progresses as a continuum and consists of a progressive sequence of states of disease severity without distinct borders. In preclinical AD, patients remain asymptomatic while biological changes begin to appear in the brain. The transition from preclinical to clinical or symptomatic AD is marked by cognitive decline, which occurs not only in association with amyloid-beta plaques which are the hallmark of AD but may also be characterized by neurofibrillary tangles of the microtubule binding protein tau and neuron loss [6, 7]. Clinical AD usually begins as mild cognitive impairment (MCI), where minor symptoms may not yet interfere with everyday activities and dementia is not yet present. AD then progresses to dementia, which is categorized as mild, moderate, or severe, based on symptom severity. The patient’s memory, language, and ability to function in daily life are increasingly impaired with worsening severity of AD [4, 7]. Progression from MCI to AD occurs in approximately 15% of patients after 2 years [8]. Understanding AD progression is important when assessing the long-term effects (including cost savings) of AD treatments designed for early states [9, 10].
Cognitive scores obtained from screening tools can be used for disease severity staging 11,12,13], based on which disease progression can be established. Screening tools that have been widely used to measure cognitive impairment include Mini-Mental State Exam (MMSE) [14], clinical dementia rating (CDR) scale [12], clinical dementia rating sum of boxes (CDR-SB) [15], and modified telephone interview for cognitive status (TICS-m). Most of these tools, e.g., MMSE, CDR, and CDR-SB, require in-person assessment, which may be impractical for elderly patients due to physical limitations. TICS-m can be administered via a telephone interview, using similar questions to the MMSE with an additional recall memory assessment portion [16].
Multi-state models can characterize the disease progression using a finite number of disease states [17, 18]. Implemented in a Bayesian hierarchical modeling framework, multi-state models have flexible model structures (e.g., non-linear or time-varying covariate effects, or including between-patient variabilities to capture the variability of the same transition across different patients), provide appropriate measures of uncertainties, and construct predictive distributions 19,20,21,22,23,24,25,26]. Both multi-state Markov models [18, 27, 28] and hidden Markov models [29, 30] have been used to explore disease progression in AD [18, 18,27,28,29,30,31]. Most of them assumed no more than 3 disease states and did not consider the variabilities across patients. In the present study, we developed a continuous-time multi-state Markov model in a Bayesian modeling framework by increasing the number of states to 5, and incorporating patient-level random effects and baseline covariates. This study aims to estimate the transition probabilities between different states of AD and to identify relevant covariates, such as demographic variables, and their impact on the transition probabilities.
Methods
Data and Population
The data used in the study were derived from the Health and Retirement Study (HRS), a representative sample of the US population containing data for Americans over 50 years old. In-depth interviews are conducted every 2 years, and respondents are selected into the HRS using a multi-stage national probability sampling to ensure national representativeness. A new cohort of individuals aged 51–56 is added every 6 years, leading to more than 43,000 individuals interviewed to date [32]. The HRS is supported by the National Institute on Aging and the Social Security Administration [33, 34], sponsored by the National Institute on Aging (grant number NIA U01AG009740) and is conducted by the University of Michigan [33]. The HRS has been approved by the Institutional Review Board at the University of Michigan. The HRS obtains informed verbal consent from voluntary participants and follows strict procedures to protect study participants from disclosure (including maintaining a Federal Certificate of Confidentiality). The public data, made available under restricted data use agreements to researchers and used in this study, are de-identified.
Cognitive performance was assessed using the education-adjusted TICS-m [16]. Since no algorithm is available to define AD severity using education-adjusted TICS-m, a crosswalk to MMSE applying equipercentile equating [14] was performed using data from the Aging, Demographics, and Memory Study (ADAMS). ADAMS is a supplemental study in HRS that gathered in-depth information on the cognitive status of the respondents and collected both TICS-m and MMSE data [35]. The MMSE cut-offs [11, 36] and their corresponding education-adjusted TICS-m cut-offs from the cross-walk were used to define MCI and AD severity states in the current analysis (Table 1).
Survey respondents who reported a clinical diagnosis of AD or had an education-adjusted TICS-m between 20 and 22 corresponding to MCI were included if they had at least one follow-up visit after baseline. A cut-off was applied on the longitudinal data, removing data collected upon “normal” state, since the modeling was focused on the disease progression from MCI onwards. Baseline was defined as the first visit/survey where respondents met the inclusion criteria. All post-baseline years of the HRS Core/Exit databases were used in the analysis for the included survey respondents.
Multi-State Markov Model
A 5-state Markov model was developed, with the 5 states being the 4 AD categories (MCI, mild AD, moderate AD, and severe AD) together with death. Death is an absorbing state since, once entered, it cannot be left. States were denoted by indices I or j when describing transitions from one state to another. As shown in Fig. 1, a patient could first transition between the 4 AD categories and finally enter the absorbing death state with certain probabilities, denoted by Pij for the transition probability from state I to state j.
The transition intensity \({V}_{ij}\) indicates the instantaneous hazard of progression from state I to state j, and all possible transition intensities of the 5-state Markov model form the transition intensity matrix \({\varvec{V}}\):
The diagonal elements of \({\varvec{V}}\) are equal to the negative sum of non-diagonal elements in the same row, as the elements of each row sum to zero. The last row of \({\varvec{V}}\) is all zeros corresponding to the absorbing death state, as it is not possible to go back to other states once in the absorbing state. The transition intensity matrix is used to calculate the transition probabilities \({P}_{ij}\). See the Supplementary Material for more details, also on a more detailed mathematical description of the multi-state Markov model.
Assuming a multi-nomial distribution for the observed number of transitions in the multi-state Markov process, we can estimate any transition probability \({P}_{ij}(t)\) through a Cox proportional hazard model for the non-diagonal elements of the transition intensity matrix. A patient-level random effect was added to each transition intensity to capture the between-patient variabilities. A list of baseline covariates was added to the above Cox model for “forward” transitions (i.e., transitions that move from less to more severe states), based on the literature [31] and clinical hypotheses: age (continuous); years of education (continuous); sex (binary, male/female); employment (binary, yes/no); nursing home residency (binary, yes/no); having hypertension (binary; yes/no); and having diabetes (binary; yes/no).
A Bayesian approach was used to fit the multi-state model using the Nimble software, an advanced platform for statistical modeling and high-performance statistical computation [37]. The ordinary differential equation was numerically solved with the R package deSolve [38]. More details can be found in the Supplementary Material.
Model Validation
An internal validation was carried out by using visual predictive checks (VPCs). Two predictions were assessed. In the first prediction, the model was used to predict the next disease state from a given current disease state, and time as in the observations (one-step prediction). In the second prediction, the model was used to predict the longitudinal trajectory of disease states given the initial disease state, and time as in the observations (longitudinal prediction). The prediction was stopped after a predicted death state. Both predictions were performed for each patient. The proportions of patients in specific disease states (i.e., the prevalence of each disease state) were calculated over time and compared with the observations.
Sensitivity Analysis
A sensitivity analysis was performed to explore how the results changed by using less informative priors, (i.e., prior information regarding model parameters given as distributions with larger standard deviations, thus assuming less certainty on the actual values of the model parameters). More details can be found in the Supplementary Material.
Results
Study Population
A total of 42,233 HRS respondents were assessed for eligibility; of these, 12,464 MCI or AD patients with at least 2 measurements of disease states were selected for analysis (Fig. 2). Seven baseline covariates were considered for estimating the transition probabilities: age, years of education, sex, employment status, nursing home status, co-morbid hypertension, and co-morbid diabetes. No imputation of missing values was performed: thus, 11,292 AD patients without missing values were included for modeling (Fig. 2). No strong correlation was observed between these 7 baseline covariates, as shown in the correlation heat map in the Supplementary Material (Figure S1). In the dataset, patients had bi-annual assessments of disease states until up to 24 years’ follow-up.
The majority of patients had MCI (69.5%), followed by mild (20.4%), moderate (9.6%), and severe (< 1%) AD. Baseline patient characteristics are summarized in Table 2. Overall, the mean (standard deviation) age of the patient population was 70.8 (9.0) years and 54.5% were female. The mean age for patients with MCI was 69.5 years, about 3–4 years younger than patients with AD whose mean age ranged from 73 to 75 years. The proportion of females increased with AD severity, from 53.3% for patients with MCI to 56.8%, 62.5%, and 67.9% for patients with mild, moderate, and severe AD, respectively. The years of education were similar across AD severity (11.4–12.1 years). Approximately 25% of patients with MCI were employed, whereas < 15% of patients with any dementia state were employed. Nursing home residence at baseline among patients with severe AD (6.5%) was more than double that of the other AD severities. Co-morbid hypertension and diabetes were present in 52.5% and 18.5% of the study population, respectively, with generally comparable rates between groups.
The transitions between different AD severity states over time are shown in Fig. 3. We observed that the number of transitions from severe to MCI or mild were small. Since this may generate unstable parameter estimates, \({V}_{41}\) (severe to MCI) and \({V}_{42}\) (severe to mild) of the transition intensity matrix (Eq. (1)) were fixed to zero.
Patient counts and percentages per disease state over time. This Sankey plot shows the number and percentage of patients in a particular disease state at baseline (time = 0 years) and subsequent bi-annual surveys (time = 2, 4, 6,… years). MCI is represented in olive green, mild AD in green, moderate AD in blue, severe AD in violet, and the absorbing death state in red. The colored curves between the time points indicate the number of patients transitioning from one state to another, e.g., from time = 0 to time = 2, approximately 290 patients transition from MCI to mild AD, approximately 90 to moderate AD, and approximately 1050 to death, while none transition to severe AD. AD Alzheimer’s disease, MCI mild cognitive impairment
Model Assessment and Validation
Hypertension and diabetes status were removed from the model due to non-significance in an initial test. After convergence of Markov chain Monte Carlo was reached, the posterior estimates were derived and can be found in the Supplementary Material (Table S1). The averaged coefficient of variation was estimated to be 9.1%, indicating a good accuracy of our statistical method.
Two VPCs were performed to compare the predicted with observed prevalence per disease state, using a one-step prediction and a longitudinal prediction, as explained in “Model validation” section. As shown in Fig. 4, the one-step prediction was well matched to the observations, indicating that this predictive model was sufficient for short-term predictions of the disease progression (such as bi-annual predictions, in line with the survey intervals of the HRS study). The results of the longitudinal prediction when only using the initial disease states and baseline covariates for the prediction are shown in Fig.I5. The longitudinal prediction in the first 20 years was similar to the observations. However, after 20 years’ follow-up, the model underestimated the prevalence of death and overestimated the prevalence of the moderate state.
Transition Probabilities
The predicted transition probabilities for time frames of 1 year and of 10 years are summarized in Table 3. These data show that the probability to transition between states within 1 year was low; however, the probability to transition increased markedly after 10 years. In the first year, the probabilities to transition from MCI or mild AD to another state were 17.1–19.9%, were 7.9% for moderate AD, and were 4.5% for severe AD, whereas, after 10 years, the probability to transition was 72.0% for MCI, 66.4% for mild AD, 50.6% for moderate AD, and 36.9% for severe AD. The probability of dying in the first year was low for all groups (< 5%). Patients with MCI had the highest probability to transition to the next state (i.e., the state of the subsequent disease severity) in the first year (12.8%), followed by patients with mild AD (5.0%), whereas the probability was < 1% for patients with moderate AD. After 10 years, the probability of transitioning to the next state was increased for all groups, and remained higher in earlier disease: 29.8% for MCI, 23.5% for mild AD, and 5.7% for moderate AD.
As shown in Fig. 6, starting from any state, the estimated probability of remaining in the same state steadily decreased over time, and the probability of death increased over time. Thus, patients would likely first experience an unstable period of 5–10 years with either more or less severe symptoms, and then eventually progress to worse disease states leading to death.
As shown in Table 4, the model results indicated that patients characterized by older age, lower years of education, without employment, or living in a nursing home were at higher risk of transition to a more severe state (forward transition). One unit increase in age at baseline (corresponding to 8 years due to standardization of this covariate during model fitting) increased the risk of transition to more severe states by 45%, the hazard ratio (HR) being 1.45 and the 95% credibility interval (CrI) being (1.42, 1.48). Each unit increase in education at baseline (corresponding to 3 years due to standardization) decreased the risk of transition to more severe states by 7% [HR (95% CrI) 0.93 (0.91, 0.94)]. Employment decreased the risk of transition to more severe states by 21%, respectively [HR (95% CrI) 0.79 (0.75, 0.82)). Living in nursing home led to a 39% increase in the risk of transition to more severe states [HR (95% CrI) 1.39 91.25, 1.55)). Female sex decreased the risk of transition to more severe states by 2%, but this decrease was not statistically significant [HR (95% CrI) 0.98 (0.95, 1.02)].
Sensitivity Analysis
The sensitivity analysis showed that less informative priors with larger standard deviations led to longer running time to reach convergence but did not indicate any major change in parameter estimates (data not shown). The derived transition probabilities were hence consistent and did not depend on the choice of priors, which was also a result of the large amount of data available.
Impact of Baseline Covariates on Transition Probabilities Over Time
The impact of the baseline variables age, years of education, sex, employment status, and nursing home status on the transition probabilities over time was explored (Figs. 7, 8, 9, 10, 11). We varied the covariate values one by one and kept the respective remaining covariate values at the median values of the study population.
Estimated transition probabilities over time by age subgroups. The results are based on the population level parameter estimates (i.e., fixed effects) of the model, with the other 4 baseline covariates set to their median values. The shaded area is the 95% credible interval of the predictions, solid lines denote age at first quartile (65 years old), and dotted lines denote age at third quartile (76 years old). MCI mild cognitive impairment
Estimated transition probabilities over time by years of education subgroups. The results are based on the population level parameter estimates (i.e., fixed effects) of the model, with the other 4 baseline covariates set to their median values. The shaded area is the 95% credible interval of the predictions, solid lines denote 0 (minimal) year of education and dotted lines denote 17 (maximal) years of education. MCI mild cognitive impairment
Estimated transition probabilities over time by sex. The results are based on the population level parameter estimates (i.e., fixed effects) of the model, with the other 4 baseline covariates set to their median values. The shaded area is the 95% credible interval of the predictions, solid lines denote female and dotted lines denote male. MCI mild cognitive impairment
Estimated transition probabilities over time by employment status. The results are based on the population level parameter estimates (i.e., fixed effects) of the model, with the other 4 baseline covariates set to their median values. The shaded area is the 95% credible interval of the predictions, solid lines denote with employment and dotted lines denote without employment. MCI mild cognitive impairment
Estimated transition probabilities over time by nursing home status. The results are based on the population level parameter estimates (i.e., fixed effects) of the model, with the other 4 baseline covariates set to their median values. The shaded area is the 95% credible interval of the predictions, solid lines denote with nursing home, and dotted lines denote without nursing home. MCI mild cognitive impairment
The estimated probabilities of staying in the same state or moving to less severe disease states were higher for younger than for older patients, while older patients had higher probabilities to transition to more severe disease states than younger patients (see Fig. 7). Similarly, the estimated probabilities of staying in the same state or moving to less severe disease states were higher for patients with more years of education than for those with fewer years of education, and the probabilities of transitioning to more severe disease states were lower for patients with more years of education than for patients with fewer years of education (see Fig. 8). Due to the non-significant effect of sex on the transition probabilities, transition probability curves for male and female patients greatly overlap and do not allow a differentiation (see Fig. 9). For employed patients or patients not living in a nursing home at baseline, the probabilities of staying in the same disease state or moving to less severe disease states were higher than for unemployed patients or patients living in a nursing home at baseline, respectively, and the probabilities of transitioning to more severe disease states were lower for employed patients and patients not living in a nursing home at baseline than for unemployed patients or patients living in a nursing home at baseline (see Figs. 10 and 11, respectively).
Discussion
The present study investigated the progression of AD in the symptomatic phase using a 5-state Markov model and data from the HRS. Four disease severity categories (MCI and mild, moderate, and severe AD) plus death comprised the 5 states considered in the model. The developed model satisfactorily described the disease progression in terms of transition probabilities between disease states, and provided a good fit to the data. Further, the model provided estimates of the effects of the 5 baseline covariates on the transitions.
In the present analysis, a high probability that patients would stay in the same disease states in the short term (i.e., 1-year transition probabilities; Table 3) was observed. However, in the long term, a lower probability of symptomatic patients maintaining their disease status and a higher probability of patients experiencing disease deterioration, leading to death, was found (i.e., 10-year transition probabilities; Table 3). This is consistent with the current understanding of AD, i.e., that it is a chronic, progressive, and essentially irreversible disease [4].
Transition probabilities to the next state (i.e., disease progression) was more pronounced in earlier AD states. Even at 1 year, patients with MCI and mild AD had transition probabilities of 12.8% and approximately 5.0% to mild and moderate AD, respectively, while transition from moderate to severe AD was < 1%. After 10 years, the probability of transition to the next state were notably higher for all groups but remained more pronounced for those with MCI or mild AD (23.5–29.8%) than for those with moderate AD (5.7%). The probability to transition from MCI to AD (mild, moderate, or severe AD) was estimated as 15.8% after 1 year and 50.6% after 10 years, which is fairly consistent with the literature, reporting, e.g., that 15% of MCI patients transition to AD after 2 years [8], and estimated as 50% after 10 years. Notably, the HRS database contains no information on biomarker testing status, and some of the MCI categorization may not be attributable to AD. It is conceivable that higher transition probabilities across MCI and AD severity stages may be observed among patients who have undergone amyloid-β confirmation or other biomarker validation.
The developed model identified patient characteristics that were associated with an increased probability of experiencing disease deterioration and death. It was observed that symptomatic patients who were older, with fewer years of education, unemployed, or living in a nursing home at baseline were at a higher risk of transition to more severe states. Our findings support that age and education level are important prognostic factors for AD [39, 40]. Nursing home status was identified as a risk factor for disease progression; however, this should be interpreted with caution due to the potential for selection bias: in general, patients with more severe and progressive AD would likely be placed in a nursing home 41,42,43]. Although patient sex was retained as a clinically important covariate in the model, the impact of sex on transition probabilities over time was not statistically significant, and negligible compared to other baseline characteristics.
The disease severity categorization, and hence the allocation of the patients into MCI, mild, moderate, or severe AD states, was based on cut-offs for the TICS-m that were derived from a cross-walk between TICS-m and MMSE. Both MMSE and TICS-m are scores that assess the cognitive abilities of the patient in several domains (including memory, recall, orientation). We recognize that our reliance on cognitive test-based determination of disease severity is a study limitation. While cognitive scores can provide guidance regarding AD severity, complete ascertainment of severity would include assessment of functional ability and, ideally, be further informed with data on amyloid-β burden, especially at the earlier stages of the disease. Specifically, our identified sample of individuals from the HRS database with MCI included MCI due to AD, as well as MCI due to other causes; the former of these groups would likely be substantially larger.
Of note, the time intervals between data collection cycles in HRS were not always equally spaced due to missing questions/interviews or other unreported reasons, but the continuous-time Markov process applied in the current analysis accommodates unequally-spaced time intervals [21]. Another advantage of our 5-state Markov model is the inclusion of a random effects parameter, which helped to capture the between-patient heterogeneity.
The 2 internal validation assessments indicated that the developed model was appropriate for predicting disease progression within 20 years of follow-up but tended to underestimate the death for long-term disease progression beyond the 20-year follow-up. The discrepancies between observations and predictions might be due to only 316 (2.8%) patients and 327 (0.7%) observations being available after 20 years of follow-up. Additional data limitations were that approximately half of the study patients had less than 4 data points, a large portion (42.1%) did not transition to death, and that the number of transitions related to the severe state was small compared to other disease states (Fig. 3). Future modeling and predicting of long-term disease progression could be improved with more sufficient longitudinal data and possibly by refining disease state definitions (e.g., merging the severe and moderate states).
A further limitation of the current model was that all the parameters in the transition intensity matrix \(V\) except \({V}_{41}\) and \({V}_{42}\) were estimated; however, the parameter estimates of \({V}_{14}\) and \({V}_{24}\) were also very small (Table S1 in the Supplementary Material). Thus, setting these additional 2 parameters to 0 could be considered to simplify the model structure. Indeed, the proportions of transitions from MCI to severe (\({V}_{14}\)) and from mild to severe (\({V}_{24}\)) were small, as can be seen in Fig. 3. In addition to that, transitions from more severe to less severe disease states were observed in our study population (e.g., 11% of patients with mild AD transitioned back to MCI in the first year, and 26% in 10 years, respectively), which could indicate limitations of patient identification using TICS-m that relied on the score cut-offs that were benchmarked using MMSE and are not validated, especially for the early stages of the disease.
The current model only included baseline covariates; inclusion of time-dependent covariates may be considered for future models to improve performance, especially given the long disease course of AD. The modeling framework proposed in this study can easily implement such a modeling structure.
Moreover, the current model estimated the overall effect of covariates not specific to the individual transitions, due to limitations of computational power and data (not all the transitions are represented by the full range of covariate values). However, if the data allow, the covariate effect \(\beta \) could be made fully transition-specific to explore how the covariates impact individual transitions.
Conclusions
This study used nationally representative HRS data from the US, providing population- and individual-level estimates of the probabilities for disease progression across MCI and clinical states of AD severity until death. Within 1 year from the initial state, the estimated probability of transition to the next AD state was greater in earlier disease: 12.8% from MCI to mild AD and 5.0% from mild to moderate AD, but < 1% from moderate to severe AD. After 10 years, the probability of transition to the next state was markedly higher for all states, but still greater in earlier disease: 29.8% from MCI to mild AD, 23.5% from mild to moderate AD, and 5.7% from moderate to severe AD. This underscores the need for timely patient identification and early management.
Baseline characteristics significantly associated with higher progression risk included older age, fewer years of education, unemployment, and nursing home stay. Our analysis provides a general approach for estimating transition probabilities between disease states and for identifying patients at high risk of disease progression, accounting for patient-level variability and enabling quantification and propagation of model uncertainty. The disease transition model can thus serve as a tool for designing individual treatment intervention schemes, and the resulting transition probabilities can be used to refine existing cost-effectiveness frameworks.
References
Rajan KB, Weuve J, Barnes LL, McAninch EA, Wilson RS, Evans DA. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020–2060). Alzheimers Dement. 2021;17(12):1966–75. https://doi.org/10.1002/alz.12362.
Wimo A, Guerchet M, Ali GC, et al. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement. 2017;13(1):1–7. https://doi.org/10.1016/j.jalz.2016.07.150.
Jutkowitz E, Kane RL, Gaugler JE, MacLehose RF, Dowd B, Kuntz KM. Societal and family lifetime cost of dementia: implications for policy. J Am Geriatr Soc. 2017;65(10):2169–75. https://doi.org/10.1111/jgs.15043.
2022 Alzheimer's disease facts and figures. Alzheimers Dement. 2022;18(4):700–789. https://doi.org/10.1002/alz.12638.
Tahami Monfared AA, Byrnes MJ, White LA, Zhang Q. The humanistic and economic burden of Alzheimer’s disease. Neurol Ther. 2022. https://doi.org/10.1007/s40120-022-00335-x.
Murphy MP, LeVine H 3rd. Alzheimer’s disease and the amyloid-beta peptide. J Alzheimers Dis. 2010;19(1):311–23. https://doi.org/10.3233/JAD-2010-1221.
Aisen PS, Cummings J, Jack CR Jr, et al. On the path to 2025: understanding the Alzheimer’s disease continuum. Alzheimers Res Ther. 2017;9(1):60. https://doi.org/10.1186/s13195-017-0283-5.
Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: mild cognitive impairment: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology. 2018;90(3):126–35. https://doi.org/10.1212/WNL.0000000000004826.
US Food and Drug Administration. Prescribing information. Aduhelm. 2021.
Cummings J, Lee G, Zhong K, Fonseca J, Taghva K. Alzheimer’s disease drug development pipeline: 2021. Alzheimers Dement (N Y). 2021;7(1):e12179. https://doi.org/10.1002/trc2.12179.
Yan X, Li F, Chen S, Jia J. Associated factors of total costs of Alzheimer’s disease: a cluster-randomized observational study in China. J Alzheimers Dis. 2019;69(3):795–806. https://doi.org/10.3233/JAD-190166.
Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr. 1997;9(Suppl 1):173–6. https://doi.org/10.1017/s1041610297004870. (discussion 177–8).
O’Bryant SE, Waring SC, Cullum CM, et al. Staging dementia using clinical dementia rating scale sum of boxes scores: a Texas Alzheimer’s research consortium study. Arch Neurol. 2008;65(8):1091–5. https://doi.org/10.1001/archneur.65.8.1091.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. https://doi.org/10.1016/0022-3956(75)90026-6.
Cedarbaum JM, Jaros M, Hernandez C, et al. Rationale for use of the clinical dementia rating sum of boxes as a primary outcome measure for Alzheimer’s disease clinical trials. Alzheimers Dement. 2013;9(1 Suppl):S45-55. https://doi.org/10.1016/j.jalz.2011.11.002.
Cook SE, Marsiske M, McCoy KJ. The use of the Modified Telephone Interview for Cognitive Status (TICS-M) in the detection of amnestic mild cognitive impairment. J Geriatr Psychiatry Neurol. 2009;22(2):103–9. https://doi.org/10.1177/0891988708328214.
Meira-Machado L, de Una-Alvarez J, Cadarso-Suarez C, Andersen PK. Multi-state models for the analysis of time-to-event data. Stat Methods Med Res. 2009;18(2):195–222. https://doi.org/10.1177/0962280208092301.
Zhang L, Lim CY, Maiti T, et al. Analysis of conversion of Alzheimer’s disease using a multi-state Markov model. Stat Methods Med Res. 2019;28(9):2801–19. https://doi.org/10.1177/0962280218786525.
Amzal B, Fu S, Angehrn Z. A generic Bayesian DES model for multi-state disease progression. Value Health. 2018;21:S382.
Shen S, Han SX, Petousis P, et al. A Bayesian model for estimating multi-state disease progression. Comput Biol Med. 2017;81:111–20. https://doi.org/10.1016/j.compbiomed.2016.12.011.
Pan SL, Chen HH. Time-varying Markov regression random-effect model with Bayesian estimation procedures: application to dynamics of functional recovery in patients with stroke. Math Biosci. 2010;227(1):72–9. https://doi.org/10.1016/j.mbs.2010.06.003.
Taghipour S, Caudrelier LN, Miller AB, Harvey B. Using simulation to model and validate invasive breast cancer progression in women in the study and control groups of the Canadian National Breast Screening Studies I and II. Med Decis Mak. 2017;37(2):212–23. https://doi.org/10.1177/0272989X16660711.
Pasanisi A, Fu S, Bousquet N. Estimating discrete Markov models from various incomplete data schemes. Comput Stat Data Anal. 2012;56(9):2609–25.
Chien CR, Lai MS, Chen TH. Estimation of mean sojourn time for lung cancer by chest X-ray screening with a Bayesian approach. Lung Cancer. 2008;62(2):215–20. https://doi.org/10.1016/j.lungcan.2008.02.020.
Musenge E. Rheumatoid arthritis disease progression in a South African cohort: Bayesian multistate chronic disease, dynamic modelling. South African Center for Epidemiological Modelling and Analayis. SACEMA; 2013.
Wu D, Rosner GL, Broemeling L. MLE and Bayesian inference of age-dependent sensitivity and transition probability in periodic screening. Biometrics. 2005;61(4):1056–63. https://doi.org/10.1111/j.1541-0420.2005.00361.x.
Herring WL, Gould IG, Fillit H, et al. Predicted lifetime health outcomes for aducanumab in patients with early Alzheimer’s disease. Neurol Ther. 2021;10(2):919–40. https://doi.org/10.1007/s40120-021-00273-0.
Yu HM, Yang SS, Gao JW, Zhou LY, Liang RF, Qu CY. Multi-state Markov model in outcome of mild cognitive impairments among community elderly residents in Mainland China. Int Psychogeriatr. 2013;25(5):797–804. https://doi.org/10.1017/S1041610212002220.
Sukkar R, Katz E, Zhang Y, Raunig D, Wyman BT. Disease progression modeling using Hidden Markov models. Annu Int Conf IEEE Eng Med Biol Soc. 2012;2012:2845–8. https://doi.org/10.1109/EMBC.2012.6346556.
Kang K, Cai J, Song X, Zhu H. Bayesian hidden Markov models for delineating the pathology of Alzheimer’s disease. Stat Methods Med Res. 2019;28(7):2112–24. https://doi.org/10.1177/0962280217748675.
Potashman M, Buessing M, Levitchi Benea M, et al. Estimating progression rates across the spectrum of Alzheimer’s disease for amyloid-positive individuals using national Alzheimer’s Coordinating Center Data. Neurol Ther. 2021;10(2):941–53. https://doi.org/10.1007/s40120-021-00272-1.
Fishxer GG, Ryaxn LH. Overview of the health and retirement study and introduction to the special issue. Work Aging Retire. 2018;4(1):1–9. https://doi.org/10.1093/workar/wax032.
Health and Retirement Study, public use dataset. Produced and distributed by the University of Michigan with funding from the National Institute on Aging (grant number NIA U01AG009740). Ann Arbor, MI. 2022. https://hrs.isr.umich.edu/about. Accessed 7 Apr 2022.
NIH National Institute on Aging. Health and Retirement Study (HRS). 2022. https://www.nia.nih.gov/research/resource/health-and-retirement-study-hrs. Accessed 7 April 2022.
Langa KM, Plassman BL, Wallace RB, et al. The aging, demographics, and memory study: study design and methods. Neuroepidemiology. 2005;25(4):181–91. https://doi.org/10.1159/000087448.
Zaudig M. A new systematic method of measurement and diagnosis of “mild cognitive impairment” and dementia according to ICD-10 and DSM-III-R criteria. Int Psychogeriatr. 1992;4(Suppl 2):203–19. https://doi.org/10.1017/s1041610292001273.
de Valpine P, Turek D, Paciorek CJ, Anderson-Bergman C, Lang DT, Bodik R. Programming with models: writing statistical algorithms for general model structures with NIMBLE. J Comput Graph Stat. 2017;26(2):403–13.
Soetaert K, Petzoldt T, Setzer RW. Solving differential equations in R: package deSolve. J Stat Softw. 2010;33:1–25.
Xu W, Tan L, Wang HF, et al. Education and risk of dementia: dose–response meta-analysis of prospective cohort studies. Mol Neurobiol. 2016;53(5):3113–23. https://doi.org/10.1007/s12035-015-9211-5.
Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–46. https://doi.org/10.1016/S0140-6736(20)30367-6.
Smith GE, O’Brien PC, Ivnik RJ, Kokmen E, Tangalos EG. Prospective analysis of risk factors for nursing home placement of dementia patients. Neurology. 2001;57(8):1467–73. https://doi.org/10.1212/wnl.57.8.1467.
Toot S, Swinson T, Devine M, Challis D, Orrell M. Causes of nursing home placement for older people with dementia: a systematic review and meta-analysis. Int Psychogeriatr. 2017;29(2):195–208. https://doi.org/10.1017/S1041610216001654.
Knopman DS, Berg JD, Thomas R, Grundman M, Thal LJ, Sano M. Nursing home placement is related to dementia progression: experience from a clinical trial. Alzheimer’s Disease Cooperative Study. Neurology. 1999;52(4):714–8. https://doi.org/10.1212/wnl.52.4.714.
Acknowledgements
Funding
Eisai provided the funding for the analysis and the manuscript, including the journal’s Rapid Service fee.
Medical Writing and/or Editorial Assistance
Laura De Benedetti, MSc, an employee of Certara, provided medical writing assistance and contributed to the development of the introduction. Additional medical writing support was provided by Kulvinder K Singh, PharmD, KKSingh, LLC. Min-ge Xie, Distinguished Professor, Rutgers University, provided advice to the statistical methodology. Medical writing was funded by Eisai Inc.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICJME) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Amir Abbas Tahami Monfared and Quanwu Zhang were responsible for the initial study concept and overall study direction and planning. All authors contributed to the conception and design of the manuscript. Amir Abbas Tahami Monfared and Quanwu Zhang provided the idea behind the article, Aastha Chandak prepared the data used for the analysis, Luyuan Qi and Shuai Fu conducted the analysis, Luyuan Qi, Shuai Fu and Noemi Hummel designed and drafted the manuscript. Amir Abbas Tahami Monfared and Quanwu Zhang provided feedback and critical analysis of the manuscript outline and the manuscript text.
Prior publication
A similar analysis, using a slightly different model, was presented at the AD/PD™ 2023 International Conference on Alzheimer’s and Parkinson’s Diseases and related neurological disorders, March 28–April 1, 2023, Gothenburg, Sweden.
Disclosures
Amir Abbas Tahami Monfared is an employee of Eisai Inc. He serves as Associate Editor for the Journal of Alzheimer's Disease and did not receive any fees or honoraria. Raymond Zhang and Quanwu Zhang are employees of Eisai Inc. Shuai Fu, Noemi Hummel, Luyuan Qi and Aastha Chandak are or were employees of Certara at the time of writing the manuscript. Luyuan Qi is now affiliated with Novartis Pharma AG, Basel, Switzerland. Kulvinder K Singh received funding from Eisai for providing medical writing support. Min-ge Xie received consultancy fees from Eisai for providing statistical advice.
Compliance with Ethics Guidelines
The HRS has been approved by the Institutional Review Board at the University of Michigan. The HRS obtains informed verbal consent from voluntary participants and follows strict procedures to protect study participants from disclosure (including maintaining a Federal Certificate of Confidentiality). The public data, made available under restricted data use agreements to researchers and used in this study, is de-identified. The authors who worked on the data analysis agreed and adhered to the conditions of the data use agreement set forth by HRS.
Data Availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Tahami Monfared, A.A., Fu, S., Hummel, N. et al. Estimating Transition Probabilities Across the Alzheimer’s Disease Continuum Using a Nationally Representative Real-World Database in the United States. Neurol Ther 12, 1235–1255 (2023). https://doi.org/10.1007/s40120-023-00498-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-023-00498-1