Abstract
Introduction
Teriflunomide is a once-daily oral immunomodulator approved for relapsing forms of multiple sclerosis (MS) or relapsing–remitting multiple sclerosis (RRMS; depending on the local label), based on extensive evidence from clinical trials and a real-world setting on efficacy, tolerability and patient-reported benefits. The TERICARE study assessed the impact of teriflunomide treatment over 2 years on health-related quality of life (HRQoL) and some of the most common and disabling symptoms of MS, such as fatigue and depression.
Methods
This prospective observational study in Spain included RRMS patients treated with teriflunomide for ≤ 4 weeks. The following patient-reported outcomes (PROs) were collected at baseline and every 6 months for 2 years: the 29-item Multiple Sclerosis Impact Scale version 2 (MSIS-29), the 21-item Modified Fatigue Impact Scale (MFIS-21), the Beck Depression Inventory (BDI-II), the Short Form (SF)-Qualiveen and the Treatment Satisfaction Questionnaire for Medication v1.4 (TSQM). Annualised relapse rate (ARR), disability progression according to the Expanded Disability Status Scale (EDSS), and no evidence of disease activity (NEDA-3) were also assessed.
Results
A total of 325 patients were analysed. Patients had a mean (SD) age of 43.2 years (10.4), a mean baseline EDSS score of 1.75 (1.5), a mean number of relapses in the past 2 years of 1.5 (0.7), and 64% had received prior disease-modifying therapy (DMT). Patients showed significant improvements in the psychological domain of MSIS-29 from 35.9 (26.6) at baseline to 29.4 (25.5) at 18 months (p = 0.004) and 29.0 (24.6) at 24 months (p = 0.002). Levels of fatigue and depression were also reduced. After 2 years of treatment with teriflunomide, ARR was reduced to 0.17 (95% CI 0.14–0.21) from the baseline of 0.42 (95% CI 0.38–0.48), representing a 60.1% reduction. Mean EDSS scores remained stable during the study, and 79.9% of patients showed no disability progression. 54.7% of patients achieved NEDA-3 in the first 12 months, which increased to 61.4% during months 12–24. Patients reported increased satisfaction with treatment over the course of the study, regardless of whether they were DMT naive or not.
Conclusion
Teriflunomide improves psychological aspects of HRQoL and maintains low levels of fatigue and depression. Treatment with teriflunomide over 2 years is effective in reducing ARR and disability progression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The heterogeneity of multiple sclerosis (MS) and the current therapeutic landscape require an integrated assessment that includes individual needs to optimise treatment benefits. Having data on patients’ symptoms, overall health and satisfaction with treatment—which are often undetected by clinicians—is a good start with regard to offering better patient-centred care. |
The objective of this study was to assess the effect of teriflunomide on health-related quality of life (HRQoL) and symptoms such as fatigue and depression, which complement effectiveness and safety outcomes for determining whether the clinical benefits of teriflunomide are reflected in patient’s lives. |
The improvements observed in the psychological domain of HRQoL and the lack of worsening of fatigue and depression over 2 years on teriflunomide reflect how MS patients perceived the clinical benefits of the treatment and how it may contribute to their general well-being. |
Moreover, the effectiveness at reducing the annualized relapse rate (ARR) and disability worsening in mildly affected patients with relapsing–remitting MS reinforces the importance of appropriate patient selection in achieving better outcomes. |
Introduction
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system (CNS) that usually debuts in young adults with a typical initial clinical course of relapses and remissions of neurological symptoms, leading to significant impairment in different aspects of patients’ lives.
Although MS remains incurable, the increasing number of available disease-modifying therapies (DMTs) with proven long-term benefits and a manageable safety profile provide patients and neurologists with a better chance of an individualised approach based on the patient’s perspective, ensuring the right drug is used by the right patient.
Teriflunomide is indicated as a once-daily oral immunomodulator for the treatment of relapsing forms of MS or relapsing–remitting multiple sclerosis (RRMS; depending on the local label). In the phase 3 clinical trials TEMSO [1], TOWER [2] and TENERE [3], teriflunomide was demonstrated to significantly reduce disease activity and risk of relapse and to delay disease progression, with these benefits being maintained for the long term in the respective extension studies [4,5,6,7,8]. These findings have also been replicated in large phase 4 [9,10,11] and other real-world [12] studies. In addition to clinical outcome measures, teriflunomide has been demonstrated to improve health-related quality of life (HRQoL) [9,10,11, 13, 14], increase the satisfaction of patients with the effectiveness, side-effect profile and convenience of treatment [3, 9,10,11,12, 14], and improve or stabilise cognitive functioning [10, 15].
Obtaining data on patient symptoms, overall health, disability and treatment satisfaction, which are often undetected by routine clinical examination, represents a good start in the move towards patient-centred management, as they help to determine the relative effectiveness of a drug and thus its effects on patient status [16]. The complexity of MS and the current therapeutic landscape require an integrated assessment that includes patients’ needs in order to optimise patient benefits.
This study aimed to assess the impact of teriflunomide treatment over 2 years on HRQoL and some of the most common and disabling symptoms of MS. Moreover, we investigated the effectiveness and safety of this treatment to better understand whether the clinical benefits of teriflunomide are reflected in the patient’s perception of their disease.
Methods
Study Design and Patients
TERICARE was a 2-year observational prospective study in patients diagnosed with RRMS from 48 centres in Spain. Patients were included in this study if they were receiving teriflunomide for no more than 4 weeks and they had no relapses within the last 30 days prior to inclusion. Both DMT-naive patients and DMT-experienced patients could enter the study.
The Ethics Committee “Corporació Sanitària Parc Taulí of Sabadell (Barcelona, Spain; reference number 2015619) approved the protocol. The study was conducted in accordance with the Declaration of Helsinki and local regulations, and all patients provided written informed consent.
Assessments
The observation period was 2 years after patient enrolment. Data collection and patient observations occurred at baseline and 6, 12, 18, and 24 months within the normal course of clinical care. Any treatment decision during the study was made at the physician’s discretion.
The primary endpoint of this study was HRQoL as measured by the 29-item Multiple Sclerosis Impact Scale version 2 (MSIS-29) [17]. Secondary assessments included changes from baseline in fatigue status measured by the Modified Fatigue Impact Scale (MFIS-21) [18], emotional functioning using the Beck Depression Inventory-II (BDI-II) [19] and urinary function as measured by the Short Form (SF)-Qualiveen [20].
Data collection also included demographics, medical history of MS, clinical relapses (the appearance of a new clinical sign/symptom or clinical worsening of a previous sign/symptom that persisted for ≥ 24 h in the absence of fever), disability progression according to EDSS scores (increase of ≥ 1 point in patients with baseline scores < 5.5, or of ≥ 0.5 points in those with baseline scores ≥ 5.5), and magnetic resonance imaging (MRI) activity at months 12 and 24 (gadolinium-enhancing T1-weighted hyperintense lesions and new or enlarging T2-weighted lesions). Patients had to rate their satisfaction with teriflunomide using the Treatment Satisfaction Questionnaire for Medication (TSQM v1.4) [21]. Adverse events (AEs) and AEs of special interest (AESIs; serious or non-serious) were collected throughout the study. An AESI is a medical concern that requires close monitoring of the patient. Predefined AESIs were an overdose, ALT levels > 3 times the upper limit of the normal range, leukocyte count < 1000 cells/μl, increased blood pressure (SBP ≥ 160 mmHg, DBP > 100 mmHg), respiratory symptoms compatible with diffuse interstitial lung disease, peripheral neuropathy, acute renal failure, hyperkalaemia, and severe skin/allergic reactions. Liver function was regularly monitored according to the summary of product characteristics (SmPC).
Statistical Analysis
Descriptive analysis included the mean, standard deviation, median, and range for continuous variables and the frequency distribution of patients for categorical variables. Patients who stopped teriflunomide were analysed until the last visit they made.
ANOVA of the change from baseline at 6, 12, 18, and 24 months in MSIS-29, MFIS-21, BDI-II, SF-Qualiveen, and EDSS scores was performed. Paired t-tests were performed to compare MSIS-29, MFIS-21, BDI-II, SF-Qualiveen, and EDSS scores between baseline and each time point after baseline.
MSIS-29 scores of 0–19 categorise as ‘no problems’, 20–39 as ‘few problems’, 40–59 as ‘moderate problems’, 60–79 as ‘quite a few problems’, and 80–100 as ‘extreme problems’ [17]. The total MFIS-21 score ranges from 0 to 84 and uses a cutoff of 38 to discriminate between fatigued and non-fatigued patients [18]. BDI-II scores for depression are classified as minimal (0–13), mild (14–19), moderate (20–28), and severe (29–63) [19]. The SF-Qualiveen scale ranges from 0 (no impact of urinary problems on QoL) to 4 (a highly adverse impact of urinary difficulty) [20].
Correlations between MFIS-21, BDI-II and SF-Qualiveen and the MSIS-29 physical and psychological components were examined by the Spearman rank correlation coefficient (r). A post-hoc analysis was performed to compare the differences in HRQoL between patients suffering relapses (as defined in the protocol) during the study and those who did not. The absolute and percent differences in MSIS-29 physical and psychological scores at month 24 between these two independent groups were analysed using the Mann–Whitney test. The annualised relapse rate (ARR) was calculated as the total number of relapses divided by the total patient time at risk of relapse (time in follow-up). Annual NEDA-3 [no evidence of disease activity, defined as no protocol-defined relapses, no Gd-enhancing lesions or new/enlarging T2 lesions, and no 6-month confirmed disability progression (as protocol defined)] was also analysed post hoc. Patients who progressed in the last 6 months of the planned 24 months of follow-up were excluded from the analysis of the period from month 12 to month 24 because they did not have an additional follow-up assessment to confirm progression.
A statistical analysis plan was generated prior to database locking. Missing data were not considered in the analysis and a significance level of 0.05 was used for statistical testing. The Statistical Package for the Social Sciences version 22.0 was used for all analyses (SPSS Inc, Chicago, IL, USA).
Results
Patient Characteristics
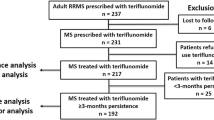
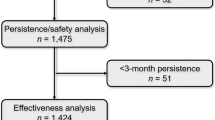
Of the 336 patients who were enrolled, 325 were analysed and 11 were excluded as screening failures. Demographics and clinical characteristics are summarised in Table 1. The mean (SD) age was 43.2 years (10.4), with a range from 36.4 to 50.5 years. Seventy-one percent were female, and the mean time since diagnosis was 7.2 years (7.3), with a range from 0.5 to 12 years. The median EDSS score was 1.5 (1–2.5), and 190 (58.5%) patients had experienced at least a relapse in the past 2 years. Sixty-four percent of patients were switched to teriflunomide from other DMTs. During the 2-year follow-up, 88 (27%) patients discontinued the study. The reasons were treatment discontinuation because of a lack of effectiveness (n = 37, 11.4%) and AE occurrence (n = 31, 9.5%), loss to follow-up (n = 6, 1.8%), investigator’s decision (n = 3, 0.9%) and patient’s willingness (n = 11, 3.4%). Patients discontinuing teriflunomide due to a lack of effectiveness were numerically younger, less likely to be relapse free and more frequently DMT-naive at baseline than patients who discontinued for safety reasons (see Table S1 in the electronic supplementary material for details).
Health-Related Quality of Life
MSIS-29 scores showed a significant reduction in the psychological impact of MS from baseline to month 18 (35.9 (26.6) vs 29.4 (25.5); p = 0.004) and month 24 (35.9 (26.6) vs 29.0 (24.6); p = 0.002). No change in physical impact was observed (Fig. 1a).
Patient-reported outcomes over 2 years: (a) MSIS-29, (b) MFIS-21, (c) BDI-II, (d) SF-Qualiveen. BDI-II Beck Depression Inventory-II, MFIS-21 21-item Modified Fatigue Impact Scale, MSIS-29 29-item Multiple Sclerosis Impact Scale, SD standard deviation. In MSIS-29, MFIS-21, BDI-II and SF-Qualiveen, lower scores are indicative of less impact
The analysis of HRQoL comparing relapsing and relapse-free patients did not show significant differences in the absolute and relative changes in MSIS-29 physical and psychological scores at month 24 (see Table S2 in the electronic supplementary material for details).
Fatigue, Depression and Urinary Function
At baseline, the patients exhibited a mean MFIS-21 score of 27.4 (21.2), which was significantly reduced at month 24 to 23.2 (20.5); p = 0.027 (Fig. 1b). The mean baseline BDI-II score of 12.9 (10.8) was significantly reduced at month 24 to 10.9 (11.2); p = 0.041 (Fig. 1c). The mean SF-Qualiveen score was 0.95 (0.8) at baseline, suggestive of no impact of urinary problems on QoL, and did not change significantly over time (Fig. 1d).
Correlation Analysis Between Fatigue, Depression, Urinary Dysfunction and HRQoL
We found a strong correlation between MFIS-21 and the MSIS-29 physical (r = 0.88; p < 0.001) and psychological (r = 0.87; p < 0.001) components over 1 year of treatment with teriflunomide (See Fig. S1 in the electronic supplementary material for details). The correlation between BDI-II and the physical domain of MSIS-29 was moderate (r = 0.71; p < 0.001), while that with the psychological MSIS-29 component was strong (r = 0.87; p < 0.001). The correlation between SF-Qualiveen and both components of MSIS-29 was moderate. The changes in the MFIS-21, BDI-II and MSIS-29 scores at month 24 were also found to be significantly correlated (see Fig. S2 in the electronic supplementary material for details).
Disease Activity
The mean number of relapses in the past 2 years was 1.5 (0.7). In the first 12 and 24 months of treatment, the mean number of relapses was 1.2 (0.5) and 1.1 (0.2), respectively. Converted to an ARR, the overall relapse rate per year was 0.43 (95% CI 0.38–0.48) at baseline, 0.22 (95% CI 0.17–0.28) at 12 months and 0.17 (95% CI 0.14–0.21) at 24 months. This represents a 60.1% reduction in the ARR after 2 years of treatment with teriflunomide (Fig. 2). Seventy-five patients (24.4%) had relapses during the study, and 232 (75.6%) were in relapse remission.
Mean EDSS scores remained stable during the study (Fig. 3a), with 75% of patients having EDSS < 2.5. At 2 years, 79.9% (191/240) of the patients did not show disability progression according to EDSS (Fig. 3b). Baseline characteristics of the patients by progression status based on EDSS after 2 years of teriflunomide use are shown in Supplementary Table S3. Except for a numerically higher proportion of DMT-naive patients among those who progressed (45.8%) compared to patients with stable EDSS (36.1%), the remaining baseline characteristics were found to be similar.
Overall, the presence of gadolinium-enhanced lesions was reported in 10.2% of patients (28/195) at month 12 and 9.2% (14/152) at month 24. The percentage of patients with new or enlarging T2-weighted lesions was 30% (62/207) at month 12 and 25.6% (41/160) at month 24.
In the first 12 months on teriflunomide, 54.7% (150/274) of patients achieved annual NEDA-3, which increased to 61.4% (140/228) during months 12–24.
Treatment Satisfaction
In patients switching to teriflunomide from other DMTs, statistically significant increases were observed in mean effectiveness, convenience, side effects, and global satisfaction score from baseline to months 18 and 24 (Fig. 4a). The increase in mean convenience was observed as early as month 6.
Mean TSQM v1.4 global treatment satisfaction, effectiveness, side effects and convenience scores. (a) Patients switching to teriflunomide from other DMTs. *t-test change from baseline (p < 0.05). ‡t-test change from baseline (p < 0.01); ANOVA of effectiveness and side effects (p < 0.05). (b) DMT-naive patients. *t-test change from baseline (p < 0.05); ANOVA of side effects (p < 0.05). DMTs disease-modifying therapies, SD standard deviation, TSQM Treatment Satisfaction Questionnaire for Medication
In DMT-naive patients, increases in mean convenience, side effects, and global satisfaction scores were observed from baseline to month 18. Increases in mean side effects remained at month 24 (Fig. 4b).
Safety
A total of 299 (92%) patients experienced at least one AE and 19 (5.8%) experienced a serious AE (there were 22 serious AEs in total), two of whom reported a serious pneumonia event that was considered to be related to teriflunomide (Table 2). Sixteen patients showed teriflunomide-related transaminase elevations of mild (eight cases) and moderate intensity (eight cases). None of these transaminase elevations were severe. No AEs were considered of special interest, and no deaths were reported during the study.
Discussion
The TERICARE study suggests that teriflunomide may have a positive effect on psychological aspects of the quality of life of patients with MS in the real-world setting. Following treatment with teriflunomide, we observed that MS patients experienced significant improvements from baseline in the MSIS-29 psychological domain. However, the perceived physical impact of disease remained unchanged over the course of the study. In this respect, we could argue that (1) subjectively perceived improvements in functional limitations may take longer than improvements in emotional well-being; (2) it is more difficult to capture improvements in a 20-item domain (physical domain) than in a 9-item (psychological domain); and (3) our patients had a low level of disability at baseline (mean EDSS 1.7), which was maintained throughout the study. EDSS has been shown to negatively correlate with quality of life in RRMS [22], and the lack of worsening in EDSS may have played a role in the stabilization of physical functioning (in other words, in the absence of deterioration). Although there is evidence that clinical relapses have a measurable effect on the quality of life of people with MS [23], we did not find differences in the changes observed in physical and psychological MSIS-29 scores between patients experiencing relapses and those in remission. This may be due to the well-controlled relapse activity in our study, although a direct analysis correlating relapses with quality of life was not performed.
When interpreting all these data, it is important to note that quality of life was generally good at inclusion, so the magnitude and meaningfulness of the observed effects on MSIS-29 scores are subject to discussion. While an increase of ≥ 7.5 in the physical MSIS-29 is considered clinically relevant [24], there is no defined threshold for the psychological scale.
Previous real-world studies are consistent with the present study in showing small improvements or stability in the psychological and physical functioning of patients treated with teriflunomide who were similar to our cohort in terms of age, disease duration, disability level and relapse activity [9,10,11, 13, 14, 25]. Therefore, the effect of teriflunomide on quality of life in different populations needs to be further studied.
Our results on fatigue and depression may help us to understand the data observed on quality of life. Patients in TERICARE were generally mildly affected by fatigue and depression at inclusion. Even so, they showed small yet significant improvements in MFIS-21 and BDI-II scores over 2 years of treatment. Although these improvements do not represent changes in the intensity categories of the scales used, they were maintained over the study and might have aided in enhancing or maintaining the quality of life. In support of this, we have shown that fatigue and depression are strongly correlated with quality of life, and the improvements noticed by patients, however small, may have influenced their perception of their health, as others have suggested [26].
The use of PROs in clinical practice is becoming increasingly important, as they provide information on the impact of a disease and its treatment from the patient’s perspective. However, to provide clinicians with a comprehensive assessment of the patient, PRO data should be combined with clinical outcomes.
This study showed that teriflunomide significantly reduced the ARR to 0.17 and slowed disability progression in patients with RRMS treated for 2 years. Although our ARR at month 24 seems to be lower than those reported in the phase 3 studies TEMSO, TOWER and TENERE (ranging from 0.26 to 0.37) [1,2,3, 27], caution is advised when comparing with clinical trials due to differences in methodology and patient populations. These results also appear favourable compared to those from the Teri-PRO and TAURUS-MS observational studies, where patients had a longer disease duration of around 11 years [10, 12]; however, ARR at month 24 in TERICARE was higher than it was in the AURELIO and Teri-REAL cohorts, with fewer relapses prior to teriflunomide [13, 14].
It is of note that most patients were free from relapses (75.6%) and disability progression (80%) during the study. This result is important because teriflunomide was the first DMT used in 36% of patients, suggesting a niche of patients who may benefit from first-line treatment with the drug, but also because it suggests that teriflunomide is an effective switch therapy for previously treated patients with a similar profile to our cohort.
NEDA-3 outcome under teriflunomide has been little explored in the clinical practice setting. Our NEDA-3 results in the first 12 months of treatment with teriflunomide were lower than the 77% and 79% reported in other observational cohorts [28, 29]; however, unlike TERICARE, those studies used a period of 3 months instead of 6 months to confirm disability progression. The increase in NEDA-3 during the following 12 months (months 12–24) reflects that the disease is stabilized on teriflunomide, unconfounded by any disease activity carried over during the time that the medication requires to reach full clinical effect.
The 27% overall study discontinuation rate in TERICARE is within the range of 24.8–41% reported in other observational studies of teriflunomide [12,13,14, 25, 30, 31]. The low rate of treatment discontinuation due to tolerability issues compared with similar studies [10, 12] may result from the good safety experience with teriflunomide in TERICARE and the substantially lower incidence of serious adverse events than previously reported (5.8% vs 13%) [10, 12]. It remains to be seen if the low disability level in our cohort and the improvements perceived in their quality of life may have favoured the rate of discontinuation due to a perceived lack of efficacy. What is clear is that the low rates of discontinuation for safety and inefficacy reasons align with the high treatment satisfaction reported by the patients in all four domains of TSQM v1.4. Consistent with other observational studies of teriflunomide, the significant increase in satisfaction following the initiation of therapy persisted over 2 years, regardless of whether patients were DMT-naive or not [9,10,11,12].
This study had several limitations to consider when interpreting its results, including the observational design, which may have introduced selection bias; the non-collection of symptomatic treatment, which may have favoured some findings; the lack of a comparison group; and the loss to follow-up. Our findings regarding the reduction in relapse rate should be interpreted with caution, as this measure may be biased by the phenomenon of regression to the mean, among other reasons, which may partially explain the observed effect. Further, since patients were treated and followed in a real-world setting, variations in medical practice are possible. Among the strengths of the study are the sample size, with complete representativeness of all regions in Spain; the 2 years of follow-up to allow the detection of changes; and the use of a standardised MS-specific questionnaire for the assessment of HRQoL that accurately identifies the priorities of MS patients.
Conclusions
The patient-reported benefits in psychological HRQoL and the lack of worsening in fatigue and depression during 2 years of treatment with teriflunomide reflect how MS patients perceived the clinical benefits of the treatment and how it may contribute to their general well-being. In addition, this study shows that teriflunomide is effective at reducing ARR and disability progression in mildly affected relapsing MS patients, reinforcing the importance of proper patient selection in achieving better clinical outcomes. The applicability of the results to wider MS populations warrants further research.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
O’Connor P, Wolinsky JS, Confavreux C, Comi G, Kappos L, Olsson TP, Benzerdjeb H, Truffinet P, Wang L, Miller A, et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med. 2011;365(14):1293–303.
Confavreux C, O’Connor P, Comi G, Freedman MS, Miller AE, Olsson TP, Wolinsky JS, Bagulho T, Delhay JL, Dukovic D, et al. Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13(3):247–56.
Vermersch P, Czlonkowska A, Grimaldi LM, Confavreux C, Comi G, Kappos L, Olsson TP, Benamor M, Bauer D, Truffinet P, et al. Teriflunomide versus subcutaneous interferon beta-1a in patients with relapsing multiple sclerosis: a randomised, controlled phase 3 trial. Mult Scler. 2014;20(6):705–16.
Confavreux C, Li DK, Freedman MS, Truffinet P, Benzerdjeb H, Wang D, Bar-Or A, Traboulsee AL, Reiman LE, O’Connor PW. Long-term follow-up of a phase 2 study of oral teriflunomide in relapsing multiple sclerosis: safety and efficacy results up to 85 years. Mult Scler. 2012;18(9):1278–89.
Freedman MS, Bar-Or A, Benamor M, Truffinet P, Poole EM. Long-term disability outcomes in patients treated with teriflunomide for up to 14 years: group-and patient-level data from the phase 2 extension study. Mult Scler J. 2017;23(3):637–8.
Maurer M, Miller A, Comi G, Kappos L, Wolinsky JS, Stangel M, McDonell R, Truffinet P, Thangavelu K, Freedman MS. Impact of long-term teriflunomide treatment on severe relapses: analysis of TEMSO and TOWER extensions. Neurology. 2017;88(16):896.
O’Connor P, Comi G, Freedman MS, Miller AE, Kappos L, Bouchard JP, Lebrun-Frenay C, Mares J, Benamor M, Thangavelu K, et al. Long-term safety and efficacy of teriflunomide: nine-year follow-up of the randomized TEMSO study. Neurology. 2016;86(10):920–30.
Sormani MP, Truffinet P, Thangavelu K, Rufi P, Simonson C, De Stefano N. Predicting long-term disability outcomes in patients with MS treated with teriflunomide in TEMSO. Neurol Neuroimmunol Neuroinflamm. 2017;4(5): e379.
Coyle PK, Khatri B, Edwards KR, Meca-Lallana JE, Cavalier S, Rufi P, Benamor M, Brette S, Robinson M, Gold R. Patient-reported outcomes in relapsing forms of MS: real-world, global treatment experience with teriflunomide from the Teri-PRO study. Mult Scler Relat Disord. 2017;17:107–15.
Coyle PK, Khatri B, Edwards KR, Meca-Lallana JE, Cavalier S, Rufi P, Benamor M, Poole EM, Robinson M, Gold R. Teriflunomide real-world evidence: global differences in the phase 4 Teri-PRO study. Mult Scler Relat Disord. 2019;31:157–64.
Coyle PK, Khatri B, Edwards KR, Meca-Lallana JE, Cavalier S, Rufi P, Benamor M, Thangavelu K, Robinson M, Gold R. Patient-reported outcomes in patients with relapsing forms of MS switching to teriflunomide from other disease-modifying therapies: results from the global Phase 4 Teri-PRO study in routine clinical practice. Mult Scler Relat Disord. 2018;26:211–8.
Kallmann BA, Tiel-Wilck K, Kullmann JS, Engelmann U, Chan A. Real-life outcomes of teriflunomide treatment in patients with relapsing multiple sclerosis: TAURUS-MS observational study. Ther Adv Neurol Disord. 2019;12:1756286419835077.
Bencsik K, Dobos E, Jobbagy Z, Birkas AJ, Kovacs K, Satori M, Lencses G, Bartok G, Losonczi E, Vecsei L, et al. Real-world evidence for favourable quality-of-life outcomes in Hungarian patients with relapsing-remitting multiple sclerosis treated for two years with oral teriflunomide: results of the Teri-REAL study. Pharmaceut (Basel). 2022;15(5):598.
Dardiotis E, Perpati G, Borsos M, Nikolaidis I, Tzanetakos D, Deretzi G, Koutlas E, Kilidireas C, Mitsikostas DD, Hadjigeorgiou G, et al. Real-world assessment of quality of life in patients with relapsing remitting multiple sclerosis treated with teriflunomide for two years: patient-reported outcomes from the AURELIO study in Greece. Neurol Ther. 2022;11(3):1375–90.
Sprenger T, Jeannette L-S, Sormani MP. Evaluation of the long-term treatment effect of teriflunomide on cognitive outcomes and association with brain volume change: data from TEMSO and its extension study. J Neurol. 2018;89(6):28.
Khurana V, Sharma H, Afroz N, Callan A, Medin J. Patient-reported outcomes in multiple sclerosis: a systematic comparison of available measures. Eur J Neurol. 2017;24(9):1099–107.
Hobart J, Lamping D, Fitzpatrick R, Riazi A, Thompson A. The Multiple Sclerosis Impact Scale (MSIS-29): a new patient-based outcome measure. Brain. 2001;124(Pt 5):962–73.
Larson RD. Psychometric properties of the modified fatigue impact scale. Int J MS Care. 2013;15(1):15–20.
Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71.
Bonniaud V, Bryant D, Parratte B, Guyatt G. Development and validation of the short form of a urinary quality of life questionnaire: SF-Qualiveen. J Urol. 2008;180(6):2592–8.
Atkinson MJ, Sinha A, Hass SL, Colman SS, Kumar RN, Brod M, Rowland CR. Validation of a general measure of treatment satisfaction, the treatment satisfaction questionnaire for medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes. 2004;2:12.
Schmidt S, Jostingmeyer P. Depression, fatigue and disability are independently associated with quality of life in patients with multiple sclerosis: results of a cross-sectional study. Mult Scler Relat Disord. 2019;35:262–9.
Healy BC, Degano IR, Schreck A, Rintell D, Weiner H, Chitnis T, Glanz BI. The impact of a recent relapse on patient-reported outcomes in subjects with multiple sclerosis. Qual Life Res. 2012;21(10):1677–84.
Phillips GA, Wyrwich KW, Guo S, Medori R, Altincatal A, Wagner L, Elkins J. Responder definition of the Multiple Sclerosis Impact Scale physical impact subscale for patients with physical worsening. Mult Scler. 2014;20(13):1753–60.
Hestvik ALK, Frederiksen JL, Nielsen HH, Torkildsen O, Eek C, Huang-Link Y, Haghighi S, Tsai JA, Kant M. Real-world study of relapsing-remitting multiple sclerosis patients treated with teriflunomide in Nordic countries: quality-of-life, efficacy, safety and adherence outcomes. Mult Scler Relat Disord. 2022;63:103892.
Green R, Cutter G, Friendly M, Kister I. Which symptoms contribute the most to patients’ perception of health in multiple sclerosis? Mult Scler J Exp Transl Clin. 2017;3(3):2055217317728301.
Kappos L, Comi G, Freedman MS. Pooled efficacy data from two phase 3 placebo-controlled trials of oral, once-daily teriflunomide. Mult Scler. 2013;19(11):74–558.
D’Amico E, Zanghi A, Callari G, Borriello G, Gallo A, Graziano G, Valentino P, Buccafusca M, Cottone S, Salemi G, et al. Comparable efficacy and safety of dimethyl fumarate and teriflunomide treatment in Relapsing-Remitting Multiple Sclerosis: an Italian real-word multicenter experience. Ther Adv Neurol Disord. 2018;11:1756286418796404.
Zhang Y, Yin H, Zhang D, Xu Y, Peng B, Cui L. Real-world outcomes of teriflunomide in relapsing-remitting multiple sclerosis: a prospective cohort study. J Neurol. 2022;269(9):4808–16.
Bucello S, Annovazzi P, Ragonese P, Altieri M, Barcella V, Bergamaschi R, Bianchi A, Borriello G, Buscarinu MC, Callari G, et al. Real world experience with teriflunomide in multiple sclerosis: the TER-Italy study. J Neurol. 2021;268(8):2922–32.
de Seze J, Devy R, Planque E, Delabrousse-Mayoux JP, Vandhuick O, Kabir M, Gherib A. Fatigue in teriflunomide-treated patients with relapsing remitting multiple sclerosis in the real-world Teri-FAST study. Mult Scler Relat Disord. 2021;47:102659.
Acknowledgements
The authors are extremely grateful to all the participating patients.
Medical Writing/Editorial Assistance
Medical writing support was provided by Isabel Caballero from Evidenze Health España, S.L.U. during the preparation of this article, and was funded by Sanofi. The journal’s fee was funded by Sanofi.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Funding
This work and the journal’s fee were funded by Sanofi.
Author information
Authors and Affiliations
Contributions
Jose E. Meca-Lallana, José María Prieto González, Ana Belén Caminero Rodríguez and Javier Olascoaga Urtaza contributed to the conception and design of the work, the acquisition and interpretation of data, and to the drafting of the manuscript. Ana María Alonso, Eduardo Durán Ferreras, Raúl Espinosa, Julio Dotor, Mercedes Romera, Adrián Ares Luque, Domingo Perez Ruiz, Carmen Calles, Miguel Ángel Hernández, Miguel Hervás García, Amelia Mendoza Rodríguez, Yasmina Berdei Montero, Nieves Téllez, Nicolás Herrera Varó, Javier Sotoca, Silvia Presas-Rodriguez, Luis A. Querol Gutierrez, Mariona Hervás Pujol, Jordi Batlle Nadal, Gisela Martín Ozaeta, Laura Gubieras Lillo, Sergio Martínez Yélamos, Lluís Ramió-Torrentà, Javier Mallada Frechin Antonio Belenguer Benavides, Francisco Gascón-Giménez, Bonaventura Casanova, Lamberto Landete Pascual, Leticia Berenguer, Laura Navarro, Montserrat Gómez Gutierrez, Carmen Durán9, Ana Rodríguez Regal, Elena Álvarez, Daniel Apolinar García-Estévez, Ana María Lopez Real, Miguel Ángel Llaneza González, María Eugenia Marzo Sola, José Luís Sánchez-Menoyo, Agustín Oterino, Ramón Villaverde González, Tamara Castillo-Triviño, Amaya Álvarez de Arcaya and Cristina Llarena contributed to the data acquisition critically for important intellectual content. All authors approved the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
Jose E. Meca-Lallana declares consulting fees, honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events, support for attending meetings, participation on advisory boards, and receipt of equipment, materials, drugs, medical writing, gifts or other services from Biogen, Sanofi, Novartis, Roche, Merck and BMS. José M. Prieto González declares consulting fees from Bayer, Biogen, Daiichi Sankyo, Genzyme Corporation, Janssen, Merck, Novartis, Sanofi, Sandoz Iberia, Teva, Roche, Almirall, Celgene; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Bayer, Biogen, Genzyme Corporation, Merck, Novartis, Sanofi, Teva, Roche and Almirall; participation on advisory boards from Bayer, Biogen, Genzyme Corporation, Merck, Novartis, Sanofi, Sandoz Ibérica, Teva, Roche, Almirall and Celgene; receipt of equipment, materials, drugs, medical writing, gifts or other services from Bayer, Biogen, Genzyme, Merck, Novartis, Sanofi and Teva. Ana B. Caminero Rodríguez declares consulting fees from Biogen, Merck Serono, Novartis, Roche, Sanofi and Teva; payment or honoraria for lectures, presentations and speakers bureaus from Almirall, Bayer, Biogen, BMS, Merck, Mylan Pharmaceuticals SL, Novartis, Roche, Sanofi, Teva; support for attending meetings from Almirall, Biogen, Merck, Novartis, Roche, Sanofi, Teva; participation on advisory boards from Biogen, Merck, Roche, Sanofi; receipt of equipment, materials, drugs, medical writing, gifts or other services from Almirall, Bayer, Biogen, Merck, Roche, Sanofi. Javier Olascoaga Urtaza declares grants or contracts from Sanofi and support for attending meetings and travel from Sanofi. Ana M. Alonso declares consulting fees from Novartis, Biogen, Sanofi and Janssen; payment for speaker from Biogen, Merck, Roche, Sanofi, Novartis, BMS, Almirall; support for attending meetings from Teva. Eduardo Durán Ferreras declares payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events, for expert testimony and for participation on advisory boards from Biogen, Sanofi, Merck, Roche, Teva, Novartis, Almirall; support for attending meetings from Biogen, Sanofi, Roche, Merck, Novartis. Julio Dotor declares payment for speaker from Sanofi, Merck, Biogen, UCB, Zambón, Almirall; support for attending meetings from Merck; participation on advisory boards from Merck and Janssen. Mercedes Romera declares honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events, support for attending meetings and participating on advisory boards from Sanofi, Biogen, Merck, Roche and Novartis. Adrián Ares Luque declares consulting fees from Biogen, Roche, Merck and Sanofi, payment or honoraria for lectures, presentations, and speakers bureaus from Biogen, Novartis, Sanofi, Merck, Roche and Teva, and support for attending meetings from Biogen and Sanofi. Carmen Calles declares payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events, payment for expert testimony, and support for attending meetings and participating on advisory boards from Teva, Merck, Sanofi, Roche, Bristol Myers, Novartis, Biogen. Miguel A. Hernández declares payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events and support for attending meetings from Sanofi, Merck, Roche, Novartis and Genzyme. Miguel Hervás García declares payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Sanofi, Merck, Novartis and Roche; support for attending meetings and travel from Merck, Novartis and Roche; participation on an advisory board from Merck. Amelia Mendoza Rodríguez declares payment or honoraria for lectures, presentations, speakers bureaus from Sanofi, Merk and Roche; support for attending meetings from Teva and Sanofi. Yasmina Berdei Montero declares payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Sanofi, Roche and Biogen; support for attending meetings from Biogen; participation on advisory board from Sanofi. Nicolás Herrera Varó has changed his affiliation during the completion of the manuscript (new affiliation: Hospital Virgen de la Concha, Zamora, Spain). He declares consulting and conference fees, honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events and support for attending meetings/travel from Roche, Merck and Genzyme; payment for expert testimony from Genzyme. Javier Sotoca has changed his affiliation during the completion of the manuscript (new affiliation: Hospital Universitari Vall d’Hebron; Barcelona, Spain). He declares consulting fees from Bayer; payments or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Merck, Sanofi, Biogen, Novartis, Roche, Almirall; payment for advisory boards from Roche and Sanofi; support for attending meetings from Roche, Biogen and Sanofi. Silvia Presas-Rodriguez declares honoraria for speakers bureaus from Biogen and Novartis, support for attending meetings from Biogen, Novartis and Roche. Luis A. Querol Gutierrez declares consulting fees from Grifols, Sanofi-Genzyme, Johnson & Johnson, Novartis, Annexon Pharmaceuticals, Takeda, Alexion, CSL Behring; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Sanofi-Genzyme, Biogen, Merck, Grifols, Roche, CSL Behring; participation on advisory boards from Roche, UCB and Grifols. Mariona Hervás Pujol declares honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Novartis; support for attending meetings from Sanofi; participation on advisory boards from Sanofi, Biogen, Almirall and Bayer. Gisela Martín Ozaeta declares payment for lectures and a chair from Biogen and Merck; support for attending meetings from Biogen. Sergio Martínez Yélamos declares grants or contracts from Novartis, Roche, Merck and Biogen; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Biogen, Roche and Novartis; support for attending meetings from Biogen, Roche, Novartis and Merck; participation on advisory board from Biogen and Novartis. Lluís Ramió-Torrentà declares consulting fees, payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events and support for attending meetings from Novartis, Roche, Merck, Sanofi, Biogen. Javier Mallada Frechin declares payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Novartis and Sanofi. Antonio Belenguer Benavides declares payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Sanofi, Biogen and Novartis; payment for expert testimony from Sanofi and Biogen; support for attending meetings from Sanofi; participation on advisory boards from Sanofi. Francisco Gascón-Giménez declares consulting fees, payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events and support for attending meetings and travel from Teva, Biogen, Almirall, Merck, Sanofi, Novartis; participation on advisory boards from Sanofi and Novartis. He is a member of the Valencia Committee Multiple Sclerosis Treatment of the Sanitary Local Government of the Region of Valencia in Spain and coordinator of the Multiple Sclerosis Group of the Valencia Neurology Society, Spain. Bonaventura Casanova declares grants or contracts from BMS, Merck and Novartis; consulting fees from Novartis, Roche and BMS; support for attending meetings from Sanofi. Lamberto Landete Pascual declares honoraria for an advisory board, educational activities and attending meetings from Sanofi, Teva, Novartis, Biogen, Roche, Merck, Bayer, Almirall, BMS, Janssen. Laura Navarro declares payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Sanofi, Biogen, Merck and Roche. Montserrat Gómez Gutierrez declares honoraria for lectures and presentations from Novartis, Merck, Sanofi, Biogen, Bial and BMS; support for attending meetings from Bial, Merck and Sanofi; participation on advisory boards from Novartis. Ana Rodríguez Regal declares consulting fees and honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Merck, Sanofi, Novartis, Biogen and Roche; participation on advisory boards from Sanofi and Merck. Elena Álvarez declares consulting fees from Almirall, Bayer, Biogen, Novartis, Sanofi, Merck, Teva, Roche; payment or honoraria for payment or honoraria for lectures, presentations, and educational events from Almirall, Bayer, Biogen, Novartis, Sanofi, Merck, Teva and Roche; support for attending meetings and travel from Almirall, Biogen, Novartis, Sanofi, Merck, Teva, Roche; participation on advisory boards from Sanofi and Merck. Daniel A. García-Estévez declares payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Sanofi and Novartis; support for attending meetings from Novartis; participation on an advisory board from Biogen. Ana M. Lopez Real declares consulting fees from Biogen, Merck, Sanofi; payment or honoraria for lectures, presentations and speakers bureaus from Roche, Biogen, Novartis, Merck and Sanofi. Miguel A. Llaneza González has changed his affiliation during the completion of the manuscript (new affiliation: Hospital Universitario Central de Asturias, Spain). He declares grants or contracts from Sanofi, Roche, Biogen, Merck, BMS, Janssen, Teva, Novartis; consulting fees from Sanofi, Roche, Biogen, Novartis, Merck, BMS, Janssen, Teva; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Sanofi, Roche, Biogen, Novartis, Merck, BMS, Teva, Janssen; payment for expert testimony from Sanofi, Novartis, BMS, Merck, Teva, Janssen, Biogen and Roche; participation on Advisory Board from Sanofi, Biogen, Janssen, Roche, BMS, Merck, Novartis, Teva. José L. Sánchez Menoyo declares grants or contracts from MS Base and Roche; honoraria for speakers bureaus from Sanofi, Merck and Novartis; support for travel from Biogen; participation on advisory boards from Merck and Sanofi. Agustín Oterino has changed his affiliation during the completion of the manuscript (new affiliation: Hospital Universitario Central de Asturias, Spain). He declares grants or contracts from Sanofi, Novartis, Merck; honoraria for speakers bureaus from Sanofi, Teva, Merck, Novartis, Almirall, Biogen, payment for expert testimony from Sanofi, Teva, Merck, Novartis, Almirall, Biogen, support for attending meetings from Merck, Sanofi, and as expert from Sanofi. Ramón Villaverde González declares consulting fees from Merck; honoraria for lectures from Merck, Roche, Biogen, Novartis, Sanofi; support for travel from Biogen and Roche. Tamara Castillo-Triviño declares consulting fees from Biogen, Sanofi, Novartis and Merck; honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Biogen, BMS, Merck, Novartis, Roche, Sanofi, Teva; support for attending meetings from Biogen, Janssen, Merck, Novartis, Roche, Sanofi, Teva. Amaya Álvarez de Arcaya declares consulting fees from Sanofi and Merck; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Biogen, Sanofi, Merck and Roche; support for attending meetings from Roche and Sanofi. Cristina Llarena declares consulting fees from Almirall, Novartis, Biogen, Roche, Genzyme/Sanofi, Teva, Merck. Raúl Espinosa, Domingo Pérez Ruiz, Nieves Téllez, Jordi Batlle Nadal, Laura Gubieras Lillo, Leticia Berenguer, Carmen Durán and María E. Marzo Sola declare that they have no competing interests.
Ethical Approval
The ethics committee “Corporació Sanitària Parc Taulí of Sabadell” (Barcelona, Spain; reference number 2015619) approved the protocol. The study was conducted in accordance with the Declaration of Helsinki and local regulations and all patients provided written informed consent.
Additional information
Prior presentation: 8th Joint ECTRIMS-ACTRIMS Meeting, 11–13 September 2020, virtual. LXXII Annual Meeting of the Spanish Society of Neurology, 23 November–3 December 2020, virtual. 37th Congress of ECTRIMS Meeting 13–15 October 2021, virtual. LXXIII Annual Meeting of the Spanish Society of Neurology, 22 November–2 December 2021, virtual.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Meca-Lallana, J.E., Prieto González, J.M., Caminero Rodríguez, A.B. et al. Effectiveness and Safety of Teriflunomide in Relapsing–Remitting Multiple Sclerosis and Improvements in Quality of Life: Results from the Real-World TERICARE Study. Neurol Ther 12, 2177–2193 (2023). https://doi.org/10.1007/s40120-023-00557-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-023-00557-7