Abstract
Introduction
There remains a critical need for precise localization of the epileptogenic foci in individuals with drug-resistant epilepsy (DRE). 18F-Fluorodeoxyglucose positron emission tomography (FDG-PET) imaging can reveal hypometabolic regions during the interval between seizures in patients with epilepsy. However, visual-based qualitative analysis is time-consuming and strongly influenced by physician experience. CortexID Suite is a quantitative analysis software that helps to evaluate PET imaging of the human brain. Therefore, we aimed to evaluate the efficacy of CortexID quantitative analysis in the localization of the epileptogenic zone in patients with temporal lobe epilepsy (TLE).
Methods
A total of 102 patients with epilepsy who underwent 18F-FDG-PET examinations were included in this retrospective study. The PET visual analysis was interpreted by two nuclear medicine physicians, and the quantitative analysis was performed automatically using CortexID analysis software. The assumed epileptogenic zone was evaluated comprehensively by two skilled neurologists in the preoperative assessment of epilepsy. The accuracy of epileptogenic zone localization in PET visual analysis was compared with that in CortexID quantitative analysis.
Results
The diagnostic threshold for the difference in the metabolic Z-score between the right and left sides of medial temporal lobe epilepsy (MTLE) was calculated as 0.87, and that for lateral temporal lobe epilepsy (LTLE) was 2.175. In patients with MTLE, the area under the curve (AUC) was 0.922 for PET visual analysis, 0.853 for CortexID quantitative analysis, and 0.971 for the combined diagnosis. In patients with LTLE, the AUC was 0.842 for PET visual analysis, 0.831 for CortexID quantitative analysis, and 0.897 for the combined diagnosis. These results indicate that the diagnostic efficacy of CortexID quantitative analysis is not inferior to PET visual analysis (p > 0.05), while combined analysis significantly increases diagnostic efficacy (p < 0.05). Among the 23 patients who underwent surgery, the sensitivity and specificity of PET visual analysis for localization were 95.4% and 66.7%, and the sensitivity and specificity of CortexID quantitative analysis were 100% and 50%.
Conclusion
The diagnostic efficacy of CortexID quantitative analysis is comparable to PET visual analysis in the localization of the epileptogenic zone in patients with TLE. CortexID quantitative analysis combined with visual analysis can further improve the accuracy of epileptogenic zone localization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Why carry out this study? |
There remains a critical need for precise localization of the epileptogenic zone in individuals with drug-resistant epilepsy (DRE). |
Visual-based qualitative analysis is time-consuming and strongly influenced by physician experience. Therefore, we aimed to evaluate the efficacy of CortexID quantitative analysis in the localization of the epileptogenic zone in patients with TLE. |
What was learned from the study? |
CortexID quantitative analysis achieves diagnostic efficacy comparable to visual analysis in the localization of the epileptogenic zone in patients with TLE. |
CortexID quantitative analysis can provide additional information in detecting the epileptogenic zone. |
Nuclear medicine physicians can choose to supplement their reading methodology with the CortexID quantitative analysis. |
Introduction
Epilepsy is a common brain disorder that results in persistent seizures, impacting patients’ quality of life [1, 2]. While anti-seizure medication can control seizures in many cases, about one-third of patients continue to experience uncontrolled seizures. For individuals with drug-resistant focal epilepsy, epilepsy surgery offers the best chance of long-term freedom from seizures. Successful surgery relies on accurately identifying the epileptogenic zone and completely removing or destroying it [1, 3,4,5].
Epileptogenesis is often marked by neuronal damage and gliosis [6], which can result in reduced brain metabolism. 18F-Fluorodeoxyglucose positron emission tomography (FDG-PET) imaging can reveal hypometabolic regions during the interval between seizures in patients with epilepsy, and a coincidence rate of up to 90% between PET localization of the epileptogenic zone and intraoperative cortical electroencephalography (EEG) can be achieved [7]. Visual-based qualitative analysis is the most widely used method for PET analysis at present. However, this approach is time-consuming, and is strongly influenced by physician experience.
Quantitative analysis with PET imaging is a promising approach for evaluating the epileptogenic zone. Visual assessment of hypometabolism using 18F-FDG PET is less accurate than quantitative assessment using voxels, machine learning, model classifiers, statistical parametric mapping (SPM), and other artificial intelligence methods [8,9,10,11]. However, these methods are cumbersome and time-consuming, and some of them need to build a normal PET image population database separately, so they are not easy to apply promptly in the clinic [12].
CortexID Suite is a quantitative analysis software developed by GE Healthcare (Chicago, IL, USA) that enables fast data processing without the need to develop or purchase additional software. The software supports information on the relative changes in the metabolism of brain FDG and amyloid images and helps to evaluate PET imaging of the human brain [13]. The software has proprietary, adaptive template alignment technology based on voxel or three-dimensional stereotactic surface projection (3D-SSP) to normalize PET images by anatomy to a standardized space [14]. In a recent study, Wang et al. [15] compared epileptic focus detection by SPM and 3D-SSP for 18F-FDG-PET brain mapping analyses and concluded that both methods can improve the detection rate, recommending the use of 3D-SPP and SPM for more complex cases. CortexID quantitative analysis software has been applied to a variety of neurological diseases, including ischemic-hypoxic encephalopathy and Alzheimer’s disease [13, 14, 16, 17]. However, there is still a lack of studies on its application in locating the epileptogenic zone.
Considering the multifactorial nature of the disease, integrating multiple levels of information is crucial for localizing the epileptogenic zone. Therefore, the aim of this study was to evaluate the efficacy of CortexID quantitative analysis in the localization of the epileptogenic zone in patients with temporal lobe epilepsy (TLE).
Materials and Methods
Data Selection and Patients
Data on patients with epilepsy who underwent 18F-FDG PET examination from August 2021 to September 2023 at the Department of Nuclear Medicine, Xijing Hospital, were retrospectively collected. This study was approved by the Medical Ethical Committee of the First Affiliated Hospital of Air Force Military Medical University. The inclusion criteria for this study were as follows: (1) meeting the diagnostic criteria for epilepsy [18]; (2) 18F-FDG PET examination performed in the interictal period; (3) clearly identified as a patient with focal epilepsy; (4) clear localization of epileptic foci; and (5) having signed an informed consent form. Exclusion criteria were as follows: (1) patients with surgical resection or stereoelectroencephalography radio-frequency thermocoagulation; (2) patients with other serious neurological impairments or neurological diseases; (3) incomplete collection of clinical data.
Location of the Epileptogenic Zone
Clinical information, including age, duration of disease, age of onset, seizure duration, seizure frequency, medication regimen, history, 24-h video EEG, brain nuclear magnetic resonance imaging (MRI), and seizure semiology, was collected from the Xijing Hospital medical record system. The assumed epileptogenic zone was evaluated comprehensively by two skilled neurologists in preoperative assessment of epilepsy based on these findings, and in the case of disagreement, a third expert was invited for discussion.
18F-FDG PET Acquisition
Image acquisition of patients was conducted using PET/MR scanners. All patients had fasted for more than 6 h, fasting blood glucose was controlled below 11.1 mmol/L, and 18F-FDG was injected intravenously. After the injection, the scan was started after 40–60 min of rest with eyes closed in a suitable temperature environment. All patients signed informed consent before the examination.
Image Analysis
Visual Analysis Method
Two experienced nuclear medicine physicians interpreted 18F-FDG PET images indicating whether the patient had a hypometabolic zone in the temporal lobe; in cases of disagreement, a third nuclear medicine physician was required to make a decision.
CortexID Quantitative Analysis
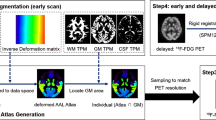
CortexID Suite quantitative analysis is a cerebral metabolic analysis software that analyzes 18F-FDG PET brain micrographs using the 3D-SSP method [19], with an adaptive template alignment technique by anatomical normalization to a standardized space. The Z-score method compares the patient’s standardized cortical peak metabolic activity with that of a healthy control group from the software’s normal group database, displaying the hypometabolic Z-score maps at eight standard angles [20, 21]. All of the above processes were automatically processed and displayed by CortexID (Fig. S1 in the Supplemental Material). In this study, the difference in the Z-score between the left and right temporal lobes was used to determine a quantitative analysis result.
Calculation and Statistical Analysis
Statistical analysis was conducted using SPSS 26.0 software and R programming language version 4.3.1. Continuous variables were first tested for conformity with normal distribution and, if not normally distributed, were described as median (interquartile spacing) [M(P25, P75)]; conformity with normal distribution was described as mean ± standard deviation (x ± SD) and compared using Student’s t-test. Otherwise, the Mann–Whitney U test was used. Categorical variables were expressed as proportions. Comparisons between the two groups were performed using the X2 or Fisher exact test, as appropriate. The area under the receiver operating characteristic (ROC) curve (AUC) area was compared using the DeLong test, and all tests were two-tailed, with p < 0.05 indicating a statistically significant difference.
Results
Construction of Diagnostic Models for TLE
Calculation of Thresholds for Quantitative Analyses of Medial Temporal Lobe Epilepsy (MTLE)
A total of 102 patients with epilepsy were included, of which nine had bilateral MTLE lesions. Therefore, a total of 93 cases were included in the diagnostic model, including 40 patients with MTLE. The ROC curves were constructed according to the assumed epileptogenic zone (Fig. 1): AUC = 0.905, Z-score threshold = 0.87 for the MTLE. The Z-score difference between the left and right MTLE was used to determine whether there were lesions in the medial temporal lobe, with the plus and minus signs representing the lateralization. An absolute value greater than the threshold value was considered as indicating the presence of lesions in the medial temporal lobe.
Construction of the MTLE Model
Univariate analysis showed statistically significant (p < 0.05) results for MRI, PET visual analysis, and CortexID quantitative analysis (Table S1 in the Supplemental Material). The multiple linear regression analysis showed that the variance inflation factor (VIF) of each variable was less than 5, indicating no collinearity issue (Table S2 in the Supplemental Material). Therefore, three factors were included in the binary logistic regression model for multifactorial analysis, indicating that the results of the PET visual analysis and CortexID quantitative analysis had an independent diagnostic basis (p < 0.05) (Table S3 in the Supplemental Material).
In this study we chose to use logistic regression and cross-validation to construct the model and calculate AUC. The results are shown in Table S4 in the Supplemental Material, indicating that the logistic regression results are superior. Therefore, logistic regression was used to construct the ROC curve, as shown in Fig. 2, and the AUC was calculated. The accuracy of the MTLE visual analysis was compared with the accuracy of each diagnostic model as shown in Table 1. Statistical results showed the following: AUC (visual analysis) = 0.922 and AUC (CortexID quantitative analysis) = 0.853, with no statistically significant difference (p = 0.136); AUC (visual analysis) = 0.922 and AUC (combination of both) = 0.971, with a statistically significant difference (p = 0.013).
Calculation of Thresholds for Quantitative Analyses of Lateral Temporal Lobe Epilepsy (LTLE)
A total of 102 patients with epilepsy were included, of which seven had bilateral LTLE lesions. Therefore, a total of 95 cases were included in the diagnostic model, among which 47 patients had LTLE. The ROC curves were constructed according to the assumed epileptogenic zone (Fig. S2 in the Supplemental Material): AUC = 0.843, Z-score threshold = 2.175 for the LTLE.
Construction of the LTLE Model
Univariate analysis showed statistically significant (p < 0.05) results for seizure duration of 1–3 min, MRI, PET visual analysis, and CortexID quantitative analysis (Table S5 in the Supplemental Material).
The multiple linear regression analysis showed that the VIF of each variable was less than 5, indicating no collinearity issue (Table S6 in the Supplemental Material). Therefore, four factors were included in the binary logistic regression model for multifactorial analysis, indicating that the results of the PET visual analysis and CortexID quantitative analysis had an independent diagnostic basis (p < 0.05) (Table S7 in the Supplemental Material).
In this study we chose to use logistic regression and cross-validation to construct the model and calculate AUC. The results are shown in Table S4 (in the Supplemental Material), indicating that the logistic regression results are superior. Therefore, logistic regression was used to construct the ROC curve, as shown in Fig. S3 in the Supplemental Material, and the AUC was calculated. The accuracy of the LTLE visual analysis was compared with each diagnostic model as shown in Table 2. Statistical results showed the following: AUC (visual analysis) = 0.842 and AUC (cortexID quantitative analysis) = 0.831, with no statistically significant difference (p = 0.808); AUC (visual analysis) = 0.842 and AUC (combination of both) = 0.897, with a statistically significant difference (p = 0.009).
Surgical Patients
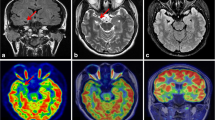
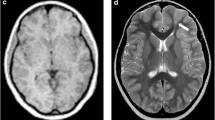
A total of 23 patients who underwent surgery were excluded from this study, which was used for the validation part of the above study. There were 29 lesions in the 23 patients (detailed clinical information can be found in Table S8 in the Supplemental Material), including six lesions outside the temporal lobe and 23 lesions in the temporal lobe (11 lesions in the medial temporal lobe and 12 lesions in the lateral temporal lobe). After follow-up (12 [6, 15] months), all of these patients had a good outcome with regard to their seizures: 21 patients with Engel I, two patients with Engel II, and no patients with Engel III–IV. Therefore, the reliability of preoperative evaluation for this group of patients is acceptable. Statistical results showed AUC (visual analysis) = 0.822 and AUC (cortexID quantitative analysis) = 0.75, and the difference was not statistically significant (p = 0.377). The sensitivity of CortexID quantitative analysis reached 100%, which was higher than that of visual analysis in Table 3 and Table S9 (in the Supplemental Material). A lesion with negative visual analysis and positive quantitative analysis is shown in Figs. 3 and 4. This patient’s PET visual analysis suggests only hypometabolic regions in the left medial temporal lobe, and CortexID quantitative analysis shows the presence of lesions in both the left medial and lateral temporal lobes. The pathological results indicated a low-grade glial neuronal tumor in the medial left temporal lobe and focal cortical dysplasia in the lateral left temporal lobe. No seizures were observed in the patient after 12 months of postoperative follow-up, and the prognosis was Engel I.
A 24-year-old patient with refractory focal epilepsy for 2 years. CortexID quantitative analysis showed a difference of 6.19 in the Z-score between the left and right sides of the medial temporal lobe of the patient, while a Z-score difference of 2.76 was found between the left and right sides of the lateral temporal lobe, indicating the presence of lesions in both the left medial and lateral temporal lobes
Ethical Approval
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. This study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Medical Ethical Committee of the First Affiliated Hospital of the Air Force Military Medical University (reference no. KY20222137).
Discussion
In this study, CortexID quantitative analysis achieved diagnostic efficacy comparable to visual analysis in the localization of the epileptogenic zone in patients with TLE. The results of postoperative pathology and follow-up suggest that CortexID quantitative analysis can provide additional information in epileptogenic zone detection.
CortexID quantitative analysis was as effective as visual analysis in diagnosing TLE in this study, suggesting that CortexID quantitative analysis is a non-inferior diagnostic modality. The results of the visual analysis are closely related to the experience of the doctor who reads the image and are highly influenced by the doctor’s subjectivity. In contrast, CortexID quantitative analysis is not affected by the subjective factors of the doctor. It is a repeatable, time-saving, and simple-to-use method. Quantitative analysis is valuable in that it can interpret images in the case of low confidence in visual analysis. Thus, it is useful in improving the speed of image reading and identifying difficult cases. Moreover, our study indicates that combining CortexID quantitative analysis with visual analysis significantly further improves the diagnostic accuracy of epileptogenic zone localization.
In follow-up data from postoperative patients, we found that quantitative analysis is more sensitive in locating the epileptogenic zone than visual analysis, consistent with previous studies. Yen-Cheng et al. [10] used a machine learning classifier to extract image features from 18F-FDG PET data and to classify normalized PET uptake with regions of interest (ROIs) from 18F-FDG PET images to identify the lateralized epileptogenic zone. Using postoperative pathology as the gold standard, they found that quantitative analysis was more sensitive than visual analysis (98.15 vs. 81.48%, respectively). All patients in our study demonstrated good outcomes (Engel I–II) during follow-up. The sensitivity of quantitative analysis in the present study was as high as 100%, which may be related to the relatively small sample of postoperative patients and the risk of selection bias. In addition, the high sensitivity in postoperative patients may indicate that using postoperative follow-up as the gold standard is more reliable.
From a clinical point of view, nuclear medicine physicians can choose to supplement their reading methodology with the CortexID quantitative analysis tool to scrutinize images through visual means. In patients with epilepsy outside the temporal lobe, we can identify the site with a large metabolic difference between the left and right sides as a reduced metabolic area, while in bilateral lesions we compare the metabolic situation of the whole brain, focusing on the area with a large difference from the whole brain. The analysis of inconsistencies and the added value of considering visually negative and quantitatively positive cases as potentially more risky adds a tool to routine assessment. Compared to other studies [22], the greatest advantage of this study is that the quantitative analysis software used is convenient to use. The software already has a database of normal human metabolism, which can quickly draw preliminary conclusions for clinical doctors. There is no need for extensive modeling and image preprocessing, nor is there a need to rebuild a normal human database.
Several limitations of this trial should be pointed out. First, the control group of CortexID quantitative analysis software used in this study comes from a database for individuals aged 42–89 years with normal imaging [23]. This database was not accurately matched with the age and gender of the patients included in our study. However, the method used in this study was designed to calculate the metabolic differences between the left and right sides rather than simply comparing the differences between each site and the normal database. Thus, poor age matching in the control group may not have had a significant impact on the analysis [12]. Furthermore, a study on pediatric patients with epilepsy found that children over 6 years of age and adults appear to have the same pattern of glucose utilization [24]. Supplementing normal control data for additional age groups may further improve the accuracy of CortexID software.
Second, this is a retrospective study that considers the assumed epileptogenic zone as the gold standard. Although postoperative pathology and follow-up data may be more accurate in the localization of the epileptogenic zone, only a small proportion of our patients received surgery. Nonetheless, the results of quantitative analysis for the surgical and nonsurgical groups are similar.
Conclusion
In conclusion, the diagnostic efficacy of CortexID quantitative analysis is comparable to PET visual analysis in the localization of the epileptogenic zone in patients with TLE. CortexID analysis is repeatable, time-saving, and simple to use, and could be used as a non-inferior diagnostic modality to improve the accuracy of preoperative epileptogenic focus localization.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Devinsky O, et al. Epilepsy. Nat Rev Dis Primers. 2018;4:18024.
Fisher RS, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475–82.
Thijs RD, et al. Epilepsy in adults. Lancet. 2019;393(10172):689–701.
Jehi L, et al. Development and validation of nomograms to provide individualised predictions of seizure outcomes after epilepsy surgery: a retrospective analysis. Lancet Neurol. 2015;14(3):283–90.
Frauscher B. Localizing the epileptogenic zone. Curr Opin Neurol. 2020;33(2):198–206.
Patel DC, et al. Neuron-glia interactions in the pathophysiology of epilepsy. Nat Rev Neurosci. 2019;20(5):282–97.
Lagarde S, et al. Relationship between PET metabolism and SEEG epileptogenicity in focal lesional epilepsy. Eur J Nucl Med Mol Imaging. 2020;47(13):3130–42.
Tan YL, et al. Quantitative surface analysis of combined MRI and PET enhances detection of focal cortical dysplasias. Neuroimage. 2018;166:10–8.
Peter J, et al. Applications of global quantitative 18F-FDG-PET analysis in temporal lobe epilepsy. Nucl Med Commun. 2016;37(3):223–30.
Shih YC, et al. Machine learning quantitative analysis of FDG PET images of medial temporal lobe epilepsy patients. Clin Nucl Med. 2022;47(4):287–93.
Guo K, et al. Morphometric analysis program and quantitative positron emission tomography in presurgical localization in MRI-negative epilepsies: a simultaneous PET/MRI study. Eur J Nucl Med Mol Imaging. 2022;49(6):1930–8.
Mendes Coelho VC, et al. Automated online quantification method for 18F-FDG positron emission tomography/CT Improves detection of the epileptogenic zone in patients with pharmacoresistant epilepsy. Front Neurol. 2017. https://doi.org/10.3389/fneur.2017.00453.
Isella V, et al. Validity of cingulate-precuneus-temporo-parietal hypometabolism for single-subject diagnosis of biomarker-proven atypical variants of Alzheimer’s disease. J Neurol. 2022;269(8):4440–51.
Bertagnoni G, et al. 18F-fluorodeoxyglucose positron-emitted tomography for predicting neurological outcome in hypoxic-ischemic encephalopathy. Brain Inj. 2021;35(10):1292–300.
Wang K, et al. Comparative study of voxel-based epileptic foci localization accuracy between statistical parametric mapping and three-dimensional stereotactic surface projection. Front Neurol. 2016;7:164.
Tripathi M, et al. Biomarker-based prediction of progression to dementia: F-18 FDG-PET in amnestic MCI. Neurol India. 2019;67(5):1310–7.
Bao YW, et al. Heterogeneity of amyloid binding in cognitively impaired patients consecutively recruited from a memory clinic: evaluating the utility of quantitative 18F-flutemetamol PET-CT in discrimination of mild cognitive impairment from Alzheimer’s disease and other dementias. J Alzheimers Dis. 2021;79(2):819–32.
Scheffer IE, et al. ILAE classification of the epilepsies: position paper of the ILAE commission for classification and terminology. Epilepsia. 2017;58(4):512–21.
Minoshima S, et al. A diagnostic approach in Alzheimer’s disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. J Nucl Med. 1995;36(7):1238–48.
Minoshima S, et al. Anatomic standardization: linear scaling and nonlinear warping of functional brain images. J Nucl Med (1978). 1994;35(9):1528–37.
Minoshima S, et al. Automated detection of the intercommissural line for stereotactic localization of functional brain images. J Nucl Med (1978). 1993;34(2):322–9.
Bacon EJ, Jin C, He D, et al. Epileptogenic zone localization in refractory epilepsy by FDG-PET: the comparison of SPM and SPM-CAT with different parameter settings. Front Neurol. 2021;12: 724680.
Knopman DS, et al. 18F-fluorodeoxyglucose positron emission tomography, aging, and apolipoprotein E genotype in cognitively normal persons. Neurobiol Aging. 2014;35(9):2096–106.
Muzik O, et al. Statistical parametric mapping: assessment of application in children. Neuroimage. 2000;12(5):538–49.
Acknowledgements
We would like to extend our gratitude to all the staff of the Neurology and Nuclear Medicine departments at Xijing Hospital. We would like to thank all the patients and their families who participated in this study.
Medical Writing and Editorial Assistance
The authors did not use any medical writing or editorial assistance for this article.
Funding
This study was funded by the Science and Technology Research and Development Program of Shaanxi Province (Grant number: 2023-YBSF-269) and the National Natural Science Foundation of China (Grant numbers: 82071449 and 82122033). The Rapid Service Fee was funded by the National Natural Science Foundation of China (Grant number: 82071449).
Author information
Authors and Affiliations
Contributions
Shuangshuang Li: conception and design of the study, data acquisition, data interpretation, and writing of the original draft. Kun Guo: data interpretation, and writing of the original draft. Yuanyuan Wang, Dianwei Wu, Yang Wang: data interpretation. Lanlan Feng, Junling Wang, Xiaoli Men: data acquisition. Lei Ma, Hua He, Fei Kang: designed and supervised the conduct of the study, critically revising the manuscript, and funding acquisition. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors Shuangshuang Li, Kun Guo, Yuanyuan Wang, Dianwei Wu, Yang Wang, Lanlan Feng, Junling Wang, Xiaoli Meng, Lei Ma, Hua He, and Fei Kang declare that there are no conflicts of interest.
Ethical Approval
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. This study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Medical Ethical Committee of First Affiliated Hospital of Air Force Military Medical University (reference no. KY20222137).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Li, S., Guo, K., Wang, Y. et al. Evaluating the Efficacy of CortexID Quantitative Analysis in Localization of the Epileptogenic Zone in Patients with Temporal Lobe Epilepsy. Neurol Ther 13, 1403–1414 (2024). https://doi.org/10.1007/s40120-024-00646-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-024-00646-1