Abstract
Introduction
Lymphocyte depletion via anti-CD52 monoclonal antibody (mAb) therapy is an effective treatment strategy for relapsing–remitting multiple sclerosis (MS) but is associated with infusion/injection-associated reactions (IARs) and autoimmune-related adverse events (AEs). Gatralimab is a next-generation humanized anti-CD52 mAb.
Methods
Two first-in-human trials were conducted in participants with progressive MS to assess the pharmacodynamics, pharmacokinetics, and safety of gatralimab administered via subcutaneous (SC) and intravenous (IV) routes, and to determine the effect of different comedication regimes on IARs to SC gatralimab. A Phase 1 trial (NCT02282826) included double-blind, placebo-controlled sequential ascending single IV (1, 3.5, and 12 mg) and SC (12, 36, and 60 mg) dose groups. A Phase 1b trial (NCT02977533) involved five groups who received SC gatralimab (36, 48, or 60 mg) and different comedications. A long-term safety (LTS) study (NCT02313285) examined safety and pharmacodynamics over 4 years.
Results
Gatralimab produced depletion of lymphocytes (dose-dependently) and CD4+ regulatory T cells, with partial repopulation to normal values by approximately 12 months. Peak serum gatralimab concentrations followed dose-proportionality and were delayed by 6.0–7.5 days following SC administration. Treatment-emergent AEs, including IARs, were reported for most participants but were generally of mild or moderate severity, and treatment-emergent serious AEs were mostly MS-related. Methylprednisolone and antihistamine comedications were associated with reduced incidence of fevers and skin and subcutaneous tissue AEs, respectively. During the LTS study, one participant (3.0%) experienced an autoimmune-related AE (Basedow’s disease), and subsequently died from pulmonary sepsis deemed unrelated to gatralimab by the investigator.
Conclusions
These data show that gatralimab achieves the desired pharmacodynamic effect of lymphocyte depletion followed by repopulation, and has an acceptable safety profile, including low risk of non-MS autoimmunity. Although gatralimab is no longer in development for MS, insights from these trials may inform the development of comedication regimes of future anti-CD52 mAbs and subcutaneous formulations of other lymphocyte-depleting mAbs.
Trial registration
NCT02282826, NCT02977533, NCT02313285.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
While highly effective at reducing relapse frequency in people with relapsing–remitting multiple sclerosis, alemtuzumab, an anti-CD52 lymphocyte-depleting monoclonal antibody, carries the risk of administration reactions and autoimmune-related adverse events. |
These trials examined the pharmacodynamics, pharmacokinetics, and safety of gatralimab, a next-generation anti-CD52 monoclonal antibody designed to provide similar efficacy as alemtuzumab with an improved safety profile in participants with progressive multiple sclerosis. |
What was learned from the study? |
Following a single intravenous or subcutaneous dose of gatralimab, lymphocytes were depleted and autoimmune-related adverse events were rare. Prophylactic glucocorticoid and antihistamine comedications reduced certain systemic and local injection-related reactions, respectively. |
These findings show that gatralimab achieves comparable lymphocyte depletion as alemtuzumab with a potentially improved safety profile. Use of prophylactic treatments may be beneficial for the management of adverse events with lymphocyte-depleting monoclonal antibodies administered subcutaneously. |
Introduction
Multiple sclerosis (MS) is a chronic inflammatory, demyelinating, and neurodegenerative disease of the central nervous system (CNS) [1, 2]. There are many approved treatments for relapsing–remitting MS (RRMS) that address acute inflammatory episodes associated with transient increases in MS-related disability (i.e., relapses) [1, 2]. One such therapy is the humanized anti-CD52 monoclonal antibody (mAb) alemtuzumab which depletes CD52-expressing lymphocytes, enabling subsequent repopulation of lymphocytes with reduced pathogenicity [3,4,5]. While highly effective at reducing relapse frequency in people with RRMS, alemtuzumab treatment is associated with several administration-related reactions and an increased risk of autoimmune conditions [6, 7].
Gatralimab (GLD52; GZ402668) was designed as a next-generation humanized anti-CD52 mAb, with the aim of providing similar efficacy to alemtuzumab while offering the possibility for reduced infusion/injection-associated reactions (IARs) and decreased immunogenicity. Gatralimab targets a CD52 epitope that overlaps with, but is distinct from, the epitope targeted by alemtuzumab [8]. Like alemtuzumab, gatralimab induces lymphocyte depletion through antibody-dependent cell-mediated cytotoxicity and complement-dependent cell-mediated cytotoxicity [9, 10]. However, preclinical evidence suggests that proinflammatory cytokine release, which may account for certain IARs [11], is less with gatralimab than with alemtuzumab [8]. Gatralimab is no longer being developed as a potential treatment for MS due to a business decision unrelated to its safety, pharmacokinetic, or pharmacodynamic profile.
IARs are common adverse events (AEs) associated with mAb therapy and would therefore be expected for all anti-CD52 treatments [12]. Mild to moderate IARs include chills, fever, mild hypotension, dyspnea, and rash, while severe reactions are associated with marked hypotension, anaphylaxis, and cardiac dysfunction [13]. Several approaches have been suggested for the management of IARs. For alemtuzumab, the approaches used to manage IARs in the Phase 3 CARE-MS trials included a combination of pharmacological therapies, infusion interruption or rate reduction, and continual patient education and support [11]. Pharmacological management of IARs to alemtuzumab involves premedication with a corticosteroid (methylprednisolone; 1000 mg) immediately prior to each infusion on the first three days of any treatment course in addition to antipyretics and/or antihistamines at the physician’s discretion [5, 11].
The present report describes two first-in-human trials that were conducted in participants with progressive MS to assess the pharmacodynamics, pharmacokinetics, and safety of gatralimab after single ascending intravenous (IV) and subcutaneous (SC) doses (Phase 1; NCT02282826), and after a single SC dose with different comedication regimes for management of IARs (Phase 1b; NCT02977533). Comedications included a corticosteroid (methylprednisolone), a non-steroidal anti-inflammatory drug (NSAID), and antihistamines (H1 and H2 antagonists).
Methods
Study Design
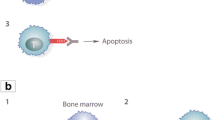
Two single-center, randomized, double-blind, placebo-controlled trials were conducted (Fig. 1). The Phase 1 trial consisted of sequential ascending single-dose studies of gatralimab administered IV (1, 3.5, and 12 mg) and SC (12, 36, and 60 mg). The Phase 1b trial involved five groups who received a single SC dose of gatralimab (36, 48, or 60 mg) and various pre-specified comedications to manage IARs. Both Phase 1 and 1b trials followed participants for 4 weeks after which they could enroll in a 4-year open-label long-term safety (LTS) study (NCT02313285).
Study design. A Phase 1. B Phase 1b. Phase 1 participants administered ≥ 12 mg of gatralimab received comedication as described in the Materials and Methods section. For cohort 1A, naproxen was administered pre-dose on D1 and every 12 h on D1 and 2. For cohort 1B, methylprednisolone was administered 24 h and 30 min pre-dose. For cohort 1C, methylprednisolone was administered 24 h and 30 min pre-dose and 24 h post-dose. For cohort 1D, methylprednisolone was administered as per cohort 1C when given alone or for two additional days (D3 and 4) when given with antihistamines. For cohort 2E, methylprednisolone was administered 24 h and 30 min pre-dose and every 24 h on D2–4 with no taper (640 mg total). Ranitidine, levocetirizine, and fexofenadine were administered once daily from D1 to D7. Fexofenadine was administered twice daily in cohort 2E if exanthema occurred. n indicates the number of participants in each group. *When sufficient pharmacological activity was demonstrated, the dose level was repeated IV in an additional 8 participants. Otherwise, dose escalation proceeded via IV administration. **After review of the pharmacologically active IV dose level (n = 12), transition to SC administration occurred.#Premedication determined after completion of cohort 1c. ##Premedication determined after SDRM. D day, IV intravenous, SC subcutaneous, SDRM Safety Data Review Meeting
Standard Protocol Approvals, Registrations, and Participant Consents
These trials were conducted in accordance with international ethics guidelines, including the Declaration of Helsinki and International Council for Harmonisation Good Clinical Practice guidelines, and all applicable laws, rules, and regulations. Written informed consent was obtained from all participants. The authors have used the Expanded Disability Status Scale (EDSS) in this article.
Study Population and Selection Criteria
Participants were adults aged 18–65 years with a diagnosis of progressive MS (i.e., MS according to the 2010 revisions to the McDonald criteria [14] and either primary progressive MS, secondary progressive MS, or progressive relapsing MS), body weight greater than 40 kg, and normal cardiovascular and laboratory parameters. Enrolment of people with progressive MS in these placebo-controlled trials was justified by evidence showing that alemtuzumab may reduce disability accrual [15] and was not expected to result in a lost treatment opportunity, since treatment options for progressive MS were limited when these trials were initiated in 2014. Key exclusion criteria were: current diagnosis of RRMS, recent use of a potentially interfering substance (including immunosuppressants or immunomodulators; Table S1), significant comorbidities (including poorly controlled hypertension, cardiovascular disease, inflammatory disorders, immunodeficiency, autoimmune disease, renal failure, liver dysfunction, cancers other than treated basal skin cell carcinoma, and active infection), previous alemtuzumab treatment, active infection, prior history of invasive fungal infection or tuberculosis, high risk of infection, and recurrent headaches, migraines, nausea, or vomiting. Participants were permitted to use stable concomitant medication during the trials provided the medications were not exclusionary at enrollment.
Treatment Protocol
Following screening for up to 4 weeks, eligible participants were randomly assigned to receive gatralimab or placebo. All investigators, study site personnel, and participants were blinded to treatment allocation. Participants were administered gatralimab or matching placebo once (i.e., on Day 1) either via IV infusion over 4 h or SC abdominal injection. To avoid viral infections, participants received 200 mg oral acyclovir twice daily from the day of gatralimab or placebo administration up to 28 days post-dose.
In the Phase 1 trial, IV dose escalation involved three groups (1, 3.5, and 12 mg gatralimab), each with four participants (3:1, gatralimab:placebo; Fig. 1A). Dose escalation commenced with the 1-mg arm and continued until sustained lymphocyte depletion through Day 10 was demonstrated. Thereafter, a fourth group of eight participants (6:2, gatralimab: placebo) received the pharmacologically active dose (12 mg). After review of the IV data, SC dose escalation occurred in three groups (12, 36, and 60 mg gatralimab), each with eight participants (6:2, gatralimab:placebo). Participants receiving ≥ 12 mg of gatralimab received premedication and/or prophylaxis to reduce IARs as follows. Methylprednisolone (125 mg IV) was administered 0.5–1 h pre-dose for the initial 12-mg IV cohort. IAR reduction for the other cohorts involved oral administration of ibuprofen (400 mg; an NSAID) at different pre-determined time points due to its short half-life. For the second 12-mg IV dose group and the 12-mg SC cohort, ibuprofen was administered pre-dose and at 2 h post-dose. For the 36-mg SC group, ibuprofen was administered at 6 and 9 h post-dose. For the 60-mg SC arm, ibuprofen was administered at 4, 8, and 12 h post-dose. All participants were able to receive ibuprofen for symptomatic management of AEs.
In the Phase 1b trial, five groups each with four participants (3:1, gatralimab:placebo) received a single SC dose of gatralimab (36, 48, or 60 mg) and different comedication regimes to manage IARs. The comedication regimens are described in Fig. 1B and included naproxen (an NSAID) or methylprednisolone with or without antihistamines (H1 and H2 antagonists).
In the LTS study, participants remained blinded for 6 months, after which the blinding was removed and participants who received placebo were discharged. Participants had monthly visits for the study duration and did not receive additional gatralimab courses.
Pharmacodynamics
Lymphocyte phenotyping was the primary pharmacodynamic measure and involved counts of lymphocytes and the CD4+ cell subpopulation with a phenotype consistent with regulatory T cells (Tregs). In the Phase 1 and 1b trials, blood samples for lymphocyte phenotyping were obtained at baseline (Day 1, pre-dose), 6 and 12 h from the start of administration, and on Days 2, 3, 4, 7, 10, 15, and 29. In the LTS study, lymphocyte counts were measured every 3 months from Month 3 to 12 and every 6 months thereafter. CD4+ Treg cells were assessed at baseline, on Day 29, and every 3 months of the LTS study from Month 3 to 12 and every 6 months thereafter.
Pharmacokinetics
Blood samples for pharmacokinetic assessments were obtained at baseline (Day 1, pre-dose) and at 2, 4, 8, 24, 36, 48, and 72 h post-dose as well as on Days 7, 10, 15, and 29. Plasma concentrations of gatralimab were determined using a validated enzyme-linked immunosorbent assay method with a lower limit of quantitation of 0.123 µg/mL. Pharmacokinetic parameters were determined using noncompartmental methods and included the maximum concentration observed (Cmax), time to reach Cmax (tmax), time of last measurable concentration (tlast), terminal half-life (t½), steady-state volume of distribution (Vss), and total clearance (CL).
Safety Assessments
Safety assessments included AEs reported by participants or observed by the investigator, laboratory tests (hematology, chemistry, prothrombin time, and urinalysis), vital signs, and physical and neurological examinations. An AE of special interest (AESI) was defined as a serious or non-serious adverse event of scientific and medical concern, specific to gatralimab, for which ongoing monitoring and rapid communication by the investigator to the study sponsor was appropriate. AESIs were: hepatic enzyme elevation, QTc ≥ 500 ms, pregnancy, overdose with gatralimab or any comedication, hypersensitivity or anaphylaxis, autoimmune cytopenias, complete blood count decreased, glomerulonephropathies, serious infections, malignancy, thyroid disorder which is clinically manifest or requires endocrinologist referral or additional testing or treatment, and cervical dysplasia and/or cervical intraepithelial neoplasia indicative of human papillomavirus infection. IARs were defined as any AE with onset during or within 24 h of gatralimab or placebo administration.
Statistical Analysis
Demographic data were recorded for all participants. Descriptive statistics were used to summarize continuous and categorical variables. For pharmacodynamic measures, mean percentage changes from baseline were determined. Pharmacokinetic analyses were performed separately for each administration route.
Results
Respondent Characteristics
Demographics and baseline disease characteristics were generally similar between trials and treatment groups, except for the mean time since first diagnosis which was higher in the IV than SC cohort in the Phase 1 trial (Table 1). All randomized participants completed their allocated trial and enrolled in the LTS study. Six participants discontinued the LTS study due to personal decision (n = 1 for the IV cohort and n = 3 for the SC cohort), AE (n = 1 for the SC cohort), or being lost to follow-up (n = 1 for the SC cohort).
Pharmacodynamics
Phase 1 Trial
Lymphocyte depletion was observed across all IV and SC cohorts and was dose-dependent for each administration route (Fig. 2A, B). The mean decrease in absolute lymphocyte count from baseline to Day 29 was 81.4%, 79.3%, and 88.5% for the 12-mg IV, 36-mg SC, and 60-mg SC cohorts, respectively. Partial lymphocyte repopulation during the Phase 1 trial was evident in the lower dose groups that showed less complete depletion. Compared with SC administration, lymphocyte depletion with IV administration was more rapid and of greater magnitude [trough lymphocyte counts ± standard deviation (SD) were 0.57 ± 0.64 at 72 h post-dose and 0.07 ± 0.05 cells/nL at 6 h post-dose for the 12-mg SC and IV groups with ibuprofen comedication, respectively].
Mean absolute lymphocyte count during Phase 1 and 1b trials (cells/nL ± SD). A Phase 1 IV cohorts, B Phase 1 SC cohort, C Phase 1b SC cohort. Lower and upper limits of normal as provided by the clinical laboratory are indicated with solid gray lines. CS corticosteroid, D day, H hour, IB ibuprofen, IV intravenous, premed premedication, prophy prophylaxis, SC subcutaneous, SD standard deviation
Participants who received IV or SC gatralimab had comparable depletion of CD4+ Treg cells (Fig. 3A, B). The mean overall decrease in CD4+ Treg cell count from baseline to Day 29 was 91.9% and 96.3% for the 12-mg IV and 60-mg SC arms, respectively. CD4+ Treg cells remained stable in the placebo groups. The proportion of Treg to total CD4+ cells increased in all gatralimab cohorts, rising from mean ± SD of 6.7% ± 1.8% at baseline to 20.2% ± 12.0% at Day 29 for the combined IV cohort and 6.8% ± 1.6% to 26.8% ± 10.8% for the combined SC cohort. Depletion was observed across other lymphocyte subpopulations (including B cells, T cells, and natural killer cells) and subsets in all groups (data not shown).
Mean absolute CD4+ Treg count during Phase 1 and 1b trials (cells/nL, ± SD). A Phase 1 IV cohorts, B Phase 1 SC cohort, C Phase 1b SC cohort. Lower and upper limits of normal as provided by the clinical laboratory are indicated with solid gray lines. CS corticosteroid, H hour, IB ibuprofen, IV intravenous, premed premedication, prophy prophylaxis, SC subcutaneous, SD standard deviation, Treg regulatory T cells
Phase 1b Trial
Lymphocyte depletion was observed in all cohorts (Fig. 2C). Participants who received placebo showed transient lymphocyte depletion at 6–12 h post-dose with complete lymphocyte repopulation by 24 h post-dose. In contrast, mean lymphocyte counts continued to decline from 6 h post-dose for participants who received gatralimab, with maximal depletion occurring around 48–72 h post-dose and remaining below normal limits through to Day 29. Maximal lymphocyte depletion was generally similar between gatralimab cohorts, with no apparent effect of dose or comedication. The mean decrease in absolute lymphocyte count from baseline to Day 29 was 90.4%, 86.7%, and 88.0% for the combined 36-, 48-, and 60-mg groups, respectively.
CD4+ Treg cells also showed substantial depletion at Day 29 relative to baseline, with mean percentage reductions of 95.2%, 92.1%, and 94.0% for the 36, 48, and 60 mg groups, respectively (Fig. 3C). CD4+ Treg cells remained stable in the placebo group. The proportion of Treg to total CD4+ cells increased in all gatralimab cohorts, rising from mean ± SD 6.7% ± 1.6% at baseline to 33.5% ± 10.6% at Day 29 for the combined cohort.
LTS Study
Total lymphocyte and Treg cell counts gradually increased throughout the LTS study in the combined gatralimab group. Mean lymphocyte count was 21.3% of that at baseline at Month 1 and rose steadily thereafter to 38.6% and 52.3% at Months 12 and 48, respectively (Fig. 4A). Mean CD4+ Treg cell count was 11.2% of that at baseline at Month 1 and 39.5% at Month 48 (Fig. 4B). Mean lymphocyte and CD4+ Treg cell counts were within the normal range by Month 12 and 18, respectively (Fig. 4A, B).
Pharmacodynamics during the LTS study. A Mean absolute lymphocyte count (cells/nL ± SD), B Mean absolute CD4+ Treg cell count (cells/nL ± SD). Lower and upper limits of normal as provided by the clinical laboratory are indicated with solid gray lines. B baseline, SD standard deviation, Treg regulatory T cells
Pharmacokinetics
Mean serum gatralimab Cmax values increased approximately dose-proportionally following IV and SC administration (Table S2). Peak serum concentrations occurred at the end of IV infusion (Table S2) and by 6.0–7.5 days following SC administration. The mean terminal half-life was approximately 11 days for the 12-mg IV dose and approximately 13 days for SC doses in the Phase 1 trial (Table S2). Gatralimab was eliminated slowly with measurable serum concentrations up to the last sampling time (28 days post-dose) (Table S2 and S3).
Safety
Phase 1 Trial
No deaths, serious treatment-emergent adverse events (TEAEs), or Grade 3 or higher AEs were reported in the Phase 1 trial (Table 2).
For the IV cohort, TEAEs were reported for 14 participants (93.3%) who received gatralimab and four participants (80.0%) who received placebo, with no apparent effect of dose. The most common TEAEs were headache, nausea, raised body temperature, and asthenia (Table 3). IARs were reported for 12 participants (80.0%) who received IV gatralimab. Three participants who received IV gatralimab experienced a treatment-emergent AESI: one participant in the 3.5-mg group reported moderately elevated liver enzymes; one participant in the 12-mg group reported mild thrombocytopenia, and one participant in the 12-mg group reported moderately elevated liver enzymes and mild thrombocytopenia. All abnormal laboratory values returned to the normal range by Day 29.
For the SC cohort, TEAEs were reported for all participants who received gatralimab or placebo. The most common TEAEs were injection site erythema, raised body temperature, headache, injection site edema, and asthenia (Table 3). IARs were reported for 16 participants (88.9%) who received SC gatralimab. One participant who received 60 mg gatralimab experienced an AESI of moderately elevated liver enzymes, with values returning to normal within 9 days. The incidence of individual TEAEs, particularly IARs, increased proportional to gatralimab dose (data not shown).
In an analysis of gatralimab dose groups showing similar levels of lymphocyte depletion (Fig. 2A, B), incidences of common systemic TEAEs were: headache (83.3%, 66.7%, and 100% for the 12-mg IV with NSAID comedication, 36-mg SC, and 60-mg SC groups, respectively), raised body temperature (16.7%, 100%, and 100%), nausea (50.0%, 0%, and 50.0%), and fatigue (16.7%, 16.7%, and 0%).
Phase 1b
No deaths were reported following gatralimab administration. Four participants (26.7%) reported severe (Grade 3 or higher) TEAEs, and one (6.7%) participant reported a treatment-emergent serious AE (clear cell renal cell carcinoma).
All participants in the gatralimab group and four participants (80.0%) in the placebo group reported TEAEs (Table 2). A total of 172 TEAEs were reported, of which 75 (43.6%) were deemed related to gatralimab administration. One participant experienced an AESI (moderately elevated liver enzymes) that was deemed unrelated to gatralimab. The most common TEAEs in the gatralimab group were injection site erythema, raised body temperature, and headache (Table 4). IARs were reported for all participants who received SC gatralimab and all were of mild or moderate severity. The incidence of skin and subcutaneous tissue disorders (injection site erythema, pruritus, and rash) was lower for participants administered antihistamine comedication. Fevers and chills were reported at a lower frequency for participants who received methylprednisolone compared to participants who received naproxen.
LTS Study
During the extension period, treatment-emergent serious AEs were reported for 20.0% of participants in the IV cohort and 57.6% of participants in the SC cohort. The most commonly reported treatment-emergent serious AEs were MS relapse (6.7% and 33.3% of participants in the IV and SC cohorts, respectively), MS (0% and 6.1%), and road traffic accident (6.7% and 3.0%). Of the 73 treatment-emergent serious AEs, one (1.4%; a urinary tract infection) was deemed possibly related to gatralimab. All participants in the IV cohort and 97.0% of participants in the SC cohort experienced a TEAE; 26.7% and 57.6% experienced a TEAE of Grade 3 or higher. The most common TEAEs were nasopharyngitis, urinary tract infection, MS relapse, and headache (Table 5). AESIs were reported for no participant in the IV cohort and 12.1% of participants in the SC cohort, including one participant (3.0%) each with Basedow’s disease, clear cell renal carcinoma, goiter, and thyroid mass.
The AESI of Basedow’s disease occurred in a 51-year-old male who received 48 mg SC gatralimab 3.4 years earlier and was deemed possibility related to gatralimab by the investigator. Approximately 1 month following diagnosis of Basedow’s disease, the participant developed fatal pulmonary sepsis during hospitalization due to myocardial infarction. The death was deemed unrelated to gatralimab by the investigator. No other deaths occurred during the LTS study. The AESI of clear cell renal carcinoma was reported for a 54-year-old male who was administered 36 mg SC gatralimab. The carcinoma was detected 28 days following gatralimab administration on investigation of elevated serum creatinine level reported on the same day. It was considered unrelated to gatralimab and resolved following nephrectomy. The single case of goiter occurred in a 41-year-old female 10 months following 12 mg SC gatralimab, was considered unlikely to be related to gatralimab, and was resolving at end of study. A thyroid mass, which was reported for a 56-year-old female who received 60 mg SC gatralimab 3.5 years earlier, was deemed unrelated to gatralimab and was unresolved at the end of the study.
Discussion
We report two first-in-human trials of gatralimab, an anti-CD52 mAb, and their 4-year LTS extension. Gatralimab produced the desired pharmacodynamic response of lymphocyte depletion followed by partial repopulation, across a range of SC and IV doses, in participants with progressive MS. Although TEAEs were experienced by almost all participants who received gatralimab, treatment-emergent serious AEs or severe (Grade 3 or higher) AEs were relatively uncommon during the Phase 1 and 1b trials, while those that occurred during the LTS study were mostly MS-related. For SC gatralimab, the profile of IARs was dependent on the comedications administered, with skin and subcutaneous tissue disorders being less common when antihistamines were administered, and fevers being less frequent with methylprednisolone comedication than with naproxen.
The safety risks accompanying anti-CD52 mAb treatment, including IARs and secondary autoimmunity, were predicted to be improved with gatralimab due to the mAb’s distinct epitope binding and potential for SC administration. In these trials, IARs were reported for the majority of participants who received gatralimab, which is similar to alemtuzumab, an anti-CD52 mAb which is approved for the treatment of RRMS via IV infusion [5, 11, 16]. However, severe IARs, which are reported for ~ 7–10% of people treated with alemtuzumab [11, 16], were not observed with gatralimab. This difference may be underscored by reduced proinflammatory cytokine release with gatralimab versus alemtuzumab as suggested by preclinical studies [8]. The profile of IARs to gatralimab was generally similar to that reported for other mAbs that are administered via both SC and IV routes [17,18,19]. Local IARs (e.g., erythema and edema) were most common following SC injection. Although certain systemic IARs (e.g., nausea and fatigue) were more frequent with IV infusion, fever was more common with SC administration.
Over 4 years of follow-up in the LTS study, nearly all participants experienced a TEAE, which is in line with the findings of other trials that included participants with moderately advanced progressive MS [20, 21]. In the LTS study, serious AEs were reported by a higher percentage of participants who received gatralimab via SC injection compared to IV infusion, though these were most commonly related to MS disease activity. Autoimmune conditions were included as an AESI in the LTS study since they are common with alemtuzumab, occurring in approximately one-third of people [7]. In these trials, only one (3.0%) participant experienced non-MS autoimmunity (Basedow’s disease) over 4 years, noting that the sample size was relatively small (n = 48) and gatralimab was administered as a single dose in contrast to alemtuzumab treatment which involves two or more doses.
Although gatralimab is no longer being developed as a potential therapy, insights from these trials may inform future trials for other anti-CD52 mAb treatments for MS. Currently, there are no established comedication regimes for subcutaneously administered anti-CD52 mAbs since those that have been developed for alemtuzumab are optimized for IV administration [5, 11]. A key challenge for the choice and timing of the prophylactic treatment of IARs for subcutaneously administered mAbs is that SC administration delays mAb absorption into the systemic circulation [22]. To address this, this Phase 1b trial examined comedication regimes that included a long-acting NSAID (naproxen) and a long-acting H1 receptor antagonist (fexofenadine), as well as repeated doses of the prophylactic treatment over multiple days. In line with a real-world study of alemtuzumab IARs [16], we found that participants who received antihistamines prior to and following SC gatralimab had reduced incidence of rash and other skin and subcutaneous tissue disorders. In addition, participants who received methylprednisolone had lower incidence of fevers compared to participants who received naproxen. These findings may inform the design of IAR-reduction strategies for future anti-CD52 mAb treatments, including alemtuzumab for which SC administration is being investigated [23].
Pharmacodynamic analyses showed that gatralimab elicited dose-dependent lymphocyte depletion when administered IV and SC. In the placebo groups, small and transient lymphocyte reductions were observed, which may be due to glucocorticoid comedication (for Phase 1b trial participants) and endogenous glucocorticoid release stimulated by the injection/infusion procedures [24]. In the gatralimab groups, maximal lymphocyte depletion was reached more rapidly after IV than SC administration, in line with the delayed absorption of SC gatralimab. However, the magnitude of lymphocyte depletion at 1 month after gatralimab administration was similar for the highest IV (12 mg; 81.4%) and SC (60 mg; 88.5%) doses. The magnitude of lymphocyte depletion 1 month following IV infusion of 12 mg gatralimab was comparable to that previously reported for alemtuzumab (88.6%) [25]. Likewise, lymphocyte repopulation kinetics for gatralimab resembled that of alemtuzumab [25], with lymphocytes and the CD4+ Treg cell subpopulation returning to normal levels approximately 12–18 months post-dose, though remaining below baseline values through to Year 4 of the LTS study. Furthermore, gatralimab increased the proportion of Treg to total CD4+ cells at 1 month relative to baseline, which is similar to that observed with alemtuzumab and viewed as potentially advantageous in providing long-lasting suppression of disease activity [26]. These findings confirm the desired biological activity of gatralimab.
The key strengths of these trials are that they followed an established study design for first-in-human studies assessing pharmacodynamics, pharmacokinetics, and safety, and one-half of participants were women, reflecting the disease population. AEs were documented meticulously, noting all individual symptoms of infusion- or injection-related local and systemic reactions, which allowed detailed comparisons. In terms of limitations, sample sizes were small, and the population was drawn from a single center, which together may limit the robustness of conclusions and generalizability to the larger MS population. In addition, the placebo group exited the LTS study at Month 6, precluding comparisons to the untreated condition for long-term safety and pharmacodynamic data. Therefore, larger trials with longer blinded treatment periods are necessary to comprehensively define the safety profile of gatralimab.
Conclusion
Gatralimab showed dose-dependent lymphocyte depletion followed by lymphocyte repopulation in participants with progressive MS. This desired pharmacodynamic effect was accompanied by an acceptable safety profile characterized by mild or moderate severity IARs that are typical of anti-CD52 treatment, though relatively few treatment-related serious AEs and autoimmune-related AEs occurred over 4 years of follow-up. Although the development of gatralimab has been discontinued, the present findings support an optimized comedication regimen with methylprednisolone, fexofenadine, and ranitidine as a strategy to reduce IARs for future anti-CD52 mAbs.
References
Gajofatto A, Benedetti MD. Treatment strategies for multiple sclerosis: when to start, when to change, when to stop? World J Clin Cases. 2015;3(7):545–55.
Attfield KE, Jensen LT, Kaufmann M, Friese MA, Fugger L. The immunology of multiple sclerosis. Nat Rev Immunol. 2022;22(12):734–50.
Rao SP, Sancho J, Campos-Rivera J, Boutin PM, Severy PB, Weeden T, et al. Human peripheral blood mononuclear cells exhibit heterogeneous CD52 expression levels and show differential sensitivity to alemtuzumab mediated cytolysis. PLoS ONE. 2012;7(6): e39416.
Gilmore W, Lund BT, Li P, Levy AM, Kelland EE, Akbari O, et al. Repopulation of T, B, and NK cells following alemtuzumab treatment in relapsing-remitting multiple sclerosis. J Neuroinflammation. 2020;17(1):189.
Sanofi Belgium. LEMTRADA summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/lemtrada-epar-product-information_en.pdf. Accessed 16 Sept 2022.
Coles AJ, Arnold DL, Bass AD, Boster AL, Compston DAS, Fernandez O, et al. Efficacy and safety of alemtuzumab over 6 years: final results of the 4-year CARE-MS extension trial. Ther Adv Neurol Disord. 2021;14:1756286420982134.
Coles AJ, Jones JL, Vermersch P, Traboulsee A, Bass AD, Boster A, et al. Autoimmunity and long-term safety and efficacy of alemtuzumab for multiple sclerosis: benefit/risk following review of trial and post-marketing data. Mult Scler. 2022;28(5):842–6.
Siders W, Wei R, Greene B, McVie-Wylie A, Bailey M, Dhawan V. GZ402668, a next-generation anti-CD52 antibody, displays decreased proinflammatory cytokine release in vitro (P3.068). Neurology. 2016;86(Supplement 16).
Margolin DH, Karimi-Anderesi N, Chirieac M, Luo X, Hueser A, Albach FN, et al. Safety, tolerability, and pharmacodynamics of intravenous and subcutaneous doses of the anti-CD52 antibody GLD52 in patients with progressive MS: a randomized, controlled, single ascending dose trial (P5.375). Neurology. 2017;88(16 Supplement).
Kasarello K, Mirowska-Guzel D. Anti-CD52 therapy for multiple sclerosis: an update in the COVID era. Immunotargets Ther. 2021;10:237–46.
Caon C, Namey M, Meyer C, Mayer L, Oyuela P, Margolin DH, et al. Prevention and management of infusion-associated reactions in the comparison of alemtuzumab and rebif® efficacy in multiple sclerosis (CARE-MS) program. Int J MS Care. 2015;17(4):191–8.
Cáceres MC, Guerrero-Martín J, Pérez-Civantos D, Palomo-López P, Delgado-Mingorance JI, Durán-Gómez N. The importance of early identification of infusion-related reactions to monoclonal antibodies. Ther Clin Risk Manag. 2019;15:965–77.
Lenz H-J. Management and preparedness for infusion and hypersensitivity reactions. Oncologist. 2007;12(5):601–9.
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302.
Coles AJ, Twyman CL, Arnold DL, Cohen JA, Confavreux C, Fox EJ, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet. 2012;380(9856):1829–39.
Vukusic S, Brassat D, de Seze J, Izquierdo G, Lysandropoulos A, Moll W, et al. Single-arm study to assess comprehensive infusion guidance for the prevention and management of the infusion associated reactions (IARs) in relapsing-remitting multiple sclerosis (RRMS) patients treated with alemtuzumab (EMERALD). Mult Scler Relat Disord. 2019;29:7–14.
Matucci A, Vultaggio A, Danesi R. The use of intravenous versus subcutaneous monoclonal antibodies in the treatment of severe asthma: a review. Respir Res. 2018;19(1):154.
Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198–207.
Bittner B, Richter W, Schmidt J. Subcutaneous administration of biotherapeutics: an overview of current challenges and opportunities. BioDrugs. 2018;32(5):425–40.
Montalban X, Hauser SL, Kappos L, Arnold DL, Bar-Or A, Comi G, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376(3):209–20.
Vermersch P, Brieva-Ruiz L, Fox RJ, Paul F, Ramio-Torrenta L, Schwab M, et al. Efficacy and safety of masitinib in progressive forms of multiple sclerosis: a randomized, phase 3, clinical trial. Neurol Neuroimmunol Neuroinflamm. 2022;9(3).
Richter WF, Bhansali SG, Morris ME. Mechanistic determinants of biotherapeutics absorption following SC administration. AAPS J. 2012;14(3):559–70.
Acevedo BR, Nos C, Baker D, Wu Y, Chirieac M, Montalban X. Lymphocyte depletion and repopulation following subcutaneous or intravenous alemtuzumab in patients with progressive multiple sclerosis: SCALA study results (P1-1.Virtual). Neurology. 2022;98(18 Supplement):2845.
Rota S, Rambaldi A, Gaspari F, Noris M, Daina E, Benigni A, et al. Methylprednisolone dosage effects on peripheral lymphocyte subpopulations and eicosanoid synthesis. Kidney Int. 1992;42(4):981–90.
Li Z, Richards S, Surks HK, Jacobs A, Panzara MA. Clinical pharmacology of alemtuzumab, an anti-CD52 immunomodulator, in multiple sclerosis. Clin Exp Immunol. 2018;194(3):295–314.
Zhang X, Tao Y, Chopra M, Ahn M, Marcus KL, Choudhary N, et al. Differential reconstitution of T cell subsets following immunodepleting treatment with alemtuzumab (anti-CD52 monoclonal antibody) in patients with relapsing-remitting multiple sclerosis. J Immunol. 2013;191(12):5867–74.
Acknowledgements
The authors would like to thank all study participants.
Medical Writing and Editorial Assistance
Medical writing assistance was provided by Conor F. Underwood, PhD, and Tina Peckmezian, PhD, of Envision Pharma Group, and was funded by Sanofi.
Author Contributions
Fredrik N. Albach was involved in the study design. Fredrik N. Albach, Christian Geier, Christian Keicher, Maximilian G. Posch, Gerald Grütz, Levent Akyüz, and Frank Wagner were the investigators. Fredrik N. Albach, Christian Geier, Levent Akyüz, Xiaodong Luo, Annaig Le-Halpere, and Philippe Truffinet analyzed the data. Fredrik N. Albach, Christian Geier, Christian Keicher, Maximilian G. Posch, Stephan J. Schreiber, Xiaodong Luo, Annaig Le-Halpere, Philippe Truffinet, and Frank Wagner contributed to the interpretation of study results. All authors participated in the drafting, critical revision, and approval of the final version of the manuscript.
Funding
This study was funded by Sanofi. The journal’s Rapid Service Fee was funded by Sanofi.
Data Availability
The datasets generated during and/or analyzed during the current study are available in the vivli repository, https://vivli.org/. Qualified researchers may request access to patient-level data and related documents. Patient-level data will be anonymized, and study documents will be redacted to protect the privacy of participants. Further details on Sanofi’s data sharing criteria and process for requesting access can be found at: https://vivli.org/.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The current affiliation for Fredrik N. Albach is: Department of Rheumatology and Clinical Immunology, Charité – Universitätsmedizin Berlin, Berlin, Germany. The current affiliation for Maximilian G. Posch is: Scirent Clinical Research and Science GmbH, Berlin, Germany. Gerald Grütz and Levent Akyüz are employees of Berlin Institute of Health/Charité Universitätsmedizin Berlin and are co-founders of CheckImmune GmbH. Xiaodong Luo, Annaig Le-Halpere, and Philippe Truffinet are employees of Sanofi and may hold shares and/or stock options in the company. Fredrik N. Albach, Christian Geier, Christian Keicher, Maximilian G. Posch, Stephan J. Schreiber, and Frank Wagner have no financial disclosures.
Ethical Approval
These trials were conducted in accordance with international ethics guidelines, including the Declaration of Helsinki and International Council for Harmonisation Good Clinical Practice guidelines, and all applicable laws, rules, and regulations. Written informed consent was obtained from all participants.
Additional information
Prior Presentation This manuscript includes work that was presented at the 7th Joint European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS)—Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Meeting, 25–28 October 2017, Paris, France.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Albach, F.N., Geier, C., Keicher, C. et al. Phase 1 Trials of Gatralimab, a Next-Generation Humanized Anti-CD52 Monoclonal Antibody, in Participants with Progressive Multiple Sclerosis. Neurol Ther (2024). https://doi.org/10.1007/s40120-024-00659-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40120-024-00659-w