Abstract
Introduction
Invasive meningococcal disease (IMD) causes significant mortality and long-term sequelae. This study assesses the potential public health impact of adolescent vaccination strategies employing MenACWY and MenC vaccines in Germany, where the existing meningococcal immunisation programme predominantly involves MenC administration in toddlers.
Methods
A dynamic transmission model was developed to simulate the carriage of five meningococcal serogroup compartments (AY/B/C/W/Other) from 2019 until 2060 within 1-year age groups from 0 to 99 years of age. IMD cases were estimated based on case-carrier ratios. The model considered vaccine effectiveness against carriage acquisition and IMD.
Results
The model predicts that introducing MenACWY adolescent vaccination could lead to a considerable reduction in IMD incidence, with the potential to prevent up to 65 cases per year and a cumulative total of 1467 cases by 2060. This decrease, mainly driven by herd effects, would result in a reduction of IMD incidence across all age groups, regardless of vaccination age. Furthermore, implementing MenACWY vaccination in adolescents is projected to decrease annual MenACWY-related IMD mortality by up to 64%, equating to an overall prevention of 156 IMD deaths by 2060. These protective outcomes are expected to culminate in approximately 2250 life years gained (LYG) throughout the model's projected time horizon. In contrast, the adoption of MenC vaccination in adolescents is predicted to have minimal influence on both IMD incidence and mortality, as well as on LYG.
Conclusion
The results of this study demonstrate that implementing MenACWY vaccination for adolescents in Germany is likely to notably reduce IMD incidence and mortality across age groups. However, the introduction of MenC adolescent vaccination shows only limited impact. Considering the extensive healthcare resources typically required for IMD management, these findings suggest the potential for economic benefits associated with the adoption of MenACWY adolescent vaccination, warranting further cost-effectiveness analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? | |
Invasive meningococcal disease (IMD) presents a significant public health challenge in Germany, with high lethality and long-term sequelae. | |
This study aimed to assess the impact of introducing MenACWY and MenC adolescent vaccination in Germany, focussing on their potential to address the unmet need in reducing IMD cases, especially from serogroups W and Y. | |
What was learned from the study? | |
The introduction of MenACWY vaccination for adolescents could lead to a substantial reduction in IMD cases and deaths, preventing up to 65 annual IMD cases and a total of 1467 IMD cases by 2060, while additional MenC vaccination showed minimal impact.Enhanced vaccine protection against carriage with MenACWY is predicted to further decrease IMD incidence, potentially preventing over 2300 cases by 2060. | |
Scenarios prohibiting co-colonization indicated strong replacement effects leading to a decrease in the number of prevented IMD cases, emphasizing the complexity of meningococcal disease dynamics. | |
The study highlights the potential public health benefits of MenACWY vaccination, including a considerable increase in life years gained, and suggests the necessity for further economic analysis to fully understand its cost-effectiveness in the context of IMD management in Germany. |
Introduction
Invasive meningococcal disease (IMD) is a severe bacterial infection caused by Neisseria meningitidis (Nm) that manifests in meningitis and/or sepsis and can lead to long-term disability or death. The pathogen colonizes the human nasopharynx without causing any symptoms, but meningococcal carriers transmit the pathogen to susceptible individuals via respiratory droplets. Carriage prevalence varies based on factors such as age, immune status and social conditions, but it is particularly high among adolescents and young adults with prevalence rates of up to 24% in Europe [1].
In Europe, IMD is caused predominantly by Nm serogroups B and C, although their incidence has been steadily decreasing in the last decade. In contrast, cases due to serogroups W and Y are rising, especially among older age groups [2, 3]. In response to these trends, various European countries have adapted their immunisation guidelines, either transitioning to MenACWY vaccination in toddlers and/or adolescents in existing programmes or introducing MenACWY vaccination campaigns among adolescents [4, 5]. Preliminary evaluations of MenACWY vaccine implementation indicate promising results, signifying a decrease in both the prevalence of Nm carriage and the incidence of IMD serogroups W and Y [6,7,8]. In Germany, MenB is the most common serogroup associated with IMD, although the proportions of MenW and MenY among IMD cases increased from 2001 to 2019 [9]. Vaccination recommendations in Germany include MenC in toddlers, and routine MenB vaccination, with the latter being introduced in early 2024 [10]. To date, MenACWY vaccination is only recommended for individuals with increased risk for IMD due to underlying conditions [4, 5]. Hence, expanding MenACWY vaccination recommendations is also discussed in Germany. Mathematical models can provide valuable insights into the potential impact of vaccination strategies on the incidence and transmission of meningococcal disease. Therefore, we developed a dynamic transmission model to evaluate various adolescent vaccination strategies in Germany. Specifically, we compared the current practice of MenC toddler vaccination alone to the potential addition of either a MenC or MenACWY vaccination for adolescents.
Methods
Model Structure
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
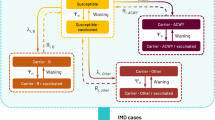
We developed a dynamic susceptible-infected-susceptible (SIS) model to estimate meningococcal carriage of five serogroup compartments (MenAY/MenB/MenC/MenW/MenO). Due to the low rate of occurrence, serogroup A was combined with serogroup Y into a single compartment, denoted as MenAY. Serogroup W was modelled as a separate compartment (MenW) to account for the recent emergence of more invasive serogroup W strains [3] despite its relatively low incidence. A compartment for meningococcal carriage of type “non-typable”/”non-ascertainable” and “other” labelled “MenO” was also added, as in the past these cases were responsible for a non-negligible share of IMD cases in Germany [11]. Individuals may get infected (i.e. become nasopharyngeal carriers) with a specific serogroup, and after recovering from carriage, return to the susceptible compartments, where they remain at risk of reinfection. To track vaccination history, we partitioned the carriage compartments into three blocks according to vaccination status: the first block encompasses all individuals without a history of MenC or MenACWY vaccination, whereas the second and third block include individuals vaccinated during toddler age and adolescence, respectively. Individuals consecutively transition through these blocks, initiating from the first (Fig. 1). The model time horizon spans from 1999 to 2060, using 1999 to 2018 as calibration phase, inclusive of a 3-year burn-in period (1999–2001), and employing 2019 to 2060 for extrapolation. The model is implemented as a system of difference equations with a time step of one day (Eq. S1–S2).
Dynamic model structure. Note: transition between susceptible and infected compartments is only displayed for block 1 but applies to all compartments. N, non-vaccinated; V1, toddler vaccinated and protected; V1U, toddler vaccinated and unprotected; V2, adolescent vaccinated and protected; V2/1, adolescent vaccinated but only toddler protected; V2U, adolescent vaccinated and unprotected; suffix “S”, susceptible; suffix “Φ”, serogroup Φ carriers. serogroup Φ symbolizes the five serogroup compartments modelled in the study. For each compartment, the above model was run separately with the respective serogroup-specific inputs
In the main analysis, we allowed for co-colonization and computed the model individually for each serogroup, incorporating serogroup-specific inputs. However, as previous models have assumed no co-colonization, an alternative analysis incorporating competitive serogroup interaction was conducted. This entailed constructing a second model version where individuals could be infected by only one serogroup at a time, thereby addressing the structural uncertainty associated with the inclusion of co-colonization (Eq. S3).
Modelled Population
The modelled population follows the German population aged 0–99 years stratified into 1200 age cohorts (i.e. monthly ageing steps). Demographic changes (i.e. mortality and migration) and the flow of age cohorts through the model over time is accounted for by the changing rate \(D\). This rate expresses the relative change in size of each age cohort during the course of one ageing step. Using population estimates and forecasts of the German Federal Statistical Office [12], the factor is calculated as follows:
with \(t\) indicating a change in time by year; \(i\), a change in age by year; \({N}^{Pop}\), the size of an age cohort at given \(t\) and \(i\); and \(s\) indicating the number of ageing steps by which a year is divided. A rate > 1 indicates that the respective age cohort grew in size during the course of an ageing step while a rate < 1 indicates a reduction in cohort size.
At the beginning of each ageing step, individuals transition from one age cohort to the next, with the cohort size adjusted in accordance with the respective demographic changing rate. All those aged 99 are assumed to die after their last ageing step in the age group of 99 years. Newborns are introduced into the model as absolute numbers to the susceptible compartment and are uniformly distributed over the pre-defined ageing steps as the model progresses in time.
Model Inputs
Carriage Transmission
For each serogroup \(\Phi\) and age group \(i\) the force of infection \({\lambda }_{{\Phi }_{t,i}}\) results from the age dependent contact behaviour, the carriage prevalence of serogroup \(\Phi\), and its transmissibility.
The transmissibility \({\varepsilon }_{{\Phi }_{i}}\) represents the serogroup- and age-specific proportion of infectious contacts leading to carriage acquisition in age group \(i\) which is estimated within the model calibration. The contact rates \({\beta }_{i,j}\) denote the average number of contacts from individuals in age group \(i\) with individuals in age group \(j\) and are informed by social contact frequencies based on the German part of the POLYMOD survey [13] as realized in Tomori et al. [14]. We assumed the force of infection to be proportional to the carrier fraction \(\frac{{C}_{{\Phi }_{t,j}}}{{N}_{t,j}}\), with \({C}_{{\Phi }_{t,j}}\) representing the carrier size of serogroup \(\Phi\) at time \(t\) in age group \(i\), and \({N}_{t,j}\) the corresponding total population.
IMD Incidence
We translated estimated carriage incidence into IMD cases via age- and serogroup-specific case–carrier ratios. These ratios were estimated using age-specific carriage prevalence derived from a meta-analysis [1], with the presumption of a 6-month carriage duration to convert prevalence into incidence [15, 16]. The serogroup distribution of carriage was extracted from a local German study in children and young adults conducted by Claus et al. [17]. We assumed the reported serogroup distribution to apply to the entire population. We obtained serogroup and age-specific IMD cases from SurvStat@RKI [18] for the period 2002–2005 and calculated a yearly average. The 2002–2005 interval was chosen as it precedes the introduction of Germany’s first meningococcal vaccine, providing a period with officially reported IMD data unaffected by vaccination impact. For each modelled serogroup we determined case–carrier ratios and applied log–log models for age-related interpolation/extrapolation (Fig. S1).
IMD Lethality
Case-fatality rates (CFR) for IMD cases were informed with data from the official German IMD surveillance. Serogroup-specific CFRs for MenB (overall CFR: 8.2%), MenC (11.9%), MenW (12.0%) and MenY (7.5%) were calculated using data from an analysis for the years 2001–2015 [19], complemented with data from the yearly national reports of infectious disease epidemiology for the years 2016–2020 [11, 20–23]. As the CFR of IMD varies by serogroup and age, we used the age stratification reported by Hellenbrand et al. [24], who reported CFRs for IMD in Germany by age group to adjust the serogroup-specific values. CFR input values combining serogroup and age group are presented in Table S1. Additionally, IMD-related deaths were converted into life years by multiplying the number of deaths in each age group by the corresponding age-specific life expectancy [25]. This approach permitted a comparison of total life years across scenarios and enabled the computation of life years gained (LYG).
Vaccination
We compared different strategies of additional adolescent vaccination to the status quo, which is MenC toddler vaccination. The adolescent vaccination strategies differed in vaccine type, vaccination age and level of carriage protection (see Table 1). Four scenarios (scenarios 1a to 2b) evaluated MenACWY vaccination strategies, whereas the remaining two focused on MenC strategies. Scenario 1a was designated as the base case scenario.
Vaccination coverage for toddlers receiving MenC vaccination up to 36 months of age was estimated at 90%, based on surveillance data indicating incomplete vaccine uptake within the first 2 years of life [26, 27]. For adolescent vaccination, we assumed that 50% of eligible individuals would be vaccinated within the vaccination time window, based on the observed human papillomavirus vaccination coverage (initial dosis) in girls in Germany [26]. We modelled vaccine effectiveness against IMD using an all-or-nothing approach for MenC and MenACWY vaccines, with both vaccines assumed to be 89% effective in vaccinees aged ≤ 10 years and 96% effective in vaccinees aged > 10 years [28].
To determine protection against carriage for the MenC vaccine, we utilised a large meningococcal carriage survey study from the UK, which reported a protective effect of 75.0% [29]. For MenACWY vaccine, we considered two sources: (i) results of a randomised controlled trial from England, which reported an effectiveness of 36.2% [30], and (ii) an observational pre-post study of the UK MenACWY vaccination program, reporting an effectiveness of 80.0% [7]. A comprehensive rationale for the selection of input parameters related to protection against carriage is presented in the supplementary material (Text S1).
We accounted for waning of vaccination effects for all serogroups using exponential decay with a mean duration of protection of 5 years for individuals aged up to 2 years, and 10 years for individuals > 2 years [28, 31,32,33]. To incorporate clearance from nasopharyngeal carriage into our model, we assumed a carriage duration of 6 months for all serogroups and estimated the clearance rate as the reciprocal of carriage duration [15].
The impact of the MenB vaccine on IMD is only considered outside the dynamic model, reducing the probability of invasive disease, due to a lack of evidence regarding the carriage effect of MenB vaccines [34]. Since 2013, the MenB vaccine has been available in Germany and was only recommended for individuals with an increased IMD risk by the standing committee on vaccination (STIKO) until early 2024 [35]. A three-dose vaccination regimen for infants and a two-dose regimen for adults were assumed [36, 37]. Based on sales data for MenB vaccine doses, we applied an annual vaccine coverage that started at 2.5% in 2014, increased to 14.5% by 2020, and remained constant thereafter. Assumed protection duration was 33 months for individuals aged up to one year and 38 months for those older than one year [38]. Vaccine effectiveness against MenB IMD was estimated to be 58% [39, 40]. A list of model inputs can be found in Table S1.
Calibration
The model was calibrated by adjusting the age- and serogroup-specific carriage transmissibility to match notified IMD cases reported in Germany from 2002 to 2018 [18]. The calibration process involved a two-stage approach: initially, a derivative-free search algorithm based on the Nelder–Mead method was employed through nloptr (R Interface to NLopt) to constrain the parameter space [41]. Subsequently, Bayesian sampling was performed via Markov chain Monte Carlo (Metropolis–Hastings algorithm) to further refine the calibrated estimates and to derive distributions of the calibrated parameters [42, 43]. Model parameters based on weak or no evidence (i.e. carriage transmissibility) were estimated within a Bayesian framework using publicly available data on yearly age- and serogroup-specific IMD cases [18]. Parameters that were well supported through data such as demographics, vaccine coverage or case-fatality rates were kept fixed within the model. We employed uniform prior distributions within plausible ranges and computed likelihood from observed and predicted age- and serogroup-specific IMD cases, assuming a Poisson distribution. The posterior distribution was subsequently derived as the product of the prior and likelihood functions.
Analysis
The public health impact of the different adolescent vaccination strategies (Table 1) was assessed by estimating IMD cases (per 100,000) and IMD deaths. Additionally, we calculated the number needed to vaccinate (NNV) to prevent one IMD case by dividing the number of persons vaccinated by the number of IMD cases prevented for each scenario. To address parameter uncertainty, a probabilistic sensitivity analysis (PSA) was performed. This analysis involved simultaneous variation of key input variables (Table S1) over their respective probability distribution to generate new sets of input parameters. In total, 5000 model realizations were computed for the status quo and the base case scenario.
Results
Calibration and Status Quo Scenario
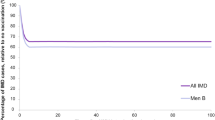
After the two-step calibration process, the model’s predictions demonstrated good fit with age- and serogroup-specific notification data from Germany. The model projections indicate a reduction in overall IMD burden from approximately 280–175 cases per year (0.33–0.23 cases per 100,000) by 2060 (Fig. S2). More specifically, model predictions for IMD cases associated with MenB, MenC and MenO show a decline, while those related to MenW and MenAY increase under the current vaccination strategy. Although MenB remains the predominant cause of IMD throughout the projection period, MenW and MenAY cumulatively account for over 50% of cases by the end of the extrapolation period. With respect to age-specific IMD cases, the model predicts an increase in cases among individuals aged 65 years and older, with a concurrent decrease in all other modelled age groups (Fig. 2).
Public Health Impact of Adolescent Vaccination Strategies
The model predicts that the base case scenario (status quo versus scenario 1a) would considerably reduce IMD incidence in Germany, with the potential to prevent up to 65 IMD cases per year and 1467 cases overall until 2060, translating into 156 prevented IMD deaths. This corresponds to a prevention of 17% of total IMD cases and 40% of MenACWY-specific cases by the end of the modelling period, resulting in a NNV of 7255. These protective effects were, to different degrees, observed across all age groups, indicating the presence of herd effects (Fig. S3). Assuming an 80% protection against carriage (scenario 2a and 2b) further amplified this impact, potentially preventing 2359 IMD cases until 2060, with up to 95 prevented IMD cases at the end of the extrapolation period (Fig. 3). In the base case scenario, the majority of prevented IMD cases were due to MenW, followed by MenAY with 853 and 576 prevented IMD cases, respectively. When assuming an 80% protection against carriage, the number of prevented MenW IMD cases increases to 1294, and the number of prevented MenAY IMD cases increases to 983. Prevented MenC IMD cases had a negligible contribution to the total number of prevented IMD cases. The introduction of MenC adolescent vaccination led to a small decrease in IMD cases, with up to 84 cases prevented over the extrapolation period. Age of vaccination had a marginal impact in all modelled scenarios. Conversion of averted IMD deaths into LYG resulted in 2250 LYG for the base case scenario and a maximum of 4222 LYG assuming 80% protection against carriage. In contrast, both MenC adolescent vaccination scenarios yielded fewer than 500 LYG, markedly less than the MenACWY scenarios yet higher when evaluated as LYG per IMD death. This discrepancy can be attributed to the model’s prediction that MenC predominantly impacts younger individuals, whereas MenAY and MenW are more prevalent in older demographics. Modifying adolescent vaccination coverage to 30% and 70% in the base case scenario resulted in the prevention of 1025 and 1776 total IMD cases, respectively, corresponding to 109 and 188 averted IMD deaths.
Public health impact of adolescent vaccination strategies: A absolute change in invasive meningococcal disease (IMD) cases over time by scenario; B total prevented IMD cases by serogroup and scenario; and C total prevented IMD deaths by serogroup and scenario. Panel B and C do not contain MenB and MenO since presented scenarios do not affect disease burden of these serogroups. Rounding to the nearest integer may have introduced an error of ± 1 to the total numbers in panel B and C
Structural Uncertainty Analysis
When restricting carriage to colonization of one serogroup at a time, the second model version predicts an increase in MenW and MenAY IMD cases and a decline in MenB and MenO cases, similar to the status quo scenario in the main analysis. However, these variations – both the rise in MenW and MenAY and the decline in MenB and MenO – are more pronounced than in the main analysis (Fig. S4). When comparing the base case with the status quo scenario, the reduction of IMD cases attributed to MenACWY (n = 1282) is offset by an increase in cases from the remaining serogroups (n = 572), resulting in major replacement effects. MenB and MenO carriers appear to occupy the niche previously dominated by MenW and MenAY carriers. Enhanced vaccine effectiveness against carriage (80% instead of 36.2%) resulted in similar trends: the prevention of 2618 MenACWY cases was offset by an additional 1856 MenB and MenO cases. As in the main analysis, introducing a MenC adolescent vaccination had a minor impact on overall IMD burden (Fig. S5).
Probabilistic Sensitivity Analysis
Considering parameter uncertainty, the base case scenario projects a 17.0% reduction [95% confidence interval (CI) 10.2–23.0%] in overall IMD cases, averting 1449 (95% CI 850–2135) cases. These estimates align with outcomes derived from deterministic analyses. The prevented cases for MenW and MenAY serogroups in the PSA were estimated to be 829 (95% CI 458–1295) and 571 (95% CI 342–832), respectively. Moreover, there was a 20.1% average reduction (95% CI 11.7–29.2%) in IMD-associated mortality, equating to 153 (95% CI 84–237) averted deaths. Further stratified, the averted deaths for MenW and MenAY were projected at 104 (95% CI 53–176) and 43 (95% CI 22–69), respectively (Fig. 4).
Results from the probabilistic sensitivity analysis: A invasive meningococcal disease (IMD) cases by serogroup over time for the status quo scenario; B IMD cases by serogroup over time for the base case scenario; C absolute change in IMD cases by serogroup comparing base case to status quo scenario; D absolute change in IMD deaths by serogroup comparing base case to status quo scenario. Note: lines depict the mean of all simulations and shaded areas show the range between the 2.5% and 97.5% quantiles
Discussion
The introduction of MenACWY vaccination in adolescents is projected by our model to substantially reduce MenAY and MenW IMD cases and associated deaths. Over the entire extrapolation period, 17% of total IMD cases and 40% of MenACWY IMD cases were averted, corresponding to a NNV to prevent one IMD case of 7255. Although this NNV appears high in relation to more prevalent diseases, it remains moderate in comparison with other meningococcal vaccination strategy models for Germany. For instance, Scholz et al. reported an NNV of 12,080 for routine infant MenB vaccination [38], while Christensen et al. reported NNV values exceeding 30,000 for various MenB strategies targeting infants to adolescents in Germany [44].
Following a rise in MenW and MenY IMD cases, countries including the UK, the Netherlands, Spain, Greece and Italy have implemented adolescent MenACWY vaccination strategies [4, 5]. Although the comprehensive evaluation of the public health impact and the vaccine effectiveness against IMD and carriage remains ongoing, preliminary data from England’s emergency adolescent MenACWY programme reported a 69% reduction in MenW IMD cases during its first year relative to trend predictions. Notably, no cases were identified in the initial vaccinated cohort, even with a vaccination coverage of 36.6% [6]. Ohm et al. assessed the long-term duration of seroprotection against Nm in adolescents who had received a single MenACWY vaccination, following priming in early childhood with MenC conjugate vaccination. Their findings indicated that in this age group, the median duration of protection against MenCWY serogroups exceeded 30 years [45]. In Germany, no routine meningococcal adolescent vaccination is currently recommended, although the RKI is evaluating potential strategies with MenC and MenACWY vaccination [46]. Based on our results, vaccines targeting MenW and MenAY should be prioritized in adolescent vaccination strategies to reduce the overall IMD burden.
We utilised the most recent Germany-specific data whenever possible and developed a dynamic transmission model to account for indirect vaccine benefits. We also addressed uncertainty in multiple dimensions. For instance, to examine structural uncertainty, we adopted two versions of meningococcal carriage transmission models: one allowing for co-colonization and another prohibiting it, due to uncertainty about the simultaneous carriage of multiple serogroups. Our findings indicate that prohibiting co-colonization results in strong replacement effects, which diminish the vaccine’s overall effectiveness against IMD, as non-targeted serogroups replace those targeted by the vaccine. This phenomenon is consistent with prior research on dynamic meningococcal carriage models [47], but has so far not been observed in countries introducing MenACWY in adolescents.
Uncertainty concerning MenACWY vaccine’s carriage protection was approached via two sources: (i) results of a randomised controlled trial from England, which reported an effectiveness of 36.2% [30], and (ii) an observational pre-post study of the UK MenACWY vaccination program, reporting an effectiveness of 80% [7]. We considered both inputs because the randomised controlled trial, while reducing potential bias in direct vaccine effectiveness estimates, was only conducted for one of the quadrivalent meningococcal vaccines available in the market. To address this uncertainty, we incorporated scenarios with carriage effectiveness data from the observational pre-post study, which employed another MenACWY vaccine.
Our model has several limitations. First, IMD cases are estimated outside the dynamic transmission model. Affected individuals remain in the modelled population and further contribute to carriage transmission, although a more accurate approach might involve removing these cases considering the IMD mortality and typical public health responses such as antibiotic prophylaxis in close contacts. However, we expect the overall impact to be minimal given the proportionally low number of IMD cases to carriers.
Additionally, the co-colonization model operates under the assumption that an individual can simultaneously experience IMD from different serogroups. Though this could inflate IMD case estimates, the small percentage of carriers developing IMD likely diminishes this effect. Another underlying assumption pertains to demographic changes, suggesting migrants possess similar carriage prevalence and serogroup distribution as the native population. Despite potential inconsistencies in this assumption [48, 49], observed and projected IMD cases, stratified by serogroup and age group, did not show notable disparities during or post the increased migration movements in 2015.
In our analysis, the potential influence of the coronavirus disease 2019 (COVID-19) pandemic containment measures on contact behaviour during 2020 to 2022 was not incorporated, potentially affecting model extrapolations for that period [50]. Nonetheless, upon evaluating reported IMD cases for Germany up to August 2023 [18], model forecasts reasonably align with observed cases in terms of serogroup distribution. Notably, MenW and MenY IMD cases showed a considerable rise, jointly representing 41% of recorded cases, with the surge predominantly attributed to MenY. The model projected a combined MenAY and MenW IMD case rate of 23%. It is significant to note that post-pandemic, there was a particular increase in MenY IMD cases, whereas MenW IMD has not returned to pre-pandemic levels. While observed MenB cases constituted 38%, the model anticipated a 56% proportion. The model also forecasted a decline in MenC IMD cases, representing 8%, though observed data indicated a more rapid decrease at 4%. Finally, MenO comprised 17% of observed cases, compared with the model’s 13%. Overall, the model effectively reflects the observed trends in IMD infections, specifically, a decline in MenB and MenC cases coupled with a rise in MenW and MenY cases.
Employing the official population projections from the German Federal Statistical Office ensures data accuracy. However, during model development, these forecasts spanned only until 2060, providing a shorter extrapolation period than other comparable models [38, 44, 47]. Considering the observed IMD infection trajectories, notably the increased prevention of IMD cases towards the end of the extrapolation period, such a restricted time horizon may underestimate the public health benefits of MenACWY adolescent vaccination.
Last, our study did not reflect the recommendation of routine immunisation against MenB in infants issued by STIKO in early 2024 [10]. We assumed steady MenB coverage of 14.5%, which is likely to be an underestimation after the recommendation and subsequent reimbursement of MenB for infants by all statutory health insurances in Germany. However, we expect minor impact on our results, as the majority of predicted IMD cases prevented by adolescent conjugate vaccination in our model is in adolescents and adults.
Conclusion
Our results suggest that introducing MenACWY adolescent vaccination in Germany could substantially reduce IMD cases across all age groups, an observation mainly attributed to indirect vaccination effects. In contrast, the addition of MenC adolescent vaccination shows minimal impact. Given the increasing incidence of MenW and MenY cases across Europe, these findings are important for the development of further meningococcal vaccination strategies. While our study provides valuable insights for public health policy formulation aimed at optimizing meningococcal vaccination benefits, it is imperative to integrate economic evaluations in subsequent analyses to provide a comprehensive basis for policymakers’ decisions.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Christensen H, May M, Bowen L, et al. Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:853–61. https://doi.org/10.1016/S1473-3099(10)70251-6.
Nuttens C, Findlow J, Balmer P et al. Evolution of invasive meningococcal disease epidemiology in Europe, 2008 to 2017. Euro Surveill. 2022. https://doi.org/10.2807/1560-7917.ES.2022.27.3.2002075.
Krone M, Gray S, Abad R et al. Increase of invasive meningococcal serogroup W disease in Europe, 2013 to 2017. Euro Surveill. 2019. https://doi.org/10.2807/1560-7917.ES.2019.24.14.1800245.
Pinto Cardoso G, Lagrée-Chastan M, Caseris M, et al. Overview of meningococcal epidemiology and national immunization programs in children and adolescents in 8 Western European countries. Front Pediatr. 2022;10:1000657. https://doi.org/10.3389/fped.2022.1000657.
Martinón-Torres F, Taha M-K, Knuf M, et al. Evolving strategies for meningococcal vaccination in Europe: overview and key determinants for current and future considerations. Pathog Glob Health. 2022;116:85–98. https://doi.org/10.1080/20477724.2021.1972663.
Campbell H, Andrews N, Parikh SR, et al. Impact of an adolescent meningococcal ACWY immunisation programme to control a national outbreak of group W meningococcal disease in England: a national surveillance and modelling study. Lancet Child Adolesc Health. 2022;6:96–105. https://doi.org/10.1016/S2352-4642(21)00335-7.
Carr JP, MacLennan JM, Plested E, et al. Impact of meningococcal ACWY conjugate vaccines on pharyngeal carriage in adolescents: evidence for herd protection from the UK MenACWY programme. Clin Microbiol Infect. 2022;28:1649.e1-1649.e8. https://doi.org/10.1016/j.cmi.2022.07.004.
Ohm M, Hahné SJM, van der Ende A, et al. Vaccine impact and effectiveness of meningococcal serogroup ACWY conjugate vaccine implementation in the Netherlands: a nationwide surveillance study. Clin Infect Dis. 2022;74:2173–80. https://doi.org/10.1093/cid/ciab791.
Gruhn S, Witte J, Greiner W, et al. Epidemiology and economic burden of meningococcal disease in Germany: a systematic review. Vaccine. 2022;40:1932–47. https://doi.org/10.1016/j.vaccine.2022.02.043.
Robert Koch-Institut. Empfehlung zur Standardimpfung von Säuglingen gegen Meningokokken der Serogruppe B und die dazugehörige wissenschaftliche Begründung. 2024. https://doi.org/10.25646/11900.
Robert Koch-Institut. Infektionsepidemiologisches Jahrbuch meldepflichtiger Krankheiten für 2018. Robert Koch-Institut. 2019.
Statistisches Bundesamt. Bevölkerung im Wandel: Annahmen und Ergebnisse der 14. Koordinierten Bevölkerungsvorausberechnung. 2019.
Mossong J, Hens N, Jit M, et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 2008;5: e74. https://doi.org/10.1371/journal.pmed.0050074.
Tomori DV, Rübsamen N, Berger T, et al. Individual social contact data and population mobility data as early markers of SARS-CoV-2 transmission dynamics during the first wave in Germany—an analysis based on the COVIMOD study. BMC Med. 2021;19:271. https://doi.org/10.1186/s12916-021-02139-6.
Christensen H, Hickman M, Edmunds WJ, et al. Introducing vaccination against serogroup B meningococcal disease: an economic and mathematical modelling study of potential impact. Vaccine. 2013;31:2638–46. https://doi.org/10.1016/j.vaccine.2013.03.034.
Beck E, Klint J, Garcia S, et al. Modelling the impact of 4CMenB and MenACWY meningococcal combined vaccination strategies including potential 4CMenB cross-protection: an application to England. Vaccine. 2020;38:7558–68. https://doi.org/10.1016/j.vaccine.2020.08.007.
Claus H, Maiden MCJ, Wilson DJ, et al. Genetic analysis of meningococci carried by children and young adults. J Infect Dis. 2005;191:1263–71. https://doi.org/10.1086/428590.
Robert Koch-Institut SurvStat@RKI 2.0: Web-basierte Abfrage der Meldedaten gemäß Infektionsschutzgesetz (IfSG).
Robert Koch-Institut. "Invasive Meningokokken- Erkrankungen 2012 – 2015". RKI-Bib1 (Robert Koch-Institut). 2016.
Robert Koch-Institut. Infektionsepidemiologisches Jahrbuch meldepflichtiger Krankheiten für 2016. Robert Koch-Institut. 2017.
Robert Koch-Institut. Infektionsepidemiologisches Jahrbuch meldepflichtiger Krankheiten für 2017. Robert Koch-Institut. 2018.
Robert Koch-Institut. Infektionsepidemiologisches Jahrbuch meldepflichtiger Krankheiten für 2019. Robert Koch-Institut. 2020.
Robert Koch-Institut. Infektionsepidemiologischse Jahrbuch meldepflichtiger Krankheiten für 2020. Robert Koch-Institut. 2021.
Hellenbrand W, Elias J, Wichmann O, et al. Epidemiology of invasive meningococcal disease in Germany, 2002–2010, and impact of vaccination with meningococcal C conjugate vaccine. J Infect. 2013;66:48–56. https://doi.org/10.1016/j.jinf.2012.09.008.
Statistisches Bundesamt. Sterbetafeln. 2023. https://www-genesis.destatis.de/genesis/online?sequenz=statistikTabellen&selectionname=12621.
Rieck T, Feig M, Siedler A. Impfquoten von Kinderschutzimpfungen in Deutschland—aktuelle Ergebnisse aus der RKI-Impfsurveillance. Epidemiol Bull. 2022;3–25.
Rieck T. KV-Impfsurveillance—Möglichkeiten und Herausforderungen der Analyse der KV-Daten. 2019.
Campbell H, Andrews N, Borrow R, et al. Updated postlicensure surveillance of the meningococcal C conjugate vaccine in England and Wales: effectiveness, validation of serological correlates of protection, and modeling predictions of the duration of herd immunity. Clin Vaccine Immunol. 2010;17:840–7. https://doi.org/10.1128/CVI.00529-09.
Maiden MCJ, Ibarz-Pavón AB, Urwin R, et al. Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J Infect Dis. 2008;197:737–43. https://doi.org/10.1086/527401.
Read RC, Baxter D, Chadwick DR, et al. Effect of a quadrivalent meningococcal ACWY glycoconjugate or a serogroup B meningococcal vaccine on meningococcal carriage: an observer-blind, phase 3 randomised clinical trial. Lancet. 2014;384:2123–31. https://doi.org/10.1016/S0140-6736(14)60842-4.
Cohn AC, MacNeil JR, Harrison LH et al. Effectiveness and duration of protection of one dose of a meningococcal conjugate vaccine. Pediatrics. 2017;139:e20162193. https://doi.org/10.1542/peds.2016-2193.
Elias J, Findlow J, Borrow R, et al. Persistence of antibodies in laboratory staff immunized with quadrivalent meningococcal polysaccharide vaccine. J Occup Med Toxicol. 2013;8:4. https://doi.org/10.1186/1745-6673-8-4.
Trotter CL, Gay NJ, Edmunds WJ. Dynamic models of meningococcal carriage, disease, and the impact of serogroup C conjugate vaccination. Am J Epidemiol. 2005;162:89–100. https://doi.org/10.1093/aje/kwi160.
Marshall HS, McMillan M, Koehler AP, et al. Meningococcal B vaccine and meningococcal carriage in adolescents in Australia. N Engl J Med. 2020;382:318–27. https://doi.org/10.1056/NEJMoa1900236.
Hellenbrand W, Koch J, Harder T, et al. Background Paper for the update of meningococcal vaccination recommendations in Germany: use of the serogroup B vaccine in persons at increased risk for meningococcal disease. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2015;58:1314–43. https://doi.org/10.1007/s00103-015-2253-z.
Ständige Impfkommission. Stellungnahme der Ständigen Impfkommission (STIKO) am Robert Koch-Institut (RKI) zum Stand der Bewertung des neuen Meningokokken-B-Impfstoffs Bexsero®. Epidemiol Bull. 2013;495–504.
Ständige Impfkommission. Aktualisierte Stellungnahme der Ständigen Impfkommission (STIKO) am Robert Koch-Institut (RKI). Epidemiol Bull. 2018;5–44.
Scholz S, Schwarz M, Beck E, et al. Public health impact and cost-effectiveness analysis of routine infant 4CMenB vaccination in Germany to prevent serogroup B invasive meningococcal disease. Infect Dis Ther. 2022;11:367–87. https://doi.org/10.1007/s40121-021-00573-w.
Ladhani SN, Andrews N, Parikh SR, et al. Vaccination of infants with meningococcal group B Vaccine (4CMenB) in England. N Engl J Med. 2020;382:309–17. https://doi.org/10.1056/NEJMoa1901229.
Vogel U, Taha M-K, Vazquez JA, et al. Predicted strain coverage of a meningococcal multicomponent vaccine (4CMenB) in Europe: a qualitative and quantitative assessment. Lancet Infect Dis. 2013;13:416–25. https://doi.org/10.1016/S1473-3099(13)70006-9.
Johnson SG. The NLopt nonlinear-optimization package. 2014.
Robert CP. The Metropolis–Hastings algorithm. 2016.
van Ravenzwaaij D, Cassey P, Brown SD. A simple introduction to Markov Chain Monte-Carlo sampling. Psychon Bull Rev. 2018;25:143–54. https://doi.org/10.3758/s13423-016-1015-8.
Christensen H, Irving T, Koch J, et al. Epidemiological impact and cost-effectiveness of universal vaccination with Bexsero(®) to reduce meningococcal group B disease in Germany. Vaccine. 2016;34:3412–9. https://doi.org/10.1016/j.vaccine.2016.04.004.
Ohm M, van Rooijen DM, Bonačić Marinović AA et al. Different long-term duration of seroprotection against Neisseria meningitidis in adolescents and middle-aged adults after a single meningococcal ACWY conjugate vaccination in The Netherlands. Vaccines. 2020;8:624. https://doi.org/10.3390/vaccines8040624.
Schönfeld V, Pozo Martin F, Scholz S et al. Auffrischimpfung gegen Meningokokken der Serogruppe C (AMSeC): Abschätzung der Effekte auf Krankheitslast und Kosten im deutschen Gesundheitssystem: Vorstellung der Studie, DGKJ—Kongress für Kinder- und Jugendmedizin, München. 2019.
Vickers DM, Anonychuk AM, de Wals P, et al. Evaluation of serogroup C and ACWY meningococcal vaccine programs: projected impact on disease burden according to a stochastic two-strain dynamic model. Vaccine. 2015;33:268–75. https://doi.org/10.1016/j.vaccine.2013.09.034.
Parikh SR, Campbell H, Bettinger JA, et al. The everchanging epidemiology of meningococcal disease worldwide and the potential for prevention through vaccination. J Infect. 2020;81:483–98. https://doi.org/10.1016/j.jinf.2020.05.079.
Pardo de Santayana C, Tin Tin Htar M, Findlow J, et al. Epidemiology of invasive meningococcal disease worldwide from 2010–2019: a literature review. Epidemiol Infect. 2023;151: e57. https://doi.org/10.1017/S0950268823000328.
Shaw D, Abad R, Amin-Chowdhury Z, et al. Trends in invasive bacterial diseases during the first 2 years of the COVID-19 pandemic: analyses of prospective surveillance data from 30 countries and territories in the IRIS Consortium. Lancet Digit Health. 2023. https://doi.org/10.1016/S2589-7500(23)00108-5.
Acknowledgements
Medical Writing, Editorial, and Other Assistance
Louise Baschet provided technical support during the model development management with funding from Sanofi.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Funding
This study and the journal’s Rapid Service fee were funded by Sanofi-Aventis Deutschland GmbH.
Author information
Authors and Affiliations
Contributions
Sebastian Gruhn, Manuel Batram, Moritz Wick, Edith Langevin, Stefan Scholz and Oliver Damm were involved in the conception or the design of the study. Sebastian Gruhn, Manuel Batram, Moritz Wick and Oliver Damm participated in the collection or generation of the data. All authors were involved in the analyses or interpretation of the data. Writing of the first draft of the manuscript was led by Sebastian Gruhn and all authors commented on previous versions of the manuscript. All authors critically reviewed the final manuscript and provided approval on the submitted version.
Corresponding author
Ethics declarations
Conflict of Interest
Moritz Wick, Edith Langevin and Oliver Damm are Sanofi employees and may hold shares and/or stock options in the company. Sebastian Gruhn, Manuel Batram, Stefan Scholz and Wolfgang Greiner received research grants from Sanofi-Aventis Deutschland GmbH. All authors declare no other financial or nonfinancial relationships and activities. Stefan Scholz has changed affiliation after the completion of the manuscript and is now an employee of Moderna Germany GmbH.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Prior Presentation: Abstract and poster presentation at the European Society for Paediatric Infectious Diseases – 41st Annual Meeting (Lisbon), and the International Society for Pharmacoeconomics and Outcomes Research – 26th Annual European Congress 2023 (Copenhagen).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Gruhn, S., Batram, M., Wick, M. et al. Modelling the Public Health Impact of MenACWY and MenC Adolescent Vaccination Strategies in Germany. Infect Dis Ther 13, 907–920 (2024). https://doi.org/10.1007/s40121-024-00958-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-024-00958-7