Abstract
Introduction
Rocuronium intravenous pain is common in induction of general anesthesia. The aim of our study was to determine the median effective dose (ED50) of prophylactic intravenous remifentanil for the prevention of rocuronium injection pain and to explore the effect of age on the ED50.
Methods
Eighty-nine adult patients undergoing elective general anesthesia, ASA I or II, regardless of gender or weight, were stratified according to age: group R1 18–44 years, group R2 45–59 years, and group R3 60–80 years. The initial dose of prophylactic remifentanil before rocuronium injection was set at 1 μg/kg lean body weight (LBW). The remifentanil doses were adjusted according to the degree of injection pain using the Dixon sequential method, with a ratio of 1.1 between adjacent doses. Injection pain was graded, and the occurrence of injection pain and adverse reactions were recorded. The ED50 and 95% confidence intervals (CIs) of remifentanil were calculated using the Dixon–Massey formula. Patients were asked whether they recalled feeling any injection pain in the post-anesthesia care unit (PACU).
Results
The ED50 (95% CIs) of prophylactic remifentanil for the prevention of rocuronium injection pain were 1.266 μg/kg (1.186–1.351 μg/kg), 1.188 μg/kg (1.065–1.324 μg/kg), and 1.070 μg/kg (1.014–1.129 μg/kg) LBW in group R1, group R2, and group R3, respectively. No adverse reactions to remifentanil occurred in any group. In PACU, 84.6, 86.7, and 85.7% of patients who experienced injection pain had memories of the pain in group R1, group R2, and group R3, respectively.
Conclusions
Prophylactic intravenous remifentanil can prevent rocuronium injection pain, and its ED50 decreases with age, with 1.266 μg/kg (18–44 years), 1.188 μg/kg (45–59 years), and 1.070 μg/kg LBW (60–80 years), respectively.
Trial Registration
ClinicalTrials.gov: NCT05217238 (registration date 18 Dec 2021).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Rocuronium injection pain, with a high incidence, may induce disappointing adverse effects. Prophylactic intravenous remifentanil can reduce rocuronium injection pain, while its dosage has usually been employed empirically. |
This study was carried out to determine the median effective dose (ED50) of prophylactic intravenous remifentanil for the prevention of rocuronium injection pain and to explore the effect of age on the ED50. |
What was learned from the study ? |
The ED50 of prophylactic remifentanil intravenous injection for rocuronium-induced injection pain prevention was 1.266 μg/kg (18–44 years), 1.188 μg/kg (45–59 years), and 1.070 μg/kg lean body weight (LBW) (60–80 years), respectively. |
The ED50 of prophylactic intravenous remifentanil to prevent rocuronium injection pain decreases with age. |
Introduction
Rocuronium, a non-depolarizing muscle relaxant, is widely used for general anesthesia induction due to the advantages of rapid onset, intermediate acting duration, satisfactory muscle relaxation, and without histamine release. However, pain occurring after intravenous injection of rocuronium is very common in the clinical setting, with an incidence ranging from 50 to 80% [1]. Intravenous injection pain is likely to cause discomfort during the induction period of general anesthesia in patients and reduce perioperative satisfaction. It can also cause dislocation or displacement of the intravenous catheter, extravasation of injected drugs, injection site swelling and delayed induction of general anesthesia, and even cardiovascular adverse events when in severe pain [2]. Prophylactic intravenous remifentanil can reduce rocuronium injection pain [3], while its dosage has been employed empirically in most previous studies. Furthermore, the doses of remifentanil may vary among different ages. There have been no reports on age-related dose of remifentanil in preventing rocuronium injection pain and its median effective dose (ED50) has not yet been determined.
Higher remifentanil doses do not inevitably decrease rocuronium injection pain more and may increase the risk of adverse reactions. In our previous study, we used the Dixon up-and-down sequential method to assess the effects of lidocaine in elevating propofol-induced injection pain [4]. The primary aim of this study was to determine the ED50 of prophylactic intravenous remifentanil for the prevention of rocuronium injection pain using the same Dixon sequential method. The second aim was to explore the effect of age factor on the ED50 of remifentanil in preventing rocuronium injection pain.
Methods
Ethics Approval
This study was approved by the Institutional Research Ethics Committee of the Affiliated Hospital of Yangzhou University, Yangzhou, China (2022-YKL1-20-002) with registration at clinicaltrials.gov (NCT05217238), and was conducted according to the principles of the Helsinki Declaration. All participants provided written informed consent.
Participants
A total of 89 patients aged 18–65 with American Society of Anesthesiologists (ASA) Class I or II status, regardless of gender or weight, who underwent elective surgery under general anesthesia were enrolled in this prospective study from December 2021 to March 2022. Exclusion criteria were allergy or contraindication to remifentanil or rocuronium; liver or kidney dysfunction; alcohol mania; long-term use of somnifacient, analgesics, or psychotropic medications; difficulty with communication; peripheral vascular disease; severe cardiovascular diseases; failed one-time cannulation in the wrist cephalic vein; and contraindication to intravenous cannulation on the wrist. According to the 2012 World Health Organization classification of age, the patients were divided into three groups: group R1, 18–44 years, group R2, 45–59 years, and group R3, 60–80 years. Because the study was adjusted for specific criteria, the recommended seven-step crossover rule was used to discontinue the inclusion of participants. Thus, we did not calculate the sample size in advance.
Anesthesia
Preoperative fasting of food for 6 h and clear liquid for 2 h were performed, and none were pre-medicated. After entering the operating theater, all patients were monitored routinely with electrocardiogram (ECG), noninvasive blood pressure measurement, and pulse oxygen saturation (SpO2) by patient monitors. A blood pressure cuff was applied on the arm contralateral to that with venous access. Venous access was established in a wrist cephalic vein with a 20-G intravenous cannula, and Ringer’s solution was transfused at a rate of 8·ml kg−1 h−1 as a carrier. For all patients, the vertical distance from the drip to the venous access was controlled at an 80-cm vertical distance. The intravenous cannula was connected to three-way stopcocks for fluid infusion and drug injection. All patients were blinded to the administered medication.
The Dixon sequential method was utilized in this study [5]. According to our preliminary as well previous studies [3], the initial dose of remifentanil (batch no. 10A07281, Yichang Renfu Pharmaceutical Co., Ltd., Hubei, China) was set at 1 μg/kg lean body weight (LBW) (calculated as \(\frac{{9270 \times {\text{TBW}}\;({\text{kg}})}}{{6680 + (216 \times {\text{BMI}}\;({\text{kg/m}}^{2} ))}}\) for males and \(\frac{{9270 \times {\text{TBW}}\;({\text{kg}})}}{{8780 + (244 \times {\text{BMI}}\;({\text{kg/m}}^{2} ))}}\) for females [6], where the total body weight (TBW) means total body weight and the BMI means body mass index. The remifentanil was drawn up with a 1-ml syringe and diluted to 5 ml with normal saline in a 5-ml syringe, and the injection time was controlled at 60 s. The rocuronium (batch no. EA2167, Zhejiang Xianju Pharmaceutical Co., Ltd., Zhejiang, China) stored at 2–8 °C was drawn up at 0.6 mg/kg TBW before anesthesia induction. One minute after remifentanil injection, rocuronium was injected at the time of 10 s. Intravenous fluid was stopped during remifentanil and rocuronium injection by using the stopcock, and after the injection was completed, the three-way stopcock was opened to continue the infusion of Ringer’s injection 8 ml·kg−1 h−1. During rocuronium injection, the patients were repeatedly asked about pain at the injection site or forearm and any other discomfort, and were observed for any arm retraction movement.
Immediately after rocuronium injection pain inquiry, propofol medium/long-chain triglyceride (MCT/LCT) emulsion 1.5–2 mg/kg, midazolam 0.05 mg/kg, and sufentanil 0.4 μg/kg were injected to perform general anesthesia induction. An endotracheal tube was inserted for mechanical ventilation. Inhaled sevoflurane (minimum alveolar concentration = 1), and intravenous infused propofol (MCT/LCT) emulsion (4–10 mg·kg−1·h−1), remifentanil (0.5–1.0 μg·kg−1·h−1) and dexmedetomidine (0.5 μg·kg−1·h−1) were continued for anesthesia maintenance. LBW was used to scale the doses of these maintaining drugs. Intraoperative intravenous rocuronium was administered intermittently to maintain moderate muscle relaxation. The depth of anesthesia was monitored with a Narcotrend anesthesia depth monitor (Monitor Technik, Germany), and Narcotrend index values was controlled at 20–46. Muscle relaxation was monitored using a muscle relaxation monitor (Datex-Ohmeda S/5, ohmeda, Inc., USA). After surgery, when the patients’ respiratory function had recovered and train-of-four (TOF) stimulation monitored T4/T1 recovered to 75%, neostigmine 0.04–0.07 mg/kg and glycopyrronium bromide 6 μg/kg were injected intravenously. Patients were transferred to a post-anesthesia care unit (PACU) after extubation. Fluctuation ranges of mean arterial pressure (MAP) and heart rate (HR) were controlled lower than 20% baseline. Ephedrine or phenylephrine was used when MAP was lower than 60 mmHg or decreased greater than 20% baseline. Atropine was used when HR was slower than 50 beats/min or decreased greater than 20% baseline.
For remifentanil dose setting following Dixon sequential method, three anesthesia team members performed the entire general anesthesia process (Fig. 1). The first anesthetist, who did not assess pain, prepared and injected remifentanil and rocuronium, and adjusted remifentanil dose according to the third anesthetist’s evaluation of patients’ injection pain. The second anesthetist, who was unaware of the remifentanil dosage, implemented oxygen supply and mechanical ventilation, and also recorded side effects of remifentanil after its injection for a 1-min period. General anesthesia induction and maintenance was continued by the third anesthetist, who was unaware of the remifentanil dose and responsible for rocuronium injection pain evaluation. When a patient reported no pain on injection (negative reaction), the remifentanil dose for the next patient was decreased, and this process was repeated until injection pain occurred. When a patient complained of pain on injection (positive reaction), the remifentanil dose was increased for the next patient, and this process was repeated until no injection pain occurred. The ratio of the adjacent remifentanil doses was 1.1. The syringes used for pretreated remifentanil were all identical. In each group, the first patient that experienced injection pain was included as the second case, with the previous patient included as the first case. The trial was terminated when seven groups defined by alternation of positive and negative reactions had been obtained in all the three groups.
Demographic data, including gender, age, weight, BMI, and ASA classification. Demographic data and the use of vasoactive drugs during anesthesia induction period were recored. Vital signs, including ECG, blood pressure, HR, and SpO2, were monitored during the perioperative period. Meanwhile, HR were recorded at the time points of just before anesthesia induction (T0), remifentanil injection completion (T1), rocuronium injection completion (T2), and anesthesia induction completion (T3), respectively. The occurrence of adverse effects of remifentanil, such as breath holding, chest wall stiffness, muscle rigidity, respiratory depression, hypotension, and bradycardia etc., rocuronium dose, and duration of surgery, were also recorded.
Rocuronium injection pain was evaluated and recorded based on a four-point rating scale, which was first published by McCrirrick and Hunter [7] (Table 1). Grades 1–3 were taken for representing injection pain.
The patients were inquired at about 1 h postoperatively when they had recovered fully in PACU whether they recalled experiencing any injection pain during general anesthesia induction. The occurrence of local adverse reactions, including itching, redness, and edema, at the venipuncture site was recorded.
Statistical Analysis
Statistical analysis was performed using SPSS version 21.0 software (IBM Corporation, Armonk, NY, USA). Continuous data with normal distributions were expressed as mean ± standard deviation and were analyzed using independent sample t tests. Categorical data were analyzed using non-parametric test. Two-sided P < 0.05 was considered a statistically significant difference.
The numbers of effective and ineffective cases for each remifentanil dose on rocuronium injection pain prevention in each group were recorded. The logarithm and the total number of cases for each remifentanil dose were calculated in each group. Meanwhile, the effective rate of rocuronium injection pain prevention and the differences between the logarithms of two adjacent remifentanil doses were calculated. The Dixon–Massey formula was used to calculate ED50 and 95% confidence interval (CIs) of remifentanil for rocuronium injection pain prevention as follows:
In the above formulas, n is the total number of cases per remifentanil dose, lgx is the remifentanil dose-specific logarithm, d is the difference between the logarithms of two adjacent doses, and p is the effective rate of pain prevention. The antilogs of the logarithms were ED50 and the corresponding 95% CI.
Results
A total of 89 patients were enrolled and all completed the study, with 27, 31, and 31 patients included in group R1, group R2, and group R3, respectively. Demographic data among the three groups were similar except for age and ASA classification (P < 0.05) (Table 2). No adverse effects of remifentanil occurred in any of the groups. All patients were hemodynamically stable during induction of general anesthesia, with no vasoactive drugs used.
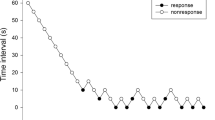
Rocuronium-induced injection pain first appeared with a remifentanil dose of 1 μg/kg LBW in all the three groups. The ED50 (95% CI) of prophylactic remifentanil intravenous injection for rocuronium-induced injection pain prevention was 1.266 (1.186–1.351) μg/kg LBW, 1.188 (1.065–1.324) μg/kg LBW and 1.070 (1.014–1.129) μg/kg LBW in group R1, group R2, and group R3, respectively. The ED50 was significantly decreased in group R2 and group R3 compared with group R1 (P < 0.05). Compared with group R2, ED50 was significantly decreased in group R3 (P < 0.05) (Table 3).
For rocuronium-induced injection pain prevention, prophylactic remifentanil was effective in 14 and ineffective in 13 cases (grade 1, n = 9; grade 2, n = 4) in group R1, effective in 16 and ineffective in 15 cases (grade 1, n = 12; grade 2, n = 3) in group R2, and effective in 17 and ineffective in 14 cases (grade 1, n = 10; grade 2, n = 4) in group R3, with no significant differences among the three groups (P > 0.05). In PACU, 11 of the 13 patients who experienced injection pain in group R1, 13 of the 15 patients who experienced injection pain in group R2, and 12 of the 14 patients who experienced injection pain in group R3 recalled injection pain, with no significant differences among the three groups (P > 0.05) (Table 4).
Compared with T0, HR increased at T2 (P < 0.05) and decreased at T3 (P < 0.05) significantly in all the groups. Compared with T1, HR increased at T2 (P < 0.05) and decreased at T3 (P < 0.05) significantly in all the groups. Compared with T2, HR decreased significantly at T3 (P < 0.05) in all the groups. There were no significant differences among the three groups (P > 0.05) (Table 5).
Sequence diagrams of prophylactic intravenous remifentanil for the prevention of rocuronium injection pain in each group are shown in Figs. 2, 3, and 4.
Discussion
In the current study, the ED50 (95% CI) of prophylactic intravenous remifentanil to prevent rocuronium-induced injection pain was determined to be 1.266 μg/kg (1.186–1.351 μg/kg), 1.188 μg/kg (1.065–1.324 μg/kg), and 1.070 μg/kg (1.014–1.129 μg/kg) LBW in group R1, group R2, and group R3, respectively. The results of this study showed that the ED50 of prophylactic intravenous remifentanil to prevent rocuronium injection pain decreased with age.
The mechanism of rocuronium-induced injection pain is still unclear and may be related to several factors [8]. The stimulation on chemoreceptors by the pH and osmotic pressure of the rocuronium solution can cause painful sensations at the injection site [9]. Kanazawa et al. [10] discovered that diluted rocuronium is effective in alleviating injection pain when administered through dorsal digital veins, which indicated that rocuronium-induced injection pain might be related to the intensity of drug stimulation on the vessels’ wall. Furthermore, rocuronium may permeate through the vascular wall to surrounding tissues, promoting histamine to be released, and creating local swelling and pain, as well as stimulating local nerve terminals to induce signal transduction, and generating central pain perception [11]. The activation of the kinin cascade system, which leads to the release of intermediate mediators such as bradykinin, may also be linked to the pain and arm withdrawal generated by rocuronium injection [9]. In addition, rocuronium is stored at a temperature of 2–8 °C. Low temperature is also a painful irritant to aggravate rocuronium injection pain. During our preliminary trial, we found that rocuronium intubating doses calculated based on LBW produced unsatisfactory muscle relax and decreased success rate of intubation, the mechanism of which might be that the rocuronium intubating doses was too low when calculated on LBW. As a result, we utilized TBW on rocuronium intubating dose calculation in our official test.
Lidocaine [12], ondansetron [13], sodium bicarbonate [14], magnesium sulfate [14], fentanyl [15], ketamine[12], or alfentanil [16] have all been used in previous studies to alleviate intravenous injection pain caused by rocuronium. These methods, however, are somewhat limited and have certain disadvantages. For example, the combination of rocuronium with lidocaine is less effective in alleviating rocuronium injection pain [12], and a mixture of rocuronium and sodium bicarbonate may form carbon dioxide bubbles [3].
Remifentanil is an esterase-metabolized opioid with rapid onset of action, ultra-short duration, and a more stable half-life than other opioids. Prophylactic intravenous remifentanil can relieve rocuronium-induced pain effectively [12]. The mechanism may be attributed to the effects of opioid action, including central and peripheral effects, with central effects predominating. Therefore, sufficient onset of action time on the central nervous system after intravenous remifentanil is required to exert its inhibitory effect on rocuronium-induced pain. The peak effect time of remifentanil is about 1 min [17], so this study chose to start rocuronium injection at 1 min later after remifentanil injection. The anesthesia depth resulting from pretreated remifentanil could increase the pain threshold and thus decrease the incidence of arm withdrawal movements [18]. Opioid receptors are present not only on the dorsal root ganglia and central terminals of primary afferent nerves but also on peripheral sensory nerve endings, which may also be the analgesic site of remifentanil. The common adverse effects of opioids (cough, chest wall stiffness, bradycardia, etc.) prevent clinicians from using them in alleviating rocuronium injection pain. Oh [19] et al. reported that prophylactic intravenous remifentanil 1 μg/kg in preventing rocuronium injection pain produced stable hemodynamic indices with a ± 20% fluctuation from the preoperative ranges with no chest wall stiffness occurring. Slow injection of opioids (> 30 s) can alleviate these adverse effects. In the current study, remifentanil was intravenously injected at a period of 60 s to avoid the occurrence of adverse reactions. Choi et al. [3] found that both remifentanil 0.5 μg/kg and 1 μg/kg were effective in inhibiting arm retraction movements induced by rocuronium injection pain. Previous studies did not consider the effect of body fat content on remifentanil effect in alleviating rocuronium-induced pain. Both fat and LBW increase in obese patients, and the increase in LBW accounted for 20–40% of the total obese body weight, which significantly affects the apparent volume of distribution of anesthetic drugs. Blood concentrations of remifentanil are approximately the same in obese patients by LBW versus normal-weight patients by TBW [20]. It is important to note that too low a dose of remifentanil may be selected in too small BMI cases and too high a dose of remifentanil may be used in too high BMI cases with possible imperfect analgesia or side effects if we calculate remifentanil dosage on the basis of TBW. The dose of remifentanil used in this study was calculated based on LBW, which is defined as the body weight without fat but including cell membranes, central nervous system, and bone marrow; therefore, the results of this study apply to patients with varying fat content [21].
In the current study, we found that the ED50 of prophylactic intravenous remifentanil to prevention rocuronium injection pain gradually decreased with age, which may be related to the following factors. First, pain sensitivity decreases with age [22]. Second, the synthesis of albumin by the liver is weakened, drug–protein binding is reduced, and the dose of analgesic drugs required is reduced with age accordingly. A smaller dose of remifentanil in aged patients can produce the same analgesic effect as in younger patients.
There were no significant changes of HR after remifentanil injection completion in all the three groups, which revealed that the doses of remifentanil used in our study were in safe dosage ranges. The limited and mild increase of HR when rocuronium injection was completed in all the groups gives us an enlightenment that efficient analgesia for rocuronium-induced pain is essential.
In this study, remifentanil adjacent doses were set at a 1.1 ratio using the classic Dixon sequential approach (n = 7 positive to negative reaction alternations). This method is suitable for ED50 determination in clinical research, as the study we have accomplished previously [4].
The study is not without limitations. First, given the existence of gender differences in pain perception, the pharmacodynamics of pain inhibition for rocuronium injections in patients of different genders needs to be further investigated. Second, the evaluation of injection pain in this study was based on the observation of patients’ behavior, which may be subjective to some extent. Therefore, we would like to employ objective nociception-guided evaluation means as well as subjective measures for future investigation.
Conclusions
In conclusion, prophylactic intravenous remifentanil can prevent rocuronium injection pain, and its ED50 decreases with age, with 1.266 μg/kg (18–44 years), 1.188 μg/kg (45–59 years), and 1.070 μg/kg LBW (60–80 years), respectively, the dose of which should be calculated according to LBW for patients with different fat content.
References
Zhang XM, Wang Q, Wang WS, et al. Large vein injection alleviates rocuronium-induced pain in gynaecologic patients. Anaesth Crit Care Pain Med. 2017;36(4):233–5.
An X, Li C, Sahebally Z, et al. Pretreatment with oxycodone simultaneously reduces etomidate-induced myoclonus and rocuronium-induced withdrawal movements during rapid-sequence induction. Med Sci Monit. 2017;23:4989–94.
Choi BI, Choi SH, Shin YS, et al. Remifentanil prevents withdrawal movements caused by intravenous injection of rocuronium. Yonsei Med J. 2008;49(2):211–6.
Tian S, Zhang D, Zhou W, et al. Median effective dose of lidocaine for the prevention of pain caused by the injection of propofol formulated with medium- and long-chain triglycerides based on lean body weight. Pain Med. 2021;22(6):1246–52.
Stocks GM, Hallworth SP, Fernando R, et al. Minimum local analgesic dose of intrathecal bupivacaine in labor and the effect of intrathecal fentanyl. Anesthesiology. 2001;94(4):593–8 (discussion 595A).
Matson JL, Wilkins J, Fodstad JC. Children with autism spectrum disorders: a comparison of those who regress vs. those who do not. Dev Neurorehabil. 2010;13(1):37–45.
McCrirrick A, Hunter S. Pain on injection of propofol: the effect of injectate temperature. Anaesthesia. 1990;45(6):443–4.
Watanabe F, Kako H, Miyazu M. Comparison of injection pain in pediatric population; original versus generic rocuronium. Saudi J Anaesth. 2020;14(1):44–7.
Cakirgoz MY, Demirel I, Duran E, et al. Gabapentin pretreatment for propofol and rocuronium injection pain: a randomized, double-blind, placebo-controlled study. Niger J Clin Pract. 2018;21(1):43–8.
Kanazawa M, Sato Boku A, Okumura Y, et al. The effect of various dilute administration of rocuronium bromide on both vascular pain and pharmacologic onset: a randomized controlled trial. BMC Anesthesiol. 2019;19(1):76.
Blunk JA, Seifert F, Schmelz M, et al. Injection pain of rocuronium and vecuronium is evoked by direct activation of nociceptive nerve endings. Eur J Anaesthesiol. 2003;20(3):245–53.
Jung KT, Kim HJ, Bae HS, et al. Effects of lidocaine, ketamine, and remifentanil on withdrawal response of rocuronium. Korean J Anesthesiol. 2014;67(3):175–80.
Memis D, Turan A, Karamanlioglu B, et al. The prevention of pain from injection of rocuronium by ondansetron, lidocaine, tramadol, and fentanyl. Anesth Analg. 2002;94(6):1517–20 (table of contents).
Turan A, Memis D, Karamanlioglu B, et al. The prevention of pain from injection of rocuronium by magnesium sulphate, lignocaine, sodium bicarbonate and alfentanil. Anaesth Intensive Care. 2003;31(3):277–81.
Oh AY, Seo KS, Goo EK, et al. Prevention of withdrawal movement associated with injection of rocuronium in children: comparison of remifentanil, alfentanil and fentanyl. Acta Anaesthesiol Scand. 2007;51(9):1190–3.
Kim JH, Kim JH, Han SH, et al. Alfentanil is comparable to remifentanil in preventing withdrawal movement following rocuronium injection. J Clin Anesth. 2009;21(1):9–12.
Yoon JR, Jeon Y, Yoo Y, et al. The analgesic effect of remifentanil on prevention of withdrawal response associated with the injection of rocuronium in children: no evidence for a peripheral action. J Int Med Res. 2010;38(5):1795–800.
Kim JY, Kim JY, Kim YB, et al. Pretreatment with remifentanil to prevent withdrawal after rocuronium in children. Br J Anaesth. 2007;98(1):120–3.
Park SH, Oh AY, Goo EK, et al. A short period of inhalation induction with sevoflurane prevents rocuronium-induced withdrawal in children. Acta Anaesthesiol Scand. 2011;55(1):87–91.
La Colla L, Albertin A, La Colla G, et al. Predictive performance of the “Minto” remifentanil pharmacokinetic parameter set in morbidly obese patients ensuing from a new method for calculating lean body mass. Clin Pharmacokinet. 2010;49(2):131–9.
Lemmens HJ. Perioperative pharmacology in morbid obesity. Curr Opin Anaesthesiol. 2010;23(4):485–91.
Yezierski RP. The effects of age on pain sensitivity: preclinical studies. Pain Med. 2012;13(Suppl 2):S27–36.
Acknowledgements
We sincerely thank all the participants and their families who participated in this study, and all the nurse staff for their help in this study.
Funding
The study was funded by Beijing Medical Award Foundation Key Project (No. YXJL-2021-0307-0632) and the Social Development Project of Yangzhou Science and Technology Bureau (No. YZ2022108) from Dr. Zhuan Zhang. No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors’ Contributions
Dongsheng Zhang and Zhuan Zhang conceived the study and contributed to the writing of the manuscript. Shiting Yan prepared and injected remifentanil. Hao Wu implemented oxygen supply and mechanical ventilation and recorded possible toxic reaction symptoms resulting from remifentanil injection. Yanlong Yu was responsible for rocuronium injection pain evaluation and general anesthetic preparation and injection. Ning Li evaluated injection pain and observed local adverse reaction occurrence in the PACU. Leyang Yu, Ying Wang and Hu Li analyzed the data. All authors read and approved the final manuscript.
Disclosures
All the authors, Shiting Yan, Hao Wu, Yanlong Yu, Ning Li, Leyang Yu, Ying Wang, Hu Li, Dongsheng Zhang, and Zhuan Zhang, declare that they have no conflicts of interest.
Compliance with Ethics Guidelines
Ethical approval (Ethical Committee 2022-YKL1-20-002) was approved by the Institutional Research Ethics Committee of the Affiliated Hospital of Yangzhou University, Yangzhou, China in December 2021. All patients provided informed consent and all procedures were conducted according to the Declaration of Helsinki.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Yan, S., Wu, H., Yu, Y. et al. Median Effective Dose of Remifentanil for the Prevention of Pain Caused by the Injection of Rocuronium: An Age-Stratified Study. Pain Ther 12, 683–694 (2023). https://doi.org/10.1007/s40122-023-00490-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-023-00490-5