Abstract
Background
While the variety of biologics (b) and targeted synthetic (ts) disease-modifying anti-rheumatic drugs (DMARDs) available for patients with psoriatic arthritis (PsA) has proved to be efficacious in randomized clinical trials, there is a growing importance to understand the benefits and potential drawbacks of these different therapies in real-world settings, which includes bio-experienced and older patients as well.
Objective
To evaluate the real-world adherence, drug survival, and discontinuation risk of bDMARDs and tsDMARDs among patients with PsA, comprising both younger and older patients.
Methods
A retrospective study using a computerized database. Treatment-naïve and treatment-experiencedpatients with PsA, younger and older than 60 years, who initiated treatment with bDMARDs [TNF-α inhibitors (TNF-αis), IL-17 inhibitors (IL-17is), IL-12/23 inhibitors (IL-12/23i)] or tsDMARDs (the PDE-4 inhibitor apremilast) during 2015–2018 were included. Adherence was assessed using the proportion of days covered (PDC) method. Time to discontinuation was analyzed using Kaplan–Meier estimates. Risk of discontinuation was estimated by Cox proportional hazard model.
Results
We identified 427 eligible patients (22.2 % were older than 60 years), utilizing 673 treatment lines. The proportion of adherent patients (PDC ≥ 0.8) was similar (62.1–66.5%) across all lines of therapy and across different biologics (70.0–72.0%), while apremilast showed the lowest, in both treatment-naïve and experienced settings (43.6% and 25.5%, respectively). The Kaplan–Meier analysis showed that in the treatment-naïve TNF-αis had higher drug survival compared with apremilast (P = 0.032). Apremilast also had the lowest drug survival in the treatment-experienced group (P < 0.0001). Kaplan–Meier analysis by age groups showed similar drug survival rates in older (≥ 60 years) and younger (age < 60 years) patients, regardless of treatment-experience status. The multivariable model showed that apremilast had increased risk for discontinuation compared with TNF-αis.
Conclusion
Adherence, drug survival and risk for discontinuation were similar for all included bDMARDs, regardless of treatment experience status, while apremilast showed lower rates and increased risk. Adherence and discontinuation rate were similar in older and younger patients. With the variety of drug modes of action available for patients with PsA, these findings may assist caregivers in selecting the appropriate treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Compared with patients using tumor necrosis factor-α (TNF-α) inhibitors, those using the phosphodiesterase-4 (PDE-4) inhibitor apremilast had increased risk for discontinuation. |
Adherence rates drug survival and risk for discontinuation were similar for all included biologic disease modifying antirheumatic drugs (DMARDs), regardless of treatment experience status, while the targeted synthetic DMARD, PDE-4 inhibitor, was associated with lower patient adherence and increased discontinuation risk. |
Adherence rates and risk for discontinuation were similar in older patients compared with younger ones, regardless of being treatment-naïve or experienced. |
With the variety of biologics and targeted synthetic DMARDs available for psoriatic arthritis, these findings may assist caregivers in selecting the appropriate treatment option, both for treatment-naïve and treatment-experienced patients, younger and older. |
1 Introduction
Psoriatic arthritis (PsA) is a chronic seronegative inflammatory arthritis occurring in approximately a third of the patients with psoriasis [1]. Men and women are equally affected and for the majority of patients rheumatic manifestations appear 7–8 years after the initial onset of psoriasis [2].
The prevalence of PsA varies widely by geographical region and is estimated to range from 0.05 to 0.67%. A study performed in Israel by Eder et al. during 2006–2015 reported a crude prevalence of 0.153% [3].
Once thought to be a relatively benign condition, it is now recognized that PsA is a systemic disease, potentially debilitating, erosive disease, that can negatively impact many aspects of daily life [4]. Repeated joint pain, chronic fatigue, decreased occupational function, as well as psychological distress and social morbidity may result in decreased quality of life (QoL) [5]. The advent of new treatments for PsA in the past decades, mainly in the form of biological disease modifying antirheumatic drugs (bDMARDs), and targeted synthetic disease modifying anti-rheumatic drugs (tsDMARDs), along with their relative early implementation in the treatment paradigm, have significantly improved patients with PsA care, quality of life and prevented disability [6, 7]. According to numerous studies, biologic agents and apremilast, have shown to ameliorate disease activity, inhibit radiographic progression and improve quality of life to a similar extent [7, 8]. In addition, their safety profile are clearly acceptable [9].
While the variety of bDMARDs and tsDMARDs available for patients with PsA has proved to be efficacious and with a good safety profile in randomized clinical trials [10], these studies included mostly relatively young and treatment-naïve patients [9, 11]. In the real-world, these drugs are used for both treatment-naïve and treatment-experienced patients and naturally encompassing all age groups. However, data on the safety and efficacy among the latter is sparse [12, 13] and novel data are lacking [14].
Drug adherence, defined as the extent to which a patient takes a drug as prescribed by the health care provider [15] and drug survival, defined as the time from initiation to discontinuation (whether due to stop or switch) of a specific therapy [16], are commonly used, indirect measures to evaluate drug efficacy and safety [17] in real-world settings. In PsA, such studies are present [18,19,20,21,22,23]; however, they are limited and often have conflicting results due to differences in drug availability originating from regional subsidization and local health insurance policies and coverage. As treatment choices continue to expand and join the growing armamentarium in PsA, there is a growing importance to understand the benefits and potential drawbacks of these different therapies.
The aim of this study was to compare the adherence and drug survival of different bDMARDs and tsDMARDs in the treatment of PsA in a real-world setting by utilizing the electronic database of a large Israeli health state-mandated health provider.
Methods
1.1 Study Sample and Data Collection
This retrospective cohort study was conducted using the computerized databases of Maccabi Healthcare Services (MHS). MHS is the second largest state-mandated health provider operating in Israel, providing healthcare services for over 2.6 million members (25% of the Israeli population), with 1.1–1.5% leaving annually. [24] The MHS databases integrate data from the MHS central laboratory, medication prescriptions and purchases throughout the MHS pharmacy network, primary care, expert consultations, hospitalizations, procedures, and socio-demographic data. Physician diagnoses are coded using the International Classification of Disease, 9th Edition (ICD-9-CM) codes as well as internal MHS codes for subclassification. In addition, MHS has developed and validated computerized registries of patients who are suffering from major chronic conditions such as cardiovascular diseases and diabetes [1, 25, 26]
The study was conducted in accordance with the protocol, applicable regulations, and guidelines governing clinical study conduct and the ethical principles that have their origin in the Declaration of Helsinki. The MHS Institutional Review Board (IRB), approved the study protocol and related documents. The MHS’s IRB waived the requirement to obtain any informed consent for this secondary analysis of existing data (approval number 0108-18-BBL, 18 December 2018).
1.2 Study Population and Follow-Up
According to the Israeli regulatory guidelines, patients with PsA are eligible for bDMARDs or tsDMARDs after failing to achieve control over their disease with two conventional synthetic (cs) DMARDs. Prior to procurement, patients are required to receive an authorization from MHS drugs authorization center, confirming they comply with the guidelines. Patients’ co-payment is the same, regardless of which bDMARD or tsDMARD they are using.
The study population included patients who, between 1 January 2015 and 31 December 2017, first purchased one or more of the following drugs for the indication of PsA: TNF-α inhibitors [adalimumab (ADA), infliximab (IFX), golimumab (GLM), etanercept (ETN), certolizumab pegol (CTZ)]; IL-12/23 inhibitor [ustekinumab (UST)]; IL-17 inhibitor [secukinumab (SEC)]; CTLA4-Ig [abatacept (ABA)]; PDE-4 inhibitor [premilast (APR)]. All patients were prescribed with these drugs for the indication of PsA only, according to the MHS drug authorization center. The first purchase was defined as the index date for the study. Included patients were adults (age ≥ 18 years) and MHS members for ≥ 12 months before and after the index date. During the study follow-up period, all the aforementioned drugs were available as first line treatment after utilizing ≥ 2 different conventional synthetic DMARDs (i.e., methotrexate, leflunomide, sulfasalazine).
Patients were followed until the earliest of the following dates: death, leaving MHS, or the end of the follow-up period (31 December 2018).
1.3 Study Variables
We included treatment-naïve patients (those with no previous purchase of any of the drugs included in the study before 1 January 2015), as well as treatment-experienced patients (those treated with any of the drugs included in the study before 1 January 2015 who switched and started treatment with any of the other drugs included in the study, between 1 January 2015 and 31 December 2017).
Initiation of a new treatment line was defined by the first purchase of the drug. For all patients, we followed all lines of therapy used during the study follow-up period and numbered them. For those defined as treatment-experienced when entering the study, we numbered the lines of therapy used during the study follow-up period, using data on bDMARDs and tsDMARD dispensed up to 10 years before entering the study.
Adherence to medical treatment was evaluated by using the proportion of days covered (PDC) method. PDC reflects the number of days covered by the dispensed drug divided by the total follow-up time for a specific line of therapy. PDC was categorized as followed: nonadherent (PDC < 40%), moderately adherent (40% ≤ PDC < 80%), or highly adherent (PDC ≥ 80%).
Drug survival was measured from the initiation of treatment (i.e., the index date) until treatment discontinuation, which was defined as the first gap of 120 days or more after the last supply date. Patients who discontinued their current drug were further classified as: switching (starting a new treatment, with any of the drugs included in the study), restarting (continued the same treatment after the ≥ 120 day’s gap), stopping (neither switching nor restarting treatment with any of the drugs included in the study).
Additional data retrieved from the database included: sociodemographic factors [age, sex, residential area socioeconomic status (SES), and smoking status]; baseline comorbidities according to MHS registries [cardiovascular disease [25], diabetes [26], hypertension [27], obesity (latest body mass index (BMI) record ≥ 30), and osteoporosis [28]]. Depression and anxiety were defined according to antidepressants and benzodiazepines dispensed 180 days before the index date. The Charlson Comorbidity Index was calculated at baseline. Additional data at baseline included disease duration, visits to a primary care physician (PCP), and being hospitalized at least once in the 180 days before the index date.
1.4 Statistical Analyses
Baseline characteristics, adherence rates, discontinuation rate, type, and time to discontinuation are presented using descriptive statistics, namely n (%), mean ± standard deviation (SD), or median and interquartile range (IQR), as appropriate. To assess differences in adherence and drug survival between older and younger patients, we used age of 60 years as the cutoff and patients were categorized by their age at treatment initiation (older, age < 60 years; younger, age ≥ 60 years) [29, 30]. Baseline characteristics are presented by age group and statistical differences between the two age groups were assessed.
Adherence was assessed by line of therapy, bio-experience status and mode of action (MoA), and bio-experience status and individual drug. Additionally, adherence rates are presented by bio-experience status and age group and differences were assessed using the Chi-squared test. Time to treatment discontinuation was analyzed and plotted using Kaplan–Meier estimates and the log rank test was used to evaluate statistical significance between MoA and between age group in treatment-naive and in treatment-experienced patients.
The risk of treatment discontinuation of each MoA was estimated separately for the treatment-naïve and the treatment-experienced patients using a multivariable Cox proportional hazards regression model. The model was adjusted for age group, sex, SES, smoking status, disease duration, various comorbidities (diabetes, hypertension, obesity, osteoporosis, depression, and anxiety), visits to the PCP, MoA, and line of therapy (only in the treatment-experienced).
Two-sided P < 0.05 was considered statistically significant. All statistical analyses were performed with IBM-SPSS V.25.0 standard statistical software for Windows and R V.3.5.
2 Results
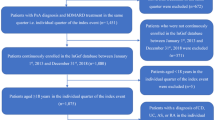
Between January 2015 and December 2017, 427 eligible patients with PsA, with 673 treatment episodes (i.e., lines of therapy), were identified. Most patients were treatment-naïve (67.9%), and the rest (32.1%) entered the study as treatment-experienced (i.e., on their second line of therapy or beyond; Fig. 1). Table 1 shows the baseline characteristics of included patients by bio-experience status. The mean age was 49.9 years (SD ± 12.2) with 22.2% (n = 95) older than 60 years (“older”) and the rest under the age of 60 years (“younger”). There was a slight female predominance (56.2%). The majority of the patients were MHS members before the index date (89.5%). The overall mean disease duration was 4.62 years (SD ± 4.16) and was longer in the bio-experienced group (7.2 ± 3.9 versus 3.4 ± 3.6, P < 0.001). Bio-experienced patients also used more lines of therapy during follow-up, with 53.3% using only one line compared with 64.8% in the bio-naïve group (P = 0.039). For the whole cohort, the most prevalent comorbidities were obesity (34.0%), hypertension (30.0%), and depression or anxiety (29.7%). Osteoporosis and depression and/or anxiety were more prevalent in the bio-experienced group (P < 0.05), which also had higher number of visits to the PCP (8.8 ± 5.4 versus 7.5 ± 5.2, P = 0.021).
2.1 Adherence to Treatment
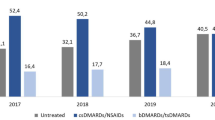
Across all lines of therapy (Fig. 2a), the proportion of highly adherent patients (PDC ≥ 0.8) was similar, ranging from 62.1 to 66.5% (P = 0.944). Similarly, the proportion of nonadherence (PDC < 0.4) was similar regardless the line of therapy examined, ranging from 16.4 to 17.0%. Analysis by treatment experience and MoA (Fig. 2b) revealed similar proportions of highly adherent patients among treatment-naïve patients using TNF-αis (72.0%) and treatment-experienced patients using TNF-αis, IL-17is, or IL-12/23i (72.0%, 71.6%, 70.4%, and 70.0%, respectively). The proportion of highly adherent patients treated with PDE-4i were lower compared with the other MoA, for both treatment-naïve and treatment-experienced patients (P <0.014). Additionally, the proportion of highly adherent patients using PDE-4i decreased from 43.6% among the treatment-naïve to 25.5% among the treatment-experienced patients. Analysis of adherence by treatment-experience and individual drug (Fig. 2c) revealed that among the treatment-naïve, GOL had the highest proportion of highly adherent patients (79.2%) followed by ETN (71.4%), ADA (69.7%), and APR (43.6%) (P < 0.001). In the treatment-experienced, the proportion of highly adherent patients ranged from 69.1 to 72.2%, for ADA, UST, GOL, SEC, and ETN, and was lower for APR (25.5%; P <0.001). Less than 20 patients received IFX and CZP. Analysis of adherence by bio-experience status and age group (Fig. S1) revealed a similar proportion of highly adherent patients rate among treatment-naïve younger and older patients (63.3% and 57.4%, respectively; P = 0.381). The proportion of highly adherent treatment-experienced patients was lower in the older (60.7%) versus the younger (66.2%; P = 0.002).
Adherence in the first 12 months, by line of therapy, mode of action, individual drugs, and treatment-experience status. A By line of therapy (n = 673 treatment lines). B By mode of action and treatment-experience status (n = 668 treatment lines; excluded in the treatment-naïve patients: IL-17is, n = 3, and excluded in the treatment-experienced patients: CTLA-4 Ig, n = 2, JAKi, n = 1). C By individual drug and treatment-experience status (n = 668 treatment lines; excluded in the treatment-naïve patients: IFX, n = 2, SEC, n = 3, and excluded in the treatment-experienced patients: ABA, n = 2, JAKi, n = 1). PDC proportion of days covered, ADA adalimumab, ETN etanercept, GOL golimumab, APR apremilast, IFX infliximab, SEC secukinumab, UST ustekinumab, ABA abatacept
2.2 Discontinuation and Drug Survival
Table 2 shows the discontinuation rate, type, and time to discontinuation in the first 12 months of follow-up, by treatment experience status, MoA, and individual drug. After 12 months, the discontinuation rate was similar across all lines of therapy (first line: 39.3%; second line: 47.9%; third line: 43.6%; fourth line and above: 50.0%; P = 0.207). As for type of discontinuation, in both treatment-naïve and treatment-experienced patients, most discontinuations were due to switching (23.8% and 31.3%, respectively). In the treatment-naïve group (n = 290), 39.3% discontinued their treatment during the first 12 months of follow-up. Patients using PDE-4i had the highest discontinuation rate (47.9%) compared with those using TNF-α (35.2%; P = 0.040). Among the latter, ADA had the highest discontinuation rate (42.1%). In the treatment-experienced group (n = 300), the overall discontinuation rate in the first 12 months was 47.3%. Patients using PDE-4i had the highest rate of discontinuation (77.3%), followed by TNF-αis (42.8%), IL-17i (38.6%), and IL-12/23i (37.9%) (P < 0.01). Among those treated with TNF-αis, ADA had the highest rate of discontinuation (53.0%), followed by ETN (38.6%) and GOL (37.0%) (P = 0.152).
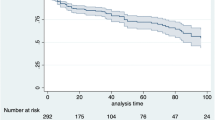
Among the treatment-naïve group, the Kaplan–Meier analysis (Fig. 3a) showed superiority for treatment with TNF-αis compared with PDE-4i (P = 0.048 by log-rank test). For the treatment-experienced group, treatment with PDE-4i had the lowest drug survival rate compared with TNF-αis, IL-17i, and IL-12/23i (P < 0.01; Fig. 3b). The Kaplan–Meier analysis by bio-experience status and age group (Fig. S2), showed similar discontinuation rates after 12 months among the older and younger patient, regardless of being treatment-naïve or treatment-experienced.
Table 3 shows the hazard ratios (HR) for treatment discontinuation in the treatment-naïve and the treatment-experienced groups, obtained from a multivariable Cox proportional hazard regression model. In the treatment-naïve group, being a smoker (ever) and having depression and anxiety and treatment with PDE-4i were associated with increased risk for treatment discontinuation [HR 2.45, 95% confidence interval (CI) 1.48–4.05; HR 1.56, 95% CI 1.02–2.38; HR 1.82, 95% CI 1.22–2.71, respectively]. Among the treatment experienced group, hypertension was associated with reduced risk for treatment discontinuation (HR 0.62, 95% CI 0.40–0.95), while obesity increased the risk for discontinuation (HR 1.58, 95% CI 1.10–2.27). Similar to treatment-naïve patients, treatment with PDE-4i was associated with increased risk for discontinuation (HR 2.95, 95% CI 1.95–4.47). Advancement in treatment lines was not associated with increased risk for discontinuation (P > 0.5), nor was treatment with IL-17i or IL-12/23i (P > 0.5). Table 4 shows the HRs for treatment discontinuation in all treatment episodes obtained from a multivariable Cox proportional hazard regression model. The results were similar to those found in the bio-naïve and bio-experienced models, with the exception of line of therapy showing that bio-experienced patients had increased risk for discontinuation compared with bio-naïve patients (HR 1.48 95% CI 1.12–1.97; P=0.006). In the model among the bio-experienced group, patients using a third, fourth, or above line of therapy did not have an increased risk for discontinuation compared to those on their second line of therapy (P > 0.05).
3 Discussion
Our study compared the patients’ adherence and drug survival of bDMARDs and tsDMARDs indicated for PsA that were available for MHS members during 2015–2018. Additionally, we examined adherence in older patients compared with younger ones. To the best of our knowledge, our results for older patients with PsA are the most comprehensive and provide useful real-world information in a mostly unexplored topic. Rates of high adherence were similar across all lines of therapy, ranging between 62.1 and 66.5%, yet adherence rate was slightly lower (60.7%) when the analysis was restricted to treatment-experienced older patients. Drug survival rates after 12 months of follow-up were similar across all lines as well. In both treatment-naïve and treatment-experienced settings, the rate of highly adherent patients was higher for bDMARDs, (TNF-αis, IL-17i, IL-12/23i) compared with that of PDE-4i (APR) (PDC > 0.8, 65.5–72.0% versus 25.5–43.6%, respectively), as were the drug survival rates (35.2–42.8% versus 47.9–77.3%, respectively). The results of the multivariable model showed that older patients had similar risk for discontinuation compared to younger ones, regardless of being treatment-naïve or experienced. Additionally, the model showed that compared with TNF-αis, patients treated with PDE-4i (APR) had increased risk for discontinuation, while those using IL-17is or IL-12/23i did not.
Previous studies reported conflicting results regarding discontinuation rates. A study by Hadad et al. [31] on patients with PsA from Clalit Health Services (CHS), Israel’s largest healthcare provider, with a similar range of treatment options, found that only 40% of patients maintained their treatment after 20 month of follow-up. Another study by Walsh et al. [32] that included 996 patients with PsA who initiated treatment with ADA, CTZ, ETN, GOL, or UST reported that only 19.7% of them remained on their index drug after 2 years. Of note, in both studies, the drug survival rates were calculated without distinguishing between treatment-naïve and treatment-experienced patients. On the contrary, and similar to our findings, Vegas et al. [33] found that among patients with PsA initiating their first bDMARDs (TNF-αis, IL-17is, or IL-12/23i), the drug survival rate after one year of follow-up was 72%. In another study by the same group [34] aiming to evaluate the persistence rate of the second biologic in patients with PsA following discontinuation of a TNF-αi, persistence rate was 42% after 1 year. In an observational study of patients with PsA treated with TNF-αis or IL-17is, the overall 12-month discontinuation rates were: 43.4% for treatment-naïve and 44.3% for treatment-experienced patients, similar to our results [21]. While we observed that the overall drug survival rates were similar for all lines of therapy, when observing the survival rates by MoA in the treatment-naïve and the treatment-experienced groups, TNF-αis had higher rates in the former. These results are consistent with the ones by Kristensen et al. [35], which also showed a decline in drug survival among TNF-αi-experienced patients with PsA who were treated with a second or third TNF-αi.
Our results showed that APR had was associated with lower patient adherence and drug survival rates, compared with other MoA, in both treatment-naïve and treatment-experienced settings. While APR was found to have limited efficacy compared to other bDMARDs [36], several studies have reported different results than ours: Ogdie et al. [37] found that in a subset of patients with PsA with milder disease involving less than five joints (“oligoarthritis”), APR is considered to be efficacious, similar to other bDMARDs; Feldman et al. [38] revealed that in treatment-naïve patients with PsA prescribed APR as first line in comparison with other bDMARDs (TNF-αis, SEC, UST) and the adherence rate after 12 months was 76.9%. A plausible explanation for the difference between these results and the ones found in our study is the difference in patients’ characteristics, mainly disease burden and the extent of joint involvement, which were unavailable in the setting of the current study. In addition, due to the fact that APR was the only orally administered drug included in the study, we cannot conclude that the observed tsDMARDs differences in adherence and persistence rates were partially or totally caused by their mode of administration.
In the current study, depression and anxiety, both prevalent in patients with PsA compared with the general population [39], were found to increase the risk for discontinuation among treatment-naïve patients with PsA. Previous reports have found that depression and anxiety may reduce the likelihood of disease remission and attenuate pain relief [40] and lower drug adherence [41]. We also observed increased risk for discontinuation in obese patients, which is a common comorbidity in PsA [42]. Other reports have indicated a negative impact of high BMI in patients with PsA on treatment response for both csDMARDs and bDMARDs [39], which may be associated with early treatment discontinuation and pursuing a new one.
PsA is not uncommon among patients over 60 year old [43], but is expected to rise in the face of increased life expectancy and improvement in diagnostic techniques [44], yet data on the topic is currently limited. Older patients with PsA in our cohort had an increased rate of cardiovascular comorbidities and cardiovascular diseases. These results are in line with those of former studies [29, 45], which also reported similar treatment strategies in younger and older patients with PsA; however, patients’ adherence and discontinuation rates were not addressed. Conversely, in a short abstract by Gönüllü et al. [46] addressing survival of only TNF-αi in the elderly (> 65 year) and younger patients with PsA, drug survival was significantly higher among the latter.
In our study the IL-12/23 inhibitor, UST, did not show superiority in terms of drug survival or patients’ adherence compared with other bDMARDs. According to findings from the BIOPURE registry, the 12-month drug survival rate for patients with PsA treated with UST was 74.4%. During this period, drug survival was significantly higher in the bio-naïve (87%) than in the TNF-αis-experienced patients (68%, P = 0.01), and bio-naïve patients had the lowest risk of UST discontinuation (HR 0.27, P = 0.01) [47]. A similar trend, also evaluating SEC, found the median drug survival for UST and SEC to be 12 and 14 months, respectively, concluding that drug survival of UST and SEC is similar to that of TNF-α inhibitors [48]. In contrast, in a real-world study based on commercial and Medicare Supplemental databases, patients using UST demonstrated longer persistence and higher adherence than either TNF-αis or tsDMARDs and comparability with IL-17is [49]. Yu et al. [50] implied that such variation in UST drug survival might express different therapeutic responses to biologic agents among different ethnic groups.
Our study has several limitations: we used administrative and computerized data, which lacked more accurate clinical information, mainly disease activity and joint distribution (polyarthritis versus oligo- and mono-arthritis). Secondly, we did not measured the impact of concomitant therapy with csDMARDs such as methotrexate, which has shown to improve drug survival and to lower discontinuation rates [51, 52]. Also, the reasons for drug discontinuation of each subject and whether it was related to loss of efficacy, adverse events, or other factors could not be retrieved from the data. Another limitation is derived from the end of the study’s follow-up period (2018) and 2023. Since 2018, additional treatment options were approved for PsA (e.g., the IL-23 inhibitors guselkumab and risankizumab, and the JAK inhibitors tofacitinib and upadacitinib). These additional therapies, as well as the treat to target approach that is now more widely used for patients with PsA, may also have an impact on drug survival [14]. The current study also has several strengths. We were able to follow patients for a long period of time and include almost all bDMARDs and tsDMARDs available for PsA, which are still widely used in 2023 [53]. We used real-world data, without any exclusion or randomization. Additionally, we were able to assess adherence, drug survival and the risk for treatment discontinuation both in treatment-naïve and treatment-experienced patients.
4 Conclusion
This real-world study shows that adherence to bDMARDs among patients with PsA is generally high, including in older patients. Drug survival was similar between older and younger patients. The adherence rates, drug survival, and the risk for discontinuation were similar across all included MoA, except for APR, which showed lower rates and increased risk for discontinuation.
In light of the variety of MoA available for patients with PsA, our findings may assist caregivers in selecting the appropriate treatment option, both for treatment-naïve and treatment-experienced patients, younger and older.
References
Ogdie A, Weiss P. The epidemiology of psoriatic arthritis. Rheum Dis Clin North Am. 2015;41:545–68.
Tillett W, Charlton R, Nightingale A, Snowball J, Green A, Smith C, et al. Interval between onset of psoriasis and psoriatic arthritis comparing the UK Clinical Practice Research Datalink with a hospital-based cohort. Rheumatology (Oxford). 2017;56:2109–13.
Eder L, Cohen AD, Feldhamer I, Greenberg-Dotan S, Batat E, Zisman D. The epidemiology of psoriatic arthritis in Israel—a population-based study. Arthritis Res Ther. 2018;20:3.
Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis. 2015;74:1045–50.
Gudu T, Gossec L. Quality of life in psoriatic arthritis. Expert Rev Clin Immunol. 2018;14:405–17.
D’Angelo S, Tramontano G, Gilio M, Leccese P, Olivieri I. Review of the treatment of psoriatic arthritis with biological agents: choice of drug for initial therapy and switch therapy for non-responders. Open Access Rheumatol. 2017;9:21–8.
Kamata M, Tada Y. Efficacy and safety of biologics for psoriasis and psoriatic arthritis and their impact on comorbidities: a literature review. Int J Mol Sci. 2020;21:1690.
Ben-Ami Shor D, Weitzman D, Dahan S, Gendelman O, Bar-On Y, Amital D, et al. Adherence and persistence with drug therapy among fibromyalgia patients: data from a large health maintenance organization. J Rheumatol. 2017;44:1499–506.
Ruyssen-Witrand A, Perry R, Watkins C, Braileanu G, Kumar G, Kiri S, et al. Efficacy and safety of biologics in psoriatic arthritis: a systematic literature review and network meta-analysis. RMD Open. 2020;6: e001117.
Dressler C, Eisert L, Pham PA, Nast A. Efficacy and safety of systemic treatments in psoriatic arthritis: a systematic review, meta-analysis and GRADE evaluation. J Eur Acad Dermatol Venereol. 2019;33:1249–60.
McInnes IB, Sawyer LM, Markus K, LeReun C, Sabry-Grant C, Helliwell PS. Targeted systemic therapies for psoriatic arthritis: a systematic review and comparative synthesis of short-term articular, dermatological, enthesitis and dactylitis outcomes. RMD Open. 2022;8: e002074.
Merola JF, Lockshin B, Mody EA. Switching biologics in the treatment of psoriatic arthritis. Semin Arthritis Rheum. 2017;47:29–37.
Ungprasert P, Thongprayoon C, Davis JM. Indirect comparisons of the efficacy of subsequent biological agents in patients with psoriatic arthritis with an inadequate response to tumor necrosis factor inhibitors: a meta-analysis. Clin Rheumatol. 2016;35:1795–803.
Gossec L, Baraliakos X, Kerschbaumer A, de Wit M, McInnes I, Dougados M, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis. 2020;79:700–12.
Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11:44–7.
Menter A, Papp KA, Gooderham M, Pariser DM, Augustin M, Kerdel FA, et al. Drug survival of biologic therapy in a large, disease-based registry of patients with psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Eur Acad Dermatol Venereol. 2016;30:1148–58.
Lin P-T, Wang S-H, Chi C-C. Drug survival of biologics in treating psoriasis: a meta-analysis of real-world evidence. Sci Rep. 2018;8:16068.
Smolen JS, Gladman D, McNeil HP, Mease PJ, Sieper J, Hojnik M, et al. Predicting adherence to therapy in rheumatoid arthritis, psoriatic arthritis or ankylosing spondylitis: a large cross-sectional study. RMD Open. 2019;5: e000585.
Aaltonen K, Heinonen A, Joensuu J, Parmanne P, Karjalainen A, Varjolahti-Lehtinen T, et al. Effectiveness and drug survival of TNF-inhibitors in the treatment of psoriatic arthritis: a prospective cohort study. Semin Arthritis Rheum. 2017;46:732–9.
Souza AF, da Silva MR, dos Santos JB, Almeida AM, Acurcio FA, Alvares-Teodoro J. Medication adherence and persistence of psoriatic arthritis patients treated with biological therapy in a specialty pharmacy in Brazil: a prospective observational study. Pharm Pract (Granada). 2021;19:2312–2312.
Oelke KR, Chambenoit O, Majjhoo AQ, Gray S, Higgins K, Hur P. Persistence and adherence of biologics in US patients with psoriatic arthritis: analyses from a claims database. J Comp Eff Res. 2019;8:607–21.
Egeberg A, Rosenø NAL, Aagaard D, Lørup EH, Nielsen M-L, Nymand L, et al. Drug survival of biologics and novel immunomodulators for rheumatoid arthritis, axial spondyloarthritis, psoriatic arthritis, and psoriasis—a nationwide cohort study from the DANBIO and DERMBIO registries. Semin Arthritis Rheum. 2022;53: 151979.
Murage MJ, Princic N, Park J, Malatestinic WN, Zhu B, Atiya B, et al. Treatment patterns and health care costs among patients with psoriatic arthritis treated with biologic or targeted synthetic disease-modifying antirheumatic drugs. JMCP. 2022;28:206–17.
The Research and Planning Administration, The National Insurance Institute, Israel, Healthfunds Membership 2018–2019. Retrieved July 12, 2024, from https://www.btl.gov.il/Publications/survey/Documents/seker317/seker_317.pdf
Shalev V, Chodick G, Goren I, Silber H, Kokia E, Heymann AD. PS2-18: the Maccabi Healthcare Services cardiovascular information system: integration of patient care data, registries, and guide. Clin Med Res. 2010;8:38.
Cahn A, Altaras T, Agami T, Liran O, Touaty CE, Drahy M, et al. Validity of diagnostic codes and estimation of prevalence of diabetic foot ulcers using a large electronic medical record database. Diabetes Metab Res Rev. 2019;35: e3094.
Leiba A, Lencovsky O, Azran C, Hadad O, Rappoprt V, Niddam NN, et al. True refractory hypertension in 2023—lessons learned from a national registry of 1.82 million adults. J Hypertens. 2024;42(Suppl 1):e163.
Goldshtein I, Shalev V, Chodick G, Chandler J, Martin NA, Ish SS. The use of clinical data repository for the establishment of an osteoporosis registry in a large health organization in Israel: epidemiologic and pharmaepidemiologic findings. Value Health. 2014;17:A389-390.
Fragoulis GE, Nikiphorou E, McInnes IB, Siebert S. Does age matter in psoriatic arthritis? A narrative review. J Rheumatol. 2022;49:1085–91.
Punzi L, Pianon M, Rossini P, Schiavon F, Gambari PF. Clinical and laboratory manifestations of elderly onset psoriatic arthritis: a comparison with younger onset disease. Ann Rheum Dis. 1999;58:226–9.
Haddad A, Gazitt T, Feldhamer I, Feld J, Cohen AD, Lavi I, et al. Treatment persistence of biologics among patients with psoriatic arthritis. Arthritis Res Ther. 2021;23:44.
Walsh JA, Cai Q, Lin I, Fitzgerald T, Pericone CD, Chakravarty SD. Real-world 2-year treatment patterns among patients with psoriatic arthritis treated with injectable biologic therapies. Curr Med Res Opin. 2020;36:1245–52.
Pina Vegas L, Penso L, Claudepierre P, Sbidian E. Long-term persistence of first-line biologics for patients with psoriasis and psoriatic arthritis in the French Health Insurance Database. JAMA Dermatol. 2022;158:513–22.
Vegas LP, Hoisnard L, Bastard L, Sbidian E, Claudepierre P. Long-term persistence of second-line biologics in psoriatic arthritis patients with prior TNF inhibitor exposure: a nationwide cohort study from the French health insurance database (SNDS). RMD Open. 2022;8: e002681.
Kristensen LE, Lie E, Jacobsson LTH, Christensen R, Mease PJ, Bliddal H, et al. Effectiveness and feasibility associated with switching to a second or third TNF inhibitor in patients with psoriatic arthritis: a cohort study from southern Sweden. J Rheumatol. 2016;43:81–7.
Reed M, Crosbie D. Apremilast in the treatment of psoriatic arthritis: a perspective review. Ther Adv Musculoskeletal. 2017;9:45–53.
Ogdie A, Liu M, Glynn M, Emeanuru K, Harrold LR, Richter S, et al. Descriptive comparisons of the effect of apremilast and methotrexate monotherapy in oligoarticular psoriatic arthritis: the corrona psoriatic arthritis/spondyloarthritis registry results. J Rheumatol. 2021;48:693–7.
Feldman SR, Pelletier CL, Wilson KL, Mehta RK, Brouillette MA, Smith D, et al. Treatment patterns and costs among biologic-naive patients initiating apremilast or biologics for psoriatic arthritis. J Comp Eff Res. 2019;8:699–709.
Patel S, Kumthekar A. Psoriatic arthritis: the influence of co-morbidities on drug choice. Rheumatol Ther. 2022;9:49–71.
Michelsen B, Kristianslund EK, Sexton J, Hammer HB, Fagerli KM, Lie E, et al. Do depression and anxiety reduce the likelihood of remission in rheumatoid arthritis and psoriatic arthritis? Data from the prospective multicentre NOR-DMARD study. Ann Rheum Dis. 2017;76:1906–10.
Betteridge N, Boehncke W-H, Bundy C, Gossec L, Gratacós J, Augustin M. Promoting patient-centred care in psoriatic arthritis: a multidisciplinary European perspective on improving the patient experience. J Eur Acad Dermatol Venereol. 2016;30:576–85.
Kumthekar A, Ogdie A. Obesity and psoriatic arthritis: a narrative review. Rheumatol Ther. 2020;7:447–56.
Deike M, Brinks R, Meller S, Schneider M, Sewerin P. Risk of psoriatic arthritis depending on age: analysis of data from 65 million people on statutory insurance in Germany. RMD Open. 2021;7: e001975.
Caso F, Tasso M, Chimenti MS, Navarini L, Perricone C, Girolimetto N, et al. Late-onset and elderly psoriatic arthritis: clinical aspects and management. Drugs Aging. 2019;36:909–25.
Gialouri CG, Evangelatos G, Iliopoulos A, Tektonidou MG, Sfikakis PP, Fragoulis GE, et al. Late-onset psoriatic arthritis: are there any distinct characteristics? A Retrospective cohort data analysis. Life (Basel). 2023;13:792.
Gönüllü E, Kalyoncu U, Yağiz B, Ateş A, Küçükşahin O, Yaşar Bilge Ş, et al. AB0355 the differences between the first preferred biological DMARD and the drug survival in geriatric and younger adult population with rheumatoid arthritis and psoriatic arthritis: treasure real-life data. Ann Rheum Dis. 2022;81(Suppl 1):1304.
Iannone F, Santo L, Bucci R, Semeraro A, Carlino G, Paoletti F, et al. Drug survival and effectiveness of ustekinumab in patients with psoriatic arthritis. Real-life data from the biologic Apulian registry (BIOPURE). Clin Rheumatol. 2018;37:667–75.
Letarouilly J-G, Sellam J, Richette P, Dieudé P, Claudepierre P, Richard CM, et al. Ab0760 Drug survival and efficacy of ustekinumab and secukinumab in psoriatic arthritis: a real-world multicentric cohort of 186 patients. Ann Rheum Dis. 2019;78(Suppl 2):1847–8.
Walsh JA, Cai Q, Lin I, Pericone CD, Chakravarty SD. Treatment persistence and adherence among patients with psoriatic arthritis who initiated targeted immune modulators in the US: a retrospective cohort study. Adv Ther. 2021;38:2353–64.
Yu S, Tsao Y-H, Tu H-P, Lan C-CE. Drug survival of biologic agents in patients with psoriatic arthritis from a medical center in southern Taiwan. Dermatol Sin. 2022;40:20.
Zhu B, Edson-Heredia E, Gatz JL, Guo J, Shuler CL. Treatment patterns and health care costs for patients with psoriatic arthritis on biologic therapy: a retrospective cohort study. Clin Ther. 2013;35:1376–85.
Zhang HF, Gauthier G, Hiscock R, Curtis JR. Treatment patterns in psoriatic arthritis patients newly initiated on oral nonbiologic or biologic disease-modifying antirheumatic drugs. Arthritis Res Ther. 2014;16:420.
Glintborg B, Di Giuseppe D, Wallman JK, Nordström DC, Gudbjornsson B, Hetland ML, et al. Uptake and effectiveness of newer biologic and targeted synthetic disease-modifying antirheumatic drugs in psoriatic arthritis: results from five Nordic biologics registries. Ann Rheum Dis. 2023;82:820–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
AbbVie Inc. (North Chicago, Illinois, USA) funded the research. AbbVie and Maccabi participated in the study design, research, analysis, data collection, interpretation of the data, review and approval of the manuscript and publication.
Conflict of interest
F. Faccin is a full-time AbbVie employee and may own AbbVie stock and/or options. V. Rosenberg, G. Chodick, and G.O. Gendelman declare that they have no conflict of interest. H. Amital received consultant fees from AbbVie for this project. After completion of the manuscript, G. Chodick changed his affiliation within Maccabi Healthcare Services, and his new affiliation is: Maccabi Healthcare Services, Tel Aviv, Israel. His other affiliation, of Tel Aviv university, remains the same.
Ethics approval consent to participate
The study was conducted in accordance with the protocol, applicable regulations, and guidelines governing clinical study conduct and the ethical principles that have their origin in the Declaration of Helsinki. The independent ethics committee and institutional review board, MHS Institutional Review Board, approved the study protocol and related documents (approval number 0108-18-BBL, 18 December 2018). MHS’s IRB waived the requirement to obtain any informed consent for this secondary analysis of existing data.
Consent for publication
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published. No honoraria or payments were made for authorship.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are not publicly available because the data that support the findings of this study originate from Maccabi Healthcare Services and restrictions apply to the availability of these data. Due to restrictions, these data can be accessed only by request to the authors and/or Maccabi Healthcare Services.
Code availability
Not applicable.
Author contribution
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by V. Rosenberg and Drs. Amital, Chodick, and Gendelman. The first draft of the manuscript was written by V. Rosenberg and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Rosenberg, V., Amital, H., Chodick, G. et al. Real-World Drug Survival of Biologics and Targeted Synthetic Disease-Modifying Anti-rheumatic Drugs Among Patients with Psoriatic Arthritis. Drugs Aging 41, 685–697 (2024). https://doi.org/10.1007/s40266-024-01136-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-024-01136-7