Abstract
Background
Immunoglobulin A nephropathy (IgAN) is a rare progressive disease that can lead to kidney failure. The current study aimed to estimate health state utility values for IgAN from a UK societal perspective.
Methods
We used the time trade-off (TTO) method to derive utility values for various health states in IgAN, defined based on chronic kidney disease (CKD) stage, proteinuria, dialysis, and nephrotic syndrome (CKD stages 1–4, proteinuria < 1 g/day vs ≥ 1 g/day; CKD stage 5, dialysis vs non-dialysis). We developed health state vignettes to describe typical symptoms and quality-of-life impairments of IgAN. Eligible participants from the UK general public completed a computer-assisted telephone interview. Estimated TTO utility values were reviewed against visual analogue scale (VAS)-derived values.
Results
In total, 200 participants were included in the study (mean age, 48.9 years; female, 59.0%). Mean (standard deviation [SD]) utility values were 0.84 (0.17) and 0.71 (0.23) for CKD stage 1/2 with proteinuria < 1 g/day and with proteinuria ≥ 1 g/day, respectively; 0.68 (0.23) and 0.61 (0.25) for CKD stage 3; and 0.55 (0.26) and 0.49 (0.27) for CKD stage 4. Mean (SD) utility of CKD stage 5 with and without dialysis was 0.38 (0.30) and 0.42 (0.28), respectively. The mean (SD) utility value of nephrotic syndrome was 0.43 (0.33).
Conclusions
Our results indicated that various IgAN health states are associated with impaired health status, with substantial utility decrements related to disease progression, elevated proteinuria, and nephrotic syndrome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Results from this study indicate that immunoglobulin A nephropathy (IgAN)-related kidney disease progression, elevated proteinuria, and nephrotic syndrome are associated with impaired health utility. |
These results aid in understanding the health utility associated with IgAN and assessing the economic value of novel therapies in patients with IgAN. |
The findings suggest that health utility of patients with IgAN may be improved by reducing elevated protein levels in urine and preventing or delaying IgAN-related decline in kidney function. |
1 Introduction
Immunoglobulin A nephropathy (IgAN) is a kidney disorder characterized by the mesangial accumulation of immune complexes containing immunoglobulin A (IgA) that leads to inflammation in glomeruli and eventually irreversible damage to the glomerular filtration barrier [1]. It is a leading cause of chronic kidney disease (CKD) and kidney failure/end-stage renal disease (ESRD) [2,3,4]. Annual incidence varies across geographic regions, reported in the range of 1 to 10 per 100,000 persons, with higher incidence in Asian countries [1,2,3].
Clinical presentation hallmarks of IgAN include hematuria, proteinuria, and progressive loss of renal function [2, 3, 5]. In less common cases (5% or less), nephrotic syndrome (defined as proteinuria >3 g/day with hypoalbuminemia, peripheral edema, and hyperlipidemia) and acute kidney injury can occur [2]. Of these symptoms, proteinuria is associated with an increased risk of disease progression and mortality [6, 7] and has been shown to significantly impair quality of life (QoL) in patients with IgAN and primary glomerulonephritis [8, 9]. Change in proteinuria has also been identified as a valid surrogate endpoint for clinical trials evaluating new treatments for IgAN [7].
The overall treatment goal for IgAN is to prevent disease progression to kidney failure through proteinuria reduction [10]. However, effective therapies for IgAN are limited [7] and supportive care remains the mainstay in IgAN treatment [2,3,4, 10]. Optimized supportive care may consist of hypertension management, use of angiotensin-converting enzyme inhibitor and angiotensin II receptor blockers, lifestyle modification, and addressing cardiovascular risks [4, 10, 11]. The Kidney Disease Improving Global Outcomes (KDIGO) guidelines should be considered only in patients who remain at high risk of disease progression after maximal supportive care [4]. However, the uncertainty over the effectiveness and safety of immunosuppressant treatment is not well supported with evidence [12, 13], and a detailed discussion of the risks and benefits of the immunosuppressant treatment should be fully evaluated for patients with IgAN [4]. Due to lack of effective treatments, some patients may eventually require renal transplantation, which is complicated by a high rate (22–44%) of disease recurrence [14,15,16]. The prognosis of IgAN, especially among high-risk patients, remains poor with existing treatments.
Recently, novel treatments have been developed to prevent disease progression and may offer promising therapies to patients with IgAN [17, 18]. To comprehensively assess the burden of IgAN and evaluate the potential benefits of new treatments, it is necessary to understand the health utility associated with the disease. While previous studies indicate that IgAN substantially reduces health-related QoL [1, 8, 9, 19], less is known about associated utility values. Utility values are often used to assess the impact of a disease on patients by measuring individuals’ preferences for different health states. Utility values constitute an important component of disease burden and provide critical inputs in economic evaluations of novel therapies. To address the gap in knowledge, our goal was to derive utility values for key health states related to IgAN from the United Kingdom (UK) societal perspective. Specifically, we estimated utility values for IgAN health states across CKD stages, proteinuria status, and dialysis status, and with nephrotic syndrome.
2 Methods
To estimate utility values for IgAN health states, we employed a vignette-based approach. This approach was used instead of EQ-5D, which is often preferred by the National Institute for Health and Care Excellence [20], because it was challenging to recruit a sufficient number of patients within a reasonable timeline due to the rarity of IgAN. In addition, EQ-5D might not be sufficiently sensitive to various IgAN health states we aimed to evaluate in this study. Specifically, time trade-off (TTO) interviews based on the developed vignettes were conducted with consenting members of the UK general population. The TTO method asks respondents about the number of years of life in a given imperfect health state they would be willing to ‘trade off’ in order to live in full health [21] and is a well-established method to measure utilities for health states across different disease areas [22].
Rare diseases such as IgAN present challenges in estimating health state utilities using preference-based approaches due to the difficulties in recruiting a sufficiently large sample of patients or caregivers within the study timeframe [23]. In addition, generic preference-based approaches may not be sensitive to certain disease attributes. Thus, vignette-based approaches may be more desirable when a study aims to isolate the utility impact of specific attributes of a disease [23]. In the case of IgAN, we aimed to estimate the utilities of various disease health states related to CKD stages, proteinuria, dialysis, and nephrotic syndrome. Given these, a vignette-based study that uses a general population is the most suitable approach for the current study. It is also a commonly used method in previous literature to derive health state utilities for rare diseases [24].
The study was reviewed and received an exemption determination from ongoing oversight by the Pearl Institutional Review Board according to FDA 21 CFR 56.104 and 45CFR46.104(b)(2): (2) Tests, Surveys, Interviews. Informed consent was obtained from all participating individuals.
2.1 Development of Health State Vignettes
We included health states describing various CKD stages (1–5), proteinuria status (defined by proteinuria < 1 g/day or ≥ 1 g/day based on the treatment target in the KDIGO guidelines [4]), dialysis status, and nephrotic syndrome. Because symptoms in CKD stage 1 and 2 are similar [25], the two CKD stages were combined into one health state.
To support study participants’ understanding of each health state, vignettes were developed to describe associated symptoms and QoL (Supplementary Table 1, see electronic supplementary material [ESM]). First, a preliminary vignette was developed for each health state based on information collected in a targeted literature review. Vignettes included descriptions of urine test outcomes, symptoms and their severities, QoL, resource use, and risks of other diseases. To ensure that the vignettes provided accurate and comprehensive descriptions of the health states, feedback on the preliminary vignettes was obtained through one-on-one web-based interviews in the UK. Five experienced nephrologists and one representative from a patient advocacy group participated in the interviews. In addition to having a child with IgAN, the patient advocacy group representative had extensive experience interacting with patients with various types of IgAN and significant research experience in this field.
2.2 Eligibility and Recruitment of Participants
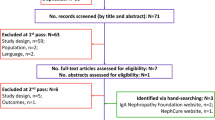
Participants were recruited through an online panel from members of the UK general public. To be included in the study, participants had to meet the following criteria: (1) at least 18 years or older, (2) able to speak and read English, and (3) able and willing to provide an informed consent.
In an initial survey, individuals who were willing to participate were screened for eligibility and asked to provide informed consent as well as information on demographics, comorbidities, and self-reported general health status on a visual analogue scale (VAS) from 0 (worst imaginable health) to 100 (best imaginable health). Eligible respondents who completed the survey were scheduled for a TTO interview. Only participants who completed both the initial screening survey and the TTO interview were compensated (consistent with fair market value) for their participation in the study. A target sample size of 200 was determined based on a margin of error < 14% of the standard deviation (SD) at a significance level of 0.05.
2.3 Time Trade-Off (TTO) Interview
Because unsupervised online surveys may compromise data quality due to decreased participant engagement, we used computer-assisted telephone interviews (CATIs) to guide participants through the TTO tasks [26]. CATIs were held over telephone/teleconference while a computerized questionnaire was administered via screen sharing between 28 January 2022 and 4 March 2022. Interviewers recorded the respondent’s answers directly into the computer. Respondents could also ask for clarification from the interviewer if they were not clear on a question.
To ensure that participants understood the concept of TTO, they first completed a training task and a practice task. Participants were then randomly assigned to view the vignettes of the IgAN health states (Supplementary Table 1, see ESM) in either increasing or decreasing severity. After reading the vignette for a health state, participants rated the value of the health state on a VAS from 0 (worst imaginable health) to 100 (best imaginable health) and responded to a series of TTO questions illustrated with visual aids (Fig. 1). Inclusion of the VAS rating prior to the TTO task is recommended by the EuroQoL protocols to validate TTO-based results [27]. In the TTO tasks, participants were repeatedly asked to compare living 10 years in an IgAN health state versus living fewer years in full health until they were indifferent between the two options. Health states worse than dead were determined by participants being indifferent between living a certain number of years in an IgAN health state versus immediate death (zero years in full health). Another example of valuing health states worse than dead is presented in Fig. 1B. The point of indifference was used to estimate the number of years of life in the IgAN health state they would be willing to give up in order to live in full health. Given that the design of TTO tasks (e.g., time horizon, structure of the hypothetical lives, iteration procedures, visual aids, and respondent training) influences how participants value health states [28], the TTO questions in this study followed the data collection procedures in the EuroQol Valuation Technology (EQ-VT) protocol [29]. The EQ-VT protocol includes TTO as one of the main valuation tasks and makes recommendations on the data collection procedures based on best practices and empirical research [27]. Following the EQ-VT protocol improves comparability between the utility values from this study and those from EQ-5D, which are commonly used in cost-effectiveness analysis models.
Prior to data collection, the initial survey and the TTO tasks were pre-tested among two eligible participants to ensure clarity of the questions and vignettes. In addition, a soft launch was conducted by collecting data from 10 participants to confirm that they interpreted the questions correctly. Feedback from the soft launch was provided to moderators to ensure optimal understanding and interpretation of the TTO questions by participants in the full launch.
2.4 Outcomes and Analysis
Sociodemographic characteristics, comorbidities, and current health status of the participants were summarized descriptively.
TTO utilities ranged from − 1 (worse than dead) to + 1 (perfect health), with the smallest difference being 0.05, and were calculated based on responses to the TTO questions. Specifically, if a health state (h) was considered better than dead, the utility value was calculated as U(h) = x/10, where x denotes the number of years in full health at the point of indifference. If a health state (h) was considered worse than dead, the utility value was calculated as U(h) = (x − 10)/10. The value of health states was also rated on a VAS from 0 (worst imaginable health) to 100 (best imaginable health) and divided by 100 to calculate VAS scores.
To evaluate the impact of proteinuria and dialysis on health utility in patients with IgAN, the utility values of proteinuria < 1 g/day versus ≥ 1 g/day for CKD stage 1 & 2, 3, and 4, as well as the utility values of dialysis versus no dialysis in CKD stage 5 were compared using paired t tests; comparisons were conducted for both TTO utility values and VAS scores.
The primary analyses removed illogical responses, that is, responses in which a participant rated a more severe health state as better than a less severe health state. Sensitivity analysis included all the illogical responses to assess the validity of VAS and TTO ratings. In addition, subgroup analyses were conducted by the order in which participants viewed the health states and by gender.
3 Results
3.1 Participant Characteristics
A total of 200 participants from the UK were included in the study (Table 1). The sample had a mean (SD) age of 48.9 (SD 15.4) years, 59.0% were female and 79.0% were white British. Most of the participants (80.5%) lived in England. The majority (60.0%) of the participants had a college or higher degree and 41.5% were working full-time.
Most commonly reported chronic comorbidities were depression (23.0%), hypertension (14.5%), diabetes (12.0%), and respiratory or lung disease (11.0%). Eight patients (4.0%) reported having kidney disease. Participants’ overall health status at the time of the survey ranged from 0 to 1 based on the VAS, with a mean (SD) of 0.72 (0.19) and a median of 0.80.
3.2 Health Utility Values
Estimated TTO utility values showed a trend of decreasing health utility with worsening CKD stage (Table 2 and Fig. 2). Among patients with proteinuria < 1 g/day, the mean (SD) TTO utility value was 0.84 (0.17), 0.68 (0.23), and 0.55 (0.26) for CKD stage 1/2, stage 3, and stage 4, respectively. A similar trend was observed among patients with proteinuria ≥ 1 g/day, with the mean (SD) utility value for CKD stage 1/2, stage 3, and stage 4 being 0.71 (0.23), 0.61 (0.25), and 0.49 (0.27), respectively.
Within CKD stages 1–4, the proteinuria < 1-g/day health state had a significantly higher TTO utility value than the proteinuria ≥ 1 g/day health state (all p < 0.0001). The utility decrement associated with proteinuria ≥ 1 g/day ranged from 0.06 in CKD stage 4 to 0.13 in CKD stage 1/2 (Fig. 2), suggesting that proteinuria had a larger impact on utility in lower CKD stages. The mean utility decrement associated with proteinuria ≥ 1 g/day across CKD stages was 0.09.
CKD stage 5 had a lower utility value than other CKD stages; mean (SD) 0.42 (0.28) and 0.38 (0.30) for patients without and with dialysis, respectively, resulting in a 0.04 utility decrement associated with dialysis (p < 0.0001, Fig. 2). The mean utility value for nephrotic syndrome was 0.43 (SD 0.33) based on the TTO responses, comparable to CKD stage 5 without dialysis. The mean (SD) utility decrement associated with nephrotic syndrome was 0.43 (0.31), 0.28 (0.25), and 0.17 (0.24) for CKD stage 1/2, stage 3, and stage 4, respectively (Table 2), suggesting that nephrotic syndrome has a larger impact on utility in lower CKD stages.
While VAS scores were consistently lower than utility values derived from TTO responses, similar trends were observed (Table 2). Importantly, decrements in TTO utility values and VAS scores associated with proteinuria ≥ 1g/day, dialysis, and nephrotic syndrome were comparable. The VAS score decrement associated with proteinuria ≥ 1g/day, compared with proteinuria < 1g/day, ranged from 0.06 in CKD stage 3 to 0.11 in CKD stage 1/2 (Fig. 3), with mean utility decrement of 0.08. The mean VAS score decrement associated with dialysis in CKD stage 5 was 0.05. The mean (SD) of the VAS score decrement associated with nephrotic syndrome was 0.39 (0.20), 0.24 (0.16), and 0.13 (0.15) for CKD stage 1/2, stage 3, and stage 4, respectively (Table 2).
3.3 Sensitivity Analysis
The TTO utility values from the sensitivity analysis that included all responses (i.e., logical and illogical responses) were generally lower compared with the corresponding values in the primary analysis, except for the utility value for CKD stage 5 without dialysis. The differences in the mean utility values between the sensitivity analysis and the primary analysis ranged from − 0.06 to 0.01 (Supplementary Table 2, see ESM). The utility decrements associated with proteinuria ≥1 g/day and nephrotic syndromes were also lower in this sensitivity analysis (Supplementary Fig. 1, see ESM). However, VAS scores in the sensitivity analysis were similar to those in the primary analysis (Supplementary Fig. 2, see ESM).
Subgroup analyses showed that the order in which health states were presented impacted the results. In all TTO utility values and most VAS scores, the values were higher (by 0.02–0.12) with decreasing severity compared with increasing severity order (Supplementary Table 3, see ESM). Overall, the values were comparable between male and female participants (Supplementary Table 4, see ESM).
4 Discussion
To the best of our knowledge, the current study is the first to estimate the utility values specifically associated with IgAN, taking into account proteinuria level and nephrotic syndrome. Previous studies have estimated utility values for different health states related to CKD, but none have specifically focused on IgAN. Due to the heterogeneity in the causes of CKD and the associated features of the underlying disease, utility values derived for the overall CKD population may not be applicable to IgAN. The current study addresses this important gap by providing specific utility values for IgAN. Additionally, there is a lack of utility values associated with proteinuria, a key target for IgAN treatment. Since proteinuria change is the primary endpoint in more recent IgAN clinical trials [30, 31], this study provides crucial inputs for the economic evaluation of new treatments for IgAN.
Overall, the findings suggest that progressive deterioration of kidney function in IgAN is associated with substantial utility decrements. Furthermore, proteinuria, nephrotic syndrome, and dialysis were associated with lower health utility values. Specifically, the mean TTO utility decrement (0.09) associated with proteinuria ≥ 1 g/day within each CKD stage was considerably higher than the utility decrement associated with dialysis within CKD stage 5 (0.04). These results support the existing evidence on QoL impairment related to proteinuria, as demonstrated in previous studies [8, 9]. The TTO utility value for nephrotic syndrome was comparable to the utility value for CKD stage 5 without dialysis, with substantial utility decrements (ranging from 0.17 in stage 4 to 0.43 in stage 1/2). The low health utility of nephrotic syndrome is also consistent with previous research demonstrating that patients with this condition had low Short Form-36 scores and a high prevalence (48%) of depression, which is similar to the rates observed in patients undergoing hemodialysis [8].
In addition, the estimated TTO utility values for various CKD stages in our study generally fall within the ranges reported in previous studies, which vary depending on the valuation methods used and geographic region. Specifically, these studies reported utility values of 0.67–0.90 for CKD stage 1/2, 0.67–0.87 for stage 3, 0.55–0.85 for stage 4, 0.54–0.85 for CKD stage 5 without dialysis, and 0.44–0.79 for hemodialysis or peritoneal dialysis [32, 33]. Among these studies, two UK studies estimated health utility values for CKD stages using the EQ-5D, with one focusing on patients in pre-dialysis stages and the other on those with kidney failure [34, 35]. The mean TTO utility value for CKD stage 1/2 with proteinuria < 1 g/day in our study (0.84) is comparable to the utility value in the UK study on the pre-dialysis population (0.85) [34]. However, the mean TTO utility values with proteinuria < 1 g/day for CKD stages 3 (0.68) and 4 (0.55), as well as CKD stage 5 without (0.42) and with (0.38) dialysis in our study are lower compared with the two UK studies [34, 35], with a difference of − 0.12, − 0.19, − 0.31, and − 0.06 for CKD stage 3, stage 4, stage 5 without and with dialysis, respectively. Interestingly, mean TTO utility values for CKD stage 3 or higher in our study are more similar to a US study that estimated utility values using the Health Utility Index-3 (HUI-3) [33]. In that study, estimated mean utility values were 0.67, 0.55, 0.54, and 0.54 for CKD stage 3, stage 4, stage 5 without dialysis, and stage 5 with dialysis, respectively [33]. However, their HUI-3 for stage 1/2 (0.67) is lower than the corresponding utility value in our study.
With the above discussion, it is important to note some key differences between a vignette-based TTO approach and an instrument-based approach. Generic instruments, such as the EQ-5D and the HUI, have been shown to lack sensitivity or sufficient symptom coverage to measure the impact of some diseases on health utility [36]. In comparison, vignettes, as used in our study, can include details on disease-specific symptoms as well as QoL impairment in multiple health states and, thus, can potentially provide more accurate estimates of health utilities for a given disease. The HUI-3 includes more domains to describe health states compared with the EQ-5D, which may partially explain why the results based on the HUI-3 for the higher CKD stages were more similar to the results from our study. However, the same US study also reported higher utility values based on TTO (ranging from 0.72 for dialysis to 0.90 for CKD stage 1/2) compared with our study, potentially due to the difference in the study population—our study was conducted with members of the general UK public while the US study included patients with renal impairment. Patients may value a disease state higher than individuals without such experience, due to adaptation to their disease state over time. The differences in TTO utility values between our study and the US study are consistent with a previous meta-analysis that found patients generally gave higher valuations than the general public [37].
One of the strengths of our study is the development of vignettes designed specifically for IgAN, accounting for the most relevant symptoms and QoL impairments. These vignettes were developed based on published literature and were refined based on feedback from nephrologists experienced in treating IgAN and a patient advocacy representative whose child had IgAN. In addition, we completed pilot testing and review of data from a limited soft launch to ensure the participants understood the various health states associated with IgAN. As recommended [27], we also used VAS scores to validate TTO-derived utility values, which showed that participants understood the TTO questions, as evidenced by a similar relative ranking of health states between VAS and TTO responses (i.e., the values decreased as the severity of the health states increased).
However, there are important limitations to our study. Although the use of vignettes was noted as a strength, the descriptions of symptoms and QoL in the vignettes are critical. Emphasizing or omitting certain aspects of the disease in the descriptions may lead to bias. We attempted to minimize such bias by developing the vignettes based on a comprehensive targeted literature review and validating them with nephrologists and a patient advocacy representative with extensive experience interacting with various patients with IgAN and significant research experience in this field. However, the development of the vignettes would have benefited more from direct patients’ involvement, which is recommended for vignette-based utility studies [23]. While this can improve the relevance of the utility results to patients and thus the economic evaluations of new treatments, experts also caution that individual patients’ experiences may not always be representative of the entire patient population [23]. Considering this and the challenges of recruiting patients with IgAN, we relied on the insights from experienced clinicians and the patient advocacy representative to ensure that patients’ perspectives were fully captured in the vignettes. Future studies may confirm or further improve the vignettes through patient interviews. Furthermore, due to the different methods and populations between this study and others in the literature, the utility values may not be directly comparable. Caution should be exercised when applying the utility values from this study to ensure that the health states match the vignettes described in this study. In addition, participants’ responses may be impacted by their understanding of the health states. To enhance the comprehension of the health states by the study participants, we described the vignettes in simple language and pre-tested with two participants prior to the data collection. Another limitation is that to manage response burden, our study only included a limited number of health states, which are not able to account for every potential symptom or level of severity and reflect the heterogenous nature of IgAN.
5 Conclusions
Our study results indicate that various IgAN health states are associated with impaired health status, with substantial utility decrements related to disease progression, elevated proteinuria, and nephrotic syndrome. These findings suggest that future treatments improving QoL in addition to clinical outcomes are desirable.
References
Kwon CS, Daniele P, Forsythe A, Ngai C. A systematic literature review of the epidemiology, health-related quality of life impact, and economic burden of immunoglobulin a nephropathy. J Health Econ Outcomes Res. 2021;8(2):36–45.
Rajasekaran A, Julian BA, Rizk DV. IgA nephropathy: an interesting autoimmune kidney disease. Am J Med Sci. 2021;361(2):176–94.
Rodrigues JC, Haas M, Reich HN. IgA nephropathy. Clin J Am Soc Nephrol. 2017;12(4):677–86.
Rovin BH, Adler SG, Barratt J, Bridoux F, Burdge KA, Chan TM, et al. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100(4):S1–276.
Wyatt RJ, Julian BA. IgA nephropathy. N Engl J Med. 2013;368(25):2402–14.
Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet. 2017;389(10075):1238–52.
Thompson A, Carroll K, Inker LA, Floege J, Perkovic V, Boyer-Suavet S, et al. Proteinuria reduction as a surrogate end point in trials of IgA nephropathy. Clin J Am Soc Nephrol. 2019;14(3):469–81.
Libório AB, Santos JPL, Minete NFA, de Alencar DC, Soares AP, Queiroz AL, et al. Proteinuria is associated with quality of life and depression in adults with primary glomerulopathy and preserved renal function. PLoS One. 2012;7(5): e37763.
Mizerska-Wasiak M, Adamczuk D, Cichoń-Kawa K, Miklaszewska M, Szymanik-Grzelak H, Pietrzyk JA, et al. Health-related quality of life in children with immunoglobulin A nephropathy—results of a multicentre national study. Arch Med Sci. 2021;17(1):84–91.
Cattran DC, Appel GB, Coppo R, Fervenza FC. IgA nephropathy: treatment and prognosis. 2022. https://www.uptodate.com/contents/iga-nephropathy-treatment-and-prognosis. Accessed 1 Dec 2022.
Floege J, Rauen T, Tang SCW. Current treatment of IgA nephropathy. Semin Immunopathol. 2021;43(5):717–28.
Rauen T, Eitner F, Fitzner C, Sommerer C, Zeier M, Otte B, et al. Intensive supportive care plus immunosuppression in IgA nephropathy. N Engl J Med. 2015;373(23):2225–36.
Vecchio M, Bonerba B, Palmer SC, Craig JC, Ruospo M, Samuels JA, et al. Immunosuppressive agents for treating IgA nephropathy. Cochrane Database Syst Rev. 2015. https://doi.org/10.1002/14651858.CD003965.pub2.
Kim YS, Moon JI, Jeong HJ, Kim MS, Kim SI, Choi KH, et al. Live donor renal allograft in end-stage renal failure patients from immunoglobulin A nephropathy. Transplantation. 2001;71(2):233–8.
Moroni G, Belingheri M, Frontini G, Tamborini F, Messa P. Immunoglobulin A nephropathy. Recurrence after renal transplantation. Front Immunol. 2019;10:1332.
Moroni G, Longhi S, Quaglini S, Gallelli B, Banfi G, Montagnino G, et al. The long-term outcome of renal transplantation of IgA nephropathy and the impact of recurrence on graft survival. Nephrol Dial Transplant. 2013;28(5):1305–14.
US Food and Drug Administration. FDA approves first drug to decrease urine protein in IgA nephropathy, a rare kidney disease. 2021. https://www.fda.gov/drugs/fda-approves-first-drug-decrease-urine-protein-iga-nephropathy-rare-kidney-disease. Accessed 1 Dec 2022.
Travere Therapeutics. Travere Therapeutics announces positive topline interim results from the ongoing phase 3 PROTECT study of sparsentan in IgA nephropathy. 2021. https://ir.travere.com/news-releases/news-release-details/travere-therapeutics-announces-positive-topline-interim-results. Accessed 1 Dec 2022.
Canetta PA, Troost JP, Mahoney S, Kogon AJ, Carlozzi N, Bartosh SM, et al. Health-related quality of life in glomerular disease. Kidney Int. 2019;95(5):1209–24.
Brazier J, Ratcliffe J, Saloman J, Tsuchiya A. Measuring and valuing health benefits for economic evaluation. Oxford: Oxford University Press; 2017.
Attema AE, Edelaar-Peeters Y, Versteegh MM, Stolk EA. Time trade-off: one methodology, different methods. Eur J Health Econ. 2013;14(Suppl 1):S53-64.
Wright DR, Wittenberg E, Swan JS, Miksad RA, Prosser LA. Methods for measuring temporary health states for cost-utility analyses. Pharmacoeconomics. 2009;27(9):713–23.
Matza LS, Stewart KD, Lloyd AJ, Rowen D, Brazier JE. Vignette-based utilities: usefulness, limitations, and methodological recommendations. Value Health. 2021;24(6):812–21.
Smith AB, Hanbury A, Ortiz B, de Zarate I, Hammes F, de Pouvourville G, Buesch K. Eliciting health state utilities for aromatic l-amino acid decarboxylase (AADC) deficiency: a vignette study in France. Patient Relat Outcome Meas. 2021;12:237–46.
Brown SA, Tyrer FC, Clarke AL, Lloyd-Davies LH, Stein AG, Tarrant C, et al. Symptom burden in patients with chronic kidney disease not requiring renal replacement therapy. Clin Kidney J. 2017;10(6):788–96.
Jiang R, Shaw J, Mühlbacher A, Lee TA, Walton S, Kohlmann T, et al. Comparison of online and face-to-face valuation of the EQ-5D-5L using composite time trade-off. Qual Life Res. 2021;30(5):1433–44.
Oppe M, Rand-Hendriksen K, Shah K, Ramos-Goñi JM, Luo N. EuroQol protocols for time trade-off valuation of health outcomes. Pharmacoeconomics. 2016;34(10):993–1004.
Lenert LA, Cher DJ, Goldstein MK, Bergen MR, Garber A. The effect of search procedures on utility elicitations. Med Decis Mak. 1998;18(1):76–83.
Oppe M, Devlin NJ, van Hout B, Krabbe PF, de Charro F. A program of methodological research to arrive at the new international EQ-5D-5L valuation protocol. Value Health. 2014;17(4):445–53.
ClinicalTrials.gov. A study of the effect and safety of sparsentan in the treatment of patients with IgA nephropathy (PROTECT). 2022. https://clinicaltrials.gov/ct2/show/NCT03762850. Accessed 1 Dec 2022.
Barratt J, Lafayette R, Kristensen J, Stone A, Cattran D, Floege J, et al. Results from part A of the multi-center, double-blind, randomized, placebo-controlled NefIgArd trial, which evaluated targeted-release formulation of budesonide for the treatment of primary immunoglobulin A nephropathy. Kidney Int. 2023;103(2):391–402.
Cooper JT, Lloyd A, Sanchez JJG, Sörstadius E, Briggs A, McFarlane P. Health related quality of life utility weights for economic evaluation through different stages of chronic kidney disease: a systematic literature review. Health Qual Life Outcomes. 2020;18(1):310.
Gorodetskaya I, Zenios S, Mcculloch CE, Bostrom A, Hsu C-Y, Bindman AB, et al. Health-related quality of life and estimates of utility in chronic kidney disease. Kidney Int. 2005;68(6):2801–8.
Jesky MD, Dutton M, Dasgupta I, Yadav P, Ng KP, Fenton A, et al. Health-related quality of life impacts mortality but not progression to end-stage renal disease in pre-dialysis chronic kidney disease: a prospective observational study. PLoS One. 2016;11(11): e0165675.
Lee AJ, Morgan CL, Conway P, Currie CJ. Characterisation and comparison of health-related quality of life for patients with renal failure. Curr Med Res Opin. 2005;21(11):1777–83.
Lin F-J, Longworth L, Pickard AS. Evaluation of content on EQ-5D as compared to disease-specific utility measures. Qual Life Res. 2013;22(4):853–74.
Peeters Y, Stiggelbout AM. Health state valuations of patients and the general public analytically compared: a meta-analytical comparison of patient and population health state utilities. Value Health. 2010;13(2):306–9.
Acknowledgements
Medical writing assistance was provided by Jipan Xie, an employee of XL Source, Inc. and funded by Travere Therapeutics, Inc. Editorial support was provided by Nucleus Global, an Inizio company, and funded by Travere Therapeutics, Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The study and open access fee were funded by Travere Therapeutics, Inc.
Conflict of interest
Mark E. Bensink is an employee of Benofit Consulting, which received consulting fees from Travere Therapeutics, Inc. Bruce Hendry is an employee of Travere Therapeutics, Inc., and holds stock/options. Zheng-Yi Zhou, Nisha C. Hazra, and Chunyi Xu are employees of Analysis Group, Inc., which has received consulting fees from Travere Therapeutics, Inc. Claire C. Sharpe is an employee of Wilkins & Sharpe, Ltd., which received consulting fees from Travere Therapeutics, Inc. Mo Zhou was an employee of Analysis Group, Inc., at the time of the study, which has received consulting fees from Travere Therapeutics, Inc.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval
The study was reviewed and received an exemption determination from ongoing oversight by the Pearl Institutional Review Board according to FDA 21 CFR 56.104 and 45CFR46.104(b)(2): (2) Tests, Surveys, Interviews.
Consent to participate
Informed consent was obtained from all participating individuals.
Consent for publication
Not applicable.
Code availability
Not applicable.
Authors’ contributions
Research idea and study design: Mark E. Bensink, Mo Zhou, Zheng-Yi Zhou, Nisha C. Hazra, Bruce Hendry, Claire C. Sharpe; data acquisition: Zheng-Yi Zhou, Mo Zhou, Nisha C. Hazra; data analysis/interpretation: Mark E. Bensink, Mo Zhou, Zheng-Yi Zhou, Nisha C. Hazra, Chunyi Xu, Bruce Hendry, Claire C. Sharpe; statistical analysis: Zheng-Yi Zhou, Mo Zhou, Nisha C. Hazra, Chunyi Xu; supervision: Mark E. Bensink, Bruce Hendry, Claire C. Sharpe. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Zhou, ZY., Bensink, M.E., Hazra, N.C. et al. Estimation of Health State Utility Values for Immunoglobulin A Nephropathy: A Time Trade-Off Analysis. PharmacoEconomics Open (2024). https://doi.org/10.1007/s41669-024-00527-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s41669-024-00527-1