Abstract
One approach for the viable production of algal biomass is to couple its production with wastewater treatment plants, power and/or industrial plants. This is intended towards the utilization of the nutrients and the CO2 in the wastewater and in the flue gases of the industry respectively by the microalgae during their growth. In the present study microalgal biomass production was conceptually coupled with a sugar factory. The potential of the wastewater and the flue gas of the factory to support the growth of the microalgae was evaluated. Likewise the possible reduction of pollution by the microalgae was studied. The outputs from the coupled process were determined using the material and energy balance approach with a spread sheet. The cultivation model shows that 12 mg of total nitrogen (TN) and 7.4 mg of total phosphorus (TP) per liter of wastewater could be transferred to the algal growth ponds. It was found that there is a nitrogen deficit in the wastewater. With the supply of makeup nutrient, 120.5 tons/year microalgal biomass could be produced from the coupled process. Application of the assumptions resulted in the reduction of chemical oxygen demand (COD) (mg O2/L) from 2200 to 447, biological oxygen demand (BOD5) (mg O2/L) from 1200 to 207, TN (mg/L) from 15 to 0.6 and, TP (mg/L) from 10 to 1.5 in the wastewater. Integration of the sugarcane processing factories with algal biomass production is important for both biomass production and bioremediation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Several important advantages associated with microalgae, such as their economic potential as a feedstock for biofuel production coupled to bioremediation, high biomass and oil productivity, diverse biodiversity, high photosynthetic efficiency [32, 44] are causing the interest in microalga-derived biofuels to rise. Furthermore the international & national agreements in shifting towards more secured renewable energy system are also other driving factors to make use of microalgae as renewable energy sources [28].
Nitrogen, phosphorus, and CO2 supplies have a considerable impact on cost, sustainability, and production siting in microalgae cultivation. Use of less expensive CO2 and nutrient supplies by coupling microalgae production with large scale facilities is, therefore, important for viable production of microalgal biomass [44]. Since microalgae can utilize various wastewaters, seawater, and other forms of produced water which cannot be introduced into the agricultural system, competition for limited land and freshwater resources will be reduced [9, 10]. Several studies are found on the utilization of wastewater for microalgae cultivation [22]. Microalgae belonging to the Scenedesmus genus are broadly explored as they can accumulate large amount of carbohydrates, lipids and proteins and can grow under non-optimal conditions, including, for example, the application of culture media enriched with wastewaters [15]. The wastewater effluent and the flue gases of sugar factories can be used as inexpensive nutrient (nitrogen and phosphorus) and CO2 sources for the growth of microalgae [10]. The possibility of coupling microalga cultivation to existing sugarcane processing factories is being currently declared in the scientific, industrial, and environmental community. Several studies are found on the matter. The possibility of collocating microalgae cultivation to sugarcane mill in Colombia have been assessed through process simulation [30]. Similar analysis on the integration of an existing sugar mill with microalgal production in Louisiana was also performed [37]. Likewise a number of studies are found on the environmental benefits of integrating microalgal production and sugarcane processing in Brazilian context [6, 34, 40].
Ethiopia is abundant in its natural resources as could be evident from the phytoplankton and other aquatic community which could bring about significant and sustainable economic changes in the country. Of the aquatic biodiversity, algae are the most important with incredible biotechnological applications. The tropical sun light and suitable weather conditions makes the country a potential site for the algal biomass production [2]. At a larger scale, this could bring about a meaningful transformation in energy system based on the microalgae resources. Currently sugar cane processing factories, both the operating ones and ongoing mega projects are/will be among the main economic sources of the country. According to data from the Ethiopian sugar corporation, it is estimated that when all the sugar and ethanol projects are completed and start to operate with their full capacity, together with the already operating factories the country will have the potential of producing more than 3 million tons of sugar per year and about 400,000 metric tons of ethanol per year [17]. Metahara Sugar factory in the South-eastern part of Ethiopia, is one of the major sugarcane processing factories in Ethiopia. The total cane cultivation area of the factory is 10230 ha and its sugar and ethanol production capacities are 130,000 tons/year and 10,873 m3/year respectively. These figures suffice the opportunity to couple algal biomass cultivation with sugar factories by utilization of resources including CO2, and wastewater generated from these factories. This integrated approach reduces both the cost of production of biofuel and the environmental pollution [5]. This integration can provide microalgae with required nutrients. Furthermore wastewater treatment systems using microalgae represent a low-cost and environment-friendly wastewater treatment alternative when compared to conventional wastewater treatment processes [29]. Other advantages include effective utilization of CO2 rich exhausts, production of biofuel and other value added products (α-Linolenic acid, docosahexaenoic acid, and docosahexaenoic acid) [25].
This present study aims to propose a viable technology for production of biofuel from microalgae through the coupling of microalgae cultivation with sugarcane processing factory in Ethiopian context, and focuses on both production of algal biomass and environmental abatement. The algae would be cultivated inexpensively using the wastewater and CO2 from the sugar factory. Metahara Sugar factory has been selected for the adoption of the presented strategy.
2 Materials and methods
The present study involves coupling of a conceptual microalgal cultivation with an actual sugar factory (Metahara Sugar factory) and then evaluation of the coupled process for its outputs and environmental abatement. In doing so, three data source viz. literature data, factory data and experimental data were employed.
2.1 Experimental works
2.1.1 Materials
The microalgae Scenedesmus (genus level) used for the experimental work was obtained from Ethiopian Biodiversity Institute, Addis Ababa, Ethiopia. All the chemical including the BG-11 and the ammonia solution were purchased from Oxoid, England.
2.1.2 Wastewater sampling
Wastewater sampling from Metahara sugar factory was carried out at the beginning, in the middle and at the end of the milling season of 2019/2020. Samples were taken from a focal point where wastewater from milling house, boiling house, factory laboratory, and factory garage mixes together. The chemical oxygen demand (COD), the biochemical oxygen demand (BOD5), the total nitrogen (TN), the total phosphorus (TP) and the oil and grease content of the wastewater was determined for the samples collected in the three seasons. The TN and TP were determined using UV/VIS Spectroscopy [4, 19]. All the other parameters were also determined according to standard methods for the examination of water and wastewater [4], 41. The result for the average values is shown in Table 1.
2.1.3 Testing the growth of microalgae in the wastewater
The growth of microalgae (Scenedesmus) in the wastewater was tested in the laboratory. The productivity of the algae, the reductions in COD and BOD5 of the wastewater and the utilization of the nutrients (TN and TP) in the wastewater were studied.
2.1.4 Inoculum preparation
The cells of Scenedesmus were cultured in deionized water using BG-11 broth (Blue-Green Medium) containing the following chemicals (g/L): 0.075, MgSO4·7H2O; K2HPO4·3H2O, 0.04; CaCl2·2H2O, 0.036; C6H8FeNO7, 0.006; C6H8O7, 0.006; C10H16N2O8, 0.001; NaNO3, 1.5; Na2CO3, 0.02; trace metal mixture which consisted of (μg/L) ZnSO4·7H2O, 0.222; H3BO3, 2.86; MnCl2·4H2O, 1.81; Na2MoO4·2H2O, 0.391; Co(NO3)2·6H2O, 0.05; and CuSO4·5H2O, 0.079 (Grobbelaar, 2013). Each 1 ml media in 1L of deionized water was sterilized at 15 psi pressure for 30 min in autoclave after adjusting its pH to 7.5. Using this media Scenedesmus was incubated under continuous artificial light (5000 lx) at 25 °C for 8 days. The fresh cultures from the BG-11 were preserved at 4 °C and were used in further study.
2.1.5 Cultivation of the microalgae in the wastewater sample
Sugar industry effluent was obtained from Metahara sugar factory, and the sample was transported to laboratory and kept in a cold room maintained at 4 °C prior to cultivation [8]. The wastewater sample was then filtered with 50 μm mesh and sterilized at 15 psi pressure for 20 min using autoclave and allowed to cool to room temperature. The experiment was conducted in duplicate using 2 L conical flasks with 1000 mL treated wastewater as growth medium with the following culture conditions: temperature 25 °C, artificial light 5000 lx (12 h. light/12 h. dark) and CO2 supply. 1 mL of Scenedesmus suspensions grown in the media (8 days old) (10 mg/mL) were taken and inoculated in the flasks containing 1000 mL of wastewater sample as the growth medium. Before the inoculation the pH of the samples were adjusted to 7.5 using a 1 molar KOH. The wastewater samples in the flasks were assigned as sample one, S1, and sample two, S2, both samples in duplicate. 2 mL of ammonia solution (NH4OH) with density of 0.91 g/mL (25% w/w) was added to S2 at the beginning of the third day to study the effect of the nutrient supply on the growth of algae, reductions in COD, BOD5 TN and TP in the wastewater. The algal growth was monitored for 20 days [5, 11].
2.1.6 Biomass determination
Sampling and measurements were carried out one time in two days interval at the same time of day. To determine the dry cell weight of the microalgae 40 mL of the sample were withdrawn and centrifuged at 1500 rpm for 15 min. Then the centrifuged samples were washed with distilled water and dried in an oven at 105 °C for 15 h. The productivity of the biomass was determined by using Eq. 2.1.
where CB is net biomass productivity (mg/mL.h), Ct is biomass concentration at time t (mg/mL), and Cto is biomass concentration at time to (mg/mL).
2.1.7 Removal of COD, BOD5, TN, and TP in the wastewater
Samples (20 mL) were taken within two days of interval to measure the removal of COD, BOD5, TN, and TP in the wastewater. Reductions in COD and BOD5 were determined according to standard methods for the examination of water and wastewater (23rd edition), part 5000 of sections 5210 and 5220 [4]. Likewise the removal of TN and TP were determined by using UV/VIS Spectroscopy [4, 19]. The procedure for the determination of TN used 2nd derivative of the absorption spectrum for NO3− that has a peak at 224 nm proportional to NO3− concentration. Samples were 1st oxidised to NO3− by persulphate digestion. Organic nitrogen standards were prepared from reagent urea, and distilled water was used as a blank. Blanks, standards and samples were scanned on the spectrophotometer between 190 and 250 nm to measure the value of NO3− peak at 224 nm. The detail procedures used for the TN determinations were according to Ferree and Shanoon [19]. In the same way TP was determined after digesting all phosphorus forms in the wastewater to the orthophosphate forms using sulfuric acid. The absorbances were read at 880 nm using reagent blank (distilled water) to zero spectrometer [4]. The removal efficiencies were determined using the following equation.
2.2 Coupling of the sugar factory with the conceptual microalgae cultivation
Important literature and actual factory process data used for the modelling and design of the process are found in Table A.1 of the online Appendix. The process is intended to produce microalgal biomass which is to be used for production of biofuel by using the factory wastes as inexpensive nutrient and CO2 sources. The process is assumed to involve primary treatment of the wastewater from sugar factory before entering the cultivation pond, selection of the microalgae, culturing of the algae in a closed system photo bioreactor (PBR), phototrophic cultivation of the algae in a pond, and harvesting of the algae.
2.2.1 Primary treatment of the wastewater from the sugar factory
It is assumed the wastewater effluent from the factory would be treated in the primary treatment plant before entering the cultivation pond to avoid sedimentation for the next treatment steps by reducing solids and increase photosynthetic efficiency in the following algae cultivation. The nutrient concentration of wastewater from the primary treatment effluent is obtained by applying the pollution reduction factors as shown in Table A.2 (see online Appendix). Retention time and over flow rate are the two main design parameters for a clarifier in a primary treatment of wastewater and usually a retention time of 1.5–2.5 h. and overflow rate of 30–50 m/day are used [31]. For the present study over flow rate of 40 m/day (1.67 m/h.) with typical depth of 4 m and retention time of 2.4 h is assumed. With a wastewater flow of 1074 m3/day, a total of ten 107.4 m3 of clarifiers, with surface of 26.85 m2 are needed. Settled solids are assumed to be removed from the bottom of tanks by sludge rakes. Scum which mainly contains the oil and grease is assumed to be swept across the tank surface by water jets. The primary treatment effluent is sent to the microalgae cultivation pond, while the sludge is removed from the bottom. The estimated compositions of the effluent and sludge after the primary treatment are shown in Table 2.
2.2.2 The characteristics of the microalgae
For the growth of the microalgae, it is assumed that carbon would be provided in the form of CO2 from the flue gases, nitrogen in the form of nitrates (NO3−), and ammonia (NH4+) from wastewater, and phosphorus in the form of orthophosphate (PO43−) from the wastewater. Nutrient deficiency in the wastewater would be supplied by makeup nutrient. It is assumed that other micronutrients such as calcium, potassium and manganese would not be limiting for the growth of microalgae and iron is naturally present in the raw water. Considering the above algal biomass content, the microalgae used in this study are assumed to have the elemental formula of (C106H181O45N15P) [5, 13] with molecular wt. % of components as follows: C, 52.69; H, 7.5; O, 29.83; N, 8.7; and P, 1.28. From this elemental analysis the C:N:P mass ratio in the microalgae is approximately 41.2:6.8:1, and this composition is used to evaluate the potential of the wastewater from factory to support the microalgae growth.
2.2.3 Cultivation of the microalgae in the pond/secondary wastewater treatment
All the underlying assumptions considering operating conditions and nutrient uptake by microalgae are shown in Table 3. The inoculated microalgae is assumed be obtained from the inoculation system which would use photo bioreactors (PBRs), covered pond, and the open lined pond [31].
2.2.4 The stoichiometric model for predicting nutrient requirements by the microalgae in the ponds
The cultivation model (Figs. 1 and 2) requires input data for the carbon, nitrogen, oxygen, hydrogen, and phosphorus based on the composition of the algae. The elemental composition of the microalgae is entered in the mass balance equations (Eqs. (2.3) to (2.8)) in the form of the mass fraction of each element. The derivation of the mass balance equations in the cultivation model is based on the following assumptions:
-
Photoautotrophic microalgae cultivation is assumed in the ponds that the flue gas from the boilers of the sugar mill is the only carbon dioxide sources; the microalgae do not get their carbon source from the wastewater. Though the microalgae could get organic carbon from the wastewater, in the present study it is considered insignificant for the simplicity of the model. The CO2 uptake efficiency factor in the ponds is entered in the mass balance equations (Eq. 2.8) to account for any losses due to off gassing from the ponds.
-
The nitrogen and phosphorus requirements of the microalgae are obtained from the primary treated wastewater of the sugar mill, and it was assumed that any nitrogen and phosphorus deficit is supplied by make nutrient.
-
All macronutrient elements (C, N, P) feeding the ponds are converted quantitatively into
-
microalgal cells, and the recycle water from the harvesting operations contains no usable C, N, and P
-
The utilization efficiency of nitrogen and phosphorus is entered to account for any losses of nitrogen and phosphorus
Equations (2.3) to (2.8) represent mass balance calculations around the cultivation/growth step.
where \({N}_{FED}\) is total amount of nitrogen in the wastewater added to the cultivation step [kg/day], \({N}_{FER}\) is total amount of nitrogen in the makeup nutrient added to the cultivation step [kg/day], \({Q}_{WW}\) is the flow rate of the primary treated water [m3/day], \({X}_{N}\) is the concentration of total nitrogen in the wastewater [-], \({P}_{FED}\) is total amount of phosphorus in the wastewater added to the cultivation step [kg/day], \({P}_{FER}\) is total amount of phosphorus in the makeup nutrient added to the cultivation step [kg/day], \({X}_{P}\) is the concentration of total phosphorus in the wastewater [-], \({{CO}_{2}}_{FED}\) is total amount of CO2 in the flue gas added to the cultivation pond [kg/day], \({M}_{wt {CO}_{2}}\) is molecular weight of CO2 [kg/k-mol], \({M}_{wt C}\) is molecular weight of carbon [kg/k-mol], \(\%C\) is mass fraction of carbon in the microalgae [-], \(\%N\) is mass fraction of nitrogen in the microalgae [-], \({N}_{TOT}\) is total amount of nitrogen to be converted to microalgae [kg/day], \({P}_{TOT}\) is total amount of phosphorus to be converted to microalgae [kg/day], \({{CO}_{2}}_{TOT}\) is total amount of CO2 to be converted to microalgae [kg/day], \({K}_{N}\) is nitrogen utilization factor in the raceway ponds [-], \({K}_{P}\) is phosphorus utilization factor in the raceway ponds [-], \({K}_{CO2}\) is CO2 utilization factor in the raceway ponds [-].
From elemental analysis of the microalga, considering one mole of microalgae, mass percentages for carbon (%C), hydrogen (%H), oxygen (%O), nitrogen (%N), and phosphorus (%P) in the microalgae are = 52.69, 7.5, 29.83, 8.7, and 1.28 respectively. Thus the C:H:O:N:P mass ratio in the microalgae is 41.03:5.84:23.23:6.77:1. The N:P ratio in the microalgae is compared with the ratio of mass of nitrogen utilized by the microalgae in the wastewater and the mass of phosphorus utilized by the microalgae in the wastewater, i.e.,\({X}_{N}{Q}_{WW} :{X}_{P}{Q}_{WW}\). Then the satisfactory condition for the microalgae to grow by utilizing all the nitrogen and the phosphorus in the wastewater was considered 4:1 ≤ \({{K}_{N}X}_{N}{Q}_{WW} :{{K}_{P}X}_{P}{Q}_{WW}\)≤ 40:1 [5]. If \({{K}_{N}X}_{N}{Q}_{WW} :{{K}_{P}X}_{P}{Q}_{WW}\) ≤ 4:1 the microalgae cannot grow by utilizing all the nitrogen and phosphorus implying that there should be added make up nitrogen, \({N}_{FER}\) while if \({{K}_{N}X}_{N}{Q}_{WW} :{{K}_{P}X}_{P}{Q}_{WW}\)≥ 40:1 there should be added a phosphorus source, \({P}_{FER}\) to avoid excess nitrogen which may retard the algal growth in the pond. In the light of this:
Once the above parameters are determined the total biomass production rate, in in the raceway ponds, the total cultivation area required, total facility foot print, and the number of ponds are determined as:
Or
where \({B}_{P}\) is total algal biomass production rate [kg/day], \({P}_{B}\) is productivity of algae in the ponds [kg/m2.day], \({A}_{TOT}\) is total cultivation area required [m2], \({A}_{TFF}\) is total facility foot print [m2], \(R\) is ratio of total area to total facility foot print [none], \({A}_{SUR}\) is surface area of a pond [m2], \({N}_{p}\) is number of ponds [none].
The amount of water consumed in the photosynthesis reaction can also be predicted using the stoichiometric model. To do so an elemental balance on hydrogen around the ponds is performed with following assumptions:
-
The hydrogen content of the nitrogen and phosphorus containing compounds in the wastewater and in the makeup nutrient is negligible
-
Sufficient water is oxidized in the photosynthesis reactions to exactly satisfy the balance around the ponds
Having these assumptions the following equations can be written:
where \({H}_{TOT}\) is total amount of hydrogen to be incorporated in to microalgae [kg/day], \({H}_{RXN}\) is amount of hydrogen which is obtained from the water reaction in the photosynthesis process [kg/day].
The \({H}_{RXN}\) is obtained from Eq. (2.16) and substituted in Eq. (2.17).
2.2.4.1 Water balance model around the cultivation step to predict water requirements
The mass balance around the cultivation step is completed by performing a water balance around the ponds (Fig. 3). \({Q}_{WW}\) is the flow rate of the primary treated water, \({Q}_{EVAP}\) is estimated using the evaporation rate given in Table 3 and the total area of the ponds which is calculated in Eq. (2.13), and \({Q}_{RXN}\) is obtained from the stoichiometric model in Sect. 2.2.4.1. \({Q}_{BIOMASS}\), \({Q}_{EFF,}\) and \({Q}_{FIL}\) are known since the concentrations of the biomass are known at the corresponding points. Hence the unknowns: \({q}_{SUPER}\), \({q}_{RECY}\), and \({q}_{blowdown}\) can be calculated (Eqs. (2.18–2.20).
where \({q}_{blowdown}\) is the blow down water [m3/day], \({Q}_{WW}\) is the primary treated water [m3/day], \({Q}_{EVAP}\) is the water lost by evaporation from the pond [m3/day], \({Q}_{RXN}\) is the water lost during reaction [m3/day], \({Q}_{BIOMASS}\) is the water changed to microalgal biomass [m3/day], \({Q}_{FILT}\) is the water going with the filtrate [m3/day], \({q}_{super}\) is the water going with the supernatant [m3/day], \({Q}_{EFF}\) is the water in the effluent coming out from the ponds [m3/day], \({q}_{recy}\) is the recycled water from the filtration unit to the ponds [m3/day].
2.2.4.2 Energy requirements for mixing paddle wheel, flue gas injection, and wastewater pumping
The energy requirements are determined by using Eqs. (2.21) to (2.24). The units of \({A}_{TOT}\),\({Q}_{WW}\), and \({Q}_{EFF}\), \({{CO}_{2}}_{FED}\) should be in ha, L/day, L/day, and kg/day respectively, and their values are known from our previous assumptions and calculations.
where \({E}_{PW}\) is total energy requirement by the paddle wheel [kW/day], \({C}_{PW}\) is energy requirement by paddle wheel [kW/ha] (See Table 3), \({E}_{PP}\) is total energy requirement for pumping primary treated wastewater to cultivation ponds [kWh/day], \({C}_{PP}\) is energy requirement for pumping primary treated wastewater to cultivation ponds [kWh/L] (See Table 3), \({E}_{PF}\) is total energy requirement for pumping effluents from ponds [kWh/day], \({C}_{PF}\) is energy requirement for pumping effluents from ponds [kWh/L] (See Table 3), \({E}_{FI}\) is total energy requirement for flue gas injection to ponds [kWh/day], \({C}_{FI}\) is energy requirement for flue gas injection to ponds [kWh/kg-CO2] (See Table 3).
2.2.5 Microalgal harvesting
After cultivation the microalgal concentration is assumed to be 0.5 g/LWW (0.05% wt.) [44], 11. It requires to be concentrated in the harvesting process which involves three steps; auto-flocculation, dissolved air flotation (DAF), and centrifugation [44]. The key assumptions for the harvesting units are shown in Table 4.
2.2.5.1 Mass balance model equations for the microalgal biomass harvesting
The schematic of the harvesting step is displayed by Fig. 4. The mass balance on microalgae is modelled as shown by Eqs. (2.25) to (2.27). From these equations the mass flow rate of biomass in all dilute, concentrate and feed stream can be calculated.
where \({M}_{{F}_{n}}\) is mass flow rate of microalgae in the feed stream to the harvesting operation’n’ [kg/day], \({M}_{{F}_{n+1}}\) is mass flow rate of microalgae in the feed stream to the harvesting operation’n + 1′ [kg/day], \({M}_{{C}_{n}}\) is mass flow rate of microalgae in the more concentrated exiting stream from the harvesting operation’n’ [kg/day], \({M}_{{D}_{n}}\) is mass flow rate of microalgae in the more dilute exiting stream from the harvesting operation’n’ [kg/day], \({r}_{n}\) is microalgal recovery rate for the harvesting operation’n’ [kg/kg].
The mass flowrate of water in each concentrate stream is calculated from the following equations.
where \({f}_{n}\) is mass fraction of microalgae in concentrate stream, \({C}_{n}\) [kg/kg]; and, \({W}_{{C}_{n}}\) is mass flow rate of water in concentrate stream [kg/day].
By rearranging Eq. (2.28) can be written as:
Then flow rate of water in the dilute stream can be calculated from the following relations:
Since the mass concentration of biomass in the effluent from the ponds is specified, \({W}_{{F}_{1}}\) is known. Thus \({W}_{{D}_{1}}\) can be calculated. It is also possible to calculate the flow rate of every concentrate and dilute stream by using the following equation:
Then the mass flowrates are converted to volumetric flowrates using the following relations:
where \({Q}_{{D}_{n}}\) = volumetric flowrate of dilute stream \({D}_{n}\) [m3/day], \(\uprho\)= is the density of the water [kg/m3].
2.2.5.2 Energy requirements in the harvesting step
In general microalgae in culture are relatively dilute- most of the water must be removed before the microalgae can be processed into fuel. In the present study, the energy requirement in the harvesting step is estimated by using Eqs. 2.33, 2.34 and 2.35.
where \({E}_{DAF}\) is total energy requirement for the DAF [kWh], \({C}_{DAF}\) is energy requirement for the DAF [kWh/m3] (See Table 4), \({Q}_{wDAF}\) is amount of water removed in the DAF [m3], \({E}_{cent}\) is total energy requirement for the cent [kWh], \({C}_{cent}\) is energy requirement for the centrifugation [kWh/m3] (See Table 4), \({Q}_{wcent}\) is amount of water removed in the centrifugation [m3], \({E}_{filt}\) is total energy requirement for the filtration [kWh], \({C}_{filt}\) is energy requirement for the filtration [kWh/kg-algae] (See Table 4), \({M}_{algaefilt}\) is amount of algae removed in the filtration [kg].
2.2.6 Evaluation of the integrated process
The integrated process was evaluated with regard to product output, energy requirement, and pollution reduction. Material and energy balances were performed using a spread sheet to determine the material and energy flows in the process.
3 Results and discussion
3.1 Microalgae cultivation in the wastewater
The growth of the microalgae in the wastewater, the reductions in BOD5 and COD in the wastewater by the microalgae, and the utilizations of the TN, and TP under different treatments have been studied. Biomass yield increased sharply up to the 16th day and then decreased due to the depletion of the nitrogen in the media (Fig. 5). It was also observed that biomass productivity significantly increased in the nutrient supplemented media by 200 mg/L h, proving the deficit of nitrogen in the wastewater. The percentage reductions in BOD5 and COD increased in the nutrient supplemented media (Fig. 6). Percentage reductions in BOD5 and COD increased respectively by 4.5% and 4% when the media was supplemented with nutrient. Increase in reductions of BOD5 and COD possibly attributes to the increase in the biomass due to the addition of nutrient to the media. The increase in biomass in turn increased the rate of production of oxygen during the photosynthesis which eventually decreased the BOD5 and COD in the wastewater. The depletion of phosphorus in the media increased by about 25% when the media was supplemented with the nutrient. This is due to the increase in biomass which requires more phosphorus (Fig. 7).
Percentage reductions of COD, BOD5, TN, and TP in the wastewater were 75, 71, 79, and 63% respectively. In other previous studies on the cultivation of microalgae (C. vulgaris) in industrial wastewater (brewery effluent) removal of 88% BOD, 82% TN and 54% TP has been reported [36]. The experimental values of the percentage reductions in BOD5 (75%), and COD (71%) were used in the modelling. The experimental removal efficiencies of both nitrogen (79%) and phosphorus (63%) (Fig. 8) were modified before used in the modelling. As the wastewater from the sugar factory would pass through a primary treatment plant, it is expected that total solids, BOD5, and COD decrease while photosynthesis increases in the ponds. Hence the above experimental values for the consumptions of nitrogen and phosphorus were modified; a loss of 5% of total nitrogen [18],Carter et al., 2012) and a loss of 20% of phosphorus was supposed in the ponds [31].
3.2 Evaluation of the coupled process
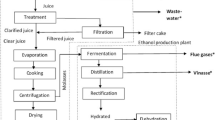
The coupled process is shown in Fig. 9. The wastewater from the sugar factory is treated in the primary wastewater treatment plant and then sent to the ponds where the microalgae are cultivated. The treated wastewater is used as a source of nutrients. The nitrogen deficit is supplied by the makeup nutrient. Photo bioreactor (PBR) is used to grow the selected algae strain before being transferred to the open pond so that the risk of contamination would be reduced. The CO2 is supplied from the sugar factory; its amount is based on the nutrient supply. The main product from the process is the algal biomass from the harvesting section. The algae lost due to the inefficiency of the harvesting is recovered by filtration. As it is found from the harvesting model no recycle water is required, hence, the whole water from the filtration unit is blown down. The sludge from the primary water treatment can be used for a production biogas in a downstream process.
The product outputs were estimated using a spread sheet by material and energy balance approach (Fig. A.1 of online Appendix) while the wastewater after treatment in the primary treatment plant and the pond is compared with a standard in order to identify whether it meets the criteria or not.
3.2.1 Microalgae cultivation in the pond
As presented in Sect. 2 and shown in Fig. 9 the Metahara sugar factory is coupled with conceptual microalgae cultivation. The wastewater from the sugar factory is assumed to be used as nitrogen and phosphorus sources, and the makeup nutrients would be used to avoid deficit. Based on underlying assumptions presented in Sect. 2 using spread sheet and material and energy balance approach the cultivation model gives the results as shown in Table 5 for the fundamental parameters including the nutrients flow, algae biomass output, algae concentration, and CO2 requirement.
The N:P mass ratio in microalgae can vary from 4:1 to 40:1 [5]. For example in a modelling study, Lardon, 2009 has used N:P ratio of 4.54:1 as low nitrogen content (nitrogen starvation condition) for C. vulgaris [27]. It has been reported that cultivation under nitrogen starvation conditions leads to a marked increase in the oil/lipid content (70–85% of dry weight), depending on the microalgae species utilized [33]. On the other hand, nitrogen limitation to increase intracellular lipid content may have negative effects on the cell growth and lipid productivity on some microalgae strains (Hsieh & Wu, 2009). Commonly nitrogen to phosphorus ratio of 15–16:1 in microalgae composition (mass ratio of 6.8–7.25:1) is taken to be the appropriate composition for the proper growth of most of the microalgal species [13]. As shown in Table 5 the cultivation model gave N:P mass ratio of 1.62:1 in the wastewater which is below the minimum ratio 4:1 and thus less than the optimum ratio. 6.8:1 is considered in this study. The experimental result for this study proves that the nutrient supplemented wastewater gave better result for the biomass productivity and phosphorus uptake.
Therefore, it is required to use a makeup nitrogen in order to meet the optimum ratio, as it is shown in the cultivation model. A makeup nitrogen (N) of 7.91 tons/year is required. Either ammonia or nitrate can be used as nitrogen sources. Since ammonia is lost from the growth media due to volatilization [3], nitrate could be better used as a nitrogen source.
From the cultivation model, it is also estimated that the total CO2 required for the microalgae cultivation is 231.3 tons/year. The flue gases either from the sugar factory or from ethanol factory can provide this CO2. If the whole flue gas had been considered to be used for microalgae cultivation it would have a potential of producing 136,850 tons/year (huge amount) but with a need of meeting the nutrient deficit not provided by the wastewater from the sugar factory. The net CO2 reduced in the process would be equal to the amount required by the algae in the ponds, which is 231.3 tons/year.
The cultivation model result gives that the total pond area for the microalgae cultivation is 1.93 ha, which is only 0.02% of the total land required for sugarcane cultivation.
As could be observed from Table 6, in the wastewater treatment (WWT) model, the wastewater from the sugar factory after primary treatment meets the requirement by Ethiopian Environmental Authority standards with respect to all the determined parameters except TSS [16] and could be discharged to the land. The wastewater after algae treatment meets the requirements for discharge in land for TN and TP. It does not meet for COD, BOD5, TSS and oil and grease. This implies that the algae treatment should be employed after biological treatment step, where activated sludge oxidizes the organic matter. Since the treated water meet the requirement to discharge to land, it could be taken that the algae have potential for bioremediation along with biomass growth. Similar analysis on environmental mitigation by integration of an existing sugarcane processing factory with microalgal production in Brazil shows that the life cycle greenhouse gas emissions could be improved by 10% [40].
The result of the cultivation model on energy requirement shows (Table 7) that mixing is the most energy intensive unit operation followed by pumping for harvesting; mixing shares 49% of the total energy demand (Fig. 10). It has been reported that one important challenge in mixing is that it would require a substantial amount of energy input to improve mixing so as to avoid poor mixing [7]. However there are improvements in the design of raceway ponds which minimizes energy consumption while achieving sufficient mixing to prevent sedimentation and dead zones [23, 24].
3.2.2 Microalgae harvesting
It is assumed that the microalgae suspension from the pond would go to auto-flocculate and then it would successively pass through DAF and centrifugation units. In the DAF, it is assumed that biodegradable flocculant, chitosan, would be used to help flocculation. Also, after dewatering, the remaining microalgae in water is assumed to be completely removed using filter. Based on the underlying assumptions given in Table 4 the main results from modelling of the different harvesting process steps are given in Table 8.
With the biomass concentration of 250 g algae/LWW (0.25 g algae/g wastewater), dry content of microalgae in the centrifugation step amounted to 98.14 tons/year (Table 8). And the corresponding water content in the microalgae was 400 m3/year. In the filtration unit, 22.61 tons/year of unrecovered algae due to inefficiencies are recovered. In the harvesting model the most energy intensive operation is found to be the centrifugation step; about 67% of the energy requirement by the harvesting process is required by the centrifugation section. It has been recommended that harvesting cost can be substantially reduced using other approaches, such as flocculation [42], as considered in the present study.
Also it needs to consider that there is 15.5% (Table A.1) excess bagasse of the total bagasse produced in the sugar factory, which is equal to 27,125 tons/year, as shown in Table 5. This bagasse has an energy content of 7893 BTU/lb, implying that it would have a potential of 14,611 MWh/year energy production. Thus the use of the excess bagasse for energy production in the process integration is significant.
4 Conclusion
Wastewater effluents and flue gases from the sugarcane processing factory have a potential to produce algal biomass which can be used for biofuel production along with an advantage of pollution reduction, including CO2 emission reduction and wastewater effluent reduction. It has been found that the nitrogen to phosphorus mass ratio in the wastewater is too low to be used sufficiently without supply of makeup nutrient. Consequently, in the coupling addition of appropriate nutrient to avoid nitrogen deficit would be required. The CO2 in the flue gases from the factory has more potential to produce algal biomass than the nutrients in the wastewater effluents. Since there are several huge factories and ongoing projects for the production of sugar and ethanol in Ethiopia, microalgae cultivation by coupling with such factories would be important so as to both increase profitability and reduce environmental pollution.
The present study has investigated the importance of the process integration for both environmental foot print and cost reduction by using the inexpressive waste resources. It can also contribute a lot by opening the way for further integrated studies including improving of algal strain and finding energy and material efficient alternative processing technologies. This way the future possible bio-refinery and environmental pollution reduction approach could be well investigated to decide the provision of such activities.
References
ANL, NREL, PNNL (2012) Renewable diesel from algal lipids: an integrated baseline for cost, emissions, and resource potential from a harmonized model. (No. ANL/ESD/12–4; NREL/TP-5100–55431; PNNL-21437). National Renewable Energy Laboratory. (NREL); Golden, CO (United States)
Asmare AM, Demessie BA, Murthy GS (2013) Theoretical estimation the potential of algal biomass for biofuel production and carbon sequestration in ethiopia. Int J Renew Energy Res 3(3):560–570. https://doi.org/10.20508/ijrer.50650
Borowitzka MA (2013) Dunaliella: biology, production, and markets. Handbook of microalgal culture: applied phycology and biotechnology, 2nd edn. Wiley, Oxford. https://doi.org/10.1002/9781118567166.ch18
Bridgewater LL (ed) (2017) Standard Methods for the examination of water and waste water, 23rd edn. APHA, AWWA and WEF, Washington
Broberg S, Andersson V, Hackl R (2011) Integrated algae cultivation for biofuels production in industrial clusters. Linköping University Electronic Press
Chagas MF, Cavalett O, Klein BC, Filho RM, Bonomi A (2016) Life cycle assessment of technologies for greenhouse gas emissions reduction in sugarcane biorefineries. Chem Eng Trans 50:421–426. https://doi.org/10.3303/CET1650071
Chiaramonti D, Prussi M, Casini D, Tredici MR, Rodolfi L, Bassi N, Zittelli GC, Bondioli P (2013) Review of energy balance in raceway ponds for microalgae cultivation: re-thinking a traditional system is possible. Appl Energy 102:101–111. https://doi.org/10.1016/j.apenergy.2012.07.040
Chinnasamy S, Bhatnagar A, Hunt RW, Das KC (2010) Microalgae cultivation in a wastewater dominated by carpet mill effluents for biofuel applications. Biores Technol 101(9):3097–3105. https://doi.org/10.1016/j.biortech.2009.12.026
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25(3):294–306. https://doi.org/10.1016/j.biotechadv.2007.02.001
Colling Klein B, Bonomi A, Maciel Filho R (2018) Integration of microalgae production with industrial biofuel facilities: a critical review. Renew Sustain Energy Rev 82:1376–1392. https://doi.org/10.1016/j.rser.2017.04.063
Craggs RJ, Heubeck S, Lundquist TJ, Benemann JR (2011) Algal biofuels from wastewater treatment high rate algal ponds. Water Sci Technol 63(4):660–665. https://doi.org/10.2166/wst.2011.100
Davis R, Markham J, Kinchin C, Grundl N, Tan E, Humbird D (2016) Process design and economics for the production of algal biomass: algal biomass production in open pond systems and processing through dewatering for downstream conversion. Natl Renew Energy Lab. https://doi.org/10.2172/1239893
Davis R, Aden A, Pienkos PT (2011) Techno-economic analysis of autotrophic microalgae for fuel production. Appl Energy 88(10):3524–3531. https://doi.org/10.1016/j.apenergy.2011.04.018
Delrue F, Setier PA, Sahut C, Cournac L, Roubaud A, Peltier G, Froment AK (2012) An economic, sustainability, and energetic model of biodiesel production from microalgae. Biores Technol 111:191–200. https://doi.org/10.1016/j.biortech.2012.02.020
Di Caprio F, Altimari P, Pagnanelli F (2018) Integrated microalgae biomass production and olive mill wastewater biodegradation: optimization of the wastewater supply strategy. Chem Eng J 349(March):539–546. https://doi.org/10.1016/j.cej.2018.05.084
EEAS (2003) Environmental protection authority and UN Industrial Development Organization. Provisional standards for industrial pollution control in Ethiopia
Ethiop Sugar Corporation (2019) Retrieved July 20, 2011, from http://www.etsugar.gov.et/index.php/en/
Fagerstone KD, Quinn JC, Bradley TH, De Long SK, Marchese AJ (2011) Quantitative measurement of direct nitrous oxide emissions from microalgae cultivation. Environ Sci Technol 45(21):9449–9456. https://doi.org/10.1021/es202573f
Ferree MA, Shannon RD (2001) Evaluation of a second derivative UV/visible spectroscopy technique for nitrate and total nitrogen analysis of wastewater samples. Water Res 35(1):327–332. https://doi.org/10.1016/S0043-1354(00)00222-0
Frank ED, Han J, Palou-Rivera I, Elgowainy A, Wang MQ (2011) Life-cycle analysis of algal lipid fuels with the GREET model. Center for Transportation Research, Energy Systems Division, Argonne National Laboratory, Oak Ridge, 11-5
Grima EM, Acie FG, Medina AR, Chisti Y (2003) Recovery of microalgal biomass and metabolites process option and economics.pdf. Biotechnol Adv 20:491–515
Guldhe A, Ansari FA, Singh P, Bux F (2017) Heterotrophic cultivation of microalgae using aquaculture wastewater: a biorefinery concept for biomass production and nutrient remediation. Ecol Eng 99:47–53. https://doi.org/10.1016/j.ecoleng.2016.11.013
Hadiyanto H, Elmore S, Van Gerven T, Stankiewicz A (2013) Hydrodynamic evaluations in high rate algae pond (HRAP) design. Chem Eng J 217:231–239. https://doi.org/10.1016/j.cej.2012.12.015
Huang J, Qu X, Wan M, Ying J, Li Y, Zhu F, Wang J, Shen G, Chen J, Li W (2015) Investigation on the performance of raceway ponds with internal structures by the means of CFD simulations and experiments. Algal Res 10:64–71. https://doi.org/10.1016/j.algal.2015.04.012
Hwang JH, Church J, Lee SJ, Park J, Lee WH (2016) Use of microalgae for advanced wastewater treatment and sustainable bioenergy generation. Environ Eng Sci 33(11):882–897. https://doi.org/10.1089/ees.2016.0132
Kadam KL (2001) Microalgae production from power plant flue gas: environmental implications on a life cycle basis. Contract. https://doi.org/10.2172/783405
Lardon L, Hélias A, Sialve B, Steyer JP, Bernard O (2009) Life-cycle assessment of biodiesel production from microalgae. Environ Sci Technol 43(17):6475–6481. https://doi.org/10.1021/es900705j
Laurens LML (2017) State of technology review—algae bioenergy. IEA Bioenergy. https://doi.org/10.1017/S0959270900002288
Liu X, Saydah B, Eranki P, Colosi LM, Greg Mitchell B, Rhodes J, Clarens AF (2013) Pilot-scale data provide enhanced estimates of the life cycle energy and emissions profile of algae biofuels produced via hydrothermal liquefaction. Bioresour Technol 148:163–171. https://doi.org/10.1016/j.biortech.2013.08.112
Lohrey C, Kochergin V (2012) Biodiesel production from microalgae: Co-location with sugar mills. Bioresour Technol 108:76–82. https://doi.org/10.1016/j.biortech.2011.12.035
Lundquist TJ, Woertz IC, Quinn NWT, & Benemann JR (2010) A realistic technology and engineering assessment of algae biofuel production. Energy (Vol. October). California
Lyon SR, Ahmadzadeh H, Murry MA (2015) Algae-based wastewater treatment for biofuel production: processes, species, and extraction methods. Biomass and biofuels from microalgae, vol 2. Springer International Publishing, Berlin, pp 95–115. https://doi.org/10.1007/978-3-319-16640-7
Mandal S, Mallick N (2009) Microalga Scenedesmus obliquus as a potential source for biodiesel production. Appl Microbiol Biotechnol 84(2):281–291. https://doi.org/10.1007/s00253-009-1935-6
Maranduba HL, Robra S, Nascimento IA, da Cruz RS, Rodrigues LB, de Almeida Neto JA (2016) Improving the energy balance of microalgae biodiesel: synergy with an autonomous sugarcane ethanol distillery. Energy 115(2016):888–895. https://doi.org/10.1016/j.energy.2016.09.061
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Renew Sustain Energy Rev 14(1):217–232. https://doi.org/10.1016/j.rser.2009.07.020
Mohsenpour SF, Hennige S, Willoughby N, Adeloye A, Gutierrez T (2020) Integrating micro-algae into wastewater treatment: A review. Sci Total Environ 752((Sept 2020)):142168. https://doi.org/10.1016/j.scitotenv.2020.142168
Moncada J, Tamayo JA, Cardona CA (2014) Integrating first, second, and third generation biorefineries: incorporating microalgae into the sugarcane biorefinery. Chem Eng Sci 118:126–140. https://doi.org/10.1016/j.ces.2014.07.035
Nappa M, Karinen P, & Hytönen E (2015) Producing lipids, biogas and fertilizer from microalgae-conceptual design and techno-economic analysis. Carbon Capture and Storage Program (CCSP): Deliverable D605
Shelef G, Sukenik A, & Green M Ganesh Saratale R, Kumar G, Banu R, Xia A, Periyasamy S, Dattatraya Saratale G (1984) A critical review on anaerobic digestion of microalgae and macroalgae and co-digestion of biomass for enhanced methane generation. Bioresour. Technol. 262, 3. Technion Research and Development Foundation ltd. Haifa, Israel (No. XK-3-03031-01). https://doi.org/10.2172/6204677
Souza SP, Gopal AR, Seabra JEA (2015) Life cycle assessment of biofuels from an integrated Brazilian algae-sugarcane biorefinery. Energy 81:373–381. https://doi.org/10.1016/j.energy.2014.12.050
Strickland JDH, & Parsons TR (1972). A practical handbook of seawater analysis. Bulletin 167. Fisheries Research Board of Canada Bulletin (2nd ed., vol. 167)
Vandamme D, Foubert I, Muylaert K (2013) Flocculation as a low-cost method for harvesting microalgae for bulk biomass production. Trends Biotechnol 31(4):233–239. https://doi.org/10.1016/j.tibtech.2012.12.005
Wiley PE, Campbell JE, McKuin B (2011) Production of biodiesel and biogas from algae: a review of process train options. Water Environ Res 83(4):326–338. https://doi.org/10.2175/106143010x12780288628615
Zewdie DT, Ali AY (2020) Cultivation of microalgae for biofuel production: coupling with sugarcane-processing factories. Energy Sustain Soc 10(1):1–16. https://doi.org/10.1186/s13705-020-00262-5
Acknowledgements
The authors would like to acknowledge the financial support from Addis Ababa University and Debre Tabor University. We are also thankful to Ethiopian Sugar Corporation and Metahara sugar factory experts for their important inputs and providing of the necessary information.
Author information
Authors and Affiliations
Contributions
The first author (DTZ) contributed to the conceptualization, data analysis, and drafting of the first version of the manuscript. Both authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zewdie, D.T., Ali, A.Y. Utilization of sugarcane factories’ wastes as inexpensive source of nutrients and CO2 for microalgal biomass production: process coupling and potential evaluation. SN Appl. Sci. 3, 297 (2021). https://doi.org/10.1007/s42452-021-04311-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-021-04311-2